Summary
Graft‐versus‐host disease (GVHD) is a major cause of transplant‐related mortality (TRM) after allogeneic haematopoietic stem cell transplantation (HSCT) and presents a challenge in haploidentical HSCT. GVHD may be prevented by ex vivo graft T‐cell depletion or in vivo depletion of proliferating lymphocytes. However, both approaches pose significant risks, particularly infections and relapse, compromising survival. A photodepletion strategy to eliminate alloreactive T cells from mismatched donor lymphocyte infusions (enabling administration without immunosuppression), was used to develop ATIR101, an adjunctive therapy for use after haploidentical HSCT. In this phase I dose‐finding study, 19 adults (median age: 54 years) with high‐risk haematological malignancies were treated with T‐cell‐depleted human leucocyte antigen‐haploidentical myeloablative HSCT followed by ATIR101 at doses of 1 × 104–5 × 106 CD3+ cells/kg (median 31 days post‐transplant). No patient received post‐transplant immunosuppression or developed grade III/IV acute GVHD, demonstrating the feasibility of ATIR101 infusion for evaluation in two subsequent phase 2 studies. Additionally, we report long‐term follow ‐up of patients treated with ATIR101 in this study. At 1 year, all 9 patients receiving doses of 0·3–2 × 106 CD3+ cells/kg ATIR101 remained free of serious infections and after more than 8 years, TRM was 0%, relapse‐related mortality was 33% and overall survival was 67% in these patients.
Keywords: haematopoietic stem cell, stem cell transplantation, graft‐versus‐host‐disease, cell therapy and immunotherapy
Haematopoietic stem cell transplantation (HSCT) using a haploidentical family donor offers the potential for cure to the approximate 20–55% of patients requiring transplant who do not have a suitable human leucocyte antigen (HLA)‐matched related or unrelated donor (Barker et al, 2010; Petersdorf, 2010; Ballen et al, 2012; Pidala et al, 2013; Ruggeri et al, 2015; Sureda et al, 2015). Although the feasibility of haploidentical stem cell transplantation has been questioned over the decades due to lethal graft‐versus‐host disease (GVHD), developments have led to a rapid increase in the use of haploidentical HSCT in recent years, challenging even matched unrelated donor as standard of care (Sureda et al, 2015; Passweg et al, 2018). Today's haploidentical transplants usually involve different ex vivo or in vivo T‐cell depletion strategies to avoid severe acute GVHD (Locatelli et al, 2017; Zeiser & Blazar, 2017; Al Malki et al, 2018), a major complication of T‐replete allogeneic HSCT (Sureda et al, 2015). Importantly, early trials of ex vivo T‐cell depletion could achieve complete engraftment without causing GVHD (Aversa et al, 1998; Martelli & Aversa, 2016). However, the reduction of T cells results in delayed immune reconstitution and may lead to lethal opportunistic infections and disease relapse in a high number of patients (Aversa et al, 1998; Di Stasi et al, 2011). Therefore, ex vivo strategies, such as selective graft depletion of alpha‐beta‐T cells or insertion of suicide genes to donor lymphocytes, have been developed to facilitate the transfer of haploidentical lymphocytes while diminishing the challenges of life‐threatening infections, GVHD, and relapse (Al Malki et al, 2018). In vivo T‐cell depletion using cyclophosphamide is an extremely simple and effective approach to facilitating haploidentical transplantation, but it is also associated with the occurrence of graft failures and higher relapse rates after reduced‐intensity conditioning (Luznik et al, 2008; Brunstein et al, 2011; Ciurea et al, 2015; Byrne & Savani, 2016; Bashey et al, 2017; McCurdy et al, 2018). In addition, the long‐term patient outcomes following many of these strategies are currently unknown (Byrne & Savani, 2016).
Donor T cells are a prerequisite of long‐lasting immunity to fight infections and relapse (Deol & Lum, 2010). Attempts at prophylactic administration of untreated donor lymphocyte infusions at doses of 3 × 104 CD3+ cells/kg or greater after T‐cell‐depleted haploidentical HSCT in the absence of relapsing cells almost always resulted in GVHD (Lewalle et al, 2003). Therefore, several groups have worked on developing selective donor lymphocyte depletion strategies to remove host‐reacting donor T cells (Garderet et al, 1999; Andre‐Schmutz et al, 2002; Guimond et al, 2002; Amrolia et al, 2006; Mielke et al, 2007; Bleakley et al, 2015). We have taken advantage of the biological alterations that occur in donor cells upon exposure to patient cells, to selectively eliminate patient‐reactive T cells (Chen et al, 2002; Guimond et al, 2002; Mielke et al, 2008). Indeed, T‐cell activation is associated with P‐glycoprotein pump inhibition, which in turn leads to intracellular accumulation of the rhodamine‐derived photosensitizer TH9402, a substrate of this pump. Alloreactive T cells with GVHD‐causing properties can then be eliminated from the donor T‐cell repertoire following exposure to visible light, whereas resting T lymphocytes capable of anti‐infection and anti‐tumour reactivity are preserved (Chen et al, 2002; Guimond et al, 2002; Mielke et al, 2008; Perruccio et al, 2008). This is a phase I clinical study to determine the maximum tolerated dose (MTD) and evaluate the safety of allodepleted T‐cell immunotherapy (ATIR101), administered without GVHD prophylaxis, in the context of CD34+‐selected haploidentical HSCT. Our data informed two phase 2 studies evaluating ATIR101 as an adjunctive infusion following T‐cell‐depleted haploidentical HSCT (Roy et al, 2018a, 2018b). A phase 3, randomized trial has also been initiated, comparing haploidentical HSCT + ATIR101 versus T‐cell replete haploidentical HSCT + post‐transplant cyclophosphamide. Herein, we also report the long‐term follow‐up (more than 8 years) of patients enrolled in this phase 1 study to show potential of ATIR101 to impact favourably on transplant‐related mortality (TRM), relapse and survival in the long‐term.
Materials and methods
Patients and donors
Adults with high‐risk haematological malignancies without possibility of transplant from an HLA‐matched sibling donor, and lacking an 6/6 HLA‐A, B and DRB1 matched unrelated donor within 2–3 months, were enrolled into this phase I, single‐centre, dose‐ranging, open‐label study (NCT00993486; see Appendix S1 for inclusion criteria). The objective was to determine the MTD and safety of ATIR101 in patients undergoing haploidentical peripheral blood HSCT with CD34+ cell selection. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and approved by the ethics committee of Hôpital Maisonneuve‐Rosemont. All patients and donors gave written informed consent. Patients meeting eligibility criteria were treated between January 2005 and August 2008 with an 8‐year median follow‐up of survivors.
Patient conditioning and transplant
Patients received myeloablative conditioning including total body irradiation (TBI) at a dose of 12 Gy, in six fractions of 2 Gy given twice daily over 3 days (starting 10 days prior to HSCT). The lungs were shielded to receive a maximum of 9 Gy. Thiotepa (5 mg/kg) was administered at 12‐h intervals on the day following TBI. Starting the next day (2 days after TBI), rabbit antithymocyte globulin 2·5 mg/kg/day (Thymoglobulin; Genzyme, Mississauga, Ontario, Canada) was infused over at least a 6‐h period for 5 days. Patients received methylprednisolone (1 mg/kg) intravenously twice daily on the antithymocyte globulin‐infusion days. Fludarabine was given at a dose of 40 mg/m2/day for 4 days starting 7 days prior to transplant. No immune suppressors were used after transplant. All donor peripheral blood CD34+ cells collected and isolated were infused on Day 0. Donor chimerism in lymphoid and myeloid compartments was measured at regular intervals before and after ATIR101 infusion (Appendix S1).
Manufacture of ATIR101 under good manufacturing practice conditions
See Appendix S1 for details of ATIR101 manufacturing. Photodepletion of host‐activated T cells in ATIR101 was evaluated immunophenotypically (CD25+ CD44+), and limiting dilution assays were used to calculate the frequencies of anti‐host and anti‐third party responding cytotoxic T‐lymphocyte precursors (CTLp), using previously described methods (Appendix S1) (Guimond et al, 2002). Cells were cryopreserved until infusion 28–42 days after HSCT.
Determination of MTD
Seven doses of ATIR101 were evaluated: 1 × 104 (L1); 5 × 104 (L2); 1·3 × 105 (L3); 3·2 × 105 (L4); 7·9 × 105 (L5); 2 × 106 (L6) and 5 × 106 (L7) T cells/kg. MTD was defined a priori as the dose of T cells whereby dose‐limiting toxicity (DLT: grade III or IV acute GVHD within 30 days of ATIR101) would occur in 33% of patients. If the first L1 patient did not experience a DLT, the following 3 patients received the L2 dose; the study would be terminated if 2 patients at the L1 dose level were to experience a DLT (Appendix S1). After L1, 18 patients were to be treated at L2–L7 (in cohorts of 3) until MTD was determined. Acute GVHD and chronic GVHD (National Institutes of Health classification) were histologically graded as published (Glucksberg et al, 1974; Filipovich et al, 2005).
Secondary variables
The secondary objective was to determine the safety of ATIR101. All adverse events (AEs) were monitored from HSCT until 30 days after ATIR101 infusion; serious adverse events (SAEs) were monitored throughout the study. Prophylaxis and monitoring for infections is described in Appendix S1.
