ABSTRACT
Introduction
Hereditary transthyretin (hATTR) amyloidosis is a progressive, degenerative disease, with peripheral neuropathy, cardiomyopathy, and other clinical manifestations. In this study we examine the impact of hATTR amyloidosis on quality of life (QOL).
Methods
Neuropathy‐specific QOL, measured with the Norfolk QOL‐Diabetic Neuropathy questionnaire, was compared between patients with hATTR amyloidosis and patients with type 2 diabetes, whereas generic QOL, measured with the 36‐item Short Form Health Survey version 2 (SF‐36v2), was compared between patients with hATTR amyloidosis, the general population, and patients with chronic diseases.
Results
Neuropathy‐specific QOL for patients with hATTR amyloidosis was nearly equivalent to that of patients with type 2 diabetes with diabetic neuropathy accompanied by a history of ulceration, gangrene, or amputation. Generic QOL was worse than that seen in the general population, with physical functioning worse than that for patients with multiple sclerosis and congestive heart failure.
Discussion
Patients with hATTR amyloidosis show significant burden on QOL, particularly in physical functioning. Muscle Nerve 60: 169–175, 2019
Keywords: ATTR amyloidosis, burden, polyneuropathy, quality of life, transthyretin amyloidosis
Abbreviations
- ADL
activities of daily living
- ANOVA
analysis of variance
- ATTR
amyloid transthyretin amyloidosis
- CD
Crohn's disease
- CHF
congestive heart failure
- DN
diabetic neuropathy
- EQ‐5D
EuroQol 5‐Dimension
- hATTR
hereditary transthyretin amyloidosis
- IBS
irritable bowel syndrome
- MCS
Mental Component Summary
- MID
minimally important difference
- mNIS
modified neuropathy impairment score
- MS
multiple sclerosis
- NHWS
National Health and Wellness Survey
- Norfolk QOL‐DN
Norfolk Quality of Life–Diabetic Neuropathy
- PCS
Physical Component Summary
- PF
physical functioning
- PRO
patient‐reported outcome
- QMNS
QualityMetric 2009 Norming Study
- QOL
quality of life
- SF‐12v2
12‐item Short Form Health Survey version 2
- SF‐36v2
36‐item Short Form Health Survey version 2
- TTR
transthyretin
Systemic amyloidoses are a set of diseases defined by the extracellular presence of misfolded protein deposits, known as amyloids, in organs and tissue.1, 2 With sufficient buildup, these deposits interfere with normal tissue structure and function, eventually leading to organ failure and death.3 Amyloid transthyretin (ATTR) amyloidosis, the most common subtype of amyloidosis, involves amyloid deposits of misfolded transthyretin (TTR) protein that is produced primarily in the liver.4, 5
A genetic mutation for developing ATTR amyloidosis can be inherited, although a nongenetic variation of the disease (wild‐type) has been identified.6, 7 Hereditary ATTR (hATTR) amyloidosis is a progressive, degenerative, and fatal disease.6, 8 Recent estimates place its worldwide prevalence at 50,000 people,8 although it is thought to be significantly underdiagnosed.
hATTR amyloidosis is a systemic disease and typically involves amyloid deposits in multiple organ systems, commonly in peripheral nerves and cardiomyocytes.4, 9 Deposit buildup in peripheral nerves leads to symptoms of peripheral neuropathy, in which patients initially experience sensory difficulties such as paresthesia, hypoesthesia, and pain in the hands and feet, that progresses proximally and eventually includes motor involvement, resulting in loss of ambulation. This pattern of progression is similar to that observed in diabetic neuropathy, although the rate of progression is much more rapid in hATTR amyloidosis. Deposits of TTR amyloid in the myocardium lead to a restrictive cardiomyopathy, which is characterized by shortness of breath, arrhythmia, and eventual cardiac failure. Patients with hATTR amyloidosis also commonly experience autonomic neuropathy, gastrointestinal, ocular, and renal dysfunction, and carpal tunnel syndrome.8
Empirical findings indicate that patients with hATTR amyloidosis carry a significant disease burden that impacts quality of life (QOL). A recent study examining 12‐item Short Form Health Survey version 2 (SF‐12v2) scores of patients with ATTR amyloidosis showed substantial burden on physical health, with scores on the Physical Component Summary (PCS) 1.7 standard deviations below those of the general United States population.10 Another study, examining patients with ATTR amyloidosis with polyneuropathy on the EuroQol 5‐Dimension (EQ‐5D) questionnaire, showed that the mean health utility score for patients was 0.50, far below the mean score for the general population (0.76).11 A clear relationship between increasing duration of symptoms of patients with ATTR amyloidosis with symptomatic polyneuropathy and worsening scores on the Norfolk Quality of Life–Diabetic Neuropathy (Norfolk QOL‐DN) questionnaire has been established.12, 13 The information lacking in the current literature, however, is a contextualization of the burden in hATTR amyloidosis, such as how it compares with the burden of other, more well‐understood medical conditions. Also unknown is the impact of hATTR amyloidosis on specific domains of QOL, as previous studies have only examined its impact via summary or total scores of QOL measures.
