Abstract
The main objective of this study was to determine baseline salt intake levels in a sample of the adult population of Shandong province and to establish the relationship between urinary sodium excretion and blood pressure. A total of 512 participants were recruited, and all the participants provided complete 24‐hour urine collections. Physical assessment and socioeconomic status of participants were collected at the same time. The mean 24‐hour urinary sodium excretion of all subjects was 228.0 ± 127.5 mmol/24 hours. Estimated salt intake was higher in obese subjects (17.6 ± 8.8 g/d) compared with overweight subjects (15.6 ± 8.0 g/d) and those with a normal BMI (13.9 ± 6.8 g/d). Likewise, urinary sodium excretion of hypertensive participants was dramatically higher than that of non‐hypertensive ones, the equivalent of 18.2 ± 9.1 g/d vs 13.3 ± 6.8 g/d. Urinary sodium was significantly associated with SBP (β = 1.08, P = .018) after adjustment for potential confounders. In summary, we found significantly high levels of salt intake in Shandong Province, particularly in obese and hypertension subjects. It is quite important to improve public education about reducing salt intake to control blood pressure among Shandong people.
Keywords: blood pressure, hypertension, salt intake, urinary sodium
1. INTRODUCTION
Hypertension, identified as the primary controllable risk factor for cardiocerebral disease and one of the most common chronic diseases, is the most important factor for worldwide morbidity and mortality,1 which accounts for about 40% of all deaths in China.2 A large number of studies in the last few decades have discovered that dietary salt intake is positively associated with blood pressure.3, 4 Similarly, there is evidence that restricting sodium consumption can be effective in reducing blood pressure, with predictable and stable substantial benefits for lowing cardiovascular mortality and health expenses.5, 6 Recently, our research showed that not only peripheral pressure, but also central aortic pressure was independently associated with increased urinary sodium excretion.7 It is a very strong possibility that proper sodium limitation is one of the most effective and economical ways to lighten the burden of hypertension.
As one of the most populous provinces in China, sodium intake among most Shandong residents considerably exceeds the recommended limit. For instance, in the INTERMAP study conducted in 2004, the average 24‐hour urinary sodium excretion of northern Chinese was 271 ± 88 mmol/24 hours8; meanwhile, the Chinese Health and Nutrition Survey in 2002 found that the average daily salt intake amount in Shandong province was 12.6 g/d,9 which was almost twice the World Health Organization (WHO) recommended level.10 In another hand, with the improvement of socioeconomic level, the Western diet characterized as high salt and high calorie has grown in popularity, which may have been exacerbated the trend for salty flavor. Effective nutrition education through radio, television, and Internet has been implemented to make the residents know more low‐salt diet knowledge and take healthy dietary practices. However, several earlier studies reported that salt intake among Shandong adults was still very high.11, 12, 13
For a long time, the 24‐hour urine collection is regarded as the gold standard to estimate daily salt intake, while it can not obtain the about 10% of dietary sodium lost through sweating, and even through exhaling and other non‐urinary routes.14 Nonetheless, the current data are scarce on urinary sodium excretion by 24‐hour urine collection in Shandong, particularly in last five years. Therefore, the main purpose of this study was to figure out the basic line level of salt consumption in a sample of the adult population. Our study also intended to explore the relationship between urinary sodium excretion by gathering a controlled 24‐hour urine collection and blood pressure.
2. METHOD
2.1. Subjects
A total of 512 participants (263 men and 249 women), ranged from 18 to 70 years, were recruited from persons undergoing health examinations in areas of Shandong province during 2017‐2018. The exclusion criteria were the following: acute illnesses or pregnancy; secondary cause of hypertension such as primary aldosteronism; hypertension emergencies; impaired renal function with plasma creatinine ≥150 μmol/L; rheumatic and autoimmune diseases; and malignancies. Written informed consent was obtained from all participants. The study protocol was approved by the ethics committee of Shandong Provincial Hospital Affiliated to Shandong University.
