Abstract
Aims
To investigate the effects and possible mechanisms of transcutaneous electrical acustimulation (TEA) combined with deep breathing training (DBT) on refractory gastroesophageal reflux disease (rGERD).
Methods
Twenty‐one patients with rGERD were recruited and randomly assigned to receive either only esomeprazole (ESO, 20 mg bid) (group A, n = 7), TEA + DBT + ESO (group B, n = 7), or sham‐TEA + DBT + ESO (group C, n = 7) in a four‐week study. The reflux diagnostic questionnaire (RDQ) score and heart rate variability (HRV) were recorded and evaluated at baseline and at the end of each treatment. Blood samples were collected for the measurement of serum acetylcholine (Ach) and nitric oxide (NO). Esophageal manometry and 24‐hour pH monitoring were performed before and after the treatment.
Results
After treatment, 1) the participants in group B had significantly lower scores of RDQ and DeMeester and increased lower esophageal sphincter pressure (LESP) than those in group C (all p < 0.05), suggesting the role of TEA; 2) low frequency band (LF)/(LF + HF) ratio in groups B and C was decreased, compared with group A (p = 0.010, p = 0.042, respectively); high frequency band (HF)/(LF + HF) ratio in B and C groups was significantly increased, compared with group A (p = 0.010, p = 0.042, respectively); 3) The serum Ach in groups B and C was significantly higher than group A (p = 0.022, p = 0.046, respectively); the serum NO in groups B and C was significantly lower than group A (p = 0.010, p = 0.027, respectively).
Conclusions
TEA combined with the DBT can effectively improve the reflux symptoms in rGERD patients by increasing LESP and reducing gastroesophageal reflux, which may be mediated via the autonomic and enteric mechanisms.
Keywords: Deep breathing training, gastroesophageal reflux, heart rate variability, low esophageal sphincter pressure, transcutaneous electrical acustimulation
INTRODUCTION
Gastroesophageal reflux disease (GERD) refers to the reflux of gastric and duodenal contents into the esophagus, which induces gastrointestinal symptoms and esophageal mucosa injury. Heartburn and regurgitation, “typical” symptoms of GERD, are considered by most physicians tantamount to GERD. Atypical symptoms include chronic cough, hoarseness, globus, dental erosions, etc., which are considered as extraesophageal manifestations of the disease 1. Impaired gastroesophageal motility with an increased number of transient lower esophageal sphincter (LES) relaxations, acidification of the esophagus, and low esophageal clearance are considered to be the most important factors in the pathogenesis of GERD 2. Gastrointestinal motility is mainly regulated by the regional enteric nervous system (ENS), but central nervous system (CNS) has a relative independent effect on it, which is the reason that gastrointestinal tract is called “little brain.” Changes of ENS and abnormal secretion of neurotransmitters will lead to gastrointestinal dysfunctions. In view of various neurotransmitters in the ENS, excitatory neurotransmitter acetylcholine (Ach) and inhibitory neurotransmitter nitric oxide (NO) are two major ones 3.
Current treatment of GERD is mainly done using proton‐pump inhibitors (PPIs), but some patients fail to respond to PPIs. According to the 2013 US GERD consensus, the following GERD patients can be diagnosed as refractory GERD (rGERD): those who are treated with PPI twice daily and do not respond to a 8‐ to 12‐week, course of acid suppression, and their GERD‐associated symptoms still exist and affect the quality of life or the GERD‐associated symptom, improvement is less than 50% 1.
Although patients with GERD typically have a good response to PPIs, the long‐term maintenance therapy to prevent recurrence is still necessary in most patients 4, 5. In fact, up to 40% of patients fail to respond to PPIs, and there is an increasing concern that long‐term PPI maintenance may cause side effects such as reduced bone mineral density bone fracture and increasing the risk of myocardial infarction (MI), which has attracted more and more attention 6, 7, 8.Theoretically, prokinetic agents can improve the ability of esophageal acid clearance by enhancing esophageal peristalsis and augmenting the tension of LES, fundamentally preventing the occurrence of GERD; however, effective target drugs have not been developed. Additionally, successful antireflux surgery, such as Nissen fundoplication, can significantly reduce the chance of recurrence, but surgical complications and mortality are still a disturbing question concerning some surgeons’ experience and technique level 9.