Efficacy evaluations included immune reconstitution and relapse rate and, in addition to the pre‐planned analysis of overall survival (OS), TRM and relapse‐related mortality (RRM) have been exploratively analysed. TRM was defined as death due to causes other than disease relapse or progression. RRM was defined as death due to disease relapse or disease progression. Patient mononuclear cell populations pre and post transplantation were evaluated using flow cytometry (Appendix S1). See Appendix S1 for a description of statistical analyses. Due to the small numbers of patients in each dose group, statistical comparisons of clinical outcomes between doses were not carried out.
Results
Patient characteristics
Nineteen adult patients (median age: 54 years; range: 20–62) with high‐risk haematological malignancies underwent haploidentical HSCT followed by infusion of a single dose of ATIR101 at a median of 31 days after transplant (range 28–40; patient and disease characteristics are provided in Table 1). At each dose level except for L1 (which included 1 patient as planned), 3 patients were given ATIR101. All patients except 1 at L7 received the planned ATIR101 dose. The majority of patients had high‐risk acute leukaemia or myelodysplastic syndrome (MDS). Importantly, 14 patients (74%) had active disease (including early relapse, partial remission or refractory disease) at time of HSCT and 10 patients (53%) had a disease history with ≥1 relapse. All donors were HLA‐A, ‐B, and ‐DR mismatched at 2–3/6 loci, except for 1 patient who received a single HLA‐DR mismatched graft.
Table 1.
Patient characteristics
| Dose level | Patient number | Patient sex (age, years) | Donor: sex, age (years), relationship to patient | Diagnosis | Disease status at HSCT | HLA matches (A, B, DR), (n/6) | CD34+ cell dose, ×106 cells/kg | CD3+ cell dose, ×104 cells/kg | Days from HSCT to ATIR101 infusion | ATIR101 dose, T cells/kg |
|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 1 | M (54) | M, 50, brother | CLL | Refractory | 4 | 8·27 | 1·09 | 32 | 1·0 × 104 |
| L2 | 2 | F (57) | F, 31, daughter | AML | Relapse 2 | 3 | 12·80 | 1·60 | 31 | 5·0 × 104 |
| L2 | 3 | F (58) | M, 27, son | AML transformed from MDS | CR1 | 4 | 12·40 | 2·00 | 33 | 5·0 × 104 |
| L2 | 4 | M (59) | M, 33, son | MDS (RA) | Refractory | 3 | 7·40 | 0·97 | 28 | 5·0 × 104 |
| L3 | 5 | M (40) | F, 34, sister | NHL relapsed post transplant | CR3 | 5 | 5·93 | 1·12 | 34 | 1·3 × 105 |
| L3 | 6 | M (58) | M, 32, son | AML transformed from MDS | PR | 3 | 13·70 | 0·65 | 28/312† | 1·3 × 105 |
| L3 | 7 | F (52) | F, 28, daughter | AML | Relapse 1 | 4 | 8·70 | 0·78 | 31 | 1·3 × 105 |
| L4 | 8 | M (55) | M, 58, brother | Acute myelofibrosis | Relapse | 3 | 7·00 | 1·04 | 28/1294† | 3·2 × 105 |
| L4 | 9 | M (21) | M, 56, father | AML | Ref‐Rel | 3 | 10·35 | 1·76 | 31 | 3·2 × 105 |
| L4 | 10 | F (61) | F, 35, daughter | AML | CR3 | 4 | 13·00 | 1·96 | 28 | 3·2 × 105 |
| L5 | 11 | M (59) | M, 22, son | AML | Relapse 1 | 3 | 13·90 | 1·43 | 40 | 7·9 × 105 |
| L5 | 12 | F (20) | M, 45, uncle | Acute biphenotypic leukaemia | Ref‐Rel | 3 | 11·20 | 1·30 | 28 | 7·9 × 105 |
| L5 | 13 | M (60) | M, 34, son | MDS: RAEB | Untreated | 4 | 12·35 | 1·40 | 28 | 7·9 × 105 |
| L6 | 14 | F (38) | M, 41, brother | MDS: RAEB | Refractory | 3 | 15·19 | 0·42 | 28 | 2·0 × 106 |
| L6 | 15 | M (37) | M, 45, brother | CML | PR‡ | 4 | 6·50 | 1·41 | 32 | 2·0 × 106 |
| L6 | 16 | F (43) | M, 39, brother | AML | CR1§ | 3 | 9·59 | 0·75 | 28 | 2·0 × 106 |
| L7 | 17 | F (54) | F, 61, sister | AML transformed from MDS | CR1 | 4 | 8·11 | 1·75 | 34 | 2·6 × 106 |
| L7 | 18 | M (44) | M, 46, brother | AML transformed from MDS | Relapse | 4 | 13·07 | 1·52 | 33 | 5·0 × 106 |
| L7 | 19 | M (62) | F, 38, daughter | CLL | PR | 3 | 10·52 | 1·95 | 28 | 5·0 × 106 |
AML, acute myeloid leukaemia; CLL, chronic lymphocytic leukaemia; CML, chronic myeloid leukaemia; CR, complete remission; F, female; HLA, human leucocyte antigen; HSCT, haematopoietic stem cell transplantation; M, male; MDS, myelodysplastic syndrome; NHL, non‐Hodgkin lymphoma relapsed after autologous stem cell transplantation; PR, partial remission; RA, refractory anaemia; RAEB, refractory anaemia with excess blasts; Ref‐Rel, refractory relapse.
Patient received a second ATIR101 infusion (same dose as first infusion).
After exposure to 3 tyrosine kinase inhibitors.
Molecular relapse.
HSCT and engraftment
After their conditioning regimen, patients received a median of 10·5 × 106 immunomagnetically isolated CD34+ cells/kg (range: 5·9–15·2 × 106) on Day 0, with a median graft T‐cell content of 1·4 × 104 CD3+ cells/kg (range: 0·4–2·0 × 104). Neutrophil (≥0·5 × 109/l) and platelet (≥20 × 109/l) engraftment were achieved rapidly at a median of 10 (range: 8–21) and 11 (range: 6–158) days, respectively. Complete (100%) and sustained donor chimerism in both myeloid and lymphoid lineages occurred in all patients within 3 weeks of transplantation, except for the L1 patient who showed 66% donor chimerism at Week 3 and died 1 month after the ATIR101 infusion due to multiple organ failure, secondary to post‐transplant lymphoproliferative disorder (Epstein–Barr virus [EBV] infection present at time of transplant). No patient experienced late graft rejection or failure.
Photodepletion of host‐reactive cells preserves functional T cells
The ATIR101 product was successfully generated for all patients. Photodepletion of activated T cells and preservation of resting T cells in the ATIR101 product were confirmed immunophenotypically for all products (Fig 1A,B). Indeed, activated CD4+ and CD8+ T cells (CD25+ CD44+) were drastically reduced, whereas the majority of resting CD4+ and CD8+ T cells were preserved. In addition, photodepletion decreased donor CTLp directed against recipient cells by a mean of 1·14 log (93% elimination; Fig 1C). In contrast, anti‐third party CTLp were preserved, indicating that ATIR101 T cells remained immunoreactive against other targets (P = 0·0008).
Figure 1.
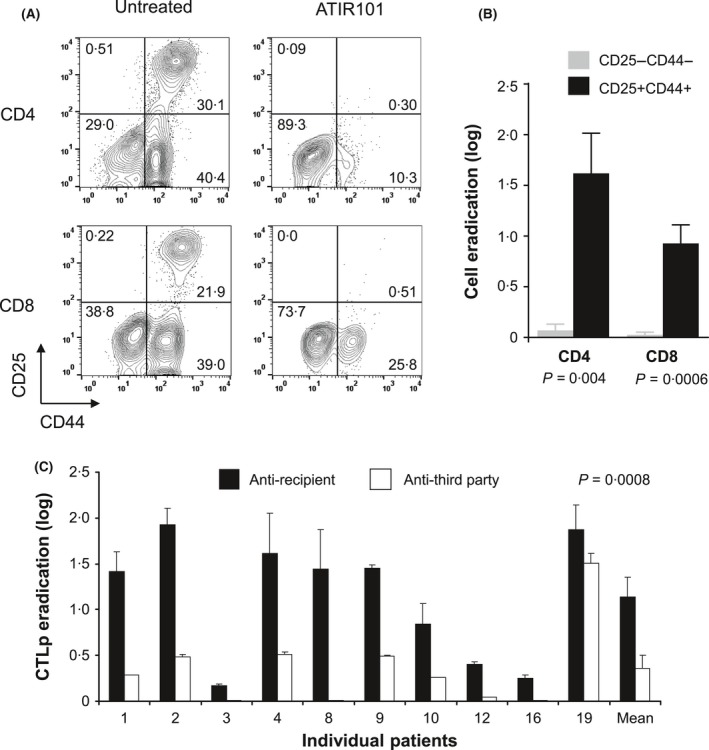
Specific elimination of alloreactive T cells and preservation of functional immune response after TH9402 photodepletion. (A) immunophenotypic analysis of resting (CD25− CD44−) and activated (CD25+ CD44+) CD4+ and CD8+ T‐cell populations in ATIR101 cell products 3 days after photodepletion (illustrative example from 1 patient). (B) compilation of cell eradication of resting (grey bar) and activated (black bar) CD4+ and CD8+ T cells after photodepletion (11 independent patient samples). Results are expressed as mean ± standard error of the mean (SEM) in logs of cell eradication relative to pre‐photodepletion samples. (C) The impact of photodepletion on the frequency of the cytotoxic T‐lymphocyte precursors (CTLp) directed against recipient (black) and third party (white) cells. Logs of eradication are shown for each evaluable patient. Last bars represent cumulative response of all patients (mean ± SEM). P‐value corresponds to paired t‐test.
