Abstract
Background:
Hispanics are the largest minority population in the United States (18%). They represent a heterogeneous and growing population. Cancer is the leading cause of death among Hispanics, yet few studies have described cancer mortality burden by specific Hispanic group nationwide.
Methods:
Cancer-related deaths from U.S. death certificates for the years 2003-2012 were analyzed for decedents identifying as Mexican, Puerto Rican, Cuban, and Central or South American. We calculated descriptive statistics including potential years of lives lost (PYLL), age-adjusted rates, standardized mortality ratios, and fitted JoinPoint regression models to evaluate annual trends by Hispanic group, using non-Hispanic Whites (NHWs) as the reference population.
Results:
We identified 287,218 cancer deaths among Hispanics and 4,570,559 among NHWs. Mortality trends were heterogeneous across Hispanic groups. Female NHWs and male Puerto Ricans had the greatest rates of adjusted PYLL per 1000 (NHWs, 19.6; Puerto Ricans, 16.5). Liver cancer was ranked among the top 5 cancer-related deaths for every Hispanic group, but not for NHWs. Stomach cancer mortality was twice as high for most Hispanic groups when compared to NHWs and especially high for Mexicans (male SMR, 2.07; 95% CI, 2.01-2.13; female SMR, 2.62; 95% CI, 2.53-2.71)
Conclusion:
We observed marked heterogeneity in cancer mortality across Hispanic groups. Several cancers affect Hispanics disproportionately compared to NHWs. Screening programs in Hispanics should be considered for stomach and liver cancer.
Impact:
Disaggregated analysis of Hispanics is needed to fully understand cancer burden among the diverse Hispanic population and is critical for cancer prevention and control efforts.
Keywords: epidemiology, cancer, vital statistics, Hispanic American, ethnic disparities
INTRODUCTION
The Hispanic population is the largest minority group in the United States and is rapidly growing[1]. 18% of the U.S. identified as Hispanic in 2016 and the population increased by 57% between 2000 and 2014[1, 2]. The rapid growth of the Hispanic population makes it essential to accurately assess health problems and uncover health disparities that affect this population. Cancer incidence and mortality appear to be steadily decreasing among the Hispanic population[3]. Factors such as acculturation, diet, physical activity, alcohol consumption, and smoking all affect the cancer burden in Hispanics[3]. Relative to the non-Hispanic white population, the Hispanic population in the U.S. is also disproportionately affected by lower income, education, and access to health care[4]. Language barriers, low health insurance coverage, low income, and unfamiliarity with dealing with insurance providers have all been shown to be obstacles of cancer screenings in the U.S.[4] In most studies, Hispanics are aggregated into a single group because Hispanics share a similar migration history, have a common language, and share many values[5]. However, specific Hispanic groups can vary significantly via genetic, cultural, behavioral, geographic, and socioeconomic factors[6-9]. For example, the poverty rate for aggregated Hispanics is 25%, but ranges from 27% for Mexicans to 18% in Cubans[10]. Behaviors such as alcohol intake also differ by Hispanic group. A recent survey found that Cuban males report drinking 8.4 drinks a week while Puerto Rican males drink 16.9 drinks a week[11]. Due to this heterogeneity in health behaviors among Hispanics, aggregation may mask important differences among specific Hispanic groups. Furthermore, studies in Asian populations have shown that disaggregated cancer statistics are essential to understanding cancer burden in a population[9, 12].
The Hispanic population presents a unique opportunity to study cancer burden because they share similar immigration experiences and have cultural commonalities, but also have key behavioral differences between them that can highlight the severity of important cancer risk factors[5]. While few studies of this kind have been done, they have shown that cancer burden is heterogeneous across Hispanic groups in specific states such as Florida[13], and California[25]. However, none of the existing studies report mortality trends over time by disaggregated Hispanic ethnicity. The goal of this paper is to report specific Hispanic cancer mortality rates and 10-year mortality trends for the first time at a national level.
MATERIALS AND METHODS
We obtained death certificate information (cause of death, age of death, sex, and race/ethnicity) from the National Center for Health Statistic (NCHS) from 2003–2012. All cancer deaths from this time period, for all ages, were identified by the “Underlying cause of death” which was coded by NCHS using International Classification of Diseases, 10th revision (ICD-10; specific codes provided in Supplementary Table 1). We chose 10 cancer sites based on their overall contribution to Hispanic mortality burden: lung, female breast, liver, colorectal, prostate, pancreas, ovary, stomach, leukemia, and Non-Hodgkin Lymphoma (NHL)[3] . Statistics for “all cancer sites” include all other cancer sites in addition to the top 10.
The study population included 243,777 Hispanic decedents, of all races, who were identified on their death certificate as Mexican, Puerto Rican, Cuban, and Central or South American and 4,579,559 non-Hispanic White (NHW) decedents who were included as the reference group. Statistics calculated for the “All Hispanic” group (N=287,218) are an aggregate of the four specific Hispanic groups as well as those who identified as “Other Hispanic”.
Statistical Analysis
Annual population denominator counts were estimated using linear interpolation and extrapolation based on age-specific population data from the 2000 and 2010 U.S. Census. Annual counts were then summed to determine total person-years for 10-year study period. Using decedent counts by sex, Hispanic group and cancer site, we estimated potential years of lives lost (PYLL), proportional cancer mortality, and age-adjusted mortality rates (AMRs). We then fitted regression models for trends in annual cancer-related mortality over the 10-year period also by sex, Hispanic group, and cancer site.
PYLL measures the impact of cancer on the potential duration of life that individuals of the given population should have, on average, in absence of death from the index cause (i.e., site-specific cancer). We calculated PYLL with respect to a fixed age limit, based on benchmarks for life expectancy of NHWs and Hispanics obtained from a report from the Center for Disease Control (CDC)[14]. For NHWs, age 81 was used as a benchmark for females, and age 76 for males. For Hispanics, age 83 was used for females, and 78 for males. The PYLL per sex and Hispanic group (for all cancer deaths) was calculated as:
where h is the fixed age limit (as defined above) and ax is the average age of death in each 5-year age group, and dx is the total number of deaths in the 5-year age group [15]. We report the crude PYLL as a rate per 1,000 person-years, where the denominator is the size of the underlying population that gave rise to the premature deaths and we also standardized this rate to the 2000 U.S. population age distribution to account for differing age distributions of the study populations.
Proportional cancer mortality, AMR, and SMR were calculated and stratified by cancer site, sex, and Hispanic group. The site-specific proportion of all cancer deaths was used to determine proportional cancer mortality. AMRs and 95% confidence intervals were calculated as deaths per 100,000 for the duration of the study period and directly standardized to the age distribution of the 2000 U.S. population. AMRs are comparable across Hispanic groups and cancer sites. SMRs were indirectly standardized by dividing the stratum-specific deaths in by the expected number of deaths (i.e., the death count for the NHW reference population). SMRs can only be compared within groups, not across groups, because they are not re-weighted by a standard distribution.