In our study we aimed to further explore the burden of hATTR amyloidosis with polyneuropathy on QOL in a clinical trial sample and provide an interpretive context through which we may better understand the QOL burden in this patient population.
METHODS
Data Sources
Norfolk QOL‐DN scores were compared among patients with hATTR amyloidosis with polyneuropathy from a clinical trial and a sample of patients with type 2 diabetes from a cross‐sectional study. SF‐36v2 scores were compared among patients with hATTR amyloidosis with polyneuropathy in a clinical trial, a general population sample, and patients with 1 of 5 chronic conditions.
NEURO‐TTR Sample
Patients with hATTR amyloidosis with polyneuropathy enrolled in the NEURO‐TTR trial (Clinical Trials ID # NCT01737398; ClinicalTrials.gov) were included in all analyses. NEURO‐TTR was a phase III, multinational, randomized, double‐blind, placebo‐controlled study of the efficacy of inotersen in patients with hATTR amyloidosis with polyneuropathy. The primary objective of NEURO‐TTR was to evaluate the efficacy of inotersen in slowing or halting nerve damage caused by TTR amyloid deposits.
Patients with hATTR amyloidosis were randomized in a 2:1 ratio to receive either a single subcutaneous injection of 1.5 ml of inotersen or placebo, respectively, 3 times in the first week and then once weekly for a 65‐week treatment period. Patients were eligible to participate in the study if they had stage 1 (ambulatory without assistance) or stage 2 (ambulatory with assistance such as a cane or walker) disease severity,14 and a neuropathy impairment score (NIS) between 10 and 130 (inclusive). Patients were stratified into treatment arms based on 3 subgroup factors: previous treatment with tafamidis or diflunisal vs. neither; stage 1 vs. stage 2 ambulatory disability; and Val30Met TTR mutation vs. non‐Val30Met TTR mutation.
The trial protocol was approved by institutional review boards or local ethics committees. All patients provided written informed consent to participate in the trial.
Type 2 Diabetes Sample
Also included in the analysis comparing Norfolk QOL‐DN scores is a sample of patients with type 2 diabetes described by Veresiu et al.15 This sample consisted of 20,469 patients from Romania (mean age 60.9 years; 53% female) who consented to participate in a cross‐sectional study for which they provided scores on the Norfolk QOL‐DN as well as self‐reported disease status. Based on the latter, patients were classified as follows: diabetes without DN (n = 6,615); diabetes with DN and no history of ulceration, gangrene, or amputations (n = 10,704); or diabetes with DN and a history of ulceration, gangrene, or amputations (n = 3,150).
General Population and Condition Benchmark Samples
Benchmark samples used for comparison of SF‐36v2 scores with the NEURO‐TTR sample were drawn from 2 separate sources. First, SF‐36v2 data were drawn from a normative general population sample collected as part of the QualityMetric 2009 Norming Study (QMNS), a probabilistic online survey of 4,040 noninstitutionalized adults in the United States.16 Along with measures of QOL, the QMNS survey included a checklist of 30 chronic conditions. In addition to the full QMNS general population sample, respondents who indicated having congestive heart failure (CHF; n = 137) or irritable bowel syndrome (IBS; n = 321) were selected for use as condition‐specific benchmarks for comparison with the NEURO‐TTR sample, due to their having overlapping symptoms with hATTR amyloidosis.
Second, SF‐36v2 scores from condition‐specific benchmark samples were drawn from Kantar Health's National Health and Wellness Survey (NHWS), an annual international online panel survey of health care in a representative sample of adults. The NHWS includes a variety of patient‐reported outcome (PRO) measures of health status and QOL, including the SF‐36v2. Respondents are asked to indicate having experienced any of dozens of different health conditions. Comparisons were made between scores of the NEURO‐TTR sample and those from NHWS respondents who indicated having experienced 1 of the following chronic health conditions, each of which shares some symptoms with hATTR amyloidosis: Crohn's disease (CD; n = 2,059); DN (n = 5,682); or multiple sclerosis (MS; n = 1,901). SF‐36v2 data from respondents in the USA who participated in the 2015 or 2016 (pooled) NHWS were included in the current analysis.