Among CD4+ cells, photodepletion decreased the number of terminally differentiated effector memory cells (TEMRA:phenotype; Fig 2D; P = 0·02), with a corresponding increase in central memory (TCM) cells (Fig 2B; P = 0·009). In contrast, in CD8+ cells, a similar decrease in TEMRA led to a relative increase in naïve cells (Fig 2H,E, respectively; both P = 0·02).
Figure 2.
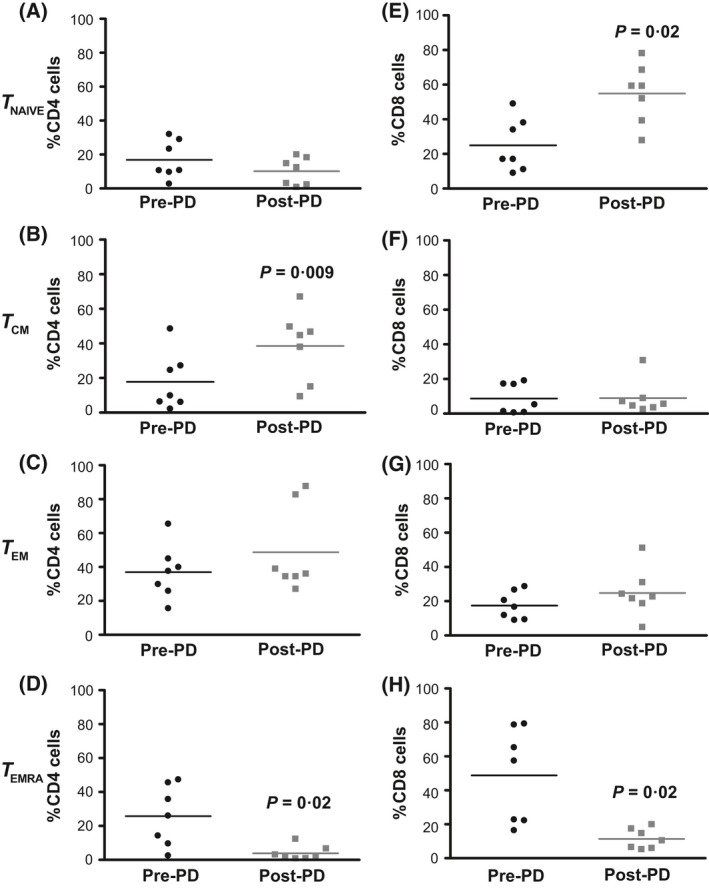
Distribution of memory T‐cell subsets in ATIR101 cells before and after photodepletion. Memory T‐cells were analysed pre‐ and post‐photodepletion (PD) by flow cytometry for CD62L, CD45RA and CD45RO expression and gated on CD4 (A–D) or CD8 (E–H) cells. CD45RO − CD45RA + CD62L+ defined TNAIVE; CD45RO + CD45RA − CD62L−, effector memory (TEM) cells; CD45RO + CD45RA − CD62L+, central memory (TCM); and CD45RO − CD45RA + CD62L−, terminally differentiated effector (TEMRA) cells. Bars represent mean values (n = 7) and P values correspond to paired t‐tests between pre‐ and post‐photodepletion results.
Safety and GVHD
Treatment‐emergent AEs in the first 30 days after ATIR101 infusion occurred in 17 patients, the most frequent being constipation (n = 6), pancytopenia (n = 4), pyrexia (n = 4), nausea (n = 3) and diarrhoea (n = 3). Besides GVHD, no treatment‐emergent AEs in this period were considered to be ATIR101 related. Overall, SAEs occurred in 17 patients, the most frequent being pyrexia (n = 10), multiple organ failure (n = 6), pancytopenia, pneumonia and recurrent leukaemia (all n = 4), and disease progression and sinusitis (both n = 3). None of the SAEs were considered to be related to the ATIR101 infusion.
Overall, 10 patients (53%) developed acute (grade I/II; n = 5) or chronic (extensive; n = 5) GVHD during the study. Five of the 10 patients with GVHD were in the 2 highest dose groups (L6 and L7), suggesting a higher probability of GVHD at higher ATIR101 doses. None of the cases of GVHD were considered to be an SAE.
The patient at ATIR101 dose L1 had grade I acute GVHD of the skin before ATIR101 infusion but no GVHD after ATIR101 administration. Therefore only 4 patients (1 each in L2 and L6; 2 in L7) developed acute GVHD post ATIR101 infusion; in these patients, this occurred late following treatment (21%; all grade II; median 102 days post HSCT). The clinical features and treatment of acute GVHD post ATIR101 are described in Table SI. Overall, 5 patients developed de novo chronic GVHD (highest severity, score 3, for skin rash in all 5 patients but without scleroderma) at a median of 144 days post HSCT (1 patient each at the L2, L4, L5, L6 and L7 doses).
Eight of the 10 patients developing GVHD responded well and rapidly to oral immunosuppressive treatment (median duration of immune suppressors was 6 months). Although 5 patients who died had GVHD at the time of death, GVHD was steroid‐sensitive in all patients, and has not been reported as the main cause of any deaths.
T‐cell recovery
Populations of T cells (CD3+, CD4+, CD8+) were measured by multiparameter flow cytometry in peripheral blood from ATIR101 infusion to 1 year post‐HSCT. Although T cells were low in the first months after HSCT, they started to recover 3–6 months after ATIR101 infusion (Fig 3A). As expected, the recovery of CD4+ (helper T cells) and CD8+ (cytotoxic T cells) T‐cell subsets was comparable to that of CD3+ cells, interestingly with higher levels of CD4+ than CD8+ cells present at 12 months (Fig 3B, C). Interestingly, patients also recovered B lymphocytes and NK cells rapidly after HSCT (Figure S1).
Figure 3.
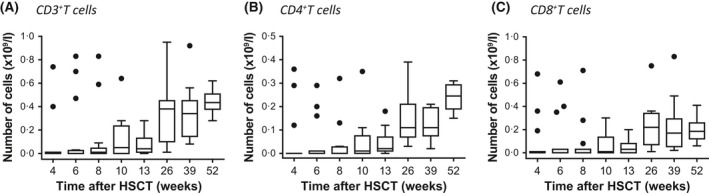
Lymphocyte reconstitution after infusion of ATIR101. Box and whisker plots of T cells (A: CD3+; B: CD4+; C: CD8+) from patients’ peripheral blood after ATIR101 infusion and through to 1 year post‐haematopoietic stem cell transplantation (HSCT). Circles indicate the outliers, as calculated by the Tukey method to identify outliers.
Infections
The proportion of patients with clinically significant infections and infections reported as SAEs, from the ATIR101 infusion until 1 year post‐HSCT, are shown in Fig 4. The figure shows patients grouped according to dose cohorts: L1–L3 (n = 7), L4–L6 (n = 9), and L7 (n = 3). All of the patients in the lower‐dose cohorts (L1–L3) and the highest‐dose cohort (L7) had at least 1 clinically significant infection. Six of the 7 patients in cohorts L1–L3 had an infection reported as an SAE (85·7%; including an EBV infection in the patient receiving the L1 dose that was active at the time of transplantation and reported as an SAE post‐transplant). The high incidence of serious infections at these lower doses of ATIR101 is probably due to having insufficient levels of T cells. All 3 patients in the L7 cohort also had infections reported as SAEs in the first year post‐HSCT. Although 8 of the 9 patients (88.9%) in cohorts L4–L6 developed at least 1 clinically significant infection (Patient 16 in L6 had no clinically significant infections after ATIR101 infusion), no infections were reported as SAEs in this group.
Figure 4.
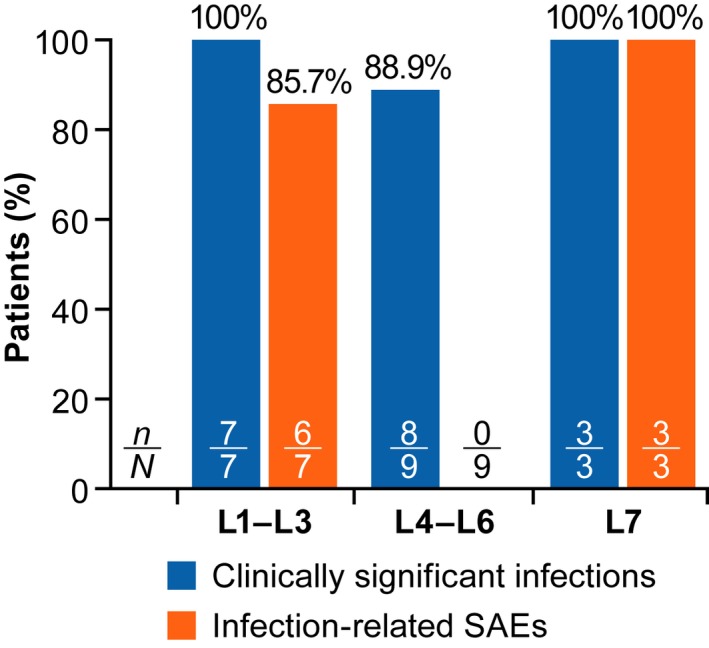
Patients with infections with onset from ATIR101 infusion to 1 year post HSCT. Proportion of patients experiencing a clinically significant infection (blue) or infection‐related serious adverse events (SAE; orange) from ATIR101 infusion to 1 year after haematopoietic stem cell transplantation. The patient at the L1 dose had active Epstein–Barr virus (EBV) infection at time of transplant, which resulted in multiple organ failure and was reported as an SAE after transplant.
