Abstract
BACKGROUND:
Biallelic variations in the dedicator of cytokinesis 8 (DOCK8) gene cause a combined immunodeficiency with eczema, recurrent bacterial and viral infections, and malignancy. Natural disease outcome is dismal, but allogeneic hematopoietic stem cell transplantation (HSCT) can cure the disease.
OBJECTIVE:
To determine outcome of HSCT for DOCK8 deficiency and define possible outcome variables.
METHODS:
We performed a retrospective study of the results of HSCT in a large international cohort of DOCK8-deficient patients.
RESULTS:
We identified 81 patients from 22 centers transplanted at a median age of 9.7 years (range, 0.7–27.2 years) between 1995 and 2015. After median follow-up of 26 months (range, 3–135 months), 68 (84%) patients are alive. Severe acute (III-IV) or chronic graft versus host disease occurred in 11% and 10%, respectively. Causes of death were infections (n = 5), graft versus host disease (5), multiorgan failure (2), and preexistent lymphoma (1). Survival after matched related (n = 40) or unrelated (35) HSCT was 89% and 81%, respectively. Reduced-toxicity conditioning based on either treosulfan or reduced-dose busulfan resulted in superior survival compared with fully myeloablative busulfan-based regimens (97% vs 78%; P = .049). Ninety-six percent of patients younger than 8 years at HSCT survived, compared with 78% of those 8 years and older (P = .06). Of the 73 patients with chimerism data available, 65 (89%) had more than 90% donor T-cell chimerism at last follow-up. Not all disease manifestations responded equally well to HSCT: eczema, infections, and mollusca resolved quicker than food allergies or failure to thrive.
CONCLUSIONS:
HSCT is curative in most DOCK8-deficient patients, confirming this approach as the treatment of choice. HSCT using a reduced-toxicity regimen may offer the best chance for survival.
Keywords: DOCK8 deficiency, HSCT, Combined immunodeficiency
INTRODUCTION
Biallelic mutations or deletions in the gene encoding the dedicator of cytokinesis 8 (DOCK8) cause a combined T- and B-lymphocyte immunodeficiency,1,2 characterized by severe and recurrent skin and systemic infections, severe allergic disease, and predisposition to malignancy,3,4 which had initially been described as the autosomal-recessive variant of hyper-IgE syndrome.5 After discovery of the causative gene in 2009, 2 larger cohorts have been published, both demonstrating the severity of this disease and its dismal outcome.3,6,7 Only about a third of patients reach the age of 30 years without hematopoietic stem cell transplantation (HSCT) and about 75% develop severe, life-threatening disease complications before the age of 20 years.6
Soon after description of the gene, 2 case reports of patients who had undergone HSCT long before genetic diagnosis was possible demonstrated the possibility of cure with HSCT.8,9 Several case reports and small case series on the outcome of HSCT with various donor types have since been published.10–17 Most of them report encouraging results, possibly skewed by publication bias. Although these reports have been helpful in directing many patients with DOCK8 deficiency to earlier HSCT, it still remains unclear which conditioning regimens or donor types will yield the best outcomes. Furthermore, some of the case reports hinted at the fact that not all disease manifestations, notably food allergies, may be equally well corrected by HSCT.8,11,18 To address these questions, larger and more comprehensive HSCT cohorts need to be studied.
On the basis of a multi-institutional retrospective chart-based review conducted on behalf of the Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation (EBMT) and the European Society for Primary Immunodeficiencies (ESID), this article reports on the largest cohort of DOCK8-deficient patients treated by HSCT so far.
METHODS
Data accrual and statistics
A case report form asking for pseudonymized chart-based data of transplanted patients was sent to authors of our previous article on the DOCK8 phenotype6 and to members of the Inborn Errors Working Party of the EBMT and ESID and was posted on the ESID Web site. The data collection was concluded on December 31, 2016. This retrospective chart review received a waiver of approval by the ethics committee of the Ludwig-Maximilians-University of Munich, Germany. German patients or their respective caregivers gave their written informed consent for inclusion in the German pediatric stem cell transplantation registry, which was approved by the ethics committee of the Medizinische Hochschule Hannover. International centers had to receive approval for data transfer from their respective ethics committee or a waiver if applicable. Kaplan-Meier survival estimates and cumulative incidence rates were compared using the log rank test (Prism 5, GraphPad, La Jolla, Calif). Other analyses used the chi-square or Fisher exact test and were accepted as significantly different at a level of P less than .05.