Acupuncture has been used to treat gastrointestinal diseases with a history of 3000 years in China, and acupuncture points (acupoints), Neiguan (PC6) and Zusanli (ST36), are commonly used for treating functional gastrointestinal diseases, including GERD 10, 11. There have been a number of reports showing that acupuncture or electroacupuncture (EA) at PC6 and/or ST36 can effectively alleviate nausea, vomiting, and other abdominal symptoms, especially for functional dyspepsia (FD) 12. Acupuncture was reported to have a significant effect on improving rGERD patients' symptoms 13. EA was found to enhance the gastric/esophageal peristalsis and accelerate gastric emptying, as delayed gastric emptying has also been shown to contribute to failure of PPI 14, 15. The newly developed transcutaneous electrical acustimulation (TEA) is a noninvasive and needleless method, with electrical stimulation delivered to acupoints via surface electrodes and is well accepted by patients 16, 17. According to a few recent preliminary studies, TEA at ST36 and PC6 seems able to improve symptoms and motility in patients with GERD 14, FD 12, 14, and postsurgical recovery 18. It has been reported that TEA can improve the symptoms of dyspepsia by enhancing vagus nerve activity and inhibiting sympathetic nerve activity 12.
The gastroesophageal junction (GEJ) includes two fundamental components: the intrinsic LES and extrinsic compression by the crural diaphragm (CD), and is the first natural barrier to acid reflux 19. Relaxation and phasic tone of the CD contribute significantly to the GEJ. There is a direct correlation between intraluminal GEJ pressure and integrated electromyography (EMG) spike activity of the CD 20. Studies have shown that CD tension of GERD patients was significantly lower than that of healthy subjects or patients with functional heartburn 21.
Deep breathing training (DBT) is thought to increase parasympathetic nervous system tone and is frequently used in many psychological, cognitive, and alternative therapies 22, 23. Such therapies have an evidence base in the treatment of chronic visceral pain syndromes such as irritable bowel syndrome. An increasing body of evidence suggests that the parasympathetic branch of the autonomic nervous system (ANS) may mediate an antihyperalgesic effect through the cholinergic anti‐inflammatory pathways 24. In recent years, open‐label studies have shown that DBT can improve the motor function of the diaphragm, enhance CD tone, and increase LES pressure (LESP), thereby increasing the antireflux barrier effect to reduce the reflux 25, 26. Recent studies reported that deep inspiratory training can significantly improve the symptoms of abdominal pain related to irritable bowel syndrome and esophageal hyperalgesia situation of patients who suffer from GERD 27. DBT brings gospel to rGERD patients.
Although TEA or DBT alone was reported to improve dyspepsia and rGERD symptoms 15, 25, it was unclear whether the combination of these two methods would have a synergistic effect for the treatment of GERD or rGERD. Accordingly, this current study was designed to explore the possible synergistic effects of TEA combined with DBT on GERD symptoms and esophageal motility and mechanisms associated with the autonomic function in patients with rGERD.
METHODS
Patients
Twenty‐one patients (male 7, female 14) with rGERD who met the 2013 US rGERD guidelines were enrolled into the study, including outpatients and inpatients seen in the Department of Gastroenterology of The First Affiliated Hospital of University of Science and Technology of China from August 2016 to February 2017 1. The patients must have more than two kinds of symptoms either typical symptoms (heartburn, regurgitation, chest pain, etc.) or atypical symptoms (belching, nausea, vomiting, etc.), and these patients must be with persisting GERD symptoms after 8–12 weeks of standard PPI therapy. Exclusion criteria were as follows: severe heart and lung diseases, diabetes, nephropathy, and other chronic diseases associated with neuropathy or gastrointestinal disorders such as ulcers, cancer, esophageal varices, etc. The protocol was approved by the Ethics Committee of Anhui Provincial Hospital (Registration No: 2016 L36), and all patients joined the study with written informed consent.
Protocol
The study was designed as a randomized controlled, single blind, prospective trial. Enrolled patients were randomly divided into three groups, 7 in group A treated with esomeprazole (ESO, 20 mg, twice daily), 7 in group B treated with TEA (30 min twice daily) + DBT (twice daily) + ESO (20 mg, twice daily), and 7 in group C with sham‐TEA (twice daily) + DBT (twice daily) + ESO (20 mg, twice daily). All patients received a four‐week treatment. There was no statistically significant difference among the patients' age, gender, weight, or height.
TEA at Zusanli (ST36) and Neiguan (PC6) maintains 25 Hz, 5 mA, and 30 min twice daily using a watch‐size stimulator (MedKinetic, Ningbo, China). The device is shown in Figure 1. It can be attached to the arm and leg. The stimulation parameters were programmed via a computer by the investigator. However, the patient was able to adjust the stimulation output current to a level that was sensible but tolerable 12, 28. Sham‐TEA was performed using the same parameters at non‐acupoints: non‐channel non‐collateral acupoint, about 2 cm away from ST36 and PC6.
Figure 1.