Eight patients were at risk for cytomegalovirus (CMV) infection, but only 3 developed CMV viraemia, which led to CMV disease in 2 cases. In 1 case, the patient had received a low ATIR101 dose (L2) and died of CMV disease. In contrast, the other patient with CMV reactivation had received a higher ATIR101 dose (L7) and responded to ganciclovir. Despite more than 90% of patients having a positive EBV serology at the time of transplant (data not shown), increasing EBV polymerase chain reaction (PCR) titres were noted in only 5 patients (26%). The first patient had received the lowest ATIR101 dose (1 × 104 CD3+ cells/kg) and died shortly thereafter. Rising EBV titres were observed in 2 patients receiving ATIR101 as the only treatment for their EBV infection. In both patients, ATIR101 infusion at doses of 3 × 105 or 2 × 106 CD3+ cells/kg resulted in a sustained complete disappearance of EBV without the addition of any rituximab (Fig 5A,B). The other 2 patients with EBV titres rising above 250 000 copies/ml received rituximab (Fig 5C,D) (Reddy et al, 2011). A single dose of rituximab was sufficient to control the viremia; in 1 of these 2 patients the elevated EBV PCR titre had already subsided at the time of rituximab administration (Fig 5D). Although T cell levels were low in all of these patients’ peripheral blood, CD3+, CD4+, and CD8+ cell levels increased in response to increasing EBV titres, suggesting a role for T‐cell expansion in the clearance of EBV infection.
Figure 5.
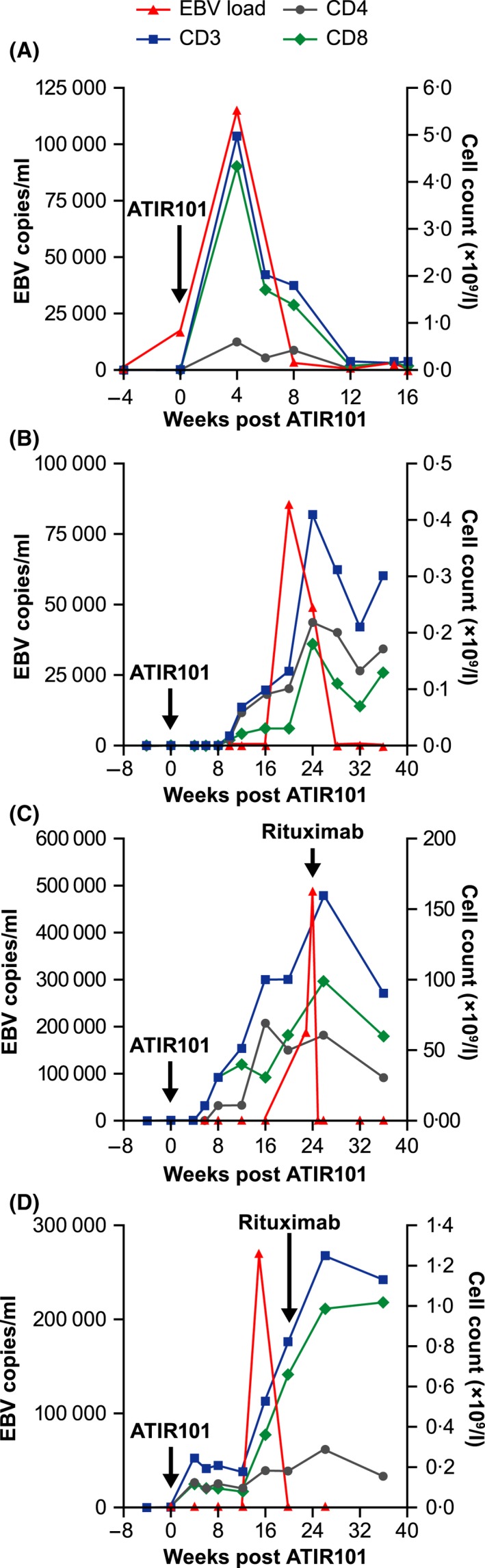
Ability of ATIR101 to control EBV reactivation. (A–D) Epstein–Barr virus (EBV) titres (red triangles) and absolute numbers of CD3+ (blue squares), CD4+ (grey circles) and CD8+ (green diamonds) cells according to the number of weeks after ATIR101 injection, each of the 4 panels depicting an individual patient. Patients in panels (A) and (B) received ATIR101 and no rituximab, whereas only patients in panels (C) and (D) received rituximab at the indicated timepoints.
Long‐term patient outcomes
Twelve of the 19 patients in this very high‐risk population died during this long‐term follow‐up (8‐year OS 37%; Fig 6A): 7 due to TRM (infections and other causes) and 5 due to relapse. In total, 8 patients relapsed after a median of 10·8 months following HSCT, with no cases of relapse occurring after 39·5 months through to more than 8 years of follow‐up. Only 1 of the patients with relapse was in complete remission (CR) at baseline (RRM at 30·8 months). Kaplan–Meier curves for OS, TRM, and RRM are shown in Fig 6A,C,E.
Figure 6.
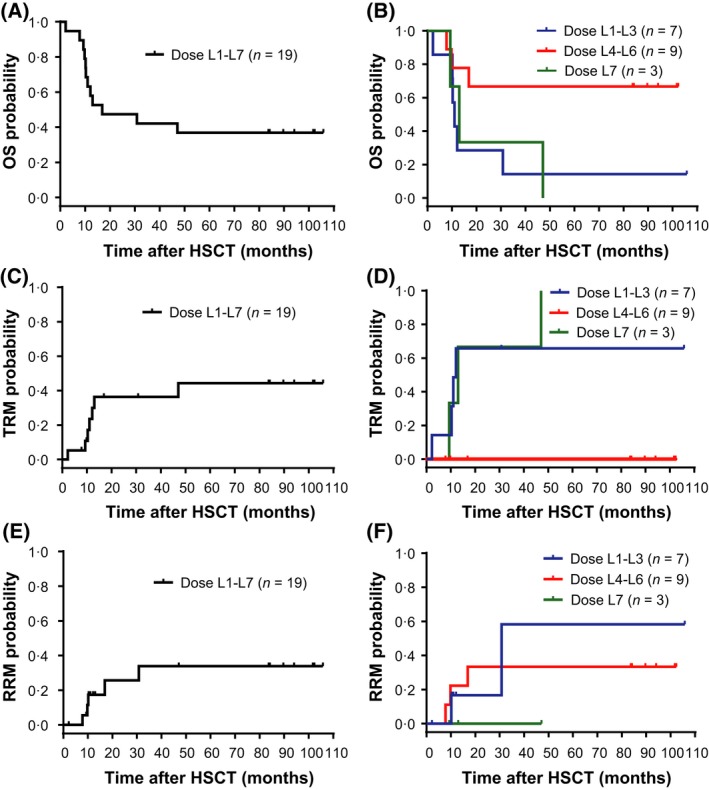
Long‐term patient outcomes. Kaplan–Meier plots for OS (A, B); TRM (C, D); and RRM (E, F) for up to 9 years post‐HSCT. Panels (A), (C), and (E) display survival and mortality for all patients who were administered ATIR101 (N = 19). Panels (B), (D), and (F) display survival and mortality separated according to the combined L1–L3 (blue line; n = 7), L4–L6 (red line; n = 9), and the L7 (green line; n = 3) ATIR101 dose cohorts. HSCT, haematopoietic stem cell transplantation; OS, overall survival; RRM, relapse‐related mortality; TRM, transplant‐related mortality.
There was no case of TRM in the 9 patients of the L4–L6 cohorts; in contrast, all 3 patients in the L7 cohort died due to TRM. The lack of TRM in the L4–L6 cohort is in line with the trend for decreased infectious episodes in cohorts L4–L6, compared with L1–L3 and L7 (Fig 4). To further explore any potential dose dependency, separate Kaplan–Meier curves for OS, TRM, and RRM were produced for the L1–L3 (n = 7), L4–L6 (n = 9) and the L7 (n = 3) cohorts (Fig 6B,D,F). There was a markedly lower TRM and higher OS in the combined L4–L6 group compared with the L1–L3 and L7 groups from 12 months after HSCT onwards. Finally, estimated 8‐year RRM was 33% in L4–L6 compared with 58% in the L1–L3 group, and was lowest in the L7 dose group (0%). Overall, long‐term follow‐up demonstrated that 67% of patients in the L4–L6 dose range survived 8 years post‐transplant, compared with 14% in L1–L3 and 0% in L7.