Patients
Included in this study were patients with a confirmed biallelic variation affecting the DOCK8 gene who underwent a first HSCT between January 1, 1995, and December 31, 2015. Partial information on 36 patients in this study was previously reported in the article by Aydin et al.6
Definitions
Unrelated donors were considered matched (MUD) if they were at least 9/10 or 10/10 HLA alleleematched. Because of the different dosing regimens for intravenous and oral busulfan, all busulfan dosages were converted to a dose equivalent to oral dosing to make them comparable. Conditioning regimens containing busulfan were considered to be myeloablative if the total dose was equivalent to an oral dose of 14 mg/kg or more, or was targeted to an area under the curve of 70.000 ng × mL/h or more and reduced intensity when the total dose was less than 14 mg/kg or targeted to an area under the curve of less than 70.000 ng × mL/h.
Graft versus host disease (GvHD) was graded according to modified Glucksberg criteria for acute GvHD and according to the National Institutes of Health consensus criteria for chronic GvHD.19,20 Severe infections were defined as sepsis, meningitis, or pneumonia requiring hospitalization and supplemental oxygen or mechanical ventilation.
The method for determining resolution of symptoms post-HSCT was left to the local physician’s discretion.
RESULTS
Patient and transplant details
Data from 81 patients (43 females, 38 males) receiving a first HSCT from 22 centers in 11 countries were included. The median age at HSCT was 9.7 years (range, 0.7–27.2 years). Donors were matched sibling donors (MSDs) in 34 transplants, matched family donors (MFDs) in 6, mismatched family donors (MMFDs) in 6, MUDs in 33, and unrelated cord blood in 2. Bone marrow was the preferred stem cell source. Bone marrow was used in 63 patients, peripheral blood stem cells in 16, and cord blood in 2. Conditioning was based on myeloablative busulfan (BUMAC) in 31 patients, whereas reduced doses of busulfan (BURIC) were used in 17 patients. A treosulfan-based regimen (TREO) was applied in 17 patients. In vitro T-cell depletion was applied in 1 MUD recipient and in 4 of the 6 MMFD recipients, whereas the other 2 MMFD recipients had posttransplant cyclophosphamide. The median follow-up after HSCT was 26 months (range, 3–135 months). More detailed patient and transplant information is given in Table I.
TABLE I.
Patient and transplant characteristics (N = 81)
| Characteristic | n |
|---|---|
| Sex | |
| Female | 43 |
| Male | 38 |
| Age (y) at HSCT, median (range) | 9.7 (0.7–27.2) |
| Donor type | |
| MSD | 34 |
| MFD | 6 |
| MUD | 32 |
| MMUD | 1 |
| HLA match | |
| 10/10 | 20 |
| 9/10 | 10 |
| 8/10 | 1 |
| 8/8 | 1 |
| 6/6 | 1 |
| MMFD | 6 |
| UCB | 2 |
| Stem cell source | |
| Bone marrow | 63 |
| PBSC | 16 |
| Cord blood | 2 |
| Conditioning | |
| Busulfan-based | 48 |
| Myeloablative | 31 |
| BU/CY | 12 |
| BU/FLU | 19 |
| Reduced* | 17 |
| Treosulfan-based (all TREO/FLU) | 17 |
| Other reduced intensity | 14 |
| With TBI (200–400 cGy) | 4 |
| Other myeloablative | 1 |
| None | 1 |
| Serotherapy used | 38 |
AUC, Area under the curve; BU/CY, busulfan cyclophosphamide; BU/FLU, busulfan fludarabine; MMUD, mismatched unrelated donor; PBSC, peripheral blood stem cell; TBI, total body irradiation; TREO/FLU, treosulfan fludarabine; UCB, unrelated cord blood.
Intravenous busulfan dose equivalent to <14 mg/kg oral dosing or busulfan tar-geted to an AUC of <70.000 ng × mL/h.