We used Joinpoint regression analysis to model cancer-related mortality trends [16]. Joinpoint analysis is used to determine if multiple “best fit” line segments of differing rates, created utilizing a Monte Carlo permutation method, are better at describing a trend rather than a single-best fit line segment. If a significant change in trend is detected (P≤0.05) a “joinpoint” is created that connects the segments of differing rates and signifies the year that the change in trend occurred. A maximum of one joinpoint was allowed due to the limited time period examined in this analysis, meaning one cancer mortality trend is limited to two-line segments if a significant change in trend occurred over the 10-year period. The estimated annual percent change (APC) was used to test the statistical significance of trends after line segments were established. PROC STDRATE in SAS version 9.4 (SAS Institute) were used to calculate direct AMRs, indirect SMRs, and adjusted PYLL rates. Trend models were fit using SEER JoinPoint software [17] and all figures were created using Microsoft Excel.
RESULTS
Top Cancer Sites
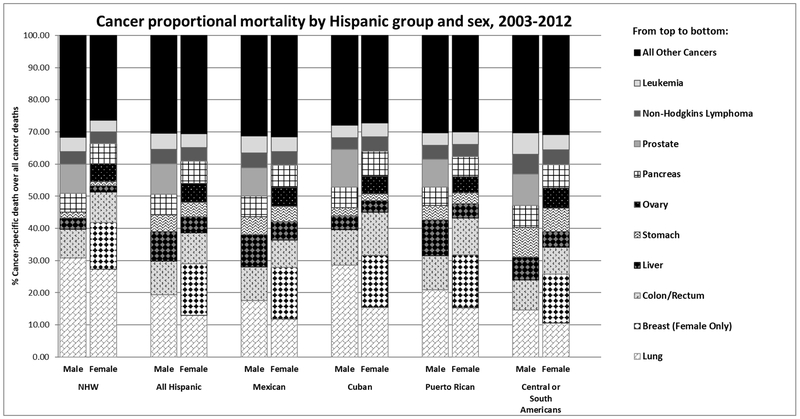
The top sites of cancer deaths ranked as proportion of all cancers (Table 1; Figure 1) among NHW males were: lung (30.8%), prostrate (9.2%), colorectal (8.9%), pancreas (5.9%), and leukemia (4.4%). Our disaggregated analysis of Hispanic cancer mortality revealed heterogeneity among Hispanic males after lung cancer, which was found to be the leading cause of cancer death in all groups. For Mexican males, these rankings were: lung (19.3%), colorectal (10.5%), liver (10.1%), prostate (9.4%), and pancreas (6.4%). For Cuban males: lung (28.6%), prostate (11.6%), colorectal (10.9%), pancreas (6.4%), and liver (4.3%). For Puerto Rican males: lung (20.9%), liver (11.2%), colorectal (10.7%), prostate (8.7%), and pancreas (5.7%). And for Central or South American males: lung (14.7%), prostate (9.9%) colorectal (9.2%), stomach (9.1%), and liver (7.2%).
Table 1:
Male and Female Top 5 cancer mortality sites ranked1 by proportion to all cancers- by ethnicity (2003-2012)
| MALE (RANK) |
NHW (n=2,389,032) |
ALL HISPANIC (N= 151,385) |
MEXICAN (n=83,236) |
CUBAN (N=16,570) |
PUERTO RICAN (N=19,034) |
CENTRAL OR SOUTH AMERICAN (N=10,521) |
|---|---|---|---|---|---|---|
| 1 | Lung 30.8% Prostate 9.2% |
Lung 19.3% Colon 10.5% |
Lung 17.6% Colon 10.4% |
Lung 28.6% Prostate 11.6% |
Lung 20.9% Liver 11.2% |
Lung 14.7% Prostate 9.9% |
| 2 | ||||||
| 3 | Colon 8.9% |
Prostate 9.4% |
Liver 10.1% |
Colon 10.9% |
Colon 10.7% |
Colon 9.2% |
| 4 | Pancreas 5.9% |
Liver 9.1% |
Prostate 8.8% |
Pancreas 6.4% |
Prostate 8.7% |
Stomach 9.1% |
| 5 | Leukemia 4.4% |
Pancreas 6.4% |
Pancreas 6.4% |
Liver 4.3% |
Pancreas 5.7% |
Liver 7.2% |
| FEMALE (RANK) |
NHW (N=2,190,527) |
ALL HISPANIC (n=135,833) |
MEXICAN (N=72,657) |
CUBAN (N=12,559) |
PUERTO RICAN (N=16,327) |
CENTRAL OR SOUTH AMERICAN (N=12,873) |
| 1 | Lung 27.3% |
Breast 15.9% |
Breast 15.9% |
Breast 16.0% |
Breast 16.4% |
Breast 15.1% |
| 2 | Breast 14.6% |
Lung 13% |
Lung 11.9% |
Lung 15.5% |
Lung 15.3% |
Lung 10.6% |
| 3 | Colon 9.4% |
Colon 9.6% |
Colon 8.6% |
Colon 13.4% |
Colon 11.5% |
Colon 8.4% |
| 4 | Pancreas 6.3% |
Pancreas 7.0% |
Pancreas 7.0% |
Pancreas 7.8% |
Pancreas 6.3% |
Pancreas 7.2% |
| 5 | Ovary 5.5% |
Ovary 5.8% |
Ovary 5.9% |
Ovary 5.4% |
Ovary 5.0% |
Stomach 7.1% |
Proportional mortality was determined by dividing site specific cancer deaths by total cancer deaths, by sex and ethnic group.
Figure 1:
Cancer proportional mortality by detailed ethnicity and sex, 2003-2012. Proportional mortality was determined by dividing site specific cancer deaths by total cancer deaths, by sex and ethnicity.
The rankings of top five sites of cancer mortality for NWH females were lung (27.3%), breast (14.6%), colorectal (9.4%), pancreas (6.3%), and ovary (5.5%). In contrast to the male findings, the top 5 sites among Hispanic females were mostly homogenous. The ranking (ranges) were breast (15.1%−16.4%), lung (10.6%−15.5%), colorectal (8.4%−13.4%), pancreas (6.3%−7.8%) and ovary (5.0%−5.8%). One exception was for Central or South American females who had stomach cancer (7.1%) in the fifth ranking rather than ovarian cancer.
Potential Years of Life Lost
Crude and adjusted PYLL’s by sex and Hispanic group are provided in Table 2. NHWs had the highest count of premature cancer deaths and age-adjusted PYLL rates (Male 16.5, Female 19.6). Puerto Ricans and Mexicans had the highest rate of age-adjusted PYLL among female Hispanics (15.7 and 15.1, respectively). Among male Hispanics, Puerto Ricans and Cubans had the highest age-adjusted PYLL rates (Puerto Rican, 16.5; Cuban 15.0).
Table 2:
Potential Years of Lives Lost (PYLL) based on a fixed life expectancy for all cancer deaths by Hispanic group and sex, 2003-2012
| Sex | Group | Premature Cancer Deaths1 |
Crude PYLL Rate per 1,000 person-years2 |
Age-Adjusted PYLL Rate per 1,000 person-years3 |
Average Age of Cancer Death4 |
|---|---|---|---|---|---|
| Female | Non-Hispanic White | 1,444,0801 | 24.7 | 19.6 | 71 |
| Female | Mexican | 64,999 | 10.0 | 15.1 | 64 |
| Female | Cuban | 9,968 | 17.5 | 14.5 | 72 |
| Female | Puerto Rican | 14,425 | 12.8 | 15.7 | 66 |
| Female | Central or South American | 11,420 | 8.5 | 9.6 | 64 |
| Male | Non-Hispanic White | 1,321,012 | 20.3 | 16.5 | 71 |
| Male | Mexican | 66,384 | 8.0 | 12.6 | 65 |
| Male | Cuban | 11,594 | 17.3 | 15.0 | 71 |
| Male | Puerto Rican | 15,702 | 12.3 | 16.5 | 65 |
| Male | Central or South American | 8,806 | 5.9 | 7.6 | 62 |
Count of “premature” cancer deaths is the total number of decedents who died before reaching the age of the fixed life expectancy.
