Despite being a well‐established research discipline, pharmacogenomics (PGx) is not yet routinely applied in patient care. Education is a crucial step for the successful implementation of PGx into the clinic. We need to offer collaborative, interprofessional approaches that encourage learning about PGx on an international level. It is especially important that PGx education enables the development of one's own thoughts and ideas to be able to understand and implement this rapidly developing field of science.
From Science to Patient Care
Pharmacogenomics is a well‐established field of science with more than 20,000 publications listed in the US National Library of Medicine National Institutes of Health (pubmed.gov) and more than 290,000 findings on Google Scholar to date for the terms “pharmacogenetics” or “pharmacogenomics.” But implementing that knowledge into clinical practice and patient care seems highly heterogeneous and sporadic, except for a few large scientific efforts. Many barriers to implementing PGx in the clinic have been identified and are currently challenged,1 such as a lack of insurance coverage, harmonization of lab structures, procedures, data, and interpretation of results. Regulatory authorities such as the US Food and Drug Administration and the European Medicines Agency incorporate PGx information relating to drug efficacy and safety into product labels. International evidence‐based guidelines for treatment adjustments based on PGx results have been produced by the Clinical Pharmacogenetics Implementation Consortium (CPIC) and others and are available through the Pharmacogenomics Knowledge Base, PharmGKB, and the CPIC website. Although PGx guidelines are published, in many countries medical specialty societies are not involved, do not recommend their use, or do not comment on their content. Increased knowledge about PGx is recognized as crucial for the implementation of PGx into clinical practice, and importantly the knowledge base within each country needs to be supported and built up to facilitate clinical implementation across multiple countries. A new generation of researchers and healthcare professionals recognize the potential value that PGx offers to patient care. Despite PGx being a prominent field of research, its implementation into clinical practice remains hampered and haphazard. Because education in PGx is crucial for successful implementation, we need to offer collaborative approaches to disseminate PGx knowledge to the future generation of healthcare professionals and to develop the knowledge and skill sets to embrace PGx implementation.
A series of PGx educational programs and concepts for use in pharmacy and medical schools have been already proposed and undertaken.2 Unfortunately, education is often not providing definitive answers regarding how PGx testing can be obtained and applied to drug therapy.
Within the European Ubiquitous Pharmacogenomics (U‐PGx) project, we performed a survey asking about general PGx knowledge in clinical practice.3 This survey was filled out by healthcare professionals and aimed to assess knowledge gaps and training needs that could be addressed by an educational program.4 The survey revealed that there is a general interest in PGx application. However, the interpretation of test results causes uncertainty, the medical knowledge is mainly limited to university centers, and it could be improved especially in postgraduate education. Therefore, educating and training healthcare professionals of independent academic institutions such as universities seems to be one of the most important steps to close the gap between the research base and patient care.
The European Perspective—Ubiquitous Pharmacogenomics (U‐PGx) Education Program
Within the U‐PGx implementation project, an educational program is offered that includes Web‐based seminars, e‐learning opportunities, and real life courses (http://upgx.eu/).
We conducted a survey among the participants of a summer school that was part of the educational program of U‐PGx. The group consisted of 49 participants from eight different countries (United Kingdom, the Netherlands, Germany, Slovenia, Greece, Italy, Portugal, and Canada) and worked in hospitals (35%), ambulatories (7.5%), and academia (57.5%). While researchers and students were commonly attending the course (51.2%), just 16.3% were physicians working in patient care, and 27.9% were pharmacists. Figure 1 a provides an overview of the availability of PGx tests in hospital settings, indications for ordering a PGx test, and locations of PGx data storage as reported by participants of the U‐PGx course.
Figure 1.