2.2. Data collection
Physical assessment of participants included weight, height, and systolic blood pressure (SBP) and diastolic blood pressure (DBP) with mercury sphygmomanometers. Blood pressure was measured three times by certified nurses using a standardized mercuric‐column sphygmomanometer on the participant in a sitting position after 15 minutes of rest, and the time interval between successive of blood pressure measurements was 2 minutes. Diagnostic criteria of hypertension were defined as SBP ≥140 mm Hg and/or DBP ≥90 mm Hg, or antihypertensive drug treatment for patient with hypertension.15 Body mass index (BMI = weight [kg]/height [m2]) was calculated, people with a BMI ≥18.5 kg/m2 to <24 kg/m2 were defined as being normal weight, those with a BMI ≥24 kg/m2 but <28 kg/m2 were defined as being overweight, and people with a BMI ≥28 kg/m2 were considered obese.16
An autonomous questionnaire was acquired about socioeconomic status and lifestyle characteristics, including degree of educational, occupation and annual family income, smoking, and alcohol consumption. Smoking was regarded as present if the subject declared current use of cigarettes or cessation of smoking in the last year. A person was defined as a current alcohol drinker if they had one or more drinks per day for women and two or more drinks per day for men at least twelve times during the past year. Specimens of fasting blood were gained from each participant after an overnight fast. Triglycerides (TG), creatinine (Cr), total cholesterol (TC), and fasting blood glucose (FBG) were measured using an automated analyzer (DXC800, Beckman‐coulter Inc).
People who agreed to participate in the study were provided a 3‐L screw‐capped plastic bottle and a 500‐ml plastic beaker and were instructed to collect a 24‐hour urine sample, discarding the first voided urine upon waking up in the morning and collecting all voided urine during the subsequent 24 hours, including the first void sample of the following morning. Upon completion of collection, trained staff recorded the urine volume in each collection container to determine the total urine volume during the 24‐hour collection period. A total of 512 samples of urine were sent to a central laboratory to determine levels of urinary sodium and creatinine. All the subjects were required to keep their daily diet habits during the day of urine collection, in the mean while to avoid strenuous exercise to reduce sweating. Complete oral and written guidance about urine specimen collection, transportation, and preservation was provided, including a caution about the common faults made during the collection. Samples were discarded if the volume of 24‐hour urine was <500 mL or its creatinine content was lower or higher than the 2 standard deviations (SD) from the population mean (male creatinine <11.3 or >1956.1 mg/d and female creatinine <8.4 or >1406.4 mmol/d).
2.3. Statistics analysis
Data are reported as means ± SD and medians for continuous variables, and proportions and percentages for categorical values. The goodness of fit to a normal distribution was evaluated using the Kolmogorov‐Smirnov test. Associations between gender and the studied variables were assessed by unpaired Student's t test for quantitative, normally distributed variables, and the Mann‐Whitney U test was used for non‐normally distributed variables. Proportions between groups were compared by the χ2 test. Analysis of variance (ANOVA) was used when more than two means were compared, followed by Tukey's post hoc test. The associations of urinary sodium with systolic and diastolic blood pressure were determined by Pearson's linear correlation coefficient. We used multivariable linear regression to examine the associations of urinary sodium output with SBP and DBP. A P‐value ≤ .05 was considered statistically significant. All statistical analyses were conducted by using SPSS (version 18.0, SPSS Inc, IBM).
3. RESULTS
The fundamental clinical data of the 512 study participants are shown in Table 1. The final population comprised 263 men and 249 women, with a mean age of 43.5 ± 10.2 years. Through comparison and analysis of baseline characteristics between genders, we found no significant differences in age, Cr, TC, TG, FBG, SBP, and DBP; however, there were significant differences in BMI, smoking, and alcohol. The prevalence of hypertension was 22.7%, smoking was 24.2%, alcohol was 22.4%, and obesity was 18.3%, respectively.
Table 1.