MTD and determination of phase 2 dose
Although ATIR101 doses of up to 5 × 106 CD3+ T cells/kg were administered in this study, MTD was not reached and no patient developed grade III/IV acute GVHD, despite the complete absence of GVHD immunoprophylaxis post‐transplant. As MTD was not reached, protocol Stage 3 to treat an additional 3 patients at the MTD was not completed (Appendix S1), and the appropriate dose for phase 2 was selected based on assessment of the doses evaluated.
Although RRM was lowest in the L7 dose group, all 3 of the patients in the L7 cohort experienced GVHD (2 grade II acute GVHD; 1 chronic GVHD), suggesting that increasing ATIR101 doses to 5·0 × 106 CD3+ cells/kg potentially led to clinically significant GVHD and a poor outcome. Indeed these patients required immunosuppressive agents and developed serious infections (Fig 4). Therefore, in light of the absence of serious infections, lower TRM and longer OS in the L4–L6 dose group, the L6 dose (2 × 106 cells/kg) was selected for subsequent phase 2 studies.
Discussion
This study evaluates a highly promising, well‐tolerated immunotherapeutic strategy allowing the delivery of high doses of ex vivo alloreactive‐depleted lymphocytes (ATIR101) from haploidentical donors early after T‐cell‐depleted transplant, in patients that did not receive post‐transplant immunosuppression as GVHD prophlaxis. Despite 5 × 106 allodepleted cells/kg being infused, none of the patients in this study developed grade III/IV acute GVHD. There were no deaths due to GVHD or GVHD cases that were classified as an SAE, and most GVHD cases responded rapidly to treatment. No other ATIR101‐related serious or non‐serious AEs were reported. As well as demonstrating the feasibility of this approach, our data also highlight the potential for long‐term disease‐free survival (more than 8 years) following haploidentical HSCT with ATIR101.
The absence of grade III/IV acute GVHD is particularly compelling in view of the major histocompatibility complex (MHC) disparity between donor and recipient, the complete absence of post‐transplant immune prophylaxis for GVHD, and the administration of T‐cell doses more than 100‐fold higher than those previously reported to cause lethal GVHD with unmanipulated T cells (Aversa et al, 1998). We believe that 3 factors contributed to the absence of grade III/IV acute GVHD or protracted chronic GVHD. First, our photodepletion process specifically eliminated more than 90% of both anti‐host activated cytotoxic precursors and CD4+ and CD8+ T cells. Second, photodepletion was previously shown to spare regulatory T cells, which may have a protective effect against GVHD (Mielke et al, 2008; Bastien et al, 2010, 2012; Koreth et al, 2011; Matsuoka et al, 2013; Martelli et al, 2014). Third, we speculate that our approach depletes both T cells reactive to HLAs encoded by the non‐shared haplotypes and T cells specific for the most immunogenic host minor histocompatibility antigens (MiHAs) presented by HLAs encoded by the shared haplotype (Perreault et al, 1998). Unlike infusions containing T lymphocytes genetically modified to alter their function, such as cells engineered with the herpes simplex thymidine kinase or caspase 9 inducible suicide genes (Ciceri et al, 2009; Zhou et al, 2015), ATIR101 cells are not genetically modified. Use of suicide gene‐transduced T cells does not prevent the occurrence of GVHD and can result in lymphodepletion upon activation of the molecular switch in patients developing GVHD. By preventing the occurrence of severe GVHD, ATIR101 may preserve lymphocytes with beneficial graft‐versu‐leukaemia (GVL) and graft‐versus‐infection effects.
Importantly, patients engrafted rapidly (median 10–11 days) and demonstrated complete donor chimerism without any late graft failure or rejection, which represents a favourable engraftment profile in comparison to most umbilical cord blood and T‐replete haploidentical HSCT strategies (Luznik et al, 2008; Brunstein et al, 2011; Scaradavou et al, 2013). All patients in cohorts L4–L6 remained free of serious infections at 1 year and the 8‐year TRM was 0% across these cohorts. For 3 of the 5 patients with rising EBV titres, EBV subsided following ATIR101 infusion as CD3+, CD4+ and CD8+ levels increased in response to infection. In general, post‐transplant T‐cell counts were initially low and started to increase 3–6 months after ATIR101 infusion. It has been reported that, in the absence of ATIR101, CD4+ T‐cell counts remain below 0·1 × 109/l for as long as 10 months following CD34+ selected grafts (Aversa et al, 1998). In addition, although CD4+ T‐cell counts usually reconstitute much later than CD8+ (Ogonek et al, 2016), it was observed that CD4+ levels were higher than CD8+ levels at 12 months, possibly accounting for potential benefits of ATIR101 infusion. Interestingly, most CD4+ and CD8+ naïve T‐cells were preserved in ATIR101 and in patients post‐transplant (Fig 2 and Figure S2) without causing severe GVHD, suggesting that anti‐host reactive T cells within this cell subset form a minority of cells and were eliminated (Anderson et al, 2003). The naive T‐cell repertoire, being approximately 100‐fold more diversified than the repertoire of memory T‐cells, (Arstila et al, 1999) is positioned to respond to a wider range of pathogens than memory T cells (Yager et al, 2008; Cicin‐Sain et al, 2010).
Though this study was not aimed at assessing survival, it is noteworthy that the included patients had particularly advanced haematological malignancies and consisted predominantly of refractory or relapsed adult leukaemia patients. Ten of the 14 patients with acute leukaemia or MDS were not in CR at time of transplant (1 of the 4 patients in CR was in molecular relapse) and 1 patient had relapsed acute myelofibrosis. Relapse occurred in 8 patients during the study (median of 10·8 months following HSCT); however, only 1 of these patients was in CR at the time of transplant. In fact, ATIR101 afforded the 9 patients in the L4–L6 cohorts an 8‐year survival rate of 67%, despite 7 of these patients having active disease at baseline. Any GVL effect related to ATIR101 most probably reflects the fact that host lymphohaematopoietic cells are highly susceptible to immune elimination by donor cells (Mori et al, 1998; Kolb et al, 2004). Thus, it is most likely that ex vivo photodepletion of ATIR101 eliminates enough host‐reactive cells to reduce the risk of grade III/IV GVHD, while potentially preserving sufficient T‐cell numbers to trigger anti‐host lymphohaematopoietic cells’ reaction and cause GVL. The fact that the in vivo milieu is a better immune‐stimulatory environment than our ex vivo culture system may thereby explain why, following T‐replete haploidentical HSCT, cyclophosphamide administered early post‐transplant may not only eliminate activated alloreactive T cells with the ability to cause GVHD but also deplete cells with GVL activity (Brunstein et al, 2011; Grosso et al, 2011; Ballen et al, 2012). This factor, along with the requirement of post‐transplant immune suppression to prevent the development of lethal GVHD, may compromise GVL effects needed to control malignant disease (Luznik et al, 2008; Brunstein et al, 2011; Ciurea et al, 2015; Rubio et al, 2015). Thus, ex vivo cell processing may provide a uniquely favourable and controlled environment to generate ‘selective’ depletion of alloreactive cells and preserve cells mediating GVL.
Formally, the MTD for ATIR101 was not reached in this dose‐escalation study; however, doses above 2 × 106 cells/kg might increase the risk of inducing GVHD, given that grade II acute GVHD was observed in 2 of the 3 patients in the highest dose cohort. As a result, the 2 × 106 cells/kg (L6) dose was selected for evaluation in two phase II clinical trials of haploidentical HSCT with ATIR101 administration post transplant (NCT01794299; NCT02500550) (Roy et al, 2018a, 2018b). Importantly, results of the first phase II study in patients with acute lymphoblastic and myeloid leukaemia in remission (unpublished observations) are in line with the present study, with absence of grade III/IV acute GVHD in the first year following ATIR101 infusion (2 × 106 cells/kg) in 23 patients and a 61% OS probability at 12 months post transplant (Roy et al, 2018a). The 12‐month OS probability in a pooled analysis of 37 patients receiving a single dose of ATIR101 in the two phase 2 studies was 58% (Roy et al, 2018b). The extensive duration of follow‐up for the present phase 1 study also highlights the potential for a durable benefit of ATIR101 treatment. In the absence of a clear standard between in vivo versus ex vivo T‐cell depletion methods, a large, phase III, randomized trial has also been initiated, comparing T‐cell‐depleted haploidentical HSCT + ATIR101 versus T‐cell replete haploidentical HSCT + post‐transplant cyclophosphamide (NCT02999854).
We demonstrate herein that photodepletion of anti‐host reactivity in the context of MHC‐mismatched cells offers a potential adjunctive treatment option allowing both anti‐infection and anti‐leukemia activity without promoting grade III/IV acute GVHD. Such an adjunct could be tested after in vivo allodepletion or other forms of haploidentical HSCT. The decreased toxicity afforded by the removal of post‐transplant immune suppression also has the potential to extend the upper age limit for transplantation and overcome comorbidities, further enabling potentially curative transplantation to more of the 20–55% of patients who do not currently have a suitable matched donor (Barker et al, 2010; Petersdorf, 2010; Ballen et al, 2012; Pidala et al, 2013; Sureda et al, 2015).
Funding
This study was supported by research funding from Kiadis Pharma and the Fonds de recherche du Québec – Santé (FRQS)‐ThéCell. Joanne McGrail, a medical writer supported by funding from Kiadis Pharma, provided drafts and editorial assistance to the authors during preparation of this manuscript.