The crude PYLL was determined by using a fixed life expectancy limit to find the difference between age of cancer death and expected age of death. For NHWs, age 81 was used as a benchmark for females, and age 76 for males. For Hispanics, age 83 was used for females, and 78 for males. PYLL’s are calculated as the difference between the age of death and the fixed age expectancy, summed across all age groups and reported as a rate per 1,000 person-years.
Age-adjusted PYLL was determined by directly standardizing the crude PYLL to the age distribution of the 2000 U.S. population.
The average age of cancer death was calculated for each sex within each Hispanic group by summing the product of category median age and age-specific death counts and then dividing by the total death count in that group.
Age-Adjusted Rates and Trend Models
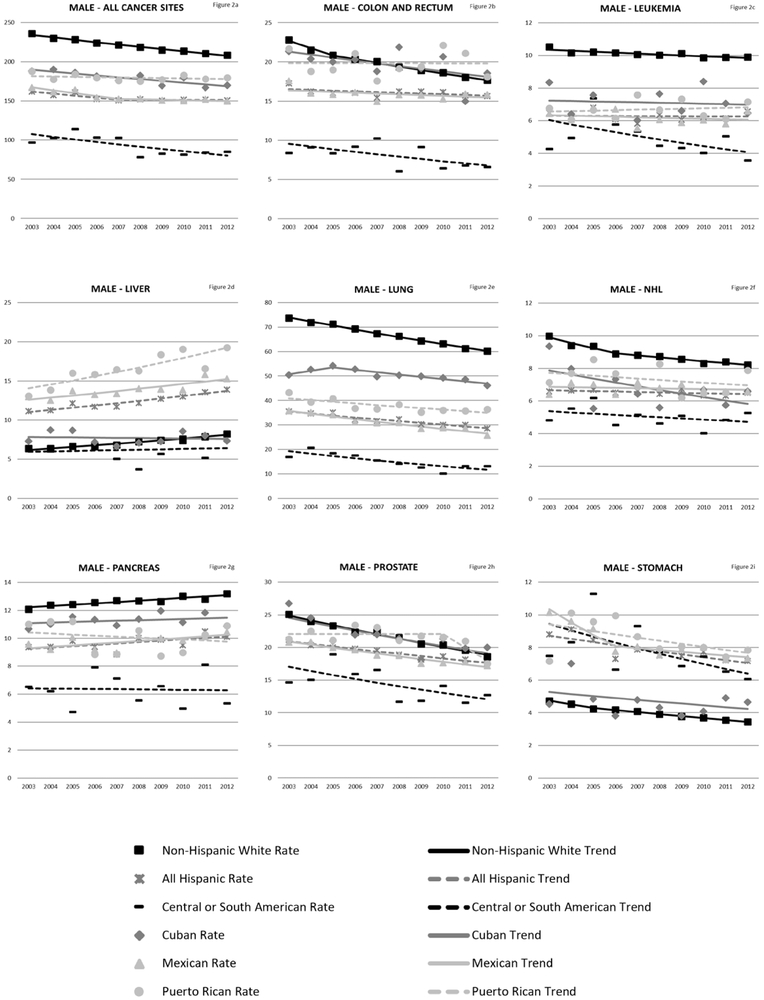
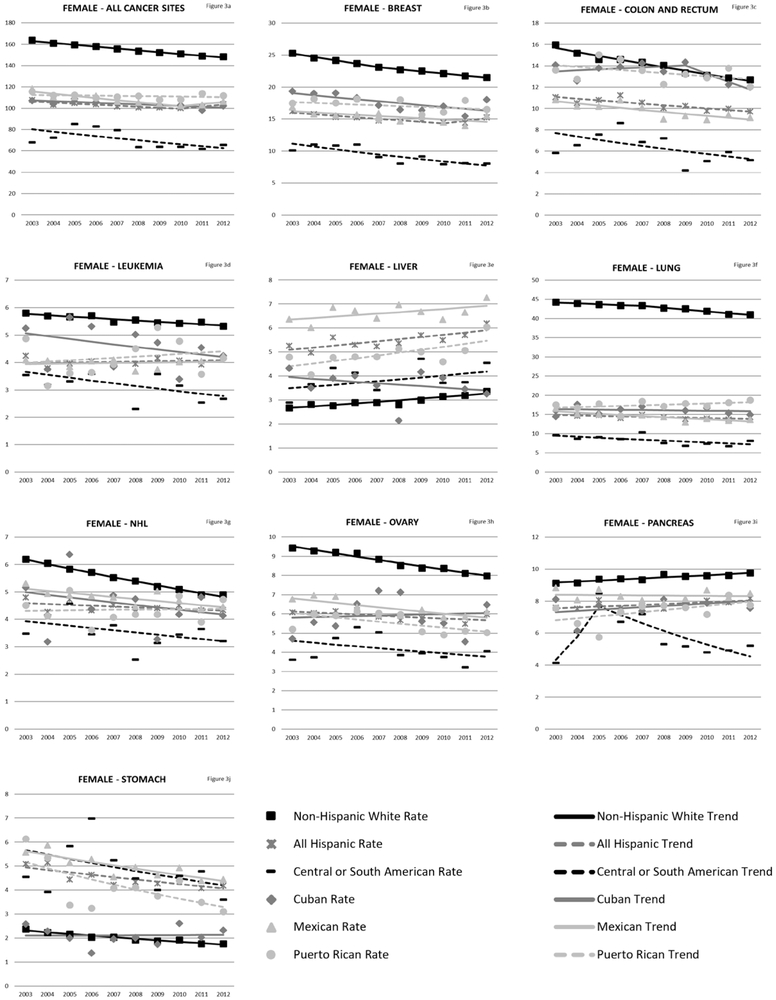
Rates of cancer mortality from any site for NHWs was 220.42/100,000 person-years (males) and 157.33/100,000 person-years (females). Hispanics (as an aggregated group) die from cancer of any site at about 70% the rate of NHWs (Table 3). Among Hispanics, Puerto Ricans (male AMR: 179.73/100,000 person-years 95% CI: 176.92–182.54; female AMR: 111.41/100,000 person-years 95% CI: 109.64–113.17) had the highest overall cancer mortality. Central and South Americans have the lowest overall cancer mortality rates (male AMR: 91.19/100,000 person-years 95% CI: 89.09–93.29; female AMR: 69.72/100,000 person years 95% CI: 68.42–71.01). Trend analyses (Figure 2a-i and Figure 3a-j) revealed that all ethnicity-specific Hispanic mortality rates of cancer at any site are stable or decreasing, but rates of liver cancer are increasing for Hispanics (overall, males and females). Annual percentage change results for the trend models are included in the Supplementary Tables 2 and 3.