Survival
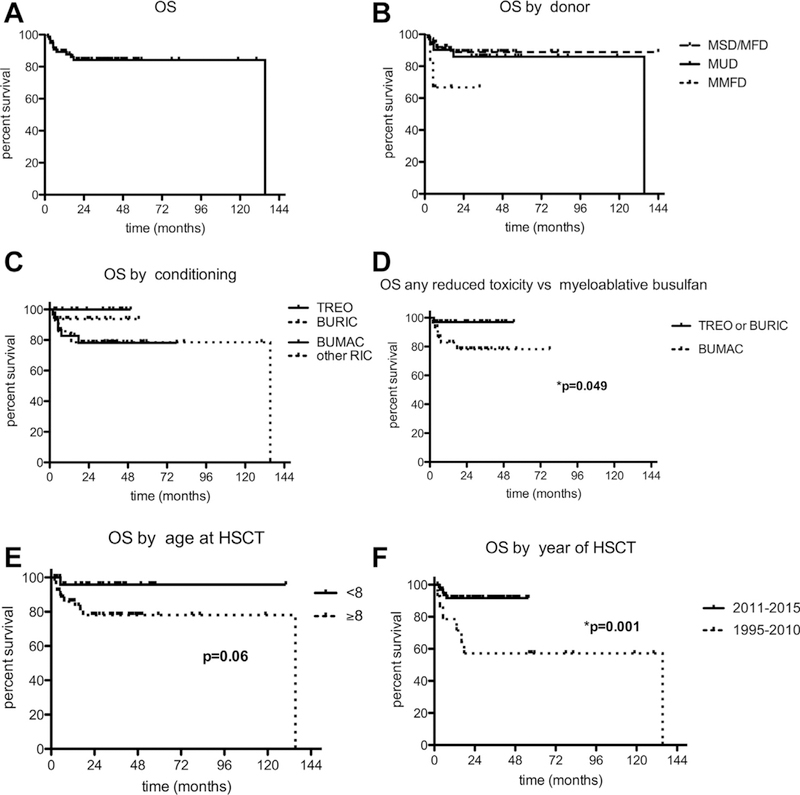
The entire cohort of 81 patients had a 2-year overall survival (OS) probability of 84% (95% CI, 73%−91%; Figure 1, A) and potential outcome variables were tested.
FIGURE 1.
Kaplan-Meier analysis of OS post-HSCT (A) of the entire cohort, (B) by donor type, (C and D) by type of conditioning, (E) by age at HSCT, and (F) by the year of HSCT. RIC, Reduced-intensity conditioning.
There was no significant survival advantage after HSCT from an MSD or MFD compared with an MUD with 2-year OS probabilities of 89% (95% CI, 73%−96%) and 86% (95% CI, 66%−95%), respectively. MMFD recipients had a 2-year OS probability of 66% (95% CI, 20%−90%), which was also not statistically different from those of the other groups (P = .18; Figure 1, B). The conditioning regimen did have an impact on HSCT outcome. Two-year OS probabilities after TREO, BURIC, BUMAC, or any other reduced-intensity regimen were 100%, 94% (95% CI, 63%−99%), 78% (95% CI, 57%−90%), and 79% (95% CI, 47%−93%) (P = .25; Figure 1, C), respectively. Using either a TREO or a BURIC regimen resulted in a significantly better OS at 97% (95% CI, 80%−100%) versus using BUMAC, which yielded an OS of 78% (95% CI, 57%−90%) (P = .049; Figure 1, D). The median age in this cohort was 9.7 years (range, 0.7–27.2 years). It was therefore prudent to test the influence of age at HSCT on survival. However, no age cutoff resulted in a significant result. There was a trend toward better survival in patients receiving their HSCT when younger than 8 years versus older, with 2-year OS of 96% (95% CI, 74%−99%) and 78% (95% CI, 63%−88%) (P = .06; Figure 1, E), respectively. Finally, the date of HSCT had a significant influence on survival. Patients transplanted between 2011 and 2015 had a 2-year OS of 92% (95% CI, 81%−96%) as compared with 57% (95% CI, 28%−78%) for those who had their HSCT between 1995 and 2010 (P = .01; Figure 1, F). Of the 13 deaths post-HSCT, the most common cause of death was infection (n = 5 patients; bacterial sepsis n = 3, unknown = 2) as well as infection associated with GvHD (n = 5; bacterial sepsis n = 2, fungal, n = 1, viral n = 2). Multiorgan failure was reported as the cause of death in 2 cases; 1 patient succumbed to a T-cell lymphoma, preexistent before HSCT, which was not driven by EBV. Virus reactivation/infection in the immediate posttransplant period occurred in 31 patients, and 2 of the deaths were associated with viral disease (cytomegalovirus and adenovirus). The frequency of virus infection/reactivation was statistically not different between surviving and deceased patients (P = .547) (Table II).