Author contributions
D.C.R., S.L., S.C., G.S., T.K., L. Bernard, C.P. and J.R. designed the study. D.C.R., S.L., S.C., G.S., T.K., L. Busque, J‐S.D., I.A., L. Bernard, N.B. and J.R. contributed to the implementation of the clinical protocol. R.S.B., K.R., S.M., A.J.B., C.P. and D.C.R. contributed to the immune analyses and interpretation of the immune data. R.S.B. and M‐C.G. contributed to statistical analyses. D.C.R., A.J.B., S.M., K.R., C.P. and J.R. contributed to clinical data analysis and writing of the manuscript. All authors edited and approved the final manuscript.
Conflict of interest disclosure
D.C.R. is an author on a patent held by the Université de Montréal and Hôpital Maisonneuve‐Rosemont and has received research support from Kiadis Pharma. He has attended advisory boards by Novartis, BMS, Paladin and Fate Therapeutics. K.R. reports consulting fees during the conduct of the study. SM discloses consultancy outside the submitted work with Novartis and MSD (personal); speaker fees from Kiadis Pharma and Miltenyi Biotec (via institution), Jazz Pharma and Cellex (personal), and Celgene (personal and via institution); travel support from Kiadis Pharma and Miltenyi Biotec (via institution) and Celgene, MSD, Gilead, Cellex, DGHO and ISCT (other); research funding from Kiadis Pharma (other); EHA registration as a speaker (other). Outside the submitted work, L.B. has attended advisory boards for Novartis, Pfizer and BMS. Outside the submitted work, J.R. has received research grants from Celgene, Janssen and Sanofi Canada in the past 36 months and honoraria for conferences by Amgen, Sanofi, Celgene and Janssen Canada in the past 36 months. S.C. and J.R. declare royalties from ExCellThera outside the submitted work. The other authors declare no competing interests.
Supporting information
Fig S1. Immune reconstitution of B and NK cells. Box and whisker plots of B lymphocytes and NK cells (A: CD19+; B: CD56+) from patients’ peripheral blood after ATIR101 infusion and through to 1 year post‐HSCT.
Fig S2. Memory T cell recovery post‐transplant. Memory T cell subtypes: central memory (TCM, dark blue), effector memory (TEM, green), terminally differentiated effector (TEMRA, red) and TNAIVE (pale blue) were evaluated within CD4 (left panels) and CD8 (right panels) T‐cells for patients administered ATIR101 at dose levels L2–L3, L4–L5 and L6–L7 over the first 36 weeks post‐ATIR101 infusion.
Table SI. Overview of acute GVHD occurring following administration of ATIR101.
Appendix S1. Supplementary methods.
Acknowledgements
The authors would like to thank Drs. Irwin Walker, Ronan Foley and Brian Leber from the Juravinski Cancer Center, Hamilton, Canada, for helpful discussions and patient care; Marieve Cossette for statistical analyses; Mireille Guérin and Jean‐Philippe Bastien for their contribution to cell assays; and the members of the Hôpital Maisonneuve‐Rosemont Cellular Therapy Laboratory, Apheresis and Stem Cell Transplantation Units as well as Radiation Oncology, Microbiology and other consultants for clinical trial support. We would like to thank Dominik Selleslag, Philippe Lewalle and Johan Maertens from AZ Sint‐Jan Brugge‐Oostende, Jules Bordet Institute, and the Catholic University of Leuven in Belgium, respectively, and Robert Negrin and Ginna Laport from Stanford University Medical Center, California, USA, for clinical trial support. In addition, we would like to thank A. John Barrett from the George Washington University, Washington DC, USA, as an originator of selective depletion and for support in data analysis and writing of the paper. Finally, we thank Edwin Wagena, Jeroen Rovers, Manfred Ruediger, Lisya Gerez and Andrew Sandler from Kiadis Pharma for their insightful comments and analyses.
Clinicaltrials.gov identifier: NCT00993486
References
- Al Malki, M.M. , Jones, R. , Ma, Q. , Lee, D. , Reisner, Y. , Miller, J.S. , Lang, P. , Hongeng, S. , Hari, P. , Strober, S. , Yu, J. , Maziarz, R. , Mavilio, D. , Roy, D.C. , Bonini, C. , Champlin, R.E. , Fuchs, E.J. & Ciurea, S.O. (2018) Proceedings from the Fourth Haploidentical Stem Cell Transplantation Symposium (HAPLO2016), San Diego, California, December 1, 2016. Biology of Blood and Marrow Transplantation, 24, 895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrolia, P.J. , Muccioli‐Casadei, G. , Huls, H. , Adams, S. , Durett, A. , Gee, A. , Yvon, E. , Weiss, H. , Cobbold, M. , Gaspar, H.B. , Rooney, C. , Kuehnle, I. , Ghetie, V. , Schindler, J. , Krance, R. , Heslop, H.E. , Veys, P. , Vitetta, E. & Brenner, M.K. (2006) Adoptive immunotherapy with allodepleted donor T‐cells improves immune reconstitution after haploidentical stem cell transplantation. Blood, 108, 1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, B.E. , McNiff, J. , Yan, J. , Doyle, H. , Mamula, M. , Shlomchik, M.J. & Shlomchik, W.D. (2003) Memory CD4+ T cells do not induce graft‐versus‐host disease. The Journal of Clinical Investigation, 112, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre‐Schmutz, I. , Le Deist, F. , Hacein‐Bey‐Abina, S. , Vitetta, E. , Schindler, J. , Chedeville, G. , Vilmer, E. , Fischer, A. & Cavazzana‐Calvo, M. (2002) Immune reconstitution without graft‐versus‐host disease after haemopoietic stem‐cell transplantation: a phase 1/2 study. The Lancet, 360, 130–137. [DOI] [PubMed] [Google Scholar]
- Arstila, T.P. , Casrouge, A. , Baron, V. , Even, J. , Kanellopoulos, J. & Kourilsky, P. (1999) A direct estimate of the human alphabeta T cell receptor diversity. Science, 286, 958–961. [DOI] [PubMed] [Google Scholar]
- Aversa, F. , Tabilio, A. , Velardi, A. , Cunningham, I. , Terenzi, A. , Falzetti, F. , Ruggeri, L. , Barbabietola, G. , Aristei, C. , Latini, P. , Reisner, Y. & Martelli, M.F. (1998) Treatment of high‐risk acute leukemia with T‐cell‐depleted stem cells from related donors with one fully mismatched HLA haplotype. The New England Journal of Medicine, 339, 1186–1193. [DOI] [PubMed] [Google Scholar]
- Ballen, K.K. , Koreth, J. , Chen, Y.B. , Dey, B.R. & Spitzer, T.R. (2012) Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood, 119, 1972–1980. [DOI] [PubMed] [Google Scholar]
- Barker, J.N. , Byam, C.E. , Kernan, N.A. , Lee, S.S. , Hawke, R.M. , Doshi, K.A. , Wells, D.S. , Heller, G. , Papadopoulos, E.B. , Scaradavou, A. , Young, J.W. & van den Brink, M.R. (2010) Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biology of Blood and Marrow Transplantation, 16, 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashey, A. , Zhang, M.J. , McCurdy, S.R. , St Martin, A. , Argall, T. , Anasetti, C. , Ciurea, S.O. , Fasan, O. , Gaballa, S. , Hamadani, M. , Munshi, P. , Al Malki, M.M. , Nakamura, R. , O'Donnell, P.V. , Perales, M.A. , Raj, K. , Romee, R. , Rowley, S. , Rocha, V. , Salit, R.B. , Solh, M. , Soiffer, R.J. , Fuchs, E.J. & Eapen, M. (2017) Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T‐cell‐replete haploidentical donor transplantation using post‐transplant cyclophosphamide. Journal of Clinical Oncology, 35, 3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien, J.P. , Krosl, G. , Therien, C. , Rashkovan, M. , Scotto, C. , Cohen, S. , Allan, D.S. , Hogge, D. , Egeler, R.M. , Perreault, C. & Roy, D.C. (2010) Photodepletion differentially affects CD4+ Tregs versus CD4+ effector T cells from patients with chronic graft‐versus‐host disease. Blood, 116, 4859–4869. [DOI] [PubMed] [Google Scholar]
- Bastien, J.P. , Roy, J. & Roy, D.C. (2012) Selective T‐cell depletion for haplotype‐mismatched allogeneic stem cell transplantation. Seminars in Oncology, 39, 674–682. [DOI] [PubMed] [Google Scholar]
