ABSTRACT
Gnotobiotic broiler chickens were used to study interactive effects of supplemented phosphorus, calcium (PCa), and phytase (Phy) on myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate) (InsP6) degradation and release of myo-inositol in the digestive tract. In 2 subsequent runs, the chickens were subjected to 1 of 4 dietary treatments with and without PCa and Phy supplementation. Sanitized eggs were hatched in 8 germfree isolators, and a minimum of 9 male Ross 308 chickens were placed in each pen (total 16 pens). Treatments implemented on day 10 included gamma-irradiated diets without (PCa−; 4.1 g P and 6.2 g Ca/kg DM) or with (PCa+; 6.9 g P and 10.4 g Ca/kg DM) monosodium phosphate and limestone supplementation and without (Phy−) or with (Phy+) 1,500 FTU Phy/kg feed in a factorial arrangement. On day 15, digesta was collected from different sections of the intestinal tract and analyzed for InsP isomers and myo-inositol. The isolators did not remain germfree, but analysis of contaminants and results of InsP degradation indicated no or minor effects of the identified contaminants. Prececal InsP6 disappearance was 42% with the PCa−Phy− treatment and 17% with PCa+Phy−. No InsP3–4 isomers were found in the digesta of the terminal ileum in PCa−Phy−. The concentration of myo-inositol in the ileal digesta from PCa−Phy− (6.1 μmol/g DM) was significantly higher than that from PCa+Phy− (1.7 μmol/g DM), suggesting rapid degradation of the lower InsP isomers by mucosal phosphatases and their inhibition by PCa. Phytase supplementation increased InsP6 disappearance and prevented inhibitory effects of PCa supplements (72% in PCa−Phy+ and 67% in PCa+Phy+). However, PCa supplementation reduced the degradation of lower InsP isomers mainly in the posterior intestinal sections in the presence of Phy, resulting in significantly lower myo-inositol concentrations. It is concluded that mucosa-derived phosphatases might significantly contribute to InsP6 degradation in broiler chickens. The potential of mucosa-derived phosphatases to degrade InsP6 and lower InsP is markedly reduced by dietary PCa supplementation.
Keywords: broiler, gnotobiotic, inositol phosphate, phytase, phytate
INTRODUCTION
Phosphorus (P) is important for the growth and well-being of poultry. In diets based on plant raw materials, P has only limited availability for non-ruminants. The main source of P is in the salt form of phytic acid [myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate); InsP6], called phytate (Eeckhout and de Paepe, 1994; Rodehutscord et al., 2016). The phosphate groups are cleaved from the phytate molecule by phytase (Phy) (myo-inositol hexaphosphate phosphohydrolases) and other phosphatases. This process hydrolyzes phytate into inositol phosphate (InsP) isomers of different phosphorylated states (InsP1–5), with myo-inositol (MI) and phosphate as the end products.
To meet the P and calcium (Ca) requirements of broiler chickens, mixtures of mineral P, limestone, and Phy are commonly added to poultry diets. When corn and soybean meal diets with low P and Ca concentrations were fed to broilers, there was high potential for InsP6 degradation, even without supplemented Phy (Applegate et al., 2003; Tamim et al., 2004; Zeller et al., 2015a). However, the addition of P and Ca reduced the degradation of InsP6 and lower InsP isomers (Shastak et al., 2014), an effect that could be partly compensated by the addition of high Phy doses (Zeller et al., 2015b; Sommerfeld et al., 2018b).
The high potential for InsP6 degradation in the absence of dietary Phy is likely attributed to endogenous Phy and other phosphatases produced by the intestinal epithelium or resident microbiota in the digestive tract of the broiler chicken. Kerr et al. (2000) found that microorganisms contributed significantly to InsP6 degradation in the ceca of broiler chickens. Other authors argued that the contribution of intestinal mucosa-derived Phy are contained within the epithelial membrane and, therefore, have minimal contribution to InsP6 degradation in the digestive tract of broilers (Rodehutscord and Rosenfelder, 2016). In one study with germfree rats, the phytate provided with feed was completely recovered in the feces (Wise and Gilburt, 1982). In a contradictory study, 54% of the phytate disappeared before the lower small intestine (Miyazawa et al., 1996). The reasons for the discrepancy between these studies is not clear. Overall, InsP6 degradation studies do not distinguish between the role of epithelial-derived or microbiota-derived enzymes.
The first objective of this study was to investigate InsP6 breakdown and, for the first time, appearance of lower InsP isomers and MI in different sections of the digestive tract of gnotobiotic broiler chickens. Our hypothesis was that InsP6 disappearance in the digestive tract is low based on the assumption that intestinal microbiota makes a large contribution to enzymatic activity. The second objective was to investigate interacting effects of supplemented P, Ca, and microbial Phy in gnotobiotic broiler chickens.
MATERIALS AND METHODS
Birds and Germfree Assembly
This work was approved by the University of Saskatchewan's Animal Research Ethics Board and adhered to the Canadian Council on Animal Care (1993, 2009) guidelines for humane animal use.
The study was carried out in 2 subsequent runs. In run 1, a total of 320 Ross 308 eggs were sanitized by emersion in 0.3% sodium hypochlorite and placed in incubators. Meanwhile, 4 germfree isolators were assembled and packed with autoclaved equipment and animal feed. Each isolator contained 2 pens (58 × 30 cm2 ground area each) and 4 pairs of gloves for handling birds and equipment. The isolators were then sealed, fumigated with formaldehyde and maintained under positive pressure with HEPA-filtered air.
After 17 days of incubation, fertile eggs were sanitized again in 0.3% sodium hypochlorite. The eggs were split into 4 groups (1 × 57 eggs and 3 × 56 eggs), and each group was aseptically placed in 1 isolator through a portal sterilized with peracetic acid (2%). Temperature was maintained at 36°C until hatching (hatching rate ranged between 63 and 75%). After hatching, the birds were sexed, and the males were assigned to 1 of 2 pens within each isolator (10 birds/pen in 2 isolators and 9 and 10 birds/pen in 2 isolators). Females were killed by cervical dislocation and removed from the isolator together with the egg shells. The birds were kept on mesh floors to avoid excreta ingestion. The birds were fed a starter diet with adequate levels of all nutrients from day 1 to day 10 and the experimental diets from day 10 to day 15. To avoid carry-over of supplemented Phy on Phy-free diets, chickens in 2 isolators per run were assigned to Phy− diets and chickens in the remaining 2 isolators assigned to Phy+ diets. Birds in each pen in each isolator were assigned to supplemented phosphorus, calcium (PCa)− or PCa+ diets. Feed and water were provided for ad libitum consumption for the duration of the experiment. The lighting program was 24 L:0 D from hatch to day 4, and 18 L:6 D from day 4 until the last day, day 15. The temperature in the isolators was set at 34°C on day 1 and was gradually decreased to 28°C on the last day.