Baseline clinical characterizes of the total population
| Male | Female | Student t/χ2 | P * | |
|---|---|---|---|---|
| No. (%) | 263 (51.4) | 249 (48.6) | 5.097 | .107 |
| Age (y) | 44.5 ± 10.9 | 43.0 ± 9.4 | 1.079 | .344 |
| BMI (kg/m2) | 25.7 ± 4.6 | 23.1 ± 3.1 | 8.599 | .000 |
| Smoking (%) yes | 114 (43.4) | 10 (4.2) | 18.983 | .000 |
| Alcohol (%) yes | 94 (35.8) | 21 (8.5) | 16.066 | .000 |
| Cr (umol/L) | 83.4 ± 15.2 | 79.1 ± 18.3 | 0.951 | .396 |
| TG (mmol/L) | 2.9 ± 0.7 | 2.5 ± 0.3 | 0.562 | .774 |
| TC (mmol/L) | 4.7 ± 0.8 | 4.5 ± 0.7 | 0.480 | .673 |
| FBG (mmol/L) | 5.96 ± 2.74 | 5.36 ± 2.59 | 0.472 | .681 |
| Office SBP (mm Hg) | 127.6 ± 20.2 | 118.3 ± 17.5 | 0.798 | .451 |
| Office DBP (mm Hg) | 80.1 ± 14.3 | 81.3 ± 12.8 | 0.512 | .718 |
| Hypertension (%) yes | 62 (23.4) | 54 (21.8) | 1.462 | .281 |
| 24‐h urinary sodium (mmol/d) | 238.2 ± 132.4 | 218.5 ± 124.9 | 8.034 | .000 |
| 24‐h urinary creatinine (mg/d) | 983.7 ± 486.2 | 707.4 ± 349.5 | 9.375 | .000 |
Abbreviations: BMI, body mass index; Cr, creatinine; DBP, diastolic blood pressure; FBG, fasting blood glucose; SBP, systolic blood pressure; TC, total cholesterol; TG, total triglycerides.
P < .05 is considered significant.
The characteristics of 24‐hour urine sodium are shown in Table 1. The mean 24‐hour urinary sodium excretion of all subjects was 228.0 ± 127.5 mmol/24 hours, corresponding to a salt intake of 13.6 g/d. The average sodium excretion between men and women was 238.2 ± 132.4 and 218.5 ± 124.9 mmol/24 hours with remarkable differences, which was roughly equivalent to 14.5 and 13.2 g/d of daily salt intake. Twenty‐four‐hour creatinine excretion was higher (983.7 ± 486.2 vs 707.4 ± 359.5, P < .001) in men than in women.
Table 2 illustrates the difference in 24‐hour urinary sodium by sociodemographic variables. Urinary sodium excretion was roughly similar among the age, education status, income levels, and occupation categories. Nonetheless, significant differences were observed among normal weight group, overweight group, and obese group. Estimated salt intake was higher in obese subjects (17.6 ± 8.8 g/d) compared with overweight subjects (15.6 ± 8.0 g/d) and those with a normal BMI (13.9 ± 6.8 g/d). Noticeable differences remain obvious even adjusted for age, sex, smoking, alcohol, SBP, and DBP (P = .001). In the same way, urinary sodium excretion of hypertensive participants was dramatically higher than that of non‐hypertensive ones, the equivalent of 18.2 ± 9.1 g/d vs 13.3 ± 6.8 g/d. Overweight and obese individuals showed elevated blood pressure than normal weight ones (138.5 ± 23.3/87.8 ± 17.3 vs 120.4 ± 19.3/80.7 ± 14.3 mm Hg). These differences still existed after controlling for age, sex, BMI, smoking, alcohol, SBP, and DBP (P = .001).
Table 2.