The wearable watch‐size stimulator. [Color figure can be viewed at wileyonlinelibrary.com]
DBT is mainly used to improve the motor function of the diaphragm, which can enhance the CD tension and increase LESP, thereby increasing the function of antireflux barrier for alleviating reflux 22, 23, 25. The patients were asked to adjust their breathing pattern into abdominal deep breathing, and keep their hands on the surface of the abdomen to fully feel the effect of the deep breath on abdominal expansion and expiratory muscle relaxation, especially focusing on the right respiration patterns of patients. The DBT used in this study was based on a modified procedure described by Thijs et al. 29. It consisted of breathing at full inspiratory capacity for 4 sec, followed by exhaling to forced expiratory vital capacity in 6 sec, and repeated at a frequency of 0.1 Hz. This entailed 6 breaths/min for 1 min every fifth minute, for 6 cycles in total, during the 30‐min duration of the acid exposure phase.
Measurements
Esophageal High‐Resolution Manometry
A water‐perfused esophageal manometric catheter with 24 pressure sensors (1 cm interval) was used to measure esophageal motility, including the upper esophageal sphincter (UES), esophageal body, and LES. All enrolled patients underwent the high‐resolution manometry (HRM) test (MedKinetic, Ningbo, China) at the beginning and the end of the treatment. After fasting for 8 hours, the esophageal catheter was inserted transnasally into the esophagus after pressure calibration. When the partial pharynx, UES, esophageal body, LES, and gastric pressure were all displayed on the screen, the catheter was fixed at the nose‐wing. Then patients were asked to lie in a supine position. After an acclimation period of a few minutes, the patient was instructed to swallow boluses of 5 mL of water, which were injected into their mouth by using a syringe 30.
24‐Hour pH Monitoring
The device recorded 24‐hour pH data by a digital data logger (Jinshan Science & Technology, Chongqing, China), including acid reflux, weak acid reflux, non‐acid reflux. Before the test, patients were asked to withdraw PPI and prokinetics for seven days. After a fast of 8 hours, The MII‐pH monitoring catheter was inserted through the nostrils to 5 cm above the LES (the LES position identified from the previous HRM test) and fixed with tape on the nose and neck. Patients were instructed to keep normal diets except acidic food or alcoholic and to record all foods and symptoms in a diary.
The analysis was performed for the DeMeester score, total reflux episodes, acid reflux episodes (pH ≤4), weak acid reflux episodes (4< pH <7), and non‐acid reflux episodes (pH ≥7). The normal range is as follows: DeMeester score ≤14.72, acid reflux ≤55 times, weak acid reflux ≤26 times, nonacid reflux ≤1 time, and total reflux episodes ≤73 times 31.
Reflux Diagnostic Questionnaire
The reflux diagnostic questionnaire (RDQ) included heartburn, regurgitation, chest pain, and sleep disturbances, and each item scale ranges from 0 to 3 32. The standard of symptoms evaluation: “0” refers to no symptoms; “1” refers to occasional symptoms (one to two days in a week); “2” refers to frequent symptoms (three to five days in a week); and “3” refers to daily symptoms. The questionnaire score over 8 indicated a positive test, and if the difference of the questionnaire scores between pretreatment and posttreatment decreased over 6, it inferred effective treatment.
Assessment of Autonomic Functions
The autonomic nervous functions were evaluated by the spectral analysis of HRV. The HRV signal was obtained from the electrocardiogram (ECG) recording (ct‐082, Hangzhou Baihui Electrocardiograms, China), and HRV data were processed by HRV analysis software (Cardiotrak Holtersystem version: 1.2.0.0, Hangzhou Baihui Electrocardiograms, China), Power spectral analysis was then performed, and the power in each frequency subband was calculated using a previously method 17. The power in the low frequency band (0.04–0.15 Hz, LF) represents mainly sympathetic activity and the power in the high frequency band (0.15–0.50 Hz, HF) stands purely for parasympathetic or vagal activity. The power ratio of LF/HF reflects the balance between the sympathetic and parasympathetic activities. The power ratio of LF/(HF + LF) represents sympathetic activity; the power ratio of HF/(HF + LF) ratio represents the parasympathetic activity.
Ach and NO Assays
Blood samples were collected in the fasting state before and at the end of the treatment in chilled EDTA and aprotinin tubes, centrifuged at 4200g and 4°C for 10 min, and stored at 4°C until extraction. Serum Ach and NO levels were determined with the corresponding commercial ELISA kits (Jiancheng Institute of Biology and Technology, Nanjing, China; Ach products batch number: 20170210; NO product batch number: 20170113).