Swabs of the peri-cloacal region of randomly selected birds were taken every 2 days and incubated in sterile soybean-casein digest broth (BBL Trypticase Soy Broth, Becton, Dickinson and Company, Sparks, MD, USA) that was maintained inside the isolators. At the end of the study, to test for bacterial contamination, a subsample of the broth was spread on Trypticase Soy Agar and incubated under both aerobic and anaerobic (10% CO2, 10% H2, 80% N2) conditions for 48 h at 37°C. Genomic DNA was extracted from multiple colonies, amplified using universal 16S rRNA gene primers, and the resulting amplicon sequenced. Identity of contaminants was determined with the use of the classifier tool available at RDP Ribosomal Database Project (http://rdp.cme.msu.edu).
In run 2, that was started 1 wk after completion of run 1, a total of 357 eggs were incubated, and 65 eggs were placed in each isolator. After hatching, all pens were stocked with 10 male birds (hatching rate was between 78 and 94%). All other procedures were conducted as described for run 1.
Diets and Treatments
Chickens were fed diets based on solvent-extracted soybean meal and corn (Table 1) because of their low intrinsic Phy activity (Eeckhout and de Paepe, 1994; Delia et al., 2011; Rodehutscord et al., 2016). This allowed us to examine enzyme activity specific to endogenous mucosa-derived phosphatases. Diets were calculated to contain adequate levels of all nutrients (except for Ca and P) according to the recommendations of the Gesellschaft für Ernährungsphysiologie (1999). Calculated concentrations of CP and ME were 251 g/kg DM and 13.5 MJ/kg DM, respectively. Titanium dioxide (5 g/kg) was included in the diets as an indigestible marker. A premix of vitamins A, D, and E was supplemented to avoid vitamin loss during feed irradiation. The study involved a 2 × 2-factorial arrangement of treatments (2 PCa levels and 2 Phy levels) and included diets without PCa (PCa−, calculated 4.1 g P/kg DM and 6.2 g Ca/kg DM) or with (PCa+, calculated 6.9 g P/kg DM and 10.4 g Ca/kg DM) monosodium phosphate and additional limestone supplementation, and without (Phy−) or with (Phy+) 1,500 FTU Phy/kg feed (modified Escherichia coli derived 6-Phy; Quantum Blue, AB Vista, Marlborough, UK). The experimental diets were produced by first mixing the main ingredients and then adding a premix of the minor ingredients. These mixes were divided into 2 parts. Each part was then supplemented with the microbial Phy product or remained without the supplement. The mash diets were sterilized by gamma irradiation (50 kGy; Nordion Inc., Laval, Quebec, Canada). Representative samples of each diet were pulverized by a vibrating cup mill (PULVERISETTE 9, Fritsch GmbH, Idar-Oberstein, Germany). The analyzed Phy activity was 1,060 and 1,250 FTU/kg feed (Table 1) and, hence, was lower than formulated but similar for respective treatments. The experimental diets contained similar concentrations of InsP5 and InsP6. Other InsP isomers were not detected in the diets.
Table 1.
Ingredient and analyzed composition of the experimental diets1 fed from day 10 to day 15.
| Ingredient, g/kg | PCa−2 | PCa+2 | ||
|---|---|---|---|---|
| Corn | 539.8 | 539.8 | ||
| Soybean meal | 399.1 | 399.1 | ||
| Soybean oil | 15.0 | 15.0 | ||
| DL-Methionine | 2.0 | 2.0 | ||
| Monosodium phosphate | – | 10.5 | ||
| Sand | 18.0 | – | ||
| Limestone | 10.0 | 19.5 | ||
| Sodium chloride | 1.0 | 1.0 | ||
| Choline chloride | 2.0 | 2.0 | ||
| Sodium bicarbonate | 3.0 | 1.0 | ||
| Vitamin/Mineral mix3 | 5.0 | 5.0 | ||
| Vitamin mix (A,D,E)4 | 0.2 | 0.2 | ||
| Titanium Dioxide | 5.0 | 5.0 | ||
| Analyzed composition | Phy− | Phy+ | Phy− | Phy+ |
| Total P, g/kg DM | 4.9 | 4.9 | 7.9 | 7.9 |
| InsP6-P, g/kg DM | 3.0 | 3.0 | 3.0 | 3.0 |
| Ca, g/kg DM | 6.5 | 6.2 | 10.5 | 10.7 |
| Myo-inositol, μmol/g DM | 0.9 | 0.9 | 0.9 | 0.9 |
| Ins(1,2,3,4,5)P55, μmol/g DM | 0.5 | 0.5 | 0.5 | 0.6 |
| Ins(1,2,4,5,6)P55, μmol/g DM | 0.9 | 1.0 | 0.9 | 1.0 |
| InsP65, μmol/g DM | 16.4 | 16.0 | 16.0 | 16.4 |
| Phytase activity, FTU/kg6 | <507 | 1,060 | <507 | 1,250 |
1Calculated composition: PCa−, 4.1 g P/kg DM and 6.2 g Ca/kg DM; PCa+, 6.9 g P/kg DM and 10.4 g Ca/kg DM; Phy−, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
2With or without supplemented 1,500 FTU/kg of a modified, Escherichia coli derived 6-phytase (Quantum Blue).
3Broiler Premix (DSM, Ayr, Ontario, Canada) provided per kg of complete diet: 11,000 IU vitamin A, 2,000 IU vitamin D, 30 IU vitamin E, 2 mg menadione, 1.5 mg thiamine, 6 mg riboflavin, 4 mg pyridoxine, 0.02 mg vitamin B12, 60 mg niacin, 10 mg pantothenic acid, 0.6 mg folic acid, 0.15 mg biotin, 10 mg copper from copper sulphate, 80 mg iron from ferrous sulphate, 80 mg manganese from manganous oxide, 0.8 mg iodine from calcium iodate, 80 mg zinc from zinc oxide, 0.3 mg selenium, 500 mg calcium carbonate.
4Vitamin A, D, and E premix (DSM, Ayr, Ontario, Canada) provided per kg complete diet: 9,000 IU Vitamin A, 2,000 IU Vitamin D3, 15 IU Vitamin E.
5No other InsP isomers were detected.