TABLE II.
Frequency of viral infections/reactivations in surviving and deceased patients
| Patients | Number of patients with virus infection/reactivation | Time point |
|
|---|---|---|---|
| Early (<day 100) |
Late (>day 100) |
||
| Surviving (68 of 81) | 25 of 68 (37%) | CMV: 15 | EBV: 2 |
| EBV: 4 | HSV: 1 | ||
| HSV: 3 | VZV: 2 | ||
| ADV: 4 | |||
| HHV6: 1 | |||
| BK: 2 | |||
| Other: 1 | |||
| Deceased (13 of 81) | 6 of 13 (42%) | CMV: 3 | CMV: 1 (persistent) |
| EBV: 2 | EBV: 1 (persistent) | ||
| HSV: 1 | ADV: 1 | ||
| HHV6: 1 | |||
ADV, Adenovirus; BK, human polyoma virus 1; CMV, cytomegalovirus; HHV6-human herpesvirus 6; HSV-herpes simplex virus; VZV-varicella zoster virus. Note. A single patient may have had multiple viruses. The frequency of virus infection/reactivation was statistically not different between surviving and deceased patients (P = .547).
In brief, HSCTs that were performed from an MSD/MFD or an MUD after TREO or BURIC conditioning after 2010 resulted in superior outcomes in this cohort.
Graft versus host disease
Acute GvHD was reported in 27 of 81 patients, resulting in a cumulative incidence of 33%. Of these, 22 (27%) had a severity of grades II to IV and 9 (11%) of grade III or IV. Of the 73 patients alive at more than 100 days post-HSCT, 7 developed chronic (10%), 3 mild, 2 moderate, and 2 severe GvHD as per the National Institutes of Health consensus criteria. In 5 of the 13 deaths, GvHD was a contributing factor (Table III).
TABLE III.
Incidences of acute and chronic GvHD
| GvHD | n |
|---|---|
| Acute GvHD | 27 of 81 (33%) |
| I | 5 (6%) |
| II | 13 (16%) |
| III | 6 (7%) |
| IV | 3 (4%) |
| II-IV | 22 (27%) |
| III-IV | 9 (11%) |
| Chronic GvHD (follow-up > 100 d) | 7 of 73 (10%) |
| Mild | 3 (4%) |
| Moderate | 2 (3%) |
| Severe | 2 (3%) |
Engraftment and chimerism
Of the 73 patients with chimerism data available at last follow-up, 64 (88%) had a global donor chimerism of 90% or higher, 1 (1%) between 80% and 90%, 4 (5%) between 20% and 80%, and 4 (5%) between 0% and 20% (Figure 2, A). Two of the 81 patients did not engraft; both died during or after the second HSCT. One had received a T- cell–depleted MMFD graft after BUMAC and 1 unrelated cord blood after nonmyeloablative conditioning. Of these 73 patients, the T-cell donor chimerism was 90% or higher in 65 patients (89%), between 80% and 90% in 4 (5%), between 20% and 80% in 3 (4%), and between 0% and 20% in 1 (1%) at last follow-up (Figure 2, B). Twenty-nine of the 31 patients (94%) receiving a BUMAC regimen had a global donor chimerism of 90% donor or higher, 1 had a chimerism of 40%, and 1 rejected. Of the 28 patients with a TREO or BURIC regimen and with chimerism data available, donor chimerism was 90% or higher in 25 (89%) patients and between 20% and 80% in 3 (11%) patients.
FIGURE 2.
Chimerism at last follow-up. Donor chimerism at last follow-up in 73 patients in whom data were available in (A) whole blood and (B) T cells. f/u, Follow-up.
Thus, engraftment in this cohort was solid, and there was no discernable effect of the intensity of the conditioning regimen on the degree of donor chimerism.
Symptom resolution post-HSCT
In early single patient reports, inconsistent resolution of DOCK8 deficiency–related symptoms after successful HSCT was described. Thus, we asked for changes in disease-related symptoms at last follow-up (median, 26 months [range, 3–135 months]).