Table 3:
Male and Female Hispanic Cancer Statistics by cancer site: age-adjusted average annual mortality rate (AMR) per 100,000 person-years and standardized mortality ratios (SMRs), 2003-2012.
| MALES | NON-HISPANIC WHITE | ALL HISPANIC | MEXICAN | CUBAN | PUERTO RICAN | CENTRAL or SOUTH AMERICAN | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Site | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 |
| All Sites | 2389032 | 220.42 (220.14-220.71) | 1 | 151385 | 153.68 (152.84-154.53) | 0.69 (0.69-0.69) | 83236 | 154.72 (153.55-155.89) | 0.69 (0.69-0.69) | 16570 | 178.62 (175.89-181.36) | 0.81 (0.8-0.82) | 19034 | 179.73 (176.92-182.54) | 0.84 (0.82-0.85) | 10521 | 91.19 (89.09-93.29) | 0.4 (0.39-0.41) |
| Lung | 736913 | 66.69 (66.54-66.85) | 1 | 29246 | 31.73 (31.34-32.12) | 0.44 (0.44-0.45) | 14634 | 30.51 (29.98-31.04) | 0.41 (0.4-0.41) | 4737 | 50.26 (48.82-51.69) | 0.75 (0.73-0.78) | 3969 | 37.59 (36.32-38.85) | 0.57 (0.55-0.59) | 1546 | 14.63 (13.79-15.47) | 0.20 (0.10-0.21) |
| Prostate | 218687 | 21.68 (21.59-21.77) | 1 | 14212 | 19.1 (18.77-19.43) | 0.89 (0.88-0.91) | 7361 | 18.69 (18.25-19.14) | 0.87 (0.85-0.89) | 1929 | 21.51 (20.54-22.47) | 1 (0.95-1.04) | 1651 | 21.32 (20.23-22.42) | 1 (0.95-1.05) | 1038 | 13.95 (13.02-14.88) | 0.64 (0.60-0.68) |
| Colon and rectum | 213040 | 19.74 (19.65-19.82) | 1 | 15892 | 16.06 (15.79-16.34) | 0.81 (0.79-0.82) | 8670 | 15.86 (15.49-16.23) | 0.8 (0.79-0.82) | 1813 | 19.54 (18.64-20.44) | 1 (0.95-1.04) | 2033 | 19.8 (18.85-20.75) | 1 (0.96-1.05) | 969 | 7.81 (7.22-8.41) | 0.41 (0.38-0.44) |
| Pancreas | 139941 | 12.66 (12.59-12.73) | 1 | 9714 | 9.72 (9.51-9.93) | 0.75 (0.74-0.77) | 5355 | 9.81 (9.52-10.1) | 0.76 (0.74-0.78) | 1057 | 11.28 (10.6-11.96) | 0.89 (0.84-0.95) | 1092 | 10.01 (9.36-10.66) | 0.81 (0.76-0.86) | 711 | 6.3 (5.75-6.84) | 0.46 (0.42-0.49) |
| Liver | 81255 | 7.17 (7.12-7.22) | 1 | 13850 | 12.45 (12.22-12.68) | 1.7 (1.67-1.73) | 8397 | 14.01 (13.68-14.34) | 1.86 (1.82-1.9) | 720 | 7.68 (7.12-8.25) | 1.07 (0.99-1.15) | 2123 | 16.71 (15.94-17.48) | 2.51 (2.41-2.62) | 763 | 6.13 (5.62-6.65) | 0.75 (0.69-0.8) |
| Stomach | 43192 | 4 (3.96-4.04) | 1 | 7996 | 7.79 (7.6-7.98) | 1.97 (1.93-2.02) | 4610 | 8.15 (7.89-8.42) | 2.07 (2.01-2.13) | 429 | 4.67 (4.22-5.11) | 1.16 (1.05-1.27) | 834 | 8.41 (7.78-9.03) | 2 (1.86-2.14) | 965 | 7.58 (7-8.16) | 1.97 (1.84-2.09) |
| Leukemia | 105913 | 10.09 (10.03-10.16) | 1 | 7615 | 6.28 (6.12-6.44) | 0.74 (0.72-0.76) | 4462 | 6.19 (5.97-6.41) | 0.78 (0.75-0.8) | 645 | 7.07 (6.52-7.62) | 0.7 (0.65-0.75) | 764 | 6.7 (6.16-7.24) | 0.74 (0.68-0.79) | 712 | 4.81 (4.36-5.27) | 0.6 (0.55-0.64) |
| NHL | 94053 | 8.84 (8.78-8.9) | 1 | 6725 | 6.54 (6.36-6.71) | 0.78 (0.76-0.8) | 3769 | 6.77 (6.52-7.01) | 0.79 (0.77-0.82) | 619 | 6.72 (6.19-7.25) | 0.76 (0.7-0.82) | 817 | 7.31 (6.75-7.86) | 0.92 (0.86-0.99) | 632 | 4.97 (4.49-5.45) | 0.62 (0.57-0.67) |
| FEMALES | NHW | ALL HISPANIC | MEXICAN | CUBAN | PUERTO RICAN | CENTRAL or SOUTH AMERICAN | ||||||||||||
| Site | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 | n | AMR1 (95% CI) | SMR2 |
| All Sites | 183994 | 157.33 (156.59-158.07) | 1 | 38978 | 106.49 (105.39-107.59) | 0.69 (0.68-0.69) | 72657 | 107.33 (106.51-108.15) | 0.7 (0.69-0.7) | 12559 | 103.87 (102.01-105.73) | 0.67 (0.66-0.68) | 16327 | 111.41 (109.64-113.17) | 0.72 (0.71-0.73) | 12873 | 69.715 (68.42-71.01) | 0.44 (0.44-0.45) |
| Breast | 597825 | 23.11 (23.02-23.19) | 1 | 21651 | 15.05 (14.84-15.26) | 0.67 (0.66-0.68) | 11572 | 15.28 (14.99-15.58) | 0.69 (0.68-0.7) | 2026 | 17.56 (16.78-18.34) | 0.76 (0.73-0.79) | 2684 | 17.02 (16.36-17.69) | 0.75 (0.72-0.78) | 1947 | 9.11 (8.67-9.54) | 0.41 (0.39-0.43) |
| Lung | 319254 | 23.11 (23.02-23.19) | 1 | 17662 | 14.32 (14.1-14.53) | 0.32 (0.32-0.33) | 8647 | 14.35 (14.03-14.66) | 0.31 (0.31-0.32) | 1947 | 16.05 (15.32-16.78) | 0.38 (0.36-0.39) | 2498 | 17.49 (16.78-18.19) | 0.4 (0.39-0.42) | 1366 | 8.12 (7.66-8.57) | 0.17 (0.16-0.18) |
| Colon and rectum | 206599 | 14.06 (14-14.12) | 1 | 13088 | 10.34 (10.16-10.52) | 0.74 (0.72-0.75) | 6252 | 9.7 (9.45-9.95) | 0.7 (0.68-0.72) | 1680 | 13.36 (12.71-14.01) | 0.95 (0.91-1) | 1880 | 13.34 (12.72-13.96) | 0.96 (0.91-1) | 1077 | 6.16 (5.77-6.56) | 0.44 (0.41-0.46) |
| Pancreas | 137154 | 9.46 (9.41-9.52) | 1 | 9560 | 7.82 (7.65-7.98) | 0.81 (0.79-0.82) | 5075 | 8.34 (8.1-8.57) | 0.86 (0.83-0.88) | 982 | 7.68 (7.19-8.17) | 0.83 (0.78-0.88) | 1022 | 7.41 (6.94-7.87) | 0.77 (0.72-0.82) | 931 | 5.7 (5.32-6.09) | 0.56 (0.53-0.6) |
| Ovary | 42249 | 2.97 (2.94-3) | 1 | 7933 | 5.87 (5.74-6.01) | 0.67 (0.66-0.69) | 4311 | 6.24 (6.04-6.44) | 0.72 (0.69-0.74) | 683 | 5.86 (5.41-6.31) | 0.66 (0.61-0.71) | 810 | 5.5 (5.11-5.89) | 0.62 (0.57-0.66) | 818 | 4.1 (3.79-4.4) | 0.48 (0.45-0.51) |