Statistical Analysis
SPSS16.0 statistical software was used to analyze data. Measurement data were expressed as mean ± standard deviation. Multiple parametric groups were compared using a one‐way analysis of variance test or nonparametric test, and pre‐ and posttreatment tests were evaluated using a paired t‐test. P values of 0.05 or less were considered to be statistically significant.
RESULTS
Effects of Treatments on Reflux Symptoms
TEA combined with DBT significantly improves GERD symptoms, whereas DBT showed no significant effect on GERD symptoms. As shown in Figure 2, the reflux symptom score was significantly reduced after each of the three treatments (p < 0.002 for all groups). However, the treatment effect was most potent in group B. After treatment, the reflux symptom score of group B was lower than that of group C (1.57 ± 0.53 vs. 2.86 ± 1.21, p = 0.018), suggesting a combined effect of TEA and DBT, and also lower that of group A (1.57 ± 0.53 vs. 3.86 ± 2.27, p = 0.023), suggesting a net TEA effect. No significant difference was noted in the symptom score after the treatment between group C and group A (2.86 ± 1.21 vs. 3.86 ± 2.27, p = 0.182), suggesting a minimal effect of sham‐TEA combined with DBT.
Figure 2.

The comparison of the reflux symptom score pre‐ and posttreatment among groups. The acid reflux symptom score of three groups decreased after treatment (*p < 0.050). There was a significant difference between TEA and sham‐TEA treatment (p < 0.050). [Color figure can be viewed at wileyonlinelibrary.com]
Effects of Treatments on LESP
TEA combined with DBT resulted in a substantial increase in LESP, and DBT also showed a significant increase in LESP. As shown in Figure 3, the LESP in group A showed no change after the PPI treatment (p = 1.0); however, a significant increase was noted in groups B (p < 0.001) and C (p < 0.001). After the corresponding treatment, the LESP in group B was significantly higher than that in group C (26.14 ± 7.06 vs. 17.57 ± 5.13, p = 0.025) and the LESP in group B was also higher than that in group A (26.14 ± 7.06 vs. 13.29 ± 5.74, p = 0.003). The results suggested that both TEA and DBT enhanced LESP, and TEA combined with DBT was more potent than DBT.
Figure 3.

The LESP of groups B and C significantly increased before and after the treatment (*p < 0.050). There was a significant difference between PPI + DBT + TEA and PPI treatment (p < 0.050). There was also a significant difference between sham‐TEA and TEA treatment (p < 0.050), and TEA group treatment increased LESP higher. [Color figure can be viewed at wileyonlinelibrary.com]
Effects of Treatments on Acid Reflux
All three methods of treatment reduced acid reflux, but TEA + DBT + PPT was the most potent method in suppressing acid reflux, suggesting a suppressive effect of TEA + DBT on acid reflux. As shown in Figure 4, DeMeester scores were significantly decreased after the three treatments, respectively (35.74 ± 5.08 vs. 24.92 ± 3.94, p = 0.003), (43.65 ± 9.29 vs. 11.17 ± 1.24, p = 0.008), and (34.80 ± 4.74 vs. 15.19 ± 0.74, p = 0.007). Importantly, after the corresponding treatment, the DeMeester score in group B was significantly lower than that in group A (11.17 ± 1.24 vs. 24.92 ± 3.94, p = 0.012) and group C (11.17 ± 3.29 vs. 15.19 ± 0.74, p = 0.019) that suggested a net TEA effect. The DeMeester score in group C was significantly lower than that in group A (15.19 ± 0.74 vs. 24.92 ± 3.94, p = 0.032).
Figure 4.
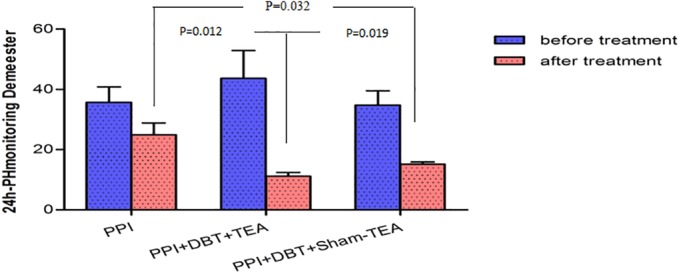
The DeMeester score of three groups significantly decreased before and after treatment (*p < 0.050). There were significant differences among three groups after treatment (p < 0.050). [Color figure can be viewed at wileyonlinelibrary.com]
Mechanisms Involving Autonomic Functions
Both TEA and DBT significantly enhanced vagal activity and suppressed sympathetic activity assessed by the spectral analysis of HRV, but TEA was more potent than DBT. As shown in Figure 5a,b, the PPI treatment did not alter LF/(LF + HF) or HF/(LF + HF) (p = 0.301), whereas both TEA+DBT + PPI and sham‐TEA+DBT + PPI significantly reduced LF/(LF + HF) and increased HF/(LF + HF). What is more, the ratio of LF/LF + HF in group A was significantly higher than that in group B (0.38 ± 0.034 vs. 0.54 ± 0.13, p = 0.010) and group C (0.41 ± 0.08 vs. 0.54 ± 0.13, p = 0.042). However, the ratio of HF/(LF + HF) in group A was significantly lower than that in group B (0.62 ± 0.034 vs. 0.46 ± 0.13, p = 0.010) and group C (0.59 ± 0.078 vs. 0.46 ± 0.13, p = 0.042).