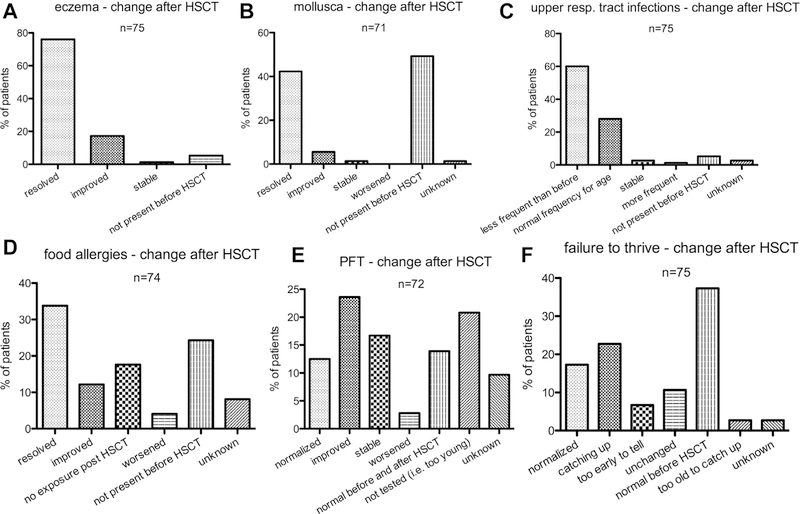
Eczema, mollusca, and recurrent upper airway infections responded very well to HSCT. Eczema was reported as resolved or improved in 70 (99%) of 71 patients who suffered from it before HSCT and mollusca in 34 (94%) of 36 patients (Figure 3, A and B). Upper airway infections were described as less frequent than before HSCT or occurring at a normal frequency for age in 66 (93%) of the 71 affected patients (Figure 3, C). Food allergies and impaired pulmonary function tests (PFTs) responded less to HSCT. Food allergies resolved or improved in 34 (61%) of 56 patients, and because 13 (23%) of 56 patients had not been exposed to their specific allergens after HSCT, resolution or improvement was observed in 34 (79%) of those 43 patients who had exposure to their respective allergens post-HSCT (Figure 3, D). Of 47 patients, impaired PFT improved or normalized in 26 (55%) patients, stabilized in 12 (26%) patients, and worsened in 2 (4%) patients (Figure 3, E). Of 47 patients who had failure-to-thrive, another frequent symptom of DOCK8 deficiency, 30 (64%) normalized or were catching up, 8 (17%) were un-changed, 2 (4%) too old to catch up (no improvement post-puberty), and in 5 (11%) it was too early after HSCT to tell (Figure 3, F). Of 12 patients with malignancies before HSCT, 11 remained in remission at last follow-up. One patient with lymphoma progressed and died. Another patient who had total body irradiation (4 Gy) as part of her conditioning developed secondary thyroid cancer 7 years after HSCT. The patient was successfully treated and remains in remission 7 years later. Finally, the treating physicians were asked whether they thought their patients had benefited from HSCT and 76 out of 81 replied. The answer was “yes, definitely” for 65 (85%) patients, “somewhat improved” for 2 (3%), “too early to tell” for 3 (4%), and “patient died” for 6 (8%).
FIGURE 3.
Correction of disease-related symptoms by HSCT. Treating physicians were asked how they rated the correction of symptoms associated with DOCK8 deficiency after HSCT.
In brief, most of the surviving patients had improvement or resolution of their disease-related symptoms.
DISCUSSION
DOCK8 deficiency, which was initially described as autosomal-recessive hyper-IgE syndrome, is a combined immunodeficiency (CID) with a high mortality rate.1,2,5 Single case reports of patients transplanted years before the causative gene had been identified showed that HSCT was curative.8,9 Two larger and partially overlapping cohorts later confirmed a poor natural disease outcome, with patient survival of about 50% at the age of 20 years in the absence of HSCT, as well as high rates of malignancy, life-threatening infections, or central nervous system events.6,7 We present the data relating to HSCT outcomes in the largest cohort of DOCK8-deficient patients to date. We found that outcomes are generally good when HSCT was performed with a reduced-toxicity regimen. All disease manifestations are potentially cured by HSCT.