24‐h urinary sodium excretion stratified by subgroups about socioeconomic status and lifestyle characteristics
| Subgroup | No. | 24‐h urine sodium (mmol/d) | P value |
|---|---|---|---|
| Age | |||
| <40 | 116 | 244.8 ± 131.5 | |
| 40‐60 | 223 | 240.1 ± 134.7 | |
| ≥60 | 173 | 238.4 ± 127.3 | .452 |
| BMI | |||
| <24 | 275 | 228.5 ± 113.7 | |
| 24‐28 | 143 | 257.2 ± 131.8 | |
| ≥28 | 94 | 289.3 ± 144.4 | .001 |
| Education | |||
| ≤Primary school | 142 | 249.3 ± 127.3 | |
| Middle‐High school | 207 | 243.2 ± 123.8 | |
| College or above | 163 | 239.5 ± 120.2 | .317 |
| Monthly income (RMB) | |||
| <3000 | 127 | 240.2 ± 121.4 | |
| 3000‐6000 | 218 | 238.6 ± 120.2 | |
| ≥6000 | 167 | 243.3 ± 122.1 | .348 |
| Occupation | |||
| Farmer | 119 | 241.8 ± 121.2 | |
| Worker | 160 | 243.5 ± 122.9 | |
| Staff | 161 | 237.4 ± 118.6 | |
| Others | 72 | 245.8 ± 124.1 | .481 |
| Hypertension | |||
| No | 401 | 218.5 ± 110.8 | |
| Yes | 111 | 299.3 ± 150.0 | .001 |
Data are presented as means ± SD. Analysis of variance (ANOVA) was used when more than two means were compared.
Correlations between blood pressure and urinary sodium were analyzed by calculating Spearman correlation coefficients. Urinary sodium excretion showed a significant positive correlation with SBP (r = .73, P < .001) and DBP (r = .62, P < .001) in all subjects (Figures 1 and 2). Table 3 presents the results of the multivariable linear regression analysis that assessed the association of sodium intake with SBP and DBP. We developed three covariate‐adjusted models. In model 1, we adjusted for age and sex; in model 2, in addition to the covariates in model 1, we adjusted for educational, income status, smoking, alcohol intake, and occupation; in model 3, we further adjusted for BMI as a continuous variable. In model 1 and model 2, urinary sodium correlated with SDP and DBP obviously (β = 1.80, 1.43, 1.06 and 0.91, respectively, all P < .05). Final results revealed that only urinary sodium was significantly associated with SBP (β = 1.08, P = .018), with an increase of 1.08 mm Hg (95% CI: 0.22‐1.97) for a 1‐SD increase in urinary sodium, while the correlation between sodium excretion and DBP disappeared (β = 0.57, P = .68) after adjustment for potential confounders.
Figure 1.
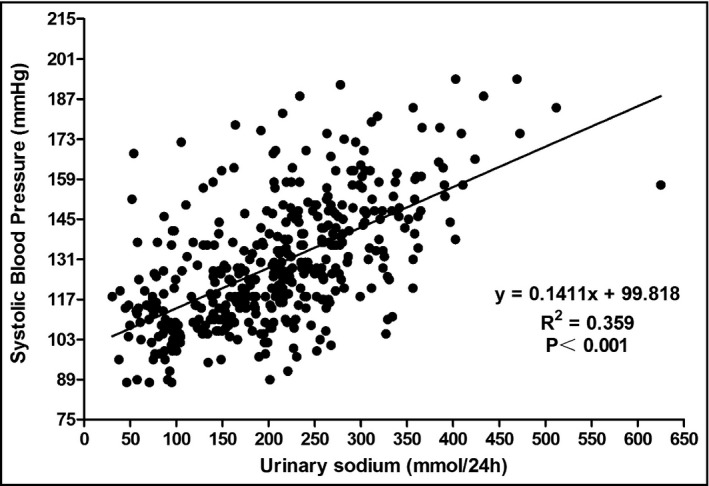
The graph showed the association of SBP with 24‐h urinary sodium
Figure 2.
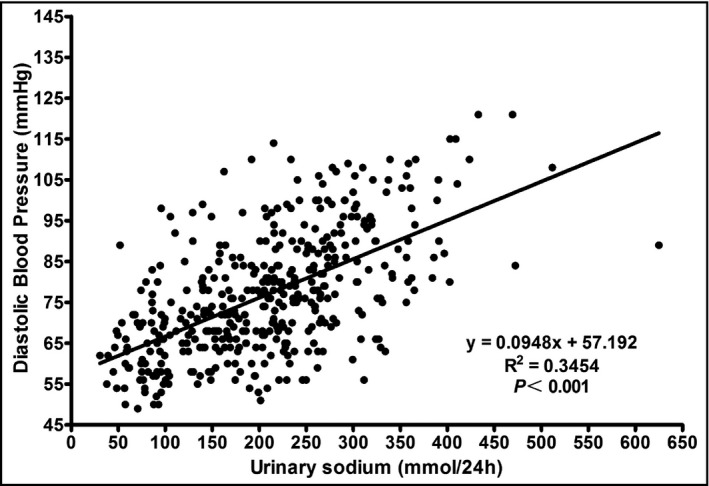
The graph showed the association of DBP with 24‐h urinary sodium
Table 3.