6Determined at pH 4.5 and 60°C.
7Below limit of detection when determined at pH 5.0 and 45°C according to Greiner and Egli (2003).
Sampling and Measurements
To standardize feed intake and filling of the birds’ crops, feeders were removed from the pens 2 h before slaughter and replaced 1 h before slaughter. Birds were sacrificed by cervical dislocation and weighed individually. Digesta from the following sections of digestive tract was collected: crop, proventriculus and gizzard together (pro+giz), duodenum and jejunum together (duo+jej), the terminal part of the ileum (last two-thirds of the section between Meckel's diverticulum and 2 cm prior the ileo-ceco-colonic junction), and both ceca. Digestive tract segments of all animals in a single pen were collected and pooled. Prior to collection, the crop was clamped with an arterial clamp to prevent emptying. The crop and pro+giz were then opened and upended, and the digesta was gently removed using a spatula without scraping the mucosa. Digesta from intestinal sections was rinsed with cold double-distilled water. All samples were immediately frozen at −80°C and later freeze-dried. Samples were pulverized by a vibrating cup mill (PULVERISETTE 9, Fritsch GmbH, Idar-Oberstein, Germany). Pulverized samples were stored in airtight containers at below 6°C until analysis.
Chemical Analysis
Ground feed samples were analyzed for DM according to the official method in Germany (method no. 3.1) (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA), 2007). Pulverized feed and digesta samples were analyzed for P, Ca, and Ti by inductively coupled plasma-optical emission spectrometry following wet digestion, using a modified method of Boguhn et al. (2009), described in detail by Zeller et al. (2015a). Extraction and measurement of InsP3−6 isomers in feed and digesta were carried out using the method of Zeller et al. (2015a) with slight modifications. Samples were extracted twice with a solution of 0.2 M EDTA and 0.1 M sodium fluoride (pH of 8; 4°C) for 30 min under agitation and were then centrifuged after each extraction at 12,000 × g for 15 min. The respective supernatants were combined, and a 1 mL sample was centrifuged at 14,000 × g for 15 min, filtered, and centrifuged again at 14,000 × g for 30 min. Filtrates were analyzed using high-performance ion chromatography and UV detection at 290 nm after a post-column reaction with Fe(NO3)3 in HClO4 using an ICS-3000 system (Dionex, Idstein, Germany). By using this methodology, separation of enantiomers is not possible and, therefore, we were unable to distinguish between the D- and L-forms. Some InsP3 isomers could not be identified because standards were unavailable. A clear discrimination of the isomers Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3 was not possible because of co-elution; therefore, in this study, we used the term InsP3x for the InsP3 isomers of unknown proportion. InsP6 was used for quantification, and correction factors for differences in detector responses for InsP3–5 were used according to Skoglund et al. (1997). Myo-inositol in feed and digesta samples was analyzed according to Sommerfeld et al. (2018a) using a gas-chromatograph/mass spectrometer after derivatization of the samples. Feed samples were analyzed for supplemented Phy activity (Enzyme Services and Consultancy, Cordova, TN, USA). Enzyme activity was measured using the analytical method of the enzyme producer (pH 4.5; 60°C), and values were converted to FTU by a validated conversion factor. Treatments without supplemented Phy were additionally analyzed for the activity of intrinsic Phy by a direct incubation method according to Greiner and Egli (2003). In brief, samples were incubated in sodium acetate buffer containing 100 μmol sodium phytate at pH 5.0 and 45°C. Liberated inorganic phosphate was measured spectrophotometrically using ammonium molybdate within an incubation period of 20–40 min.
Calculations and Statistical Analysis
The disappearance of InsP6, P, and Ca was calculated based on the analyzed concentration of InsP6, P, Ca, and Ti in the feed and digesta. The following generally accepted equation was used:
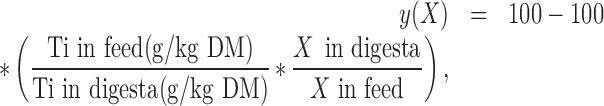 |
where y(X) is the disappearance of X in %, and X is InsP6 or P or Ca in g/kg DM. InsP6 disappearance was not calculated for pro+giz and ceca. In pro+giz, the marker does not properly represent the particles owing to different particle sizes (Zeller et al., 2015a). Only a small proportion of the digesta enters the ceca, specifically small or soluble particles (Svihus et al., 2013), suggesting that InsP6 disappearance in relation to intake cannot be accurately calculated in the ceca.
The concentration of disappeared P and Ca(y) was calculated as follows:
 |
where y is in g/kg DM, P or Ca disappearance is in %, and P or Ca content in feed is in g/kg DM.
All data were subjected to a 2-factorial analysis of variance using the MIXED procedure of the software package SAS (version 9.3; SAS Institute Inc., Cary, North Carolina). The pen was considered as the experimental unit. The following model was chosen:
 |
where Yijkl is the response variable, μ is the overall mean, αi is the fixed effect of PCa addition i, βj is the fixed effect of Phy addition j, run is the random effect of the experimental run k, isolator is the random effect of the isolator l, and ϵijkl is the residual error. Statistical significance was declared at P < 0.05.
RESULTS AND DISCUSSION
Germfree Status
The aim of the present study was to investigate InsP degradation in germfree broiler chickens and understand the contribution of mucosa-derived phosphatases to InsP degradation. However, the isolators in both runs did not remain germfree. Two contaminants were detected using DNA sequencing. In run 1, Enterococcus faecalis was found in 3 out of 4 isolators and a Pseudomonas sp. was found in 2 isolators. In run 2, E. faecalis was found in 2 isolators, and a Pseudomonas sp. was found in all 4 isolators. Contamination was likely caused by bacterial penetration of the eggshell prior to sterilization. Pseudomonas aeruginosa, for example, is a common egg spoilage organism (Berrang et al., 1999).
Enterococcus faecalis is not known to produce Phy (Yanke et al., 1998), but some Pseudomonas species are known to produce Phy (Young-Hoon et al., 2002). Because it was not possible to specify the Pseudomonas species in the present study, it cannot be ruled out that the isolators and birds were contaminated with Phy producers. However, a Pseudomonas sp. was not found in the 2 isolators with Phy− treatments in run 1. The SEM of the results from the Phy− treatments in the isolators with and without Pseudomonas sp. contamination was similar to that in previous experiments with conventional birds, and, thus, we concluded with caution that the effect of contamination was not significant. Therefore, InsP degradation detected in the Phy− treatments can be ascribed to mucosa-derived Phy and other phosphatases. Consequently, the focus of the following discussion is related to mucosa-derived enzymes.