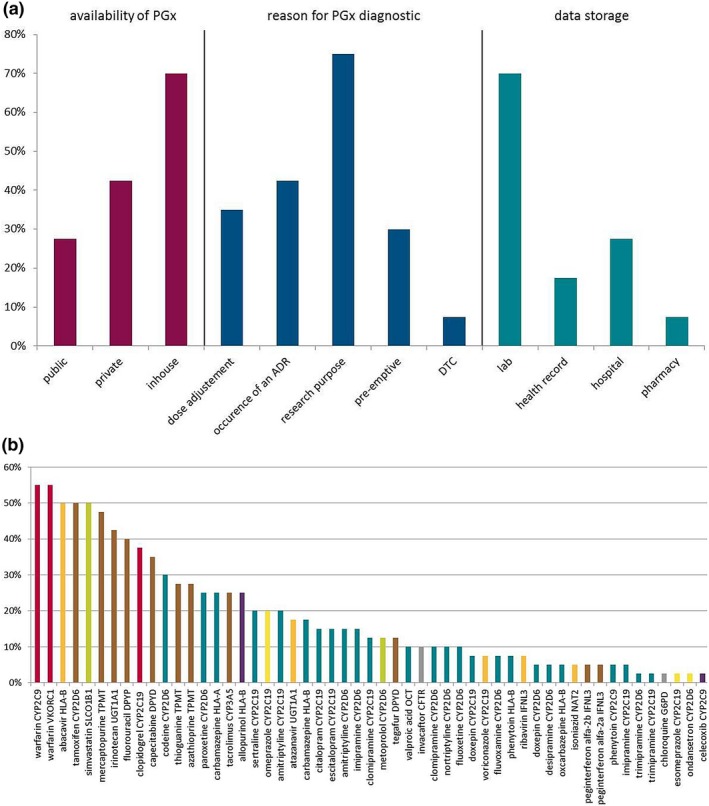
Use and availability of pharmacogenetic tests in clinical practice in Europe: data from the survey done in the Ubiquitous Pharmacogenomics project. (a) Answers to a survey concerning availability of pharmacogenomics (PGx) test (red), reason for ordering a PGx test (blue), and PGx test data storage (turquoise). Participants were able to choose more than one answer. Public: health insurances, public health system; private: companies; in‐house: hospital, laboratory; DTC: direct‐to‐consumer. (b) Importance of drug–gene pairs as rated by participants. Percentages of participants ranking the 10 most important drug–gene pairs from a list of 56 pairs of drug–gene pairs available as Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines are shown. The color of each bar indicates the class of the drug in the drug–gene pairs: red: antithrombotics; orange: antiinfectives; brown: antineoplastic and immunomodulating agents; turquoise: drugs acting on the central nervous system; green: drugs acting on the cardiovascular system; purple: drugs acting on the musculoskeletal system; yellow: drugs acting on the alimentary tract and metabolism; gray: other drugs like antiparasitic products and drugs acting on the respiratory system. ADR, adverse drug reaction; CFTR, cystic fibrosis transmembrane conductance regulator; CYP, cytochrome P450; DPYD, dihydropyrimidine dehydrogenase; HLA, human leukocyte antigen; IFNL3, interleukin 28B; NAT2, N‐acetyltransferase 2; OCT, organic cation transporter; SLCO1B1, solute carrier organic anion transporter family member 1B1; TPMT, thiopurine methyltransferase; UGT1A1, uridine diphosphate glucuronosyltransferase; VKORC1, vitamin K epoxide reductase complex subunit 1. Responder rate: n = 40 (82%, total: 49 participants from eight different countries (United Kingdom, the Netherlands, Germany, Slovenia, Greece, Italy, Portugal, and Canada).
Survey responses of this sample indicated that, within Europe, the utility of PGx is mainly limited to the research domain, with the local laboratory that generated the PGx data responsible for storage of the PGx results; these characteristics are consistent with commonly described barriers to PGx implementation to patient care.1 Despite the interest in PGx in young healthcare professionals that participated in the U‐PGx course, there is still a lack of translation of PGx knowledge from the laboratory and research structures into the clinic.
We also investigated the perceived importance, attributed by the course participants, for several established drug–gene pairs that have CPIC guidelines available. Figure 1 b shows how the drug–gene pairs were rated by the participants according to their perceived importance.
When considering drug classes, antithrombotic and antineoplastic and immunomodulating agents were ranked the most important, presumably because these drugs are associated with severe adverse drug reactions such as bleeding or leucopenia. The large number of drug–gene pairs concerning drugs acting on the central nervous system can also be appreciated from Figure 1 b, as numerous drugs that act on the central nervous system are metabolized by highly polymorphic cytochrome P450 enzymes (especially CYP2D6 and CYP2C19).