Association between urinary electrolytes and blood pressure with multivariable linear regression
| Variables | SBP | DBP | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | P | β | 95% CI | P | |
| Model 1 | 1.80 | 0.85‐2.85 | .002 | 1.06 | 0.35 to 1.75 | .006 |
| Model 2 | 1.34 | 0.42‐2.15 | .007 | 0.91 | 0.28 to 1.56 | .008 |
| Model 3 | 1.08 | 0.22‐1.97 | .018 | 0.57 | −0.05 to 1.22 | .68 |
Model 1 adjusted for age and sex. Model 2 adjusted for all factors in model 1 plus educational, income status, smoking, alcohol intake, and occupation. Model 3 adjusted for all factors in model 2 plus BMI as a continuous variable.
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
4. DISCUSSION
In this study, we determined that a highly positive correlation between urinary sodium and blood pressure, and obesity status was an important influential factor when this association was verified. Concordant with previous studies,17, 18 salt consumption in men was higher than that in women in Shandong, potentially due to the higher whole food intake by men and the differences in food pattern between men and women, which was expressly performed as higher BMI men than women (25.7 ± 4.6 vs 23.1 ± 3.1 kg/m2). In our study, even if the amount of sodium did not vary significantly regarding age, education, income level, and occupation status, an excess of salt intake was observed in obese individuals (see Table 2). It was easily identified that overweight and obese subgroup subjects consumed obviously higher amounts of sodium than normal weight ones, even after modification for age and blood pressure. The association of excessive salt intake with obesity is well known, but the biological mechanisms behind this phenomenon are not completely clear. Consuming too much salt is an underlying risk factor for obesity,19 which is correlated with obesity through energy intake such as increasing the intake of sugary drinks that have any added caloric sweetener and the coexistence of high salt and energy‐dense junk food in diet with poor quality.20 In addition, Song et al have suggested a hypothesis of a vicious cycle where excessive salt intake induces adipocyte hypertrophy and fat accumulation.21
The markedly positive relationship between 24‐hour sodium excretion and systolic and diastolic blood pressure is well established in many studies.17, 22 It also clearly indicates that the correlation between salt intake and blood pressure level is highly influenced by adjustment for BMI. Multivariable linear regression analysis suggested the relevance between salt intake and blood pressure was remarkably attenuated when adjusting for BMI; that is, urinary sodium was still associated with SBP, whereas the correlation between sodium excretion and DBP disappeared (see Table 3). The part that the BMI status played in the association between sodium intake and BP remains controversial. Similar to our research, Yan et al reported that the relationships between BP and both urinary sodium and potassium might be modified by BMI status in Chinese adults.23 Conversely, in the Dietary Approaches to Stop Hypertension (DASH)‐Sodium Trial, no evidence was found that blood pressure responses were heightened among obese participants compared with nonobese participants.24
The results of the current study have essential enlightenment for public health in China as hypertension could be largely prevented through the implementation of lifestyle changes such as a healthy diet, physical activity, and quitting smoking and alcohol. Hypertension was present in 22.7% of our sample; therefore, there is an emergency requirement for a population‐wide reduction in sodium intake, not only among hypertensive individuals. Generally speaking, the high salt consumption is mainly due to the high ingestion of soy sauce and the tradition of adding a large amount of salt to the food during cooking in China.25 Just the opposite, in European and Northern American diets, an estimated 75% of sodium intake comes from non‐discretionary source such as processed or restaurant‐prepared foods.26 However, it must be noted that China has undergone a transformation to “Western‐style” diet in recent decades, which is characterized by greater consumption of processed foods, fats, sodium, and sugars. Therefore, efforts to reduce sodium from diets in this population should focus on both discretional salt uses during home cooking and processed foods such as Western fast food, salted steak, and baked sausages.