In general, patients with CID are thought to have a survival advantage if transplanted as children.21 This may be in part due to the development of comorbidities related to primary immunodeficiency that occur over time and the desire to have an earlier intervention to prevent more significant disease complications. As the previous study by Aydin et al6 demonstrated, most patients with DOCK8 deficiency will develop a life-threatening infection, central nervous system event, or malignancy by the age of 20 years.6 In this study, with a median age at HSCT of almost 10 years, we were not able to identify an ideal age range for HSCT in DOCK8 deficiency. A much larger study will be needed to make such a recommendation. It is still possible that HSCT has a favorable risk-benefit ratio in adolescents or young adults with DOCK8 deficiency. Out of 16 patients with an age at HSCT of 16 years or higher in our cohort, 14 survived, which is in line with recent reports on good HSCT outcomes in adolescents and young adults with primary immunodeficiencies.22,23 In a disease like DOCK8 deficiency, which is characterized by severe systemic and cutaneous viral infections, it is expected that preexisting viral disease in the host will result in more infectious complications during and after HSCT. In this cohort this was not the case. Only 2 of the 13 HSCT-associated deaths were in part attributed to viral disease and only 33% of patients experienced viral reactivation/infection. This means that although special attention should still be placed on prevention of virus infections after HSCT, the presence of preexisting viral disease should not be an exclusion criterion for transplant. The reported incidence of severe acute and chronic GvHD in this cohort is low, given the high load of viral disease in DOCK8 deficiency. This may be caused by the fact that about half of the donors were MSD or MFD. This study showed no impact on OS, with 9/10 or 10/10 MUD compared with MSD/MFD. Our data may suggest that outcome after haploidentical HSCT in DOCK8 deficiency is inferior. However, 2 deaths in this very small group (n = 6) occurred in the 1990s, and all 4 patients transplanted with modern in vitro or in vivo T-cell depletion strategies (TCRαβ/CD19-depletion or posttransplant cyclophosphamide; n = 2 each) survived. This encouraging outcome after MMFD HSCT in DOCK8 deficiency is in line with recent case reports.13,16,17
This large multicenter patient series allows the analysis of the impact of different HSCT strategies on outcome. Although various conditioning regimens were reported, ranging from fully myeloablative to an unconditioned stem cell infusion in 1 patient,24 it was possible to compare fully myeloablative busulfan-based regimens to reduced-toxicity regimens based on either busulfan or treosulfan, demonstrating a significant survival benefit for these reduced regimens. The fact that 89% of patients achieved more than 90% donor chimerism with these regimens indicates that regimens based on reduced doses of busulfan with or without pharmacokinetic monitoring or treosulfan—as they are currently recommended by the Inborn Errors Working Party of EBMT and ESID—may preferentially be used for patients with DOCK8 deficiency.25 It remains to be explored in the future whether patients with specific pre-HSCT comorbidities would require conditioning regimens with the degree of myeloablation and immunosuppression tailored to their specific needs.
This study comprehensively analyzes the correction of all disease-related manifestations in DOCK8 deficiency by HSCT. As expected from previous smaller case series, eczema and mollusca resolved or improved in almost all affected patients. The fact that food allergies only slowly regress after successful HSCT could be confirmed here, which may be explained by the long-lived nature of host-derived, IgE-producing plasma cells.18 Impaired PFTs before HSCT did not improve or normalize in 30% of the patients within the relatively short follow-up period. This argues strongly for a strategy of transplanting patients before permanent lung damage has developed, because this may negatively impact not only their quality of life but also long-term survivorship. Most of the preexisting malignancies remained in remission after HSCT and only 1 patient developed thyroid cancer after HSCT, which may also have been caused by the irradiation-containing conditioning. This suggests that the strong predisposition toward malignancy in DOCK8 deficiency is corrected or at least improved by HSCT, although long-term follow-up is still limited. This may be especially true for the malignancies of B-cell origin. Whether this also remains true for the cancers of epithelial origin, which are more frequent in DOCK8 deficiency than in other CIDs,6 remains to be evaluated in larger cohorts with a longer follow-up. The hope is that with good immune reconstitution and better control of human papillomavirus infection, the incidence of human papillomaviruserelated squamous cell carcinomas will decrease. In our previously published cohort of 136 DOCK8-deficient patients, 12.5% of patients were reported to have autoimmunity.6 In this current cohort, none of the patients were reported to have had autoimmunity as a post-HSCT complication or as a cause of death. Because of this relative infrequency, we did not investigate resolution of autoimmunity after HSCT.