- Bleakley, M. , Heimfeld, S. , Loeb, K.R. , Jones, L.A. , Chaney, C. , Seropian, S. , Gooley, T.A. , Sommermeyer, F. , Riddell, S.R. & Shlomchik, W.D. (2015) Outcomes of acute leukemia patients transplanted with naive T cell‐depleted stem cell grafts. The Journal of Clinical Investigation, 125, 2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein, C.G. , Fuchs, E.J. , Carter, S.L. , Karanes, C. , Costa, L.J. , Wu, J. , Devine, S.M. , Wingard, J.R. , Aljitawi, O.S. , Cutler, C.S. , Jagasia, M.H. , Ballen, K.K. , Eapen, M. , O'Donnell, P.V. & Blood and Marrow Transplant Clinical Trials Network (2011) Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA‐mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood, 118, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, M. & Savani, B.N. (2016) The devil is in the T cells: relapsing after haploidentical hematopoietic cell transplantation. Bone Marrow Transplantation, 51, 915–918. [DOI] [PubMed] [Google Scholar]
- Chen, B.J. , Cui, X. , Liu, C. & Chao, N.J. (2002) Prevention of graft‐versus‐host disease while preserving graft‐versus‐leukemia effect after selective depletion of host‐reactive T cells by photodynamic cell purging process. Blood, 99, 3083–3088. [DOI] [PubMed] [Google Scholar]
- Ciceri, F. , Bonini, C. , Stanghellini, M.T. , Bondanza, A. , Traversari, C. , Salomoni, M. , Turchetto, L. , Colombi, S. , Bernardi, M. , Peccatori, J. , Pescarollo, A. , Servida, P. , Magnani, Z. , Perna, S.K. , Valtolina, V. , Crippa, F. , Callegaro, L. , Spoldi, E. , Crocchiolo, R. , Fleischhauer, K. , Ponzoni, M. , Vago, L. , Rossini, S. , Santoro, A. , Todisco, E. , Apperley, J. , Olavarria, E. , Slavin, S. , Weissinger, E.M. , Ganser, A. , Stadler, M. , Yannaki, E. , Fassas, A. , Anagnostopoulos, A. , Bregni, M. , Stampino, C.G. , Bruzzi, P. & Bordignon, C. (2009) Infusion of suicide‐gene‐engineered donor lymphocytes after family haploidentical haemopoietic stem‐cell transplantation for leukaemia (the TK007 trial): a non‐randomised phase I‐II study. The Lancet. Oncology, 10, 489–500. [DOI] [PubMed] [Google Scholar]
- Cicin‐Sain, L. , Smyk‐Pearson, S. , Currier, N. , Byrd, L. , Koudelka, C. , Robinson, T. , Swarbrick, G. , Tackitt, S. , Legasse, A. , Fischer, M. , Nikolich‐Zugich, D. , Park, B. , Hobbs, T. , Doane, C.J. , Mori, M. , Axthelm, M.K. , Lewinsohn, D.A. & Nikolich‐Zugich, J. (2010) Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. Journal of Immunology, 184, 6739–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea, S.O. , Zhang, M.J. , Bacigalupo, A.A. , Bashey, A. , Appelbaum, F.R. , Aljitawi, O.S. , Armand, P. , Antin, J.H. , Chen, J. , Devine, S.M. , Fowler, D.H. , Luznik, L. , Nakamura, R. , O'Donnell, P.V. , Perales, M.A. , Pingali, S.R. , Porter, D.L. , Riches, M.R. , Ringden, O.T. , Rocha, V. , Vij, R. , Weisdorf, D.J. , Champlin, R.E. , Horowitz, M.M. , Fuchs, E.J. & Eapen, M. (2015) Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood, 126, 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deol, A. & Lum, L.G. (2010) Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treatment Reviews, 36, 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi, A. , Tey, S.K. , Dotti, G. , Fujita, Y. , Kennedy‐Nasser, A. , Martinez, C. , Straathof, K. , Liu, E. , Durett, A.G. , Grilley, B. , Liu, H. , Cruz, C.R. , Savoldo, B. , Gee, A.P. , Schindler, J. , Krance, R.A. , Heslop, H.E. , Spencer, D.M. , Rooney, C.M. & Brenner, M.K. (2011) Inducible apoptosis as a safety switch for adoptive cell therapy. The New England Journal of Medicine, 365, 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovich, A.H. , Weisdorf, D. , Pavletic, S. , Socie, G. , Wingard, J.R. , Lee, S.J. , Martin, P. , Chien, J. , Przepiorka, D. , Couriel, D. , Cowen, E.W. , Dinndorf, P. , Farrell, A. , Hartzman, R. , Henslee‐Downey, J. , Jacobsohn, D. , McDonald, G. , Mittleman, B. , Rizzo, J.D. , Robinson, M. , Schubert, M. , Schultz, K. , Shulman, H. , Turner, M. , Vogelsang, G. & Flowers, M.E. (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. Diagnosis and staging working group report. Biology of Blood and Marrow Transplantation, 11, 945–956. [DOI] [PubMed] [Google Scholar]
- Garderet, L. , Snell, V. , Przepiorka, D. , Schenk, T. , Lu, J.G. , Marini, F. , Gluckman, E. , Andreeff, M. & Champlin, R.E. (1999) Effective depletion of alloreactive lymphocytes from peripheral blood mononuclear cell preparations. Transplantation, 67, 124–130. [DOI] [PubMed] [Google Scholar]
- Glucksberg, H. , Storb, R. , Fefer, A. , Buckner, C.D. , Neiman, P.E. , Clift, R.A. , Lerner, K.G. & Thomas, E.D. (1974) Clinical manifestations of graft‐versus‐host disease in human recipients of marrow from HL‐A‐matched sibling donors. Transplantation, 18, 295–304. [DOI] [PubMed] [Google Scholar]
- Grosso, D. , Carabasi, M. , Filicko‐O'Hara, J. , Kasner, M. , Wagner, J.L. , Colombe, B. , Cornett Farley, P. , O'Hara, W. , Flomenberg, P. , Werner‐Wasik, M. , Brunner, J. , Mookerjee, B. , Hyslop, T. , Weiss, M. & Flomenberg, N. (2011) A 2‐step approach to myeloablative haploidentical stem cell transplantation: a phase 1/2 trial performed with optimized T‐cell dosing. Blood, 118, 4732–4739. [DOI] [PubMed] [Google Scholar]
- Guimond, M. , Balassy, A. , Barrette, M. , Brochu, S. , Perreault, C. & Roy, D.C. (2002) P‐glycoprotein targeting: a unique strategy to selectively eliminate immunoreactive T cells. Blood, 100, 375–382. [DOI] [PubMed] [Google Scholar]
- Kolb, H.J. , Schmid, C. , Barrett, A.J. & Schendel, D.J. (2004) Graft‐versus‐leukemia reactions in allogeneic chimeras. Blood, 103, 767–776. [DOI] [PubMed] [Google Scholar]
- Koreth, J. , Matsuoka, K. , Kim, H.T. , McDonough, S.M. , Bindra, B. , Alyea, E.P. III , Armand, P. , Cutler, C. , Ho, V.T. , Treister, N.S. , Bienfang, D.C. , Prasad, S. , Tzachanis, D. , Joyce, R.M. , Avigan, D.E. , Antin, J.H. , Ritz, J. & Soiffer, R.J. (2011) Interleukin‐2 and regulatory T cells in graft‐versus‐host disease. The New England Journal of Medicine, 365, 2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewalle, P. , Triffet, A. , Delforge, A. , Crombez, P. , Selleslag, D. , De Muynck, H. , Bron, D. & Martiat, P. (2003) Donor lymphocyte infusions in adult haploidentical transplant: a dose finding study. Bone Marrow Transplantation, 31, 39–44. [DOI] [PubMed] [Google Scholar]
- Locatelli, F. , Merli, P. , Pagliara, D. , Li Pira, G. , Falco, M. , Pende, D. , Rondelli, R. , Lucarelli, B. , Brescia, L.P. , Masetti, R. , Milano, G.M. , Bertaina, V. , Algeri, M. , Pinto, R.M. , Strocchio, L. , Meazza, R. , Grapulin, L. , Handgretinger, R. , Moretta, A. , Bertaina, A. & Moretta, L. (2017) Outcome of children with acute leukemia given HLA‐haploidentical HSCT after alphabeta T‐cell and B‐cell depletion. Blood, 130, 677–685. [DOI] [PubMed] [Google Scholar]
- Luznik, L. , O'Donnell, P.V. , Symons, H.J. , Chen, A.R. , Leffell, M.S. , Zahurak, M. , Gooley, T.A. , Piantadosi, S. , Kaup, M. , Ambinder, R.F. , Huff, C.A. , Matsui, W. , Bolanos‐Meade, J. , Borrello, I. , Powell, J.D. , Harrington, E. , Warnock, S. , Flowers, M. , Brodsky, R.A. , Sandmaier, B.M. , Storb, R.F. , Jones, R.J. & Fuchs, E.J. (2008) HLA‐haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high‐dose, posttransplantation cyclophosphamide. Biology of Blood and Marrow Transplantation, 14, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli, M.F. & Aversa, F. (2016) Haploidentical transplants using ex vivo T‐cell depletion. Seminars in Hematology, 53, 252–256. [DOI] [PubMed] [Google Scholar]
- Martelli, M.F. , Di Ianni, M. , Ruggeri, L. , Falzetti, F. , Carotti, A. , Terenzi, A. , Pierini, A. , Massei, M.S. , Amico, L. , Urbani, E. , Del Papa, B. , Zei, T. , Iacucci Ostini, R. , Cecchini, D. , Tognellini, R. , Reisner, Y. , Aversa, F. , Falini, B. & Velardi, A. (2014) HLA‐haploidentical transplantation with regulatory and conventional T‐cell adoptive immunotherapy prevents acute leukemia relapse. Blood, 124, 638–644. [DOI] [PubMed] [Google Scholar]