A main strength of our study was the collection of timed 24‐hour urine specimens from population under strict quality control. We excluded 24‐hour urine collection with a small volume, which was validated less than 500 mL per day and collection intervals that were not within 24 hours.27 Furthermore, the most important exclusion criterion was creatinine excretion, which has already been proved to be an effective standard and been used widely in many previous studies.19, 28 This study also had some limitations. First, the levels of sodium and potassium in dietary consumption should be dealt with jointly when exploring their association with blood pressure, and it is very regretful that urinary potassium levels were not measured in the current study. Another potential disadvantage of our study was the method we used to evaluate the completeness of 24‐hour urine collection. We did not employ an objective biomarker of completeness such as para‐aminobenzoic acid (PABA). Third, urinary sodium was estimated by a single 24‐hour urine collection, which may be less accurate compared with two or three successive days collection due to the daily individual variability. However, a single urine measurement is identified as a more reliable measure of sodium intake than diet recall.29 Furthermore, considering the limited number of participants in this study, it is important to recognize that our study does not have enough statistical power for subgroup analysis. Also, our results need to be validated on a larger number of patients, probably in a multicenter study.
5. CONCLUSION
In summary, we found distinctly high amount of salt intake in Shandong Province, particularly in obese and hypertension subjects. Our results indicated that BMI status had an influence on the association between sodium intake and blood pressure. Dietary salt consumption among Shandong adults was overtaken excessively for a long time and changed little during the past 10 years. Reducing the population levels of salt intake is challenging, and a coordinate effective effort by governments and healthcare professional organizations is required to initiate lifestyle intervention program campaigns and control the hypertension by means of lowering salt consumption. Meanwhile, it is necessary to collaborate with restaurants and food industries to reduce excessive salt. Such public health approaches can be simple, at low cost and effective.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
The authors' contributions are as follows: N.S. and X.H. designed the study; W.W., M.L., L.C., S.J., and Y.C. conducted the research; W.H. and W.W. analyzed the data and wrote the first draft of the manuscript; N.S. and X.H. revised the paper. Wang Wei, as co‐first author, was involved in the conducting, analyzing, and reporting of the study. All authors read and approved the final manuscript.
ACKNOWLEDGMENT
We gratefully acknowledge the numerous patients, investigators, fellows, and research coordinators who participated in the present study.
Han W, Wang W, Sun N, et al. Relationship between 24‐hour urinary sodium excretion and blood pressure in the adult population in Shandong, China. J Clin Hypertens. 2019;21:1370–1376. 10.1111/jch.13644
Funding information
This study was supported by Projects of Medical and Health Technology Development Program in Shandong Province (NO. 2018WS270) and Key Research and Development project of Shandong Province (NO. 2016WSB01058).
REFERENCES
- 1. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ministry of Health . Annual report on health statistics. Beijing: Peking Union Medical College Publishing House; 2010. [Google Scholar]
- 3. Intersalt Cooperative Research Group . Intersalt: an international study of electrolyte excretion and blood pressure. Results of 24 hour urinary sodium and potassium excretion. BMJ. 1988;297:319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adrogué HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356:1966‐1978. [DOI] [PubMed] [Google Scholar]
- 5. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta‐analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 6. Wang M, Moran AE, Liu J, et al. A meta analysis of effect of dietary salt restriction on blood pressure in Chinese adults. Glob Heart. 2015;10(291‐299):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han W, Han X, Sun N, Chen Y, Jiang S, Li M. Relationships between urinary electrolytes excretion and central hemodynamics, and arterial stiffness in hypertensive patients. Hypertens Res. 2017;40:746‐751. [DOI] [PubMed] [Google Scholar]