InsP Degradation Along the Digestive Tract of Gnotobiotic Broiler Chickens
To our knowledge, for the first time, we show the degradation pathway of InsP in the digestive tract of gnotobiotic broiler chickens. Overall, the results demonstrate a high potential contribution by mucosa-derived enzymes to InsP degradation in broiler chickens. The results cast into doubt our hypothesis that microbiota-derived Phy and other phosphatases contribute more to InsP degradation than mucosa-derived enzymes.
Crop
In the crop, InsP6 disappearance was overall low when Phy was not supplemented to the feed (Table 2). In Phy− treatments, there was a 7 and 4% disappearance of InsP6 in PCa− and PCa+ treatments, respectively. InsP6 disappearance increased with Phy supplementation. In Phy+ treatments there was a 6 and 17 percentage points increase in InsP6 disappearance in PCa− and PCa+ treatments, respectively (P = 0.001). The isomers Ins(1,2,5,6)P4 and InsP3x only appeared in the crop when Phy was supplemented, and they were more concentrated in the PCa+ diets (Table 3; P < 0.05). The concentration of MI in the crop was lower in the PCa+ treatments than in the PCa− treatments (P = 0.003).
Table 2.
Effect of P, and Ca (PCa), and phytase (Phy) supplementation on InsP6, P and Ca disappearance up to the crop, duodenum and jejunum (Duo+Jej), and terminal ileum of broiler chickens fed the experimental diets from day 10 to day 151.
| Dietary treatment2 | P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| PCa− | PCa+ | |||||||
| Phy− | Phy+ | Phy− | Phy+ | Pooled SEM | PCa | Phy | PCa × Phy | |
| InsP6 disappearance | ||||||||
| Crop, % | 7 | 13 | 4 | 21 | 2.74 | 0.386 | 0.001 | 0.063 |
| Duo+Jej, % | 35b | 72a | 11c | 63a | 3.04 | <0.001 | <0.001 | 0.040 |
| Ileum, % | 42b | 72a | 17c | 67a | 2.17 | <0.001 | <0.001 | 0.001 |
| P disappearance | ||||||||
| Duo+Jej, % | 6 | 34 | 33 | 44 | 6.16 | 0.007 | 0.006 | 0.149 |
| Duo+Jej, g/kg DM | 0.3 | 1.6 | 2.6 | 3.5 | 0.35 | <0.001 | 0.003 | 0.500 |
| Ileum, % | 55c | 73a | 60b | 72a | 1.28 | 0.070 | <0.001 | 0.015 |
| Ileum, g/kg DM | 2.7 | 3.6 | 4.8 | 5.7 | 0.08 | <0.001 | <0.001 | 0.834 |
| Ca disappearance | ||||||||
| Duo+Jej, % | 49 | 54 | 34 | 41 | 2.01 | <0.001 | 0.003 | 0.476 |
| Duo+Jej, g/kg DM | 3.2b | 3.3b | 3.5b | 4.4a | 0.17 | 0.001 | 0.006 | 0.024 |
| Ileum, % | 69 | 75 | 57 | 58 | 1.77 | <0.001 | 0.021 | 0.055 |
| Ileum, g/kg DM | 4.5 | 4.7 | 6.0 | 6.2 | 0.17 | <0.001 | 0.219 | 0.854 |
1Data are given as treatment means; n = 4 pens.
2Calculated composition: PCa−, 4.1 g P/kg DM and 6.2 g Ca/kg DM; PCa+, 6.9 g P/kg DM and 10.4 g Ca/kg DM; Phy−, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
a–cMeans within a row not showing a common superscript differ (P < 0.05).
Table 3.
Effect of P, and Ca (PCa), and phytase (Phy) supplementation on concentrations of InsP isomers and myo-inositol (μmol/g DM) in the crop and in proventriculus and gizzard of gnotobiotic broiler chickens fed the experimental diets from day 10 to day 151.
| Dietary treatment2 | P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| PCa− | PCa+ | |||||||
| Phy− | Phy+ | Phy− | Phy+ | Pooled SEM | PCa | Phy | PCa × Phy | |
| Crop | ||||||||
| InsP6 | 15.0 | 13.9 | 14.9 | 12.9 | 0.31 | 0.059 | <0.001 | 0.097 |
| Ins(1,2,4,5,6)P5 | 0.9 | 0.6 | 0.9 | 0.5 | 0.03 | 0.427 | <0.001 | 0.131 |
| Ins(1,2,3,4,5)P5 | 0.5 | 0.5 | 0.6 | 0.6 | 0.02 | 0.001 | 0.293 | 0.293 |
| Ins(1,2,3,4,6)P5 | 0.2 | <LOQ3 | 0.2 | n.d.4 | 0.04 | 0.391 | – | – |
| Ins(1,2,5,6)P4 | n.d. | 1.0 | n.d. | 2.5 | 0.25 | 0.001 | – | – |
| InsP3x5 | n.d. | 0.2 | n.d. | 0.5 | 0.03 | 0.003 | – | – |
| Myo-inositol | 1.0 | 1.0 | 0.8 | 0.9 | 0.03 | 0.003 | 0.165 | 0.474 |
| Proventriculus+Gizzard | ||||||||
| InsP6 | 9.0 | 2.7 | 8.9 | 1.8 | 0.19 | 0.022 | <0.001 | 0.057 |
| Ins(1,2,4,5,6)P5 | 0.4 | 0.3 | 0.5 | 0.2 | 0.04 | 0.169 | <0.001 | 0.063 |
| Ins(1,2,3,4,5)P5 | 0.3 | 0.8 | 0.3 | 0.5 | 0.07 | 0.066 | <0.001 | 0.066 |
| Ins(1,2,5,6)P4 | n.d. | 3.2 | n.d. | 3.3 | 0.21 | 0.528 | – | – |
| InsP3x5 | n.d. | 0.7 | n.d. | 0.9 | 0.30 | 0.161 | – | – |
| Myo-inositol | 0.6b | 1.0a | 0.4c | 0.6b | 0.05 | <0.001 | <0.001 | 0.023 |
1Data are given as treatment means; n = 4 pens.
2Calculated composition: PCa−, 4.1 g P/kg DM and 6.2 g Ca/kg DM; PCa+, 6.9 g P/kg DM and 10.4 g Ca/kg DM; Phy−, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
3 <LOQ, not quantifiable in the majority of samples.
4n.d., not detectable in the majority of samples.
5At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
a–cMeans within a row not showing a common superscript differ (P < 0.05).