| Liver | 29096 | 2.01 (1.99-2.03) | 1 | 6877 | 5.52 (5.38-5.65) | 1.78 (1.74-1.83) | 4122 | 6.65 (6.44-6.87) | 2.11 (2.05-2.17) | 458 | 3.6 (3.26-3.93) | 1.27 (1.15-1.38) | 713 | 4.95 (4.58-5.32) | 1.67 (1.54-1.79) | 639 | 3.84 (3.53-4.16) | 1.18 (1.09-1.27) |
| Stomach | 79045 | 5.56 (5.52-5.6) | 1 | 6015 | 4.46 (4.34-4.58) | 2.33 (2.28-2.39) | 3415 | 4.92 (4.74-5.09) | 2.62 (2.53-2.71) | 260 | 2.1 (1.84-2.36) | 1.04 (0.91-1.17) | 565 | 4.04 (3.69-4.38) | 1.99 (1.83-2.16) | 922 | 4.73 (4.4-5.06) | 2.57 (2.4-2.73) |
| Leukemia | 121526 | 8.72 (8.67-8.77) | 1 | 5999 | 4.03 (3.92-4.14) | 0.8 (0.78-0.82) | 3308 | 3.99 (3.84-4.14) | 0.85 (0.82-0.88) | 547 | 4.54 (4.15-4.94) | 0.8 (0.73-0.87) | 641 | 4.17 (3.83-4.5) | 0.79 (0.73-0.86) | 622 | 3.12 (2.86-3.39) | 0.62 (0.57-0.67) |
| NHL | 80204 | 5.47 (5.43-5.51) | 1 | 5599 | 4.44 (4.32-4.56) | 0.83 (0.81-0.85) | 3007 | 4.75 (4.57-4.93) | 0.89 (0.86-0.92) | 556 | 4.51 (4.12-4.89) | 0.8 (0.73-0.86) | 617 | 4.33 (3.98-4.69) | 0.83 (0.76-0.89) | 585 | 3.49 (3.19-3.79) | 0.64 (0.59-0.69) |
Average annual mortality rates (AMRs) are reported per 100,000 person-years. The numerator of the rate was determined by the total number of cancer deaths over the 10-year period in each sex-, Hispanic group- and site-specific stratum. The denominator is the 10-year sum of the sex- and Hispanic group-specific U.S population based on census data. Rates were directly standardized to the 2000 U.S. population age distribution to render them comparable across stratum.
Standardized mortality ratios (SMRs) were created by dividing the sum of the 10-year sex-, Hispanic group- and site-specific deaths by the “expected” number of deaths, as defined by the sum of the 10-year deaths for the sex- and site-specific NHW population (the reference population).
Figure 2:
Cancer mortality trends (men, 2003-2012). These trends were modeled using Joinpoint regression of the age-adjusted annual morality rates by cancer site and ethnic group. A maximum of two segments were allowed per stratum. Corresponding annual percentage change results from the JoinPoint models are included in the Supplementary Tables 2 and 3.
Figure 3:
Cancer mortality trends (women, 2003-2012). These trends were modeled using Joinpoint regression of the age-adjusted annual morality rates by cancer site and ethnic group. A maximum of two segments were allowed per stratum. Corresponding annual percentage change results from the JoinPoint models are included in the Supplementary Tables 2 and 3.
Cancers of the Digestive Tract
Liver cancer was ranked among the top 5 causes of cancer-related deaths for Mexican, Puerto Rican, and Central or South American males, but was not a top ranked cancer for NHWs. . In both males and females, the highest liver cancer mortality in comparison to NHWs were among Puerto Ricans (male SMR, 2.51; 95% CI, 2.41–2.62; female SMR, 1.67; 95% CI, 1.54–1.79). Trends in liver cancer death rates among males have been increasing for all Hispanic groups except for Cubans and Central or South Americans with the highest increase in Puerto Ricans (Puerto Rican APC, 3.5; 95% CI, 1.8–5.3; Mexican, 2.2; 95% CI, 1.2–3.2; all Hispanic, 2.5; 95% CI, 1.9–3.1; NHW APC, 3.1; 95% CI, 2.7–3.1;). Among females, increased liver cancer mortality trends were observed in Puerto Ricans and all Hispanics combined (Puerto Rican APC, 2.4; 95% CI, 0.4–4.5; all Hispanic APC, 1.7; 95% CI, 0.6–2.8).
Colorectal cancer ranked among the top 5 causes of cancer deaths for the entire population. However, Hispanics have lower colorectal cancer deaths than NHWs with the lowest rates for Mexican and Central or South Americans (male Mexican AMR, 15.90; 95% CI, 15.49–16.23; male Central or South AMR, 7.81; 95% CI, 7.22–8.41; female Mexican AMR, 9.70; 95% CI, 9.45–9.95; female Central or South AMR, 6.16; 95% CI, 5.77–6.56).
Stomach cancer ranked among the top 5 cancer deaths for Central or South Americans. However, AMRs were at least twice as high in all Hispanic groups apart from Cubans. The highest stomach cancer mortality rates were among Mexicans (male AMR, 8.15; 95% CI, 7.89–8.42; female AMR, 2.62; 95% CI, 2.53–2.71). Cubans had the lowest stomach cancer mortality that largely resembled NHWs (male SMR, 1.16; 95% CI, 1.05–1.27); female SMR, 1.04; 95% CI; 0.91–1.17). Trends in stomach cancer mortality are stable or decreasing for all populations.
Overall, pancreatic cancer mortality is lower in all Hispanic groups compared to NHWs, with the lowest mortality observed in Central or South Americans (male SMR 0.46; 95% CI, 0.42–0.49; female SMR, 0.56; 95% CI, 0.53–0.60). Among males, trends in pancreatic cancer have been increasing for NHWs and Mexicans (NHWs APC, 0.80; 95% CI, 0.50–1.0; Mexican APC, 1.1; 95% CI, 0.0–2.2). Female pancreatic cancer mortality is also increasing for NHWs and all Hispanics combined (NHWs APC, 0.70; 95% CI, 0.50–0.90; all Hispanic APC, 0.70; 95% CI, 0.0–1.4).