- 8. Zhao L, Stamler J, Yan LL, et al. INTERMAP Research Group. Blood pressure differences between northern and southern Chinese: role of dietary factors: the International Study on Macronutrients and Blood Pressure. Hypertension. 2004;43:1332‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li LM, Rao KQ, Kong LZ, et al. Technical working group of China national nutrition and health survey. A description on the Chinese national nutrition and health survey in 2002. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:478‐484. [PubMed] [Google Scholar]
- 10. Guideline: Sodium Intake for Adults and Children. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 11. Zhang JY, Yan LX, Tang JL, et al. Estimating daily salt intake based on 24 h urinary sodium excretion in adults aged 18–69 years in Shandong, China. BMJ Open. 2014;4(7):e005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu J, Wang M, Chen Y, et al. Estimation of salt intake by 24‐hour urinary sodium excretion: a cross‐sectional study in Yantai, China. BMC Public Health. 2014;14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han W, Sun N, Wang H. Relationship between 24‐hour urinary sodium potassium excretion and blood pressure and arterial stiffness in hypertension patients. J hypetens. 2012;30:e323. [Google Scholar]
- 14. Lerchl K, Rakova N, Dahlmann A, et al. Agreement between 24‐hour salt ingestion and sodium excretion in a controlled environment. Hypertension. 2015;66:850‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu LS. 2010 Chinese guidelines for the management of hypertension. Chin J Cardiol. 2011;39:579‐615. [PubMed] [Google Scholar]
- 16. Bureau of Disease Control and Prevention . China's Ministry of Health. Chinese Guideline on Adult Overweight and Obesity Prevention. Beijing: People's Medical Publishing House; 2006. [Google Scholar]
- 17. Zhou BF, Stamler J, Dennis B, et al. INTERMAP Research Group. Nutrient intakes of middle‐aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens. 2003;17:623‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li JH, Xu AQ, Lu ZL, et al. Dietary sodium intake and its impact factors in adults of Shandong province. Biomed Environ Sci. 2014;27:564‐566. [DOI] [PubMed] [Google Scholar]
- 19. Ge Z, Guo X, Chen X, et al. Association between 24 h urinary sodium and potassium excretion and the metabolic syndrome in Chinese adults: the Shandong and Ministry of Health Action on Salt and Hypertension (SMASH) study. Br J Nutr. 2015;113:996‐1002. [DOI] [PubMed] [Google Scholar]
- 20. Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity? Hypertension. 2015;66:843‐849. [DOI] [PubMed] [Google Scholar]
- 21. Song HJ, Cho YG, Lee HJ. Dietary sodium intake and prevalence of overweight in adults. Metabolism. 2013;62:703‐708. [DOI] [PubMed] [Google Scholar]
- 22. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297(6644):319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan L, Bi Z, Tang J, et al. Relationships between blood pressure and 24‐hour urinary excretion of sodium and potassium by body mass index status in Chinese adults. J Clin Hypertens (Greenwich). 2015;17:916‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vollmer WM, Sacks FM, Ard J, et al. DASH‐Sodium Trial Collaborative Research Group. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH‐sodium trial. Ann Intern Med. 2001;135:1019‐1028. [DOI] [PubMed] [Google Scholar]
- 25. Liu L, Liu L, Ding Y, et al. Ethnic and environmental differences in various markers of dietary intake and blood pressure among Chinese Han and three other minority peoples of China: results from the WHO Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study. Hypertens Res. 2001;24:315‐322. [DOI] [PubMed] [Google Scholar]
- 26. Mattes RD, Donnelly D. Relative contributions of dietary sodium sources. J Am Coll Nutr. 1991;10:383‐393. [DOI] [PubMed] [Google Scholar]
- 27. Han W, Sun N, Chen Y, Wang H, Xi Y, Ma Z. Validation of the spot urine in evaluating 24‐hour sodium excretion in Chinese hypertension patients. Am J Hypertens. 2015;28:1368‐1375. [DOI] [PubMed] [Google Scholar]
- 28. Ge Z, Zhang J, Chen X, et al. Are 24 h urinary sodium excretion and sodium: potassium independently associated with obesity in Chinese adults? Public Health Nutr. 2016;19:1074‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santos JA, Webster J, Land M‐A, et al. Dietary salt intake in the Australian population. Public Health Nutr. 2017;20:1887‐1894. [DOI] [PMC free article] [PubMed] [Google Scholar]