Figure 5.

a. The ratio of LF/(LF + HF) of groups B and C significantly decreased before and after treatment (*p < 0.050). After treatment, there was a significant difference between PPI + DBT + TEA and PPI treatment (p < 0.050), and there was also a significant difference between PPI + DBT + sham‐TEA and PPI treatment (p < 0.050). b. The ratio of HF/(LF + HF) of groups B and C significantly decreased before and after treatment (*p < 0.050). After treatment, there was a significant difference between PPI + DBT + TEA and PPI treatment (p < 0.050), and there was also a significant difference between PPI + DBT + sham‐TEA and PPI treatment (p < 0.050). [Color figure can be viewed at wileyonlinelibrary.com]
Mechanisms Involving Enteric Neurotransmitters
Both TEA and DBT enhanced the release of Ach. As can be seen from Figure 6a, the content of serum Ach was significantly increased after the treatment in all three groups (A: 60.97 ± 7.01 vs. 64.04 ± 7.48, p = 0.041; B: 61.07 ± 8.25 vs. 71.27 ± 7.90, p = 0.000; C: 62.93 ± 6.39 vs. 71.66 ± 4.84, p = 0.000, respectively). After four weeks of the treatment, the content of serum Ach in group C was higher than that in group A (71.68 ± 4.84 vs. 64.04 ± 7.48, p = 0.046) and the content of Ach in group B was also significantly higher than that in group A (74.13 ± 6.92 vs. 64.04 ± 7.48, p = 0.022).
Figure 6.

a. The content of serum Ach of three groups significantly increased before and after treatment (*p < 0.050). After treatment, there was a significant difference between PPI + DBT + TEA and PPI treatment (p < 0.050), and there was also a significant difference between PPI + DBT + sham‐TEA and PPI treatment (p < 0.050). b. The content of serum NO of three groups significantly decreased before and after treatment (*p < 0.050). After treatment, there was a significant difference between PPI + DBT + TEA and PPI treatment (p < 0.050), and there was also a significant difference between PPI + DBT + sham‐TEA and PPI treatment (p < 0.050). [Color figure can be viewed at wileyonlinelibrary.com]
Both TEA and DBT significantly suppressed serum NO. From Figure 6b, it was obvious that the content of serum NO was significantly reduced after the treatment in all three groups (A: 114.09 ± 7.67 vs. 103.86 ± 6.28, p = 0.009; B:117.90 ± 10.17 vs. 73.33 ± 7.68, p = 0.000; C:108.82 ± 9.54 vs. 81.65 ± 6.20, p = 0.002). After the four‐week treatment, the content of NO in group C and group B was significantly lower than that in group A (81.65 ± 6.20 vs. 103.86 ± 6.28, p = 0.027; 73.33 ± 7.68 vs. 103.86 ± 6.28, p = 0.010). However, there was no statistical difference between group B and group C (73.33 ± 7.68 vs. 81.65 ± 6.20, p = 0.417).
DISCUSSION
In this study, we found that TEA combined with DBT significantly increased LESP, reduced acid reflux, and improved clinical manifestations of GERD. Although DBT and PPI also improved GERD, the combined treatment of TEA was mostly effective in almost every aspect of the studied parameters. The improvement of reflux is believed to be attributed to the elevation of the LESP mediated via the autonomic and enteric mechanisms.
Currently, many rGERD patients still suffered from reflux symptoms because of ineffective medical treatments, whose possible mechanism has not been elucidated 33, 34. The weaken antireflux barrier and enteric nervous dysfunctions and autonomic dysfunctions may play major roles in rGERD. Improving LES function, esophageal peristalsis and enteric and autonomic nervous activities were considered as new potential therapeutic targets.