Both the QMNS and NHWS studies utilized enrolled panels, with respondents providing written consent and agreeing to the use of their responses in group‐level analyses. Data collection for both was performed by survey companies through their existing approved panels, following their own procedures. Consequently, no specific ethics committee or institutional review board approval was required.
Assessment Instruments
Neuropathy‐related QOL was assessed using the Norfolk QOL‐DN, a PRO measure assessing the impact of several aspects of DN on patients’ QOL.17 The Norfolk QOL‐DN yields a total score based on all 35 items (score range from −4 to 136), and scores on 5 subscales capturing symptoms associated with damage to nerve fibers: physical functioning/large‐fiber neuropathy (15 items; score range −4 to 56); activities of daily living (ADL; 5 items; score range 0–20); symptoms (8 items; score range 0–32); small‐fiber neuropathy (4 items; score range 0–16); and autonomic neuropathy (3 items; score range 0–12). In all cases, higher scores indicate worse functioning.
Generic QOL was assessed using the SF‐36v2 (with 4‐week recall), a 36‐item PRO measure of functional health and well‐being.16 Responses to items are used to compute scores for 8 domains of QOL: physical functioning; role limitations due to physical health (role‐physical); bodily pain; general health; vitality; social functioning; role limitations due to emotional health (role‐emotional); and mental health. Two summary scores, the PCS and Mental Component Summary (MCS), capturing global physical health and global mental health, respectively, can be calculated using weighted scores from the 8 domains.
All SF‐36v2 domains and summary scores can be calculated as T‐scores using norm‐based methods, standardized to a mean of 50 and a standard deviation of 10 in the general population. Higher SF‐36v2 scores reflect better QOL.
Group‐level minimally important difference (MID) values have been established for the SF‐36v2 using both distribution‐based and anchor‐based methods.16 Defined as the smallest differences in scores that patients would consider as beneficial and for which a clinician would recommend adjusting patients’ care, MID threshold values can facilitate interpretation of whether group‐level differences are clinically meaningful.18 The established MID threshold value is 3 points for physical functioning, role‐physical, bodily pain, social functioning, and mental health domains, as well as for MCS. The MID threshold value is 2 points for general health and vitality domains, as well as for PCS, and 4 points for the role‐emotional domain.
Analysis of Burden of hATTR Amyloidosis on Neuropathy‐Related QOL
To estimate the burden of hATTR amyloidosis on patients’ neuropathy‐related QOL, descriptive analyses were conducted in which baseline Norfolk QOL‐DN scores from the NEURO‐TTR sample were compared with scores for type 2 diabetes patients in the study by Veresui et al.15
Analysis of Burden of hATTR Amyloidosis on Generic QOL
To estimate the burden of hATTR amyloidosis on patients’ QOL, post‐hoc analyses were conducted in which baseline SF‐36v2 scores from NEURO‐TTR patients were compared with scores from the general population and chronic condition–specific benchmark samples. For each comparison, data from the benchmark samples were adjusted to match the age and gender distribution of the NEURO‐TTR sample using ordinary least‐squares regression methods. Univariate analysis of variance (ANOVA) models were used to test for statistically significant differences in each of the domain and summary mean scores between the NEURO‐TTR sample and adjusted mean scores for each of the benchmark samples. The magnitude of burden was interpreted using 3 different criteria: statistical significance of mean differences (α = 0.05); group‐level MID threshold values to indicate clinical significance; and Cohen's d standardized mean differences to estimate magnitude of effect size, the latter of which were interpreted according to Cohen's published guidelines (d = 0.2, small effect; d = 0.5, medium effect; d = 0.8, large effect).19
To provide further interpretive context on the burden of physical functioning, item‐level comparisons between the NEURO‐TTR sample and benchmark samples were conducted for each of the 10 physical functioning items on the SF‐36v2. These physical functioning items, each referring to one of a variety of common physical activities, have 3 response options: “Limited a lot”; “Limited a little”; or “Not limited at all.” In this analysis, the percentage of patients who chose the “Limited a lot” option was tallied and descriptively compared between the NEURO‐TTR sample and each of the benchmark samples.
In addition to comparisons across benchmark samples, the magnitude of burden relative to the general population was examined between subgroups of patients in the NEURO‐TTR sample defined by clinically important characteristics. Separate comparisons between the NEURO‐TTR sample and age‐ and gender‐adjusted general population scores were made between patients with early (≤50 years) vs. late (>50 years) age of symptom onset; patients with stage 1 vs. stage 2 ambulatory disability; and patients with cardiomyopathy vs. those without cardiomyopathy.