Lung Cancer
Lung cancer was the leading cause of cancer-related deaths in male and female NHWs, and for Hispanic males and the second leading cause of cancer-related deaths in Hispanic females. Lung cancer caused about 30% of cancer deaths among male NHWs and male Cubans (Figure 1). With the exception of Cubans, all Hispanic groups have about half the lung cancer mortality compared to NHWs. Among males, Central or South Americans and Mexicans had the lowest lung cancer mortality when compared to NHWs (Central or South SMR, 0.20; 95% CI, 0.10–0.21; Mexican SMR, 0.41; 95% CI, 0.40–0.42). Lung cancer mortality trends for men were significantly decreasing for all populations starting in 2005, but stable or increasing across all years for females.
Female Breast and Ovarian Cancer
Breast cancer was the leading cause of death in Hispanic females accounting for about 16% of all cancer deaths and the second leading cause of death in NHWs (15% of deaths). Overall, female Hispanics had lower cancer deaths than NHWs. Cubans had the highest rates of breast cancer mortality among the Hispanic groups (AMR, 17.56; 95% CI, 16.78–18.34) while Central or South Americans had the lowest (AMR, 9.11; 95% CI, 8.67–9.54). For most groups, breast cancer mortality trends were stable or decreasing.
For all females, ovarian cancer accounted for 5% to 7% of cancer deaths. Hispanic ovarian cancer mortality rates were lower than NHWs for all Hispanic groups with Central or South Americans reporting the lowest ovarian cancer mortality rates (AMR, 4.10; 95 % CI, 3.79–4.40). Ovarian cancer mortality trends were stable or decreasing for all populations.
Non-Hodgkin Lymphoma (NHL) and Leukemia
All Hispanics overall had lower NHL cancer mortality than NHWs (All Hispanic male SMR, 0.78; 95% CI, 0.76–0.80; All Hispanic female SMR, 0.83; 95% CI, 0.81–0.85). Most Hispanic groups reported similar NHL cancer mortality rates, though Central or South Americans had the lowest NHL mortality rates (male AMR, 4.97; 95% CI, 4.49–5.45; female AMR, 3.49; 95% CI, 3.19–3.79).
Leukemia mortality was homogenous across Hispanic groups and compared to NHWs (All Hispanic male SMR, 0.74; 95% CI, 0.72–0.76; All Hispanic female SMR, 0.80; 95% CI, 0.78–0.82). Among Hispanics, Cubans had the highest leukemia mortality rates (male AMR, 7.07; 95% CI, 6.52–7.62; female AMR, 4.54; 95% CI, 4.15–4.94) while Central or South Americans had the lowest (male AMR, 4.81; 95% CI, 4.36–5.27; female AMR, 3.12; 95% CI, 2.86–3.39).
Prostate
Prostate cancer mortality rates were homogenous across all Hispanic groups and overall lower than NHWs (All Hispanic SMR, 0.89; 95% CI, 0.88–0.91). Cubans have the highest rates of prostate cancer deaths (AMR, 21.51; 95% CI, 20.54–22.47), though these were still similar to NHWs (SMR, 1.0; 95% CI, 0.95–1.04). Central or South Americans had the lowest prostate cancer mortality rates (AMR, 4.97; 95% CI, 4.49–5.45). Trend analysis shows that prostate cancer was stable or decreasing for all Hispanic populations.
DISCUSSION
This is the first study to describe trends in cancer mortality by specific Hispanic group for the leading 10 causes of cancer deaths nationwide. Consistent with reports from the American Cancer Society and Pinheiro, our results confirmed that for most cancer sites, mortality rates were lower in aggregate Hispanics when compared to NHWs[3, 18] . All-cause cancer mortality rates for aggregate Hispanics were about one-third that of NHWs. We also observed that most cancer mortality trends are declining in both males and females. Liver cancer mortality is a notable exception as mortality trends are increasing for most Hispanic populations. The disaggregation of cancer mortality showed distinct heterogeneity for all cancers and most site-specific cancers across all Hispanic groups.
The decreased cancer burden observed in Hispanics remains surprising due to the increased health disparities Hispanics face in comparison to NHWs. This phenomenon is known as the Hispanic Paradox and has been well characterized in other studies[19, 20] . The phenomenon is not limited to cancer mortality and has also been seen in cardiovascular disease (CVD) mortality as well[21]. Explanations for the Hispanic paradox include the immigration of healthier individuals into the U.S., the out migration of ill individuals to their country of origin, i.e., the Salmon bias, and (for incidence studies) data linkage errors in cancer registries[22] . However, none of these explanations fully account for the cancer mortality advantage seen in Hispanics[23, 24]. A recent study analyzing Hispanic groups in Texas and California suggests that the advantage may be driven by low cancer mortality rates among foreign born Hispanics[25].
In line with other findings, we found that Hispanics were disproportionately affected by cancers related to infectious agents though our study showed that mortality burden of these cancers differ by Hispanic group[26]. Liver cancer mortality disproportionately affects Hispanics and the disaggregated analysis showed that Puerto Ricans and Mexicans drive most of this disproportionality among males. Indeed, Pinheiro, et al. recently reported four-fold increases in Puerto Rican liver cancer mortality compared to NHWs in New York State[27]. This disparity may be driven by lower SES, higher body mass index (BMI) and alcohol consumption seen in these two Hispanic groups[10, 28, 29]. These higher mortality trends may indicate a need for culturally tailored programs for alcohol addiction and weight loss. Previous studies have shown a need for substance abuse research and intervention studies conducted with attention to heterogeneity among Hispanic groups [29-31]. The increases in liver cancer deaths may also indicate a need to prioritize Hepatitis C (HCV) screening and Hepatitis B (HBV) vaccination. HCV is estimated to cause over 50% of all liver cancer incidents and one study reported a 17-fold increase risk for liver cancer among HCV-infected patients[7, 32]. HCV exposure also varies significantly among Hispanic populations, with the highest prevalence in Puerto Ricans, 11.6%, while the exposure among Central or South Americans is as low as 0.4%[33]. Cuba has a high rate of HBV vaccination (99% of children), which may mitigate liver cancer mortality as 15% of liver cancer incidence is attributed to HBV infection [7, 34]. Our trend analyses also show that liver cancer mortality has been significantly increasing for all Puerto Rican and Mexican men, but have remained stable for Mexican women, Cubans, and Central or South Americans.
The higher stomach cancer mortality seen in Central and South Americans, Mexicans, and Puerto Ricans may be associated with increased H. Pylori infection seen in the Hispanic populations[35] . Though male stomach cancer mortality trends are declining in NHWs, the mortality trends for Mexicans, Puerto Ricans, and Cubans are stable. Incident cases of stomach cancer, primarily among young Hispanic men, have also been recently increasing[36]. The combination of stable mortality rates and increasing incident stomach cancer warrant an increased need for screening programs especially among the aforementioned young male populations.