TEA is a noninvasive and needleless method, which can be self‐administrated by patients 16, 17. After four weeks of TEA+DBT treatment, the RDQ and DeMeester scores were significantly lower than that of PPI (ESO) alone and sham‐TEA + DBT. Meanwhile, the LESP was also significantly higher than that of ESO alone and sham‐TEA + DBT. A few previous studies have found that electrical stimulation of the LES or the stomach can increase LESP that serves as a barrier of reflux 12, 35. Liu et al. found that TEA at both Neiguan (PC6) and Zusanli (ST36) in patients with FD was effective in relieving distention and improving dyspepsia by suppressing sympathetic activity and enhancing vagal tone 12. In addition, DBT has been reported to improve the abdominal pressure, the function of esophageal gastric junction, and the pressure of LES 36, 37. Deep inspiration was mainly composed of diaphragm contraction and relaxation, and especially the descending diaphragmatic dome strengthens the pressure of LES. The relevant data of our study also supported this effect.
Gastrointestinal physiological functions play an important role in rGERD patients, which mainly depends on the regulation of the CNS, ANS, and ENS. In this study, the autonomic nerve function was assessed based on the cardiac autonomic function using the spectral analysis of HRV. Using this method, a few previous studies have shown a postprandial increase in sympathetic/parasympathetic ratio and decrease in parasympathetic activity 38. These meal‐induced changes in cardiac autonomic functions suggested that the cardiac autonomic function can be used as a surrogate of the gastrointestinal autonomic function 39. Some studies reported that changes in the autonomic nerve function are associated with the heartburn induced by esophageal acid perfusion 40. Our study showed that TEA combined with DBT could enhance vagal/parasympathetic activity and inhibit sympathetic activity, which might have led to enhanced esophageal motility and reduced reflux.
NO is the major inhibitory neurotransmitter in the ENS, which is associated with LES relaxation, hyperpolarization of esophageal annular muscle, and esophageal smooth muscle termination reaction. Chang et al, reported that acupuncturing at Zusanli (ST36) could improve the serum NO content in rabbits after ethanol infusion 41. The level of NO in patients treated with TEA + DBT was significantly lower than those in the PPI alone and the sham‐TEA group, while there was no significant change between the PPI alone and the sham‐TEA group. The result suggests that NO inhibition may be associated with enhanced esophageal motility.
Ach plays an important role in promoting gastrointestinal smooth muscle contraction and peristalsis, and the level of Ach was bound up with vagus nerve activity. In this study, the level of Ach in patients treated with TEA + DBT was significantly higher than that in the other two groups, suggesting that increased Ach might be responsible in elevating LESP and reducing reflux. Chua YC et al. reported that deep inspiration could excite the vagus nerve and stimulate the releasing of Ach in blood, which could regulate the body inflammation and pain signal, improve esophageal hyperalgesia in GERD, promote emptying, and have a positive effect on the non‐acid reflux patients with chest pain and normal DeMeester score 42. These findings were consistent with our findings.
In summary, TEA combined with DBT may effectively alleviate reflux in rGERD patients by increasing LESP medicated via the autonomic and enteric mechanisms.
Authorship Statement
Yue Yu, Ruiling Wei, and Jiande D. Z. Chen planned the study; Ruiling Wei, Zhi Liu, Jiaqin Xu, and Chao Xu conducted the study; Ruiling Wei, Zhi Liu, Jiaqin Xu, and Chao Xu contributed to the collection and interpretation of the data; and Yue Yu, Ruiling Wei, and Jiande D. Z. Chen contributed to draft the manuscript.
COMMENT
This authors use non‐invasive and non‐pharmaceutical methods for treating a common and important clinical condition. Transcutaneous electrical acupuncture and deep breathing techniques decrease acid reflux, increase lower esophageal sphincter pressure and most importantly reduce the symptoms of resistant gastroesophageal reflux. There is also the appropriate changes in serum acetylcholine and nitric oxide.
William Snape, MD
San Francisco, CA, USA
Comments not included in the Early View version of this paper.
Acknowledgements
The study was supported by External Science and Technology Cooperation Planning Projects of Anhui Province of China (1604b0602021).
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: External Science and Technology Cooperation Planning Projects of Anhui Province of China (1604b0602021).
Conflict of Interest: The authors reported no conflict of interest.
Contributor Information
Yue Yu, Email: yuyuemd@ustc.edu.cn.
Jiande D. Z. Chen, Email: jiandedzchen@gmail.com.