The crop showed some disappearance of InsP6 in Phy− treatments. The crop epithelium is not known to secrete enzymes (Svihus, 2014), the feed components were chosen for their negligibly low intrinsic Phy activity (Eeckhout and de Paepe, 1994; Delia et al., 2011; Rodehutscord et al., 2016), and the feed was irradiated prior to usage. Enzymatic assays determined that intrinsic Phy activity was not only below the limit of detection, but the assay showed not even a minimal response. Hence, intrinsic plant Phy activity did not contribute to InsP6 degradation in this study. It is possible that the low InsP6 disappearance measured in the crop was due to experiment or laboratory variations.
The increase in InsP6 disappearance with Phy supplementation in the crop was much lower than that previously noted for conventional birds. One study showed a 66 percentage points increase in InsP6 disappearance in a low PCa diet with 1,500 FTU Phy supplementation (Sommerfeld et al., 2018b). This discrepancy might be due to the absence of lactobacillaceae in the present study, which is the most abundant bacterial family in the crop (Witzig et al., 2015). The discrepancy might be further explained by the pH levels in the crop. Lack of lactic acid production might have caused the pH levels to be higher than those found in conventional birds. Higher pH could have reduced the solubility of phytate or let to a shift of pH value out of the optimum pH for the supplemented Phy, which is 3.5 to 5.0 (Menezes-Blackburn et al., 2015). Further, lactic acid is known to chelate Ca and, thus, potentiate the activity of Phy in the crop. To better understand the differences between studies, the pH and lactate in future experiments should be measured in all digestive tract sections. Higher concentrations of Ins(1,2,5,6)P4 and InsP3x and lower concentration of MI in the PCa+ treatments suggest that PCa supplementation decreased degradation of lower InsP already in the crop.
Proventriculus and Gizzard
InsP6 disappearance was not calculated for pro+giz because of no proper representation by the marker (Zeller et al., 2015a), but concentrations of InsP can be compared between the treatments. InsP6 concentration in pro+giz was decreased by either Phy or PCa supplementation (Table 3; P < 0.001 and P = 0.022, respectively). The decrease of InsP6 concentration due to Phy supplementation was more pronounced in the pro+giz than in the crop. This can be explained by the lower, more favorable pH level in this section. The concentration of Ins(1,2,4,5,6)P5 was lower and that of Ins(1,2,3,4,5)P5 was higher in the Phy+ treatments than in the Phy− treatments (P < 0.001, respectively). Ins(1,2,5,6)P4 and InsP3x were only measured in the Phy+ treatments. Phy supplementation increased MI concentration in the PCa+ treatment less than in the PCa− treatment (P = 0.023 for the interaction effect). The small difference in MI concentration between Phy− and Phy+ treatments demonstrates an inability to degrade InsP1 to MI or a rapid exit of MI with the liquid phase from the pro+giz to the small intestine.
Small Intestine
Up to the duo+jej and terminal ileum, InsP6 disappearance was 35 and 42% in PCa−Phy− treatment, and 11 and 17% in PCa+Phy− treatment, respectively (Table 2). InsP6 disappearance was low in PCa+ diets but increased with Phy supplementation to a greater extent than that in PCa− diets, resulting in a PCa × Phy interaction (P < 0.05). In the duo+jej, the concentrations of InsP6 and Ins(1,2,3,4,5)P5 were affected by the PCa × Phy interaction (Table 4; P = 0.031 and P < 0.001, respectively). InsP6 concentration was reduced by Phy supplementation to a greater extent when PCa was supplemented. Ins(1,2,3,4,5)P5 concentration was increased by Phy to a greater extent when PCa was supplemented. Ins(1,2,3,4,6)P5 only appeared in the absence of added Phy. Ins(1,2,5,6)P4 was only measured in Phy+ treatments and was significantly higher in the PCa+ treatment. The concentrations of Ins(1,2,3,4)P4 and InsP3x were the highest in PCa+Phy+. The concentration of MI increased in Phy+ treatments and decreased in PCa+ treatments. Disappearance of P (% and g/kg DM) up to the duo+jej increased by both Phy and PCa supplementation (Table 2; P < 0.05). Disappearance of Ca (%) up to the duo+jej decreased in PCa+ (P < 0.001) and increased in Phy+ (P = 0.003) treatments. Disappearance of Ca (in g/kg DM) only increased in PCa+Phy+ treatment.
Table 4.
Effect of P, and Ca (PCa), and phytase (Phy) supplementation on concentrations of InsP isomers and myo-inositol (μmol/g DM) in the duodenum and jejunum, terminal ileum, and ceca of gnotobiotic broiler chickens fed the experimental diets from day 10 to day 151.