Although this is the largest cohort of transplanted DOCK8-deficient patients published to date, there are limitations of this study. Its retrospective and multicenter design may implicate a bias in selecting conditioning regimens for individual patients on the basis of their clinical conditions. The relatively small number of patients, incomplete chimerism data, and lack of immunological parameters post-HSCT did not allow us to analyze the impact of lineage-specific chimerism and immunological reconstitution on clinical outcome and symptom resolution. Ideally, these should be studied in a prospective manner. An increasing number of reports of vascular abnormalities including vasculitis have been reported in DOCK8 deficiency, which was not systematically assessed in this cohort. The outcome and long-term prognosis of patients with these complications should be addressed in future studies. It may also be possible that individuals with biallelic DOCK8 variations and an extremely mild clinical phenotype who do not require HSCT may be discovered, even if no current publication suggests this.
CONCLUSIONS
This study confirms that patients with DOCK8 deficiency can expect excellent survival and disease correction if transplanted with modern HSCT strategies. We believe that the overall encouraging results of this analysis will be helpful for patient counseling and for guiding clinical decision making in future DOCK8-deficient patients.
What is already known about this topic?
Biallelic variations in the DOCK8 gene cause a combined immunodeficiency with dismal natural disease outcome, which can be treated by allogeneic hematopoietic stem cell transplantation.
What does this article add to our knowledge?
Hematopoietic stem cell transplantation with a reduced-toxicity conditioning results in excellent survival and disease correction, regardless of donor type.
How does this study impact current management guidelines?
The encouraging results of this analysis may be helpful for patient counseling and for guiding clinical decision making in future DOCK8-deficient patients.
Acknowledgments
H. B. Gaspar reports other support from Orchard Therapeutics outside the submitted work. M. H. Albert reports grants from GSK and other support from GSK, Medac, CSL Behring, and Merck & Co. outside the submitted work. M. Hoenig reports personal fees from CSL Behring outside the submitted work. O. C. Ciocarlie reports grants from Servier during the conduct of the study.
H.B.G. is supported by Great Ormond Street Children’s Charity and received contributions from the UCL/Great Ormond Street National Institute for Health Research Biomedical Research Centre for this project. D.D.H., A.F.F., N.N.S., and H.C.S. are supported in part by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases and National Cancer Institute, National Institutes of Health. R.G. received grant support from National Institutes of Health/National Institute of Allergy and Infectious Diseases (grant no. 5R01AI100315). The research of A.R.G. was supported by the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle Hospitals National Health Service Foundation Trust and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health. Data cut-off for patients treated at National Institutes of Health for hematopoietic stem cell transplantation was March 1, 2015.
Abbreviations used
- BUMAC
Myeloablative busulfan
- BURIC
Reduced-dose busulfan
- CID
Combined immunodeficiency
- DOCK8
Dedicator of cytokinesis 8
- EBMT
European Group for Blood and Marrow Transplantation
- ESID
European Society for Primary Immunodeficiencies
- GvHD
Graft versus host disease
- HSCT
Hematopoietic stem cell transplantation
- MFD
Matched family donor
- MMFD
mismatched family donor
- MSD
Matched sibling donor
- MUD
Matched unrelated donor
- OS
Overall survival
- PFT
Pulmonary function test
- TREO
Treosulfan-based regimen
Footnotes
Conflicts of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol 2009;124:1289–1302.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med 2009;361:2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Q, Jing H, Su HC. Recent advances in DOCK8 immunodeficiency syndrome. J Clin Immunol 2016;36:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biggs CM, Keles S, Chatila TA. DOCK8 deficiency: insights into pathophysiology, clinical features and management. Clin Immunol 2017;181: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renner ED, Puck JM, Holland SM, Schmitt M, Weiss M, Frosch M, et al. Autosomal recessive hyperimmunoglobulin E syndrome: a distinct disease entity. J Pediatr 2004;144:93–9. [DOI] [PubMed] [Google Scholar]