REFERENCES
- 1. Philip OK, Lauren BG, Marcelo FV. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–328. [DOI] [PubMed] [Google Scholar]
- 2. Morozov S, Isakov VA, Konovalova M. Fiber‐enriched diet helps to control symptoms and improves esophageal motility in patients with non‐erosive gastroesophageal reflux disease. World J Gastroenterol 2018;24:2291–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gershon MD. Development of the enteric nervous system: a genetic guide to the perplexed. Gastroenterology 2018;154:478–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roman S, Mion F. Refractory GERD, beyond proton pump inhibitors. Curr Opin Pharmacol 2018;43:99–103. [DOI] [PubMed] [Google Scholar]
- 5. Jensen RT. Consequences of long‐term proton pump blockade: insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol 2006;98:4–19. [DOI] [PubMed] [Google Scholar]
- 6. Hershcovici T, Fass R. Step‐by‐step management of refractory gastresophageal reflux disease. Dis Esophagus 2013;26:27–36. [DOI] [PubMed] [Google Scholar]
- 7. Ozdil K, Kahraman R, Sahin A et al. Bone density in proton pump inhibitors users: a prospective study. Rheumatol Int 2013;33:2255–2260. [DOI] [PubMed] [Google Scholar]
- 8. Shah NH, Lependu P, Bauer‐Mehren A et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One 2015;10:e0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patti MG. An evidence‐based approach to the treatment of gastroesophageal reflux disease. JAMA Surg 2016;151:73–78. [DOI] [PubMed] [Google Scholar]
- 10. Chen JDZ, Ni M, Yin J. Electroacupuncture treatments for gut motility disorders. Neurogastroenterol Motil 2018;30:e13393. [DOI] [PubMed] [Google Scholar]
- 11. Yin J, Chen JD. Gastrointestinal motility disorders and acupuncture. Auton Neurosci 2010;157:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu S, Peng S, Hou X, Ke M, Chen J. Transcutaneous electroacupuncture improves dyspeptic symptoms and increases high frequency heart rate variability in patients with functional dyspepsia. Neurogastroenterol Motil 2010;20:1204–1211. [DOI] [PubMed] [Google Scholar]
- 13. Dickman R, Schiff E, Holland A et al. Clinical trial: acupuncture vs. doubling the proton pump inhibitor dose in refractory heartburn. Aliment Pharmacol Ther 2007;26:1333–1344. [DOI] [PubMed] [Google Scholar]
- 14. Xu S, Hou X, Zha H, Gao Z, Zhang Y, Chen JD. Electroacupuncture accelerates solid gastric emptying and improves dyspeptic symptoms in patients with functional dyspepsia. Dig Dis Sci 2006;51:2154–2159. [DOI] [PubMed] [Google Scholar]
- 15. Meng LN, Chen S, Chen JD, Jin HF, Lu B. Effects of transcutaneous electrical acustimulation on refractory gastroesophageal reflux disease. Evid Based Complement Alternat Med 2016;2016:8246171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Z, Zhang N, Xu F, Yin J, Dai N, Chen JD. Ameliorating effect of transcutaneous electroacupuncture on impaired gastric accommodation induced by cold meal in healthy subjects. J Gastroenterol Hepatol 2016;31:561–566. [DOI] [PubMed] [Google Scholar]
- 17. Zhang N, Song G, Chen J et al. Ameliorating effects and autonomic mechanisms of needle‐less transcutaneous electrical stimulation at ST36 on stress‐induced impairment in gastric slow waves. J Gastroenterol Hepatol 2015;30:1574–1581. [DOI] [PubMed] [Google Scholar]
- 18. Zhang B, Xu F, Hu P et al. Needleless transcutaneous electrical acustimulation: a pilot study evaluating improvement in post‐operative recovery. Am J Gastroenterol 2018;113:1026–1035. [DOI] [PubMed] [Google Scholar]
- 19. Nicodème F, Lin Z, Pandolfino JE, Kahrilas PJ. Esophagogastric junction pressure morphology: comparison between a station pull‐through and real‐time 3D‐HRM representation. Neurogastroenterol Motil 2013;25:e591–e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mittal RK, Rochester DF, McCallum RW. Sphincteric action of the diaphragm during a relaxed lower esophageal sphincter in humans. Am J Physiol 1989;256:G139–G144. [DOI] [PubMed] [Google Scholar]
- 21. Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High‐resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol 2007;102:1056–1063. [DOI] [PubMed] [Google Scholar]
- 22. Palsson OS, Whitehead WE. Psychological treatments in functional gastrointestinal disorders: a primer for the gastroenterologist. Clin Gastroenterol Hepatol 2013;11:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zautra AJ, Fasman R, Davis MC, Craig AD. The effects of slow breathing on affective responses to pain stimuli: an experimental study. Pain 2010;149:12–18. [DOI] [PubMed] [Google Scholar]