Other studies have confirmed that CRC incidence and mortality have been decreasing since 1998 and this has mainly been attributed to screening guidelines[37, 38]. We found that CRC mortality has been significantly decreasing for NHWs since 2002, but the trends in the Hispanic populations appear to be decreasing at a smaller rate or remaining stable over time. This may be due to a combination of lower CRC screening rates, higher BMI, and higher prevalence of diabetes seen in Hispanics[4, 30, 39]. This is particularly concerning for Puerto Rican and Cuban males as their CRC-related mortality rates appear to have surpassed NHWs as of 2009. Increased CRC mortality in Puerto Rican and Cubans may be associated with their higher acculturation to the U.S[18]. CRC incidence in young Hispanics is also increasing in parts of the U.S.[40]. It is feared that continued adoption of U.S. health behaviors will worsen diet and exercise practices in the Hispanic populations, potentially increasing mortality rates if adherences to CRC screening guidelines are not improved[40,41].
Breast cancer mortality is lower in Hispanic females than in NHWs. This mortality advantage has been linked to higher parity, earlier age of childbirth, and higher prevalence of breastfeeding seen in Hispanics[3, 41]. However, Hispanics are more likely to be negative for estrogen and progesterone tumor receptors, making breast cancer treatment more difficult than in NHWs[43]. Though this likely worsens survival for Hispanics, it is surprising that mortality is far lower. Nonsignificant differences in tumor receptor status have been reported among Hispanics groups, with Puerto Ricans having the highest risk of being double negative[43]. Nativity also plays a role in burden as foreign born Hispanics have lower breast cancer than U.S born[25, 41]. Cubans and Puerto Ricans have the highest rates of breast cancer mortality among the Hispanic population. This higher mortality has been linked to previously mentioned risks such as acculturation, alcohol consumption, and BMI. In agreement with our findings, a study utilizing the National Health Interview Survey to examine breast cancer risk similarly found heterogeneity by disaggregated Hispanic population [41] .
Patterns of lung cancer mortality are lower in Hispanics overall when compared to NHWs. Cuban males have the highest lung cancer mortality among Hispanics followed by Puerto Ricans though mortality trends for all male groups are declining. Cubans and Puerto Ricans report far higher smoking prevalence and daily cigarette use than the other Hispanic groups[42]. This largely explains the increased lung cancer mortality they experience. Increases use of tobacco and alcohol are indicators of acculturation to the U.S. This may raise caution for the Mexican and Central or South Americans; as these groups increase their time in the U.S. and their level of acculturation, trends in lung cancer mortality may also increase. Our study reinforces that tobacco control efforts should be culturally targeted to particularly reach Hispanics[42]. The variation of lung cancer deaths across the Hispanic population also highlights the importance of disaggregated data. Results for the All Hispanic group may reflect the highest rates, which are among Cubans, who are also among the highest smokers across all Hispanic groups. Indeed, aggregation of Hispanics may hide the true underlying trends and result in misleading interpretation for specific Hispanic groups.
Cancer profiles are also unsurprisingly different by sex, with females showing less cancer mortality than males in all cancer combined and site specific cancers. This is a finding common in most cancer studies and has been attributed to differences in behaviors such as smoking, drinking, BMI, and more[3, 26, 42]. Our study offers a unique look at trends of site specific cancer over the study period. A comparison of lung cancer mortality trends among Hispanics shows that mortality trends for males are rapidly decreasing while mortality trends for females have remained constant. This may be because men picked up smoking behaviors before women so their male trends had time to peak and then decline.
Our PYLL analysis highlights the considerably younger age of cancer-related deaths among the Hispanic groups, which may be worrisome considering that the PYLL is a measure of social and economic loss from premature death[43]. Expressed as an age-adjusted rate, PYLL’s are still higher among NHWs, but the difference is not as dramatic as noted in the AMR calculations, the latter being more heavily impacted by the mortality burden among the elderly, non-working members of society. Since Hispanics are the largest minority population in the U.S. and rapidly increasing in size, these impact measures could have important economic and demographic implications for the U.S. overall.
Limitations
Our study relies on death records which are subject to inaccurate recording race/ethnicity which may lead to misclassification. However, studies have shown the recording of Hispanic origin on death records is valid for mortality studies[13, 44]. Hispanic populations vary geographically. States with fewer Hispanics and a lower Hispanic diversity are subject to more misclassification as state medical officials may not be accustomed to accurately recording the decedent’s specific Hispanic group [23]. Therefore, our statistics of mortality at a national level may not be representative at a state level. There may also be misclassification introduced into the study from the combination of Central or South Americans as these two groups carry their own heterogeneity and carry Dominican misclassification. Denominator counts from census data may suffer from inaccuracies as population counts are only recorded each decade and only account for those who respond [45]. Our use of interpolation methods for estimating the population sizes from 2000–2010 and extrapolation methods after 2010 should improve the precision and accuracy of yearly denominator counts. However, in both numerators (cancer deaths) and denominators (populations), the amount of decedents classified as “other Hispanic” may also impact the rates reported in this study. Nativity status was not considered in this study because the numbers of U.S. born Hispanics are small for Cubans and Central or South Americans. However, studies have shown there are significant differences between foreign-born and U.S. born Hispanic cancer burden for most cancer sites[25, 46, 47]. The study also did not account for racial differences between Hispanic Whites and Hispanic Blacks. Studies have not adequately investigated Hispanic ethnicity due to the underreporting of black and mixed Hispanics making this limitation an opportunity for innovative research. Benchmarks for the calculation of PYLLs use Hispanics as a whole, while unknown differences in life expectancy may exist between the distinct Hispanic groups. Lastly, ten years is a short follow-up time though our trends give substantial insight on the long term trajectories of cancer burden.
Conclusion
The results of our paper highlight the cancer burden of specific Hispanic groups. Future studies are needed to determine the relative importance of the many potential and important risk factors in driving cancer mortality in these specific Hispanic groups. A comprehensive understanding of cancer burden is essential to guide our treatment and prevention strategies, especially in Hispanics as they represent a heterogeneous and growing segment of the U.S. population.