- Matsuoka, K. , Koreth, J. , Kim, H.T. , Bascug, G. , McDonough, S. , Kawano, Y. , Murase, K. , Cutler, C. , Ho, V.T. , Alyea, E.P. , Armand, P. , Blazar, B.R. , Antin, J.H. , Soiffer, R.J. & Ritz, J. (2013) Low‐dose interleukin‐2 therapy restores regulatory T cell homeostasis in patients with chronic graft‐versus‐host disease. Science Translational Medicine, 5, 179ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy, S.R. , Zhang, M.J. , St Martin, A. , Al Malki, M.M. , Bashey, A. , Gaballa, S. , Keesler, D.A. , Hamadani, M. , Norkin, M. , Perales, M.A. , Reshef, R. , Rocha, V. , Romee, R. , Solh, M. , Urbano‐Ispizua, A. , Waller, E.K. , Fuchs, E.J. & Eapen, M. (2018) Effect of donor characteristics on haploidentical transplantation with posttransplantation cyclophosphamide. Blood Advances, 2, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, S. , Rezvani, K. , Savani, B.N. , Nunes, R. , Yong, A.S. , Schindler, J. , Kurlander, R. , Ghetie, V. , Read, E.J. , Solomon, S.R. , Vitetta, E.S. & Barrett, A.J. (2007) Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25‐depleted allotransplantation in elderly patients and association with acute graft‐versus‐host disease. Blood, 110, 1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke, S. , Nunes, R. , Rezvani, K. , Fellowes, V.S. , Venne, A. , Solomon, S.R. , Fan, Y. , Gostick, E. , Price, D.A. , Scotto, C. , Read, E.J. & Barrett, A.J. (2008) A clinical‐scale selective allodepletion approach for the treatment of HLA‐mismatched and matched donor‐recipient pairs using expanded T lymphocytes as antigen‐presenting cells and a TH9402‐based photodepletion technique. Blood, 111, 4392–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, T. , Nishimura, T. , Ikeda, Y. , Hotta, T. , Yagita, H. & Ando, K. (1998) Involvement of Fas‐mediated apoptosis in the hematopoietic progenitor cells of graft‐versus‐host reaction‐associated myelosuppression. Blood, 92, 101–107. [PubMed] [Google Scholar]
- Ogonek, J. , Kralj Juric, M. , Ghimire, S. , Varanasi, P.R. , Holler, E. , Greinix, H. & Weissinger, E. (2016) Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Frontiers in Immunology, 7, 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg, J.R. , Baldomero, H. , Bader, P. , Basak, G.W. , Bonini, C. , Duarte, R. , Dufour, C. , Kroger, N. , Kuball, J. , Lankester, A. , Montoto, S. , Nagler, A. , Snowden, J.A. , Styczynski, J. , Mohty, M. & European Society for Blood and Marrow Transplantation (EBMT) . (2018) Is the use of unrelated donor transplantation leveling off in Europe? The 2016 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplantation, 53, 1139 – 1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault, C. , Roy, D.C. & Fortin, C. (1998) Immunodominant minor histocompatibility antigens: the major ones. Immunology Today, 19, 69–74. [DOI] [PubMed] [Google Scholar]
- Perruccio, K. , Topini, F. , Tosti, A. , Carotti, A. , Aloisi, T. , Aversa, F. , Martelli, M.F. & Velardi, A. (2008) Photodynamic purging of alloreactive T cells for adoptive immunotherapy after haploidentical stem cell transplantation. Blood Cells, Molecules & Diseases, 40, 76–83. [DOI] [PubMed] [Google Scholar]
- Petersdorf, E.W. (2010) The World Marrow Donor Association: 20 years of international collaboration for the support of unrelated donor and cord blood hematopoietic cell transplantation. Bone Marrow Transplantation, 45, 807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidala, J. , Kim, J. , Schell, M. , Lee, S.J. , Hillgruber, R. , Nye, V. , Ayala, E. , Alsina, M. , Betts, B. , Bookout, R. , Fernandez, H.F. , Field, T. , Locke, F.L. , Nishihori, T. , Ochoa, J.L. , Perez, L. , Perkins, J. , Shapiro, J. , Tate, C. , Tomblyn, M. & Anasetti, C. (2013) Race/ethnicity affects the probability of finding an HLA‐A, ‐B, ‐C and ‐DRB1 allele‐matched unrelated donor and likelihood of subsequent transplant utilization. Bone Marrow Transplantation, 48, 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, N. , Rezvani, K. , Barrett, A.J. & Savani, B.N. (2011) Strategies to prevent EBV reactivation and posttransplant lymphoproliferative disorders (PTLD) after allogeneic stem cell transplantation in high‐risk patients. Biology of Blood and Marrow Transplantation, 17, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, D.C. , Walker, I. , Maertens, J. , Lewalle, P. , Olavarria, E. , Selleslag, D. , Lachance, S. , Bönig, H. & Mielke, S. (2018a) Donor lymphocytes depleted of alloreactive T‐cells product (ATIR101) can be administered to haploidentical HSCT without causing severe GVHD: final 2‐year follow‐up of phase 2 study. In: The 44th Annual Meeting of the European Society for Blood and Marrow Transplantation: Physicians Oral Session Bone Marrow Transplantation. https://www.nature.com/articles/s41409-018-0318-y.pdf, Supplement, Abstract, O142. [Google Scholar]
- Roy, D.C. , Walker, I. , Maertens, J. , Lewalle, P. , Olavarria, E. , Beguin, Y. , Selleslag, D. , Wagner, E.M. , Bonig, H. , Bos, G. & Mielke, S. (2018b) Efficacy and safety of a single dose of donor lymphocytes depleted of alloreactive T‐cells (ATIR101) following T‐cell‐depleted haploidentical HSCT: a pooled analysis of two phase II studies. Blood, 132, 120. [Google Scholar]
- Rubio, M.T. , Labopin, M. , Blaise, D. , Socie, G. , Contreras, R.R. , Chevallier, P. , Sanz, M.A. , Vigouroux, S. , Huynh, A. , Shimoni, A. , Bulabois, C.E. , Caminos, N. , Lopez‐Corral, L. , Nagler, A. & Mohty, M. (2015) The impact of graft‐versus‐host disease prophylaxis in reduced‐intensity conditioning allogeneic stem cell transplant in acute myeloid leukemia: a study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Haematologica, 100, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri, A. , Labopin, M. , Sanz, G. , Piemontese, S. , Arcese, W. , Bacigalupo, A. , Blaise, D. , Bosi, A. , Huang, H. , Karakasis, D. , Koc, Y. , Michallet, M. , Picardi, A. , Sanz, J. , Santarone, S. , Sengelov, H. , Sierra, J. , Vincent, L. , Volt, F. , Nagler, A. , Gluckman, E. , Ciceri, F. , Rocha, V. , Mohty, M. , Eurocord, Cord Blood Committee of Cellular Therapy and Immunobiology working party‐EBMT & ALWP‐EBMT study . (2015)Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia, 29, 1891 – 1900. [DOI] [PubMed] [Google Scholar]
- Scaradavou, A. , Brunstein, C.G. , Eapen, M. , Le‐Rademacher, J. , Barker, J.N. , Chao, N. , Cutler, C. , Delaney, C. , Kan, F. , Isola, L. , Karanes, C. , Laughlin, M.J. , Wagner, J.E. & Shpall, E.J. (2013) Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood, 121, 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureda, A. , Bader, P. , Cesaro, S. , Dreger, P. , Duarte, R.F. , Dufour, C. , Falkenburg, J.H. , Farge‐Bancel, D. , Gennery, A. , Kroger, N. , Lanza, F. , Marsh, J.C. , Nagler, A. , Peters, C. , Velardi, A. , Mohty, M. & Madrigal, A. (2015) Indications for allo‐ and auto‐SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplantation, 50, 1037–1056. [DOI] [PubMed] [Google Scholar]
- Yager, E.J. , Ahmed, M. , Lanzer, K. , Randall, T.D. , Woodland, D.L. & Blackman, M.A. (2008) Age‐associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. The Journal of Experimental Medicine, 205, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiser, R. & Blazar, B.R. (2017) Pathophysiology of chronic graft‐versus‐host disease and therapeutic targets. The New England Journal of Medicine, 377, 2565–2579. [DOI] [PubMed] [Google Scholar]
- Zhou, X. , Dotti, G. , Krance, R.A. , Martinez, C.A. , Naik, S. , Kamble, R.T. , Durett, A.G. , Dakhova, O. , Savoldo, B. , Di Stasi, A. , Spencer, D.M. , Lin, Y.F. , Liu, H. , Grilley, B.J. , Gee, A.P. , Rooney, C.M. , Heslop, H.E. & Brenner, M.K. (2015) Inducible caspase‐9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood, 125, 4103–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Immune reconstitution of B and NK cells. Box and whisker plots of B lymphocytes and NK cells (A: CD19+; B: CD56+) from patients’ peripheral blood after ATIR101 infusion and through to 1 year post‐HSCT.
Fig S2. Memory T cell recovery post‐transplant. Memory T cell subtypes: central memory (TCM, dark blue), effector memory (TEM, green), terminally differentiated effector (TEMRA, red) and TNAIVE (pale blue) were evaluated within CD4 (left panels) and CD8 (right panels) T‐cells for patients administered ATIR101 at dose levels L2–L3, L4–L5 and L6–L7 over the first 36 weeks post‐ATIR101 infusion.
Table SI. Overview of acute GVHD occurring following administration of ATIR101.
Appendix S1. Supplementary methods.