- 24. Bonaz B, Picq C, Sinniger V, Mayol JF, Clarencon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti‐inflammatory pathway. Neurogastroenterol Motil 2013;25:208–221. [DOI] [PubMed] [Google Scholar]
- 25. Sun X, Shang W, Wang Z, Liu X, Fang X, Ke M. Short‐term and long‐term effect of diaphragm biofeedback training in gastroesophageal reflux disease: an open‐label, pilot, randomized trial. Dis Esophagus 2016;29:829–836. [DOI] [PubMed] [Google Scholar]
- 26. Casale M, Sabatino L, Moffa A et al. Breathing training on lower esophageal sphincter as a complementary treatment of gastroesophageal reflux disease (GERD): a systematic review. Eur Rev Med Pharmacol Sci 2016;20:4547–4552. [PubMed] [Google Scholar]
- 27. Botha C, Farmer AD, Nilsson M et al. Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut 2015;64:611–617. [DOI] [PubMed] [Google Scholar]
- 28. Shuai X, Xie P, Liu J, Xiang Y, Li J, Lan Y. Different effects of electroacupuncture on esophageal motility and serum hormones in cats with esophagitis. Dis Esophagus 2008;21:170–175. [DOI] [PubMed] [Google Scholar]
- 29. Thijs RD, Wieling W, van den Aardweg JG, van Dijk J. Respiratory countermaneuvers in autonomic failure. Neurology 2007;69:582–585.17679677 [Google Scholar]
- 30. Choi WS, Kim TW, Kim JH et al. High‐resolution manometry and globus: comparison of globus, gastroesophageal reflux disease and normal controls using high‐resolution manometry. Journal of Neurogastroenterol Motility 2013;19:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ang D, Ang TL, Teo EK et al. Is impedance pH monitoring superior to the conventional 24‐h pH meter in the evaluation of patients with laryngorespiratory symptoms suspected to be due to gastroesophageal reflux disease? J Dig Dis 2011;12:341–348. [DOI] [PubMed] [Google Scholar]
- 32. Rey E, Barceló M, Zapardiel J, Sobreviela E, Muñoz M, Díaz‐Rubio M. Is the reflux disease questionnaire useful for identifying GERD according to the Montreal definition? BMC Gastroenterology 2014;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao Y, Liang M, Peng S, Zhang N, Chen M. Tailored therapy for the refractory GERD patients by combined multichannel intraluminal impedance‐pH monitoring. J Gastroenterol Hepatol 2016;31:350–354. [DOI] [PubMed] [Google Scholar]
- 34. Kahrilas PJ, Keefer L, Pandolfino JE. Patients with refractory reflux symptoms: what do they have and how should they be managed? Neurogastroenterol Motil 2015;27:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xing JH, Lei Y, Chen JD. Gastric electrical stimulation (GES) with parameters for morbid obesity elevates lower esophageal sphincter (LES) pressure in conscious dogs. Obes Surg 2005;15:1321–1327. [DOI] [PubMed] [Google Scholar]
- 36. Chaves RCDM, Suesada M, Polisel F, Sá CCD, Navarro‐Rodriguez T. Respiratory physiotherapy can increase lower esophageal sphincter pressure in GERD patients. Respir Med 2012;106:1794–1799. [DOI] [PubMed] [Google Scholar]
- 37. Nobre e Souza MA, Lima MJ, Martins GB et al. Inspiratory muscle training improves antireflux barrier in GERD patients. Am J Physiol Gastrointest Liver Physiol 2013;305:G862–G867. [DOI] [PubMed] [Google Scholar]
- 38. Bruinstroop E, Fleur SEL, Ackermans MT et al. The autonomic nervous system regulates postprandial hepatic lipid metabolism. Am J Physiol Endocrinol Metab 2013;304:E1089–E1096. [DOI] [PubMed] [Google Scholar]
- 39. Ding ZL, Wang ZF, Sun XH, Ke MY. Therapeutic mechanism of diaphragm training at different periods in patients with gastroesophageal reflux disease. National Medical Journal of China 2013;93:3215–3219. [PubMed] [Google Scholar]
- 40. Chen CL, Orr WC. Autonomic responses to heartburn induced by esophageal acid infusion. J Gastroenterol Hepatol 2004;19:922–926. [DOI] [PubMed] [Google Scholar]
- 41. Yang J, Yan J, Wang C. The effect of acupuncture at Neiguan (PC6) pretreatment on the expression of blood serum NO, NOS, and adenosine in rabbits with MIR. J Tradit Chin Med 2014;23:1209–1211. [Google Scholar]
- 42. Chua YC, Ng KS, Sharma A et al. Randomised clinical trial: pregabalin attenuates the development of acid‐induced oesophageal hypersensitivity in healthy volunteers ‐ a placebo‐controlled study. Aliment Pharmacol Ther 2012;35:319–326. [DOI] [PubMed] [Google Scholar]