| Dietary treatment2 | P-values | |||||||
|---|---|---|---|---|---|---|---|---|
| PCa− | PCa+ | |||||||
| Phy− | Phy+ | Phy− | Phy+ | Pooled SEM | PCa | Phy | PCa × Phy | |
| Duodenum+Jejunum | ||||||||
| InsP6 | 17.1b | 7.8d | 24.9a | 11.1c | 0.90 | <0.001 | <0.001 | 0.031 |
| Ins(1,2,4,5,6)P5 | 0.3 | 0.4 | 1.1 | 1.2 | 0.06 | <0.001 | 0.104 | 0.668 |
| Ins(1,2,3,4,5)P5 | 0.7c | 1.6b | 1.0c | 3.8a | 0.15 | <0.001 | <0.001 | <0.001 |
| Ins(1,2,3,4,6)P5 | 0.3 | n.d.3 | 0.5 | n.d. | 0.04 | 0.080 | – | – |
| Ins(1,2,5,6)P4 | n.d. | 0.8 | <LOQ4 | 4.8 | 0.84 | 0.002 | – | – |
| Ins(1,2,3,4)P4 | 0.3 | 0.3 | n.d. | 0.8 | 0.05 | <0.001 | 0.625 | – |
| InsP3x5 | 0.3 | 0.3 | n.d. | 2.0 | 0.21 | <0.001 | 0.853 | – |
| Myo-inositol | 11.6b | 19.5a | 6.9c | 11.7b | 0.53 | <0.001 | <0.001 | 0.017 |
| Ileum | ||||||||
| InsP6 | 25.6b | 12.1c | 37.4a | 15.7c | 1.83 | 0.001 | <0.001 | 0.034 |
| Ins(1,2,4,5,6)P5 | 0.3 | 0.4 | 1.3 | 1.4 | 0.10 | <0.001 | 0.388 | 0.900 |
| Ins(1,2,3,4,5)P5 | 0.9c | 2.0b | 1.7b | 5.0a | 0.13 | <0.001 | <0.001 | <0.001 |
| Ins(1,2,3,4,6)P5 | 0.5 | n.d. | 0.6 | n.d. | 0.12 | 0.511 | – | – |
| Ins(1,2,5,6)P4 | n.d. | 0.8 | 0.2 | 5.9 | 0.67 | <0.001 | <0.001 | – |
| Ins(1,2,3,4)P4 | <LOQ | 0.3 | 0.2 | 0.8 | 0.04 | <0.001 | <0.001 | – |
| Ins(1,5,6)P3 | <LOQ | <LOQ | <LOQ | 0.2 | 0.05 | – | – | – |
| InsP3x5 | n.d. | n.d. | n.d. | 1.9 | 0.70 | – | – | – |
| Myo-inositol | 6.1b | 12.2a | 1.7d | 3.4c | 0.47 | <0.001 | <0.001 | 0.001 |
| Ceca | ||||||||
| InsP6 | 20.4 | 7.0 | 29.6 | 9.1 | 1.95 | 0.005 | <0.001 | 0.050 |
| Ins(1,2,4,5,6)P5 | 0.3 | 0.3 | 1.2 | 1.1 | 0.08 | <0.001 | 0.536 | 0.358 |
| Ins(1,2,3,4,5)P5 | 0.9c | 1.4b | 1.4b | 3.5a | 0.15 | <0.001 | <0.001 | <0.001 |
| Ins(1,2,3,4,6)P5 | 0.4 | n.d. | 0.6 | n.d. | 0.04 | 0.035 | – | – |
| Ins(1,2,5,6)P4 | n.d. | 0.5 | 0.2 | 4.7 | 0.32 | <0.001 | <0.001 | – |
| Ins(1,2,3,4)P4 | n.d. | 0.2 | 0.2 | 0.7 | 0.04 | <0.001 | <0.001 | – |
| Ins(1,5,6)P3 | 0.3 | 0.3 | 0.4 | 0.3 | 0.03 | 0.256 | 0.256 | 0.698 |
| InsP3x5 | n.d. | n.d. | n.d. | 1.6 | 0.50 | – | – | – |
| Myo-inositol | 11.7b | 29.4a | 2.1c | 4.0b,c | 2.80 | <0.001 | 0.004 | 0.015 |
1Data are given as treatment means; n = 4 pens.
2Calculated composition: PCa−, 4.1 g P/kg DM and 6.2 g Ca/kg DM; PCa+, 6.9 g P/kg DM and 10.4 g Ca/kg DM; Phy−, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
3n.d., not detectable in the majority of samples.
4 <LOQ, not quantifiable in the majority of samples.
5At least one of the following isomers: Ins(1,2,6)P3, Ins(1,4,5)P3, Ins(2,4,5)P3.
a–dMeans within a column not showing a common superscript differ (P < 0.05).
In the terminal ileum, InsP6 and Ins(1,2,3,4,5)P5 concentrations were affected by the PCa × Phy interaction (Table 4; P = 0.034 and P < 0.001, respectively). InsP6 concentration was decreased by Phy supplementation and higher when PCa was supplemented, but only in the absence of the added Phy. The concentration of Ins(1,2,3,4,5)P5 was increased with PCa or Phy supplementation, whereas the concentration of Ins(1,2,4,5,6)P5 was only increased when PCa was supplemented. Ins(1,2,3,4,6)P5 was only measured in the absence of the Phy. Ins(1,2,5,6)P4 and Ins(1,2,3,4)P4 were not quantifiable in the PCa−Phy− treatment and the concentration of both increased with Phy or PCa supplementation (P < 0.001, respectively). Ins(1,5,6)P3 and InsP3x were only quantifiable in PCa+Phy+ treatment. The ileal concentration of MI was increased by Phy and decreased by PCa supplementation. Phy supplementation increased disappearance of P (%) up to the terminal ileum to a greater extent in the PCa− diet (Table 2; P = 0.015). Disappearance of P (g/kg DM) was increased by both Phy and PCa supplementation (P < 0.001, respectively). Disappearance of Ca (%) up to the terminal ileum was decreased by PCa supplementation (P < 0.001) and increased by Phy supplementation (P = 0.021). Disappearance of Ca (g/kg DM) was increased by PCa supplementation (P < 0.001).
In conventional broiler experiments, prececal InsP6 disappearance from corn-soybean-based diets low in P and Ca and without Phy supplementation ranged between 56 and 67% (Shastak et al., 2014; Zeller et al., 2015b; Sommerfeld et al., 2018b). The prececal InsP6 disappearance in the present gnotobiotic study was only slightly lower (42%). As the birds were only 15 days old, it might be expected that the InsP6 disappearance would have been higher in older birds (Singh, 2008) and thus even closer to birds from conventional experiments. Moreover, the analyzed InsP isomer pattern, including MI, in all treatments corresponds surprisingly well to that found for similar diets in conventional broilers (Sommerfeld et al., 2018b). Because diets were irradiated and broilers harbored only 1 or 2 bacterial species, these observations suggest a considerable contribution of the mucosa-derived Phy and other phosphatases to InsP degradation. A high potential of the mucosa-derived Phy to degrade phytate has been shown by Maenz and Classen (1998), who reported a substantial phytate hydrolysis by preparations of chicken small intestinal brush border membrane vesicles. The Phy activity in these preparations was the highest in the duodenum and decreased toward the ileum. In the present study, InsP6 disappearance from Phy− treatments slightly increased between duo+jej (35% in PCa−, 11% in PCa+) and the terminal ileum (42% in PCa−, 17% in PCa+), which is in accordance with the results of Maenz and Classen (1998) and is the inverse of the expected microbial population density along the intestinal tract. According to Shinoda and Yoshida (2004), the intestinal microbiota had no effect on the mucosal Phy and alkaline phosphatase activity comparing germfree and conventionalized rats. This might allow transferring the present finding of a high potential of the mucosa-derived Phy to conventional birds. In both the duo+jej and ileum, InsP6 disappearance was increased by Phy supplementation and this addition compensated for the negative effects of P and Ca supplementation on InsP6 degradation. This effect was also described in a study using conventional broilers by Sommerfeld et al. (2018b). The negative effect of P and Ca supplementation on InsP6 degradation might have occurred due to end product inhibition of the endogenous Phy by the supplemented P with subsequent precipitation of the accumulated InsP6 by Ca (Angel et al., 2002).