Although revealed by a small sample, these findings are in line with the perceived meaning of drug–gene pairs as rated by members of the American Society for Clinical Pharmacology and Therapeutics in 2010.5 However, a survey addressing a larger and broader population including more physicians would be desirable.
For the U‐PGx summer school, a comprehensive curriculum was developed that focuses on PGx knowledge, skills, and attitude towards PGx. Table 1 provides the curriculum and didactic goals of the course illustrating that health professionals need to acquire knowledge and skills to empower them to practice evidence‐based precision medicine.
Table 1.
Educational needs identified and addressed in a 4‐day U‐PGx course
| Learning goal | Learning objective | Educational strategy | Learning domain |
|---|---|---|---|
| Drug distribution and tolerance affected by PGx | Participant learns about the potential influence of PGx on phase I and II enzymes | Teacher‐based instruction | Knowledge |
| Participant learns about the potential influence of PGx on HLA genes and transporters | Teacher‐based instruction | Knowledge | |
| Concepts of PGx‐guided therapy | Participant understands ways of how PGx knowledge can guide treatment decisions | Teacher‐based instruction | Knowledge |
| Selection of patients to genotype | Participant can identify cases where a PGx test should be done | Case‐based learning | Skills |
| Participant can appraise critical benefits of PGx tests and risks of treating without a PGx test | Case‐based learning | Attitudes | |
| Participant can identify cases when it is reasonable to request a PGx test | Case‐based learning | Skills | |
| Interdisciplinary collaboration | Participant can discuss PGx topics within an interdisciplinary team | Case‐based learning | Skills |
| Participant can appraise different levels of knowledge about PGx and develop strategies within a team | Case‐based learning | Attitudes | |
| Methods of genotyping | Participant learns about different genotyping techniques and their influence on test results | Teacher‐based instruction | Knowledge |
| PGx databases and resources | Participant learns about available databases and other clinically relevant resources such as PharmGKB or PharmVar to inform self on clinically relevant PGx knowledge | Teacher‐based instruction with online presentation | Knowledge |
| Interpretation of test results | Participant learns to translate common genotypes into phenotypes | Case‐based learning | Skills |
| Participant considers the integration of genotype results with co‐medications and comorbidities | Case‐based learning | Skills | |
| Ethical and legal aspects of PGx | Participant understands ethical and legal aspects pertaining to PGx | Teacher‐based instruction | Knowledge/Attitudes |
| Ethical considerations | Participant learns strategies on how to inform patients about genotyping in different situations, such as direct‐to‐consumer genotyping or genome project genotyping | Case‐based learning | Attitudes |
| Participant learns how to obtain informed consent for genotyping in different situations, such as direct‐to‐consumer genotyping or genome project genotyping | Case‐based learning | Attitudes | |
| Clinical impact of structures affected by polymorphisms on drug treatment | Participant learns about and understands important and common sequence variations that impact drug therapy | Teacher‐based instruction | Knowledge |
| PGx‐based treatment modifications | Participant can appraise dose modifications and contraindications | Case‐based learning | Skills/Attitudes |
| Participant learns to use PGx test results within patient's context when making treatment decisions | Case‐based learning | Skills | |
| History of PGx | Participants can integrate their PGx knowledge in scientific developments of the last years | Teacher‐based instruction | Skills/Knowledge |
| Implementation of PGx | Participant learns about examples and structures from clinical reality for successful implementation of PGx into patient care | Teacher‐based instruction | Knowledge |
HLA, human leukocyte antigen; PGx, pharmacogenomics; PharmGKB, Pharmacogenomics Knowledge Base; PharmVar, Pharmacogene Variation; U‐PGx, ubiquitous pharmacogenomics.