- 6.Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options—a review of 136 patients. J Clin Immunol 2015;35:189–98. [DOI] [PubMed] [Google Scholar]
- 7.Engelhardt KR, Gertz ME, Keles S, Schaffer AA, Sigmund EC, Glocker C, et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol 2015;136:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bittner TC, Pannicke U, Renner ED, Notheis G, Hoffmann F, Belohradsky BH, et al. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr 2010;222:351–5. [DOI] [PubMed] [Google Scholar]
- 9.Gatz SA, Benninghoff U, Schutz C, Schulz A, Honig M, Pannicke U, et al. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant 2011;46:552–6. [DOI] [PubMed] [Google Scholar]
- 10.McDonald DR, Massaad MJ, Johnston A, Keles S, Chatila T, Geha RS, et al. Successful engraftment of donor marrow after allogeneic hematopoietic cell transplantation in autosomal-recessive hyper-IgE syndrome caused by dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol 2010;126:1304–1305.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlogis V, Galambrun C, Chambost H, Lamoureux-Toth S, Petit P, Stephan JL, et al. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol 2011;128:420–422.e2. [DOI] [PubMed] [Google Scholar]
- 12.Boztug H, Karitnig-Weiss C, Ausserer B, Renner ED, Albert MH, Sawalle-Belohradsky J, et al. Clinical and immunological correction of DOCK8 deficiency by allogeneic hematopoietic stem cell transplantation following a reduced toxicity conditioning regimen. Pediatr Hematol Oncol 2012;29:585–94. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh S, Schuster FR, Adams O, Babor F, Borkhardt A, Comoli P, et al. Haploidentical stem cell transplantation in DOCK8 deficiency—successful control of pre-existing severe viremia with a TCR α/β/CD19-depleted graft and antiviral treatment. Clin Immunol 2014;152:111–4. [DOI] [PubMed] [Google Scholar]
- 14.Cuellar-Rodriguez J, Freeman AF, Grossman J, Su H, Parta M, Murdock H, et al. Matched related and unrelated donor hematopoietic stem cell transplantation for DOCK8 deficiency. Biol Blood Marrow Transplant 2015;21: 1037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Herz W, Chu JI, van der Spek J, Raghupathy R, Massaad MJ, Keles S, et al. Hematopoietic stem cell transplantation outcomes for 11 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol 2016;138:852–859.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman AF, Shah NN, Parta M, Su HC, Brofferio A, Gradus-Pizlo I, et al. Haploidentical related donor hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for DOCK8 deficiency. J Allergy Clin Immunol Pract 2016;4:1239–12342.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah NN, Freeman AF, Su H, Cole K, Parta M, Moutsopoulos NM, et al. Haploidentical related donor hematopoietic stem cell transplantation for dedicator-of-cytokinesis 8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant 2017;23:980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Happel CS, Stone KD, Freeman AF, Shah NN, Wang A, Lyons JJ, et al. Food allergies can persist after myeloablative hematopoietic stem cell transplantation in dedicator of cytokinesis 8-deficient patients. J Allergy Clin Immunol 2016; 137:1895–1898.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15:825–8. [PubMed] [Google Scholar]
- 20.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I, the 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant 2015;21:389–401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slatter MA, Gennery AR. Hematopoietic cell transplantation in primary immunodeficiency—conventional and emerging indications. Expert Rev Clin Immunol 2018;14:103–14. [DOI] [PubMed] [Google Scholar]
- 22.Albert MH, Hauck F, Wiebking V, Aydin S, Notheis G, Koletzko S, et al. Allogeneic stem cell transplantation in adolescents and young adults with primary immunodeficiencies. J Allergy Clin Immunol Pract 2018;6:298–301.e2. [DOI] [PubMed] [Google Scholar]
- 23.Fox TA, Chakraverty R, Burns S, Carpenter B, Thomson K, Lowe D, et al. Successful outcome following allogeneic hematopoietic stem cell transplantation in adults with primary immunodeficiency. Blood 2018;131:917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Mousa H, Hawwari A, Alsum Z. In DOCK8 deficiency donor cell engraftment post-genoidentical hematopoietic stem cell transplantation is possible without conditioning. J Allergy Clin Immunol 2013;131:1244–5. [DOI] [PubMed] [Google Scholar]
- 25.Inborn Errors Working Party. Updated! EBMT/ESID guidelines for haematopoietic stem cell transplantation for PI; 2017. Available from: https://esid.org/Working-Parties/Inborn-Errors-Working-Party-IEWP/Resources/UPDATED!-EBMT-ESID-GUIDELINES-FOR-HAEMATOPOIETIC-STEM-CELL-TRANSPLANTATION-FOR-PI. Accessed April 29, 2018.