The appearance of lower InsP isomers in the duo+jej and terminal ileum suggests degradation of lower InsP isomers along the small intestine that was inhibited by dietary PCa supplementation. Similarly, MI concentrations in both the duo+jej and ileum decreased by the addition of PCa and increased by the addition of Phy. These results further support the findings of studies that showed a reduced endogenous Phy activity with Ca supplementation (McCuaig et al., 1972; Applegate et al., 2003) and a tendency toward a reduction due to increased luminal P concentrations (Huber et al., 2015).
In the duo+jej segment, the isomer Ins(1,2,3,4,6)P5 was only measured in Phy− treatments. Because this InsP5 isomer did not occur in the feed, we assume that InsP6 degradation by a 5-Phy already took place to some extent in the upper intestinal tract. In the Phy+ treatments, concentration of Ins(1,2,3,4,5)P5, the main InsP5 isomer in the degradation pathway of the added Phy, was higher than that in Phy− treatments in both the duo+jej and terminal ileum. Similar results were shown in several previous studies (Zeller et al., 2015a,b; Sommerfeld et al., 2018a,b). Despite the non-significant differences in InsP6 concentrations between the Phy+ treatments, the concentrations of Ins(1,2,3,4,5)P5 and Ins(1,2,5,6)P4 were significantly higher in PCa+Phy+ compared to PCa−Phy+ treatment, and Ins(1,5,6)P3 and InsP3x were only present in PCa+Phy+ treatment. This demonstrates an impact of the degradation of these InsP5 and InsP4 isomers in the presence of PCa resulting in the appearance of InsP3 isomers and a lower MI concentration. Inhibition of the supplemented Phy or the intestinal phosphatases or both was more pronounced in the Phy supplemented treatment possibly because of a generally higher level of lower InsP isomers.
The prececal P disappearance was increased in Phy+ and PCa+ treatments. Because the latter reduced InsP degradation, the increased P disappearance in this treatment was likely due to the high digestibility of the mineral P, whereas the increase noted with Phy addition was likely due to improved P utilization from InsP6. The increase in prececal InsP6, P and Ca disappearance with Phy supplementation did not result in higher weight gain of broilers, however PCa+ treatment resulted in heavier birds (Table 5; P = 0.002). This suggests the P and Ca released by the Phy was either insufficient or too imbalanced to improve performance. The effect of PCa supplementation on the disappearance of P was significantly greater (mean of PCa− = 3.2 g/kg DM, mean of PCa+ = 5.3 g/kg DM up to the terminal ileum) than the effect of Phy supplementation (mean of Phy− = 3.8 g/kg DM, mean of Phy+ = 4.7 g/kg DM) (Table 2).
Table 5.
Effect of P, and Ca (PCa), and phytase (Phy) supplementation on body weight (in grams) of gnotobiotic broiler chickens fed the experimental diets from day 10 to day 151.
| Dietary treatment2 | P-values | ||||||
|---|---|---|---|---|---|---|---|
| PCa− | PCa+ | ||||||
| Phy− | Phy+ | Phy− | Phy+ | Pooled SEM | PCa | Phy | PCa × Phy |
| 444 | 450 | 487 | 482 | 20.9 | 0.002 | 0.910 | 0.545 |
1Data are given as treatment means; n = 4 pens.
2Calculated composition: PCa−, 4.1 g P/kg DM and 6.2 g Ca/kg DM; PCa+, 6.9 g P/kg DM and 10.4 g Ca/kg DM; Phy−, 0 FTU/kg; Phy+, 1,500 FTU/kg as fed.
Ceca
InsP6 disappearance in the ceca was not calculated. As reviewed by Svihus et al. (2013), only about 20% of the digesta enters the ceca, mainly small and soluble particles. Therefore, the digesta and marker likely do not enter the ceca in the same ratio as in the terminal ileum. However, the InsP isomer and MI pattern in the ceca provides valuable information of the activity of resident microorganisms. The treatment effect on InsP isomers and MI concentrations was similar to that in the ileum (Table 4). In the study of Zeller et al. (2015a), lower InsP6 levels and a change in the pattern of InsP isomers between the ileum and ceca content were noted when no Phy was supplemented. The present results show no such changes, supporting our assertion that the 2 bacterial contaminants identified in the gnotobiotic birds did not influence InsP degradation. The relevance of InsP isomers and MI produced in the ceca for the animal, possibly provided by retrograde movement of digesta (Sacranie et al., 2005), is not known thus far.
To conclude, the present study on gnotobiotic broiler chickens demonstrates a substantial contribution of mucosa-derived Phy and other phosphatases to prececal degradation of InsP6. Supplementation of P and Ca reduced the activity of the mucosa-derived enzymes. Our results confirm that mucosal Phy activity decreases from the duodenum to ileum. The InsP and MI pattern in all digestive tract sections was similar to the results of studies on conventional broiler chickens.
ACKNOWLEDGMENTS
The authors express their gratitude to Jason Marshall for his substantial help in preparing and monitoring the experimental setup. We are grateful for the doctoral scholarship of the Studienstiftung des deutschen Volkes awarded to Vera Sommerfeld.