What Education Should Address
Education of healthcare professionals: knowledge, skills, attitudes
Education in PGx should focus on knowledge and skills development, such as how to interpret test results and how to put the results into context when making treatment decisions. In addition, healthcare professionals’ attitudes, which means thoughts and views that may change the use of PGx in patient care, need to be addressed. In a globalized world, we need to foster attitudes towards implementing PGx, such as considerations when a PGx test is reasonable or should be done independent of ethnic background or reimbursement features of single countries, and even if it is not covered by available treatment guidelines. These skills and attitudes might be the crucial step for implementation as most clinics do not offer a preemptive approach. Therefore, implementation also means anticipation of clinical situations when testing might be required. Furthermore, education needs to help bridge the gap between PGx treatment guidelines and clinical reality, for instance by training healthcare practitioners how to interpret and use a genotype‐predicted phenotype in the context of interacting drugs and comorbidities that also affect drug disposition (e.g., hepatic disease, chronic kidney disease). The available PGx guidelines provide already excellent and reliable information on drug–gene interactions and the need for treatment modification. However, those guidelines are tools and, even though easily available, we need to offer education on how to use those tools.
Education of patients in health competence
It should not be neglected that the general population's awareness about PGx is increasing, which is expected to push conservative healthcare professions towards greater use of PGx. Therefore, information initiatives that target both patients and the wider public should be considered to stimulate healthcare professionals’ interest in PGx as a beneficial byproduct. To that end, the U‐PGx project provides valuable educational materials that are freely available (http://upgx.eu/).
An interdisciplinary approach between physicians and pharmacists on the one side and research personal and allied healthcare professionals on the other side is essential for tackling the most difficult questions regarding the application of PGx and to offer opportunities to learn from each other. Interdisciplinary education has been shown to enhance learning in the healthcare setting, and PGx should not be an exception.
Interdisciplinary Work
In our own experience, it is highly beneficial, appreciated, and indeed encouraged to work through interdisciplinary collaborations that foster our own thinking, recognize the multiple aspects involved in making a treatment decision, and thus do not always simply offer a single concrete answer. It is preferable in PGx education that lecturers engage and encourage teamwork among and between specialties. As pointed out, technical and conceptual developments in PGx are ongoing. Therefore, besides imparting knowledge to empower the understanding of PGx testing and treatment concepts, we need education that equips individuals with the skills to develop their own thoughts and ideas. Thereby, healthcare professionals might be enabled to understand and apply this rapidly developing field of science in a safe and informed manner. Thus, interdisciplinary collaboration and personal contact with the patient remain central tenets of any comprehensive PGx implementation program. Science needs to connect closely to clinic, and the healthcare setting should not be afraid of science to overcome the gap between the two of them. The specialty of clinical pharmacology might be particularly well suited to linking these fields to accelerate the successful translation of PGx from the bench to the bed.
Funding
This project has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 668353.
Conflict of Interest
The authors declared no competing interests for this work.
Acknowledgments
We would like to thank all U‐PGx summer school speakers who enriched the U‐PGx summer school with enthusiastic and brilliant talks: Evangelina Tsermpini, Richard Turner, Vita Dolžan, Jesse Swen, Rachel Hubbart, Leigh Jackson, Jürgen Brockmöller, David Gurwitz, and Erika Cecchin. We also thank the supporting team at the Federal Institute for Drugs and Medical Devices in Bonn. Last but not least, we thank all participants of the U‐PGx summer school, for an inspiring week, their interest, enthusiasm, and active participation.
[The copyright line for this article was changed on 06 September 2019 after original online publication].
References
- 1. Shuldiner, A.R. et al The pharmacogenomics research network translational pharmacogenetics program: overcoming challenges of real‐world implementation. Clin. Pharmacol. Ther. 94, 207–210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dunnenberger, H.M. et al Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu. Rev. Pharmacol. Toxicol. 55, 89–106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Wouden, C.H. et al Implementing pharmacogenomics in Europe: design and implementation strategy of the Ubiquitous Pharmacogenomics Consortium. Clin. Pharmacol. Ther. 101, 341–358 (2017). [DOI] [PubMed] [Google Scholar]
- 4. Just, K.S. , Steffens, M. , Swen, J.J. , Patrinos, G.P. , Guchelaar, H.J. & Stingl, J.C. Medical education in pharmacogenomics‐results from a survey on pharmacogenetic knowledge in healthcare professionals within the European pharmacogenomics clinical implementation project Ubiquitous Pharmacogenomics (U‐PGx). Eur. J. Clin. Pharmacol. 73, 1247–1252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Relling, M. & Klein, T. CPIC: Clinical Pharmacogenetics Implementation Consortium of the pharmacogenomics research network. Clin. Pharmacol. Ther. 89, 464–467 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