Supplementary Material
Acknowledgements:
S.M. Zamora was funded from the Cancer Epidemiology and Education in Special Population (CEESP) program by the National Cancer Institute (R25 CA112383); CT was funded by the National Institute for Advancing Translational Sciences (KL2TR001444) and National Cancer Institute (U54CA132384); KGH, LPP, and JH were funded from the National Institute on Minority Health and Health Disparities (R01 MD007012)
Footnotes
Conflict(s) of interest: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Colby SL, Projections of the Size and Composition of the U.S. Population:2014 to 2016 in Current Population Reports, U.S.C. Bureau, Editor. 2014: Washington DC. [Google Scholar]
- 2.Flores A, How the U.S. Hispanic Population is Changing, in Fact Tank. 2017, Pew Research Center. [Google Scholar]
- 3.American Cancer Society, Cancer Facts and Figures for Hispanics/Latinos 2015–2017. [Google Scholar]
- 4.Jandorf L, et al. , Understanding the Barriers and Facilitators of Colorectal Cancer Screening Among Low Income Immigrant Hispanics. J Immigr Minor Health, 2010. 12(4): p. 462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burchard EG, et al. , Latino Populations: A Unique Opportunity for the Study of Race, Genetics, and Social Environment in Epidemiological Research. American Journal of Public Health, 2005. 95(12): p. 2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration, Hispanic Subgroups Differ in Rates of Substance Use Treatment Need and Receipt in The National Survey on Drug Use and Health Report; 2013. [Google Scholar]
- 7.Kuniholm MH, et al. , Prevalence of Hepatitis C Virus Infection in US Hispanic/Latino Adults: Results From the NHANES 2007–2010 and HCHS/SOL Studies. J Infect Dis, 2014. 209(10): p. 1585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swenson KK, et al. , Recognition and evaluation of oncology-related symptoms in the emergency department. Ann Emerg Med, 1995. 26(1): p. 12–7. [DOI] [PubMed] [Google Scholar]
- 9.Thompson CA, et al. , The burden of cancer in Asian Americans: a report of national mortality trends by Asian ethnicity. Cancer Epidemiol Biomarkers Prev, 2016. 25(10): p. 1371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motel S and Patten E, The 10 Largerst Hispanic Origin Groups: Characteristics, Rankings, Top Counties, in V. Economics and Health Insurance. 2012, Pew Research Center. [Google Scholar]
- 11.Vaeth PAC, Caetano R, and Rodriguez LA, The Hispanic Americans Baseline Alcohol Survey (HABLAS): The association between acculturation, birthplace and alcohol consumption across Hispanic national groups. Addictive behaviors, 2012. 37(9): p. 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson CA, et al. , Patient and provider characteristics associated with colorectal, breast, and cervical cancer screening among Asian Americans. Cancer Epidemiol Biomarkers Prev, 2014. 23(11): p. 2208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro PS, et al. , Cancer Incidence in First Generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and New Latinos. Cancer Epidemiology Biomarkers & Prevention, 2009. 18(8): p. 2162–2169. [DOI] [PubMed] [Google Scholar]
- 14.Arias E, United States life tables by Hispanic origin. Vital Health Stat 2, 2010(152): p. 1–33. [PubMed] [Google Scholar]
- 15.Esteve J, Benhamou E, and Raymond L, Statistical Methods in Cancer Research Volume IV: Descriptive Epidemiology. Vol. 128 1994, Lyon, France: International Agency for Research on Cancer. [PubMed] [Google Scholar]
- 16.Hyune‐Ju K, et al. , Permutation tests for joinpoint regression with applications to cancer rates. Statistics in Medicine, 2000. 19(3): p. 335–351. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, et al. , Permutation tests for joinpoint regression with applications to cancer rates. Stat Med, 2000. 19(3): p. 335–51. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro PS, et al. , Cancer Mortality in Hispanic Ethnic Groups. Cancer Epidemiol Biomarkers Prev, 2017. 26(3): p. 376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palloni A and Arias E, Paradox lost: Explaining the hispanic adult mortality advantage. Demography, 2004. 41(3): p. 385–415. [DOI] [PubMed] [Google Scholar]
- 20.Markides KS and Coreil J, The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep, 1986. 101(3): p. 253–65. [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez F, et al. , Disaggregation of Cause-Specific Cardiovascular Disease Mortality Among Hispanic Subgroups. JAMA cardiology, 2017. 2(3): p. 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franzini Luisa, J.C.R., Keddie Arlene M., Understanding the Hispanic Health Paradox. Ethn Dis, 2001. 11(3). [PubMed] [Google Scholar]
- 23.Arias E, et al. , The Hispanic Mortality Advantage and Ethnic Misclassification on US Death Certificates. Am J Public Health, 2010. 100(Suppl 1): p. S171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraído-Lanza AF, et al. , The Latino mortality paradox: a test of the “salmon bias” and healthy migrant hypotheses. American Journal of Public Health, 1999. 89(10): p. 1543–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinheiro PS, et al. , High cancer mortality for US-born Latinos: evidence from California and Texas. BMC Cancer, 2017. 17: p. 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torre LA, et al. , Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 2015. 65(2): p. 87–108. [DOI] [PubMed] [Google Scholar]
- 27.Pinheiro PS, et al. , Cancer Site-Specific Disparities in New York, Including the 1945–1965 Birth Cohort’s Impact on Liver Cancer Patterns. Cancer Epidemiol Biomarkers Prev, 2018. 27(8): p. 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albrecht SS, Ethnic Differences in Body Mass Index Trajectories from Adolescence to Adulthood: A Focus on Hispanic and Asian Subgroups in the United States. 2013. 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrero EG, et al. , Disparities in Latino substance use, service use, and treatment: Implications for culturally and evidence-based interventions under health care reform. Drug and Alcohol Dependence, 2013. 133(3): p. 805–813. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez J, et al. , Substance Abuse Prevalence and Treatment Among Latinos and Latinas. Journal of ethnicity in substance abuse, 2007. 6(2): p. 115–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells Kenneth, et al. , Ethnic Disparities in Unmet Need for Alcoholism, Drug Abuse, and Mental Health Care. American Journal of Psychiatry, 2001. 158(12): p. 2027–2032. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention, CDC Fact Sheet: Viral Hepatitis and Liver Cancer. 2016. [Google Scholar]
- 33.El-Serag HB, Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology, 2012. 142(6): p. 1264–1273.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed G and Galindo M, Cuba’s National Immunization Program. [DOI] [PubMed] [Google Scholar]
- 35.Everhart JE, et al. , Seroprevalence and Ethnic Differences in Helicobacter pylori Infection among Adults in the United States. The Journal of Infectious Diseases, 2000. 181(4): p. 1359–1363. [DOI] [PubMed] [Google Scholar]
- 36.Merchant SJ, et al. , A Rising Trend in the Incidence of Advanced Gastric Cancer in Young Hispanic Men. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 2017. 20(2): p. 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ries LAG MD, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK SEER Cancer Statistics Review, 1975–2005, N.C. Institute, Editor. 2008: Bethesda, MD. [Google Scholar]
- 38.Smith RA, et al. , Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin, 2018. 68(4): p. 297–316. [DOI] [PubMed] [Google Scholar]
- 39.He J, et al. , The association of diabetes with colorectal cancer risk: the Multiethnic Cohort. British Journal of Cancer, 2010. 103(1): p. 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang DY, et al. , Rising incidence of colorectal cancer among young Hispanics in Texas. Journal of clinical gastroenterology, 2017. 51(1): p. 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banegas MP, et al. , Risk of developing invasive breast cancer in Hispanic women: A look across Hispanic subgroups. Cancer, 2013. 119(7): p. 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan RC, et al. , Smoking among US Hispanic/Latino adults: The Hispanic Community Health Study/Study of Latinos. American journal of preventive medicine, 2014. 46(5): p. 496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardner JW and Sanborn JS, Years of potential life lost (YPLL)--what does it measure? Epidemiology, 1990. 1(4): p. 322–9. [DOI] [PubMed] [Google Scholar]
- 44.Arias E, et al. , The validity of race and Hispanic origin reporting on death certificates in the United States. Vital Health Stat 2, 2008(148): p. 1–23. [PubMed] [Google Scholar]
- 45.Lariscy JT, Differential Record Linkage by Hispanic Ethnicity and Age in Linked Mortality Studies: Implications for the Epidemiologic Paradox. Journal of aging and health, 2011. 23(8): p. 1263–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez F, et al. , Nativity Status and Cardiovascular Disease Mortality Among Hispanic Adults. Journal of the American Heart Association, 2017. 6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang ET, et al. , Gastric Cancer Incidence among Hispanics in California: Patterns by Time, Nativity, and Neighborhood Characteristics. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 2012. 21(5): p. 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.