REFERENCES
- Angel R., Tamim N. M., Applegate T. J., Dhandu A. S., Ellestad L. E.. 2002. Phytic acid chemistry: influence on phytin-phosphorus availability and phytase efficacy. J. Appl. Poult. Res. 11:471–480. [Google Scholar]
- Applegate T. J., Angel R., Classen H. L.. 2003. Effect of dietary calcium, 25-hydroxycholecalciferol, or bird strain on small intestinal phytase activity in broiler chickens. Poult. Sci. 82:1140–1148. [DOI] [PubMed] [Google Scholar]
- Berrang M. E., Cox N. A., Frank J. F., Buhr R. J.. 1999. Bacterial penetration of the eggshell and shell membranes of the chicken hatching egg: a review. J. Appl. Poult. Res. 8:499–504. [Google Scholar]
- Boguhn J., Baumgärtel T., Dieckmann A., Rodehutscord M.. 2009. Determination of titanium dioxide supplements in different matrices using two methods involving photometer and inductively coupled plasma optical emission spectrometer measurements. Arch. Anim. Nutr. 63:337–342. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care 1993. Guide to the Care and Use of Experimental Animals, Volume 1 2nd ed Canadian Council on Animal Care, Ottawa, ON, Canada. [Google Scholar]
- Canadian Council on Animal Care 2009. CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing. Canadian Council on Animal Care, Ottawa, ON, Canada. [Google Scholar]
- Delia E., Tafaj M., Männer K.. 2011. Total phosphorus, phytate and phytase activity of some cereals grown in Albania and used in non-ruminant feed rations. Biotechnol. Biotechnol. Equip. 25:2587–2590. [Google Scholar]
- Eeckhout W., de Paepe M.. 1994. Total phosphorus, phytate-phosphorus and phytase activity in plant feedstuffs. Anim. Feed Sci. Technol. 47:19–29. [Google Scholar]
- Gesellschaft für Ernährungsphysiologie 1999. Empfehlungen zur Energie- und Nährstoffversorgung der Legehennen und Masthühner (Broiler). 1st ed DLG Verlag, Frankfurt am Main, Germany. [Google Scholar]
- Greiner R., Egli I.. 2003. Determination of the activity of acidic phytate-degrading enzymes in cereal seeds. J. Agric. Food Chem. 51:847–850. [DOI] [PubMed] [Google Scholar]
- Huber K., Zeller E., Rodehutscord M.. 2015. Modulation of small intestinal phosphate transporter by dietary supplements of mineral phosphorus and phytase in broilers. Poult. Sci. 94:1009–1017. [DOI] [PubMed] [Google Scholar]
- Kerr M. J., Classen H. L., Newkirk R. W.. 2000. The effects of gastrointestinal tract micro-flora and dietary phytase on inositol hexaphosphate hydrolysis in the chicken. Poult. Sci. 79(Suppl. 1):11. [Google Scholar]
- Maenz D. D., Classen H. L.. 1998. Phytase activity in the small intestinal brush border membrane of the chicken. Poult. Sci. 77:557–563. [DOI] [PubMed] [Google Scholar]
- McCuaig L. W., Davies M. I., Motzok I.. 1972. Intestinal alkaline phosphatase and phytase of chicks: effect of dietary magnesium, calcium, phosphorus and thyroactive casein. Poult. Sci. 51:526–530. [DOI] [PubMed] [Google Scholar]
- Menezes-Blackburn D., Gabler S., Greiner R.. 2015. Performance of seven commercial phytases in an in vitro simulation of poultry digestive tract. J. Agric. Food Chem. 63:6142–6149. [DOI] [PubMed] [Google Scholar]
- Miyazawa E., Iwabuchi A., Yoshida T.. 1996. Phytate breakdown and apparent absorption of phosphorus, calcium and magnesium in germfree and conventionalized rats. Nutr. Res. 16:603–613. [Google Scholar]
- Rodehutscord M., Rosenfelder P.. 2016. Update on phytate degradation pattern in the gastrointestinal tract of pigs and broiler chickens. Pages 15–32 in Phytate Destruction. Walk C. L., Kühn I., Stein H. H., Kidd M. T., Rodehutscord M., eds. Wageningen Academic Publishers, Wageningen. [Google Scholar]
- Rodehutscord M., Rückert C., Maurer H. P., Schenkel H., Schipprack W., Bach Knudsen K. E., Schollenberger M., Laux M., Eklund M., Siegert W., Mosenthin R.. 2016. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch. Anim. Nutr. 70:87–107. [DOI] [PubMed] [Google Scholar]
- Sacranie A., Iji P. A., Choct M.. 2005. Reflux of digesta and its implications for nutrient digestion and bird health. Aust. Poult. Sci. Symp. 17:171–174. [Google Scholar]
- Shastak Y., Zeller E., Witzig M., Schollenberger M., Rodehutscord M.. 2014. Effects of the composition of the basal diet on the evaluation of mineral phosphorus sources and interactions with phytate hydrolysis in broilers. Poult. Sci. 93:2548–2559. [DOI] [PubMed] [Google Scholar]
- Shinoda S., Yoshida T.. 2004. Effects of intestinal microflora and dietary phytate on intestinal phytase activity in germfree and conventionalized rats. Biosci. Microflora 23:31–35 [Google Scholar]
- Skoglund E., Carlsson N.-G., Sandberg A.-S.. 1997. Determination of isomers of inositol mono- to hexaphosphates in selected foods and intestinal contents using high-performance ion chromatography. J. Agric. Food Chem. 45:431–436. [Google Scholar]
- Singh P. 2008. Significance of phytic acid and supplemental phytase in chicken nutrition: a review. Worlds Poult. Sci. J. 64:553–580. [Google Scholar]
- Sommerfeld V., Künzel S., Schollenberger M., Kühn I., Rodehutscord M.. 2018a. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 97:920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., Schollenberger M., Kühn I., Rodehutscord M.. 2018b. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 97:1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svihus B. 2014. Function of the digestive system. J. Appl. Poult. Res. 23:306–314. [Google Scholar]
- Svihus B., Choct M., Classen H. L.. 2013. Function and nutritional roles of the avian caeca: a review. Worlds Poult. Sci. J. 69:249–264. [Google Scholar]
- Tamim N. M., Angel R., Christman M.. 2004. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 83:1358–1367. [DOI] [PubMed] [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA) 2007. Handbuch der landwirtschaftlichen Versuchs und Untersuchungsmethodik (VDLUFA–Methodenbuch), vol. III. in Die Chemische Untersuchung von Futtermitteln. 1st ed VDLUFA, Darmstadt, Germany. [Google Scholar]
- Wise A., Gilburt D. J.. 1982. Phytate hydrolysis by germfree and conventional rats. Appl. Environ. Microbiol. 43:753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig M., Camarinha-Silva A., Green-Engert R., Hoelzle K., Zeller E., Seifert J., Hoelzle L. E., Rodehutscord M.. 2015. Spatial variation of the gut microbiota in broiler chickens as affected by dietary available phosphorus and assessed by T-RFLP analysis and 454 pyrosequencing. PLoS One 10:e0143442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanke L. J., Bae H. D., Selinger L. B., Cheng K.-J.. 1998. Phytase activity of anaerobic ruminal bacteria. Microbiology. 144:1565–1573. [DOI] [PubMed] [Google Scholar]
- Young-Hoon K., Gwon M.-N., Yang S.-Y., Park T.-K., Kim C.-G., Kim C.-W., Song M.-D.. 2002. Isolation of phytase-producing Pseudomonas sp. and optimization of its phytase production. J. Microbiol. Biotechnol. 12:279–285. [Google Scholar]
- Zeller E., Schollenberger M., Kühn I., Rodehutscord M.. 2015a. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 4:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Witzig M., Shastak Y., Kühn I., Hoelzle L. E., Rodehutscord M.. 2015b. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 94:1018–1029. [DOI] [PubMed] [Google Scholar]