RESULTS
Sample Characteristics
Baseline Norfolk QOL‐DN and SF‐36v2 data were collected from a total of 172 patients with hATTR amyloidosis enrolled in the safety set of the NEURO‐TTR study. Sample demographics and selected disease characteristics of this sample are presented in Table S1 in the Supplementary Material (available online).
Analysis of Burden of hATTR Amyloidosis on Neuropathy‐Related QOL
Norfolk QOL‐DN mean total score (Table 1) was 48.4 points for the NEURO‐TTR sample, which was much higher (worse) than scores from type 2 diabetes patients in the study by Veresui et al.15 who had no DN or DN and no history of ulceration, gangrene, or amputation, but quite similar to those who had DN accompanied by ulceration, gangrene, or amputation. Similar patterns were observed for most Norfolk QOL‐DN subscales: mean scores for the NEURO‐TTR sample were most similar to those for patients with DN and ulceration, gangrene, and amputation for physical functioning/large‐fiber neuropathy, ADLs, symptoms, and small‐fiber neuropathy. Scores on the autonomic subscale were closest to those for patients with DN and no history of ulceration, gangrene, or amputation.
Table 1.
Mean (standard error) scores of the Norfolk QOL‐DN questionnaire subscales for hATTR and type 2 diabetes patient samples.
| Subscale | NEURO‐TTR trial (n = 172) | Veresiu et al.15 | ||
|---|---|---|---|---|
| Diabetes without DN (n = 6,615) | Diabetes with DN, without ulceration, gangrene, amputation (n = 10,704) | Diabetes with DN, with ulceration, gangrene, amputation (n = 3,150) | ||
| Total | 48.4 (2.08) | 13.7 (0.23) | 34.9 (0.24) | 50.4 (0.49) |
| PF/large fiber | 24.3 (1.12) | 7.9 (0.13) | 18.8 (0.13) | 25.6 (0.26) |
| ADL | 6.3 (0.44) | 1.19 (0.04) | 3.5 (0.04) | 5.9 (0.10) |
| Symptoms | 10.6 (0.47) | 2.8 (0.05) | 8.0 (0.05) | 11.2 (0.11) |
| Small fiber | 5.1 (0.33) | 0.8 (0.03) | 2.8 (0.03) | 4.9 (0.08) |
| Autonomic | 2.1 (0.21) | 1.0 (0.02) | 2.0 (0.02) | 3.2 (0.05) |
ADL, activities of daily living; DN, diabetic neuropathy; PF, physical functioning.
Analysis of Burden of hATTR Amyloidosis on Generic QOL
Burden of hATTR Amyloidosis Relative to General Population Norms
When compared with age‐ and gender‐adjusted general population norms, mean baseline scores from the NEURO‐TTR sample showed considerable QOL burden in all physical domains and for PCS (P < 0.001 for all; Fig. 1). The NEURO‐TTR sample exhibited the greatest deficits in physical functioning and role‐physical domains (Cohen's d ≥ 0.74 for both), with deficits greater than 10 points when compared with the general population. Considerable burden was also observed for the perception of general health domain, which had a deficit of 8 points (d = 0.53). Mean differences between the NEURO‐TTR sample and the general population exceeded the MID thresholds for all mental‐based domains except for mental health and MCS.
Figure 1.
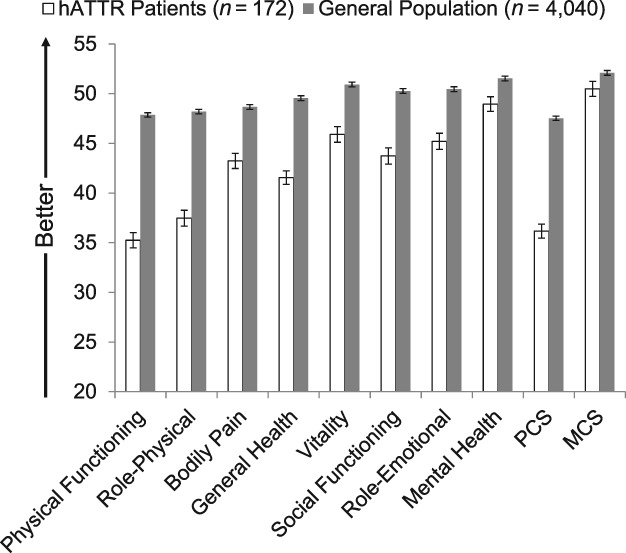
Mean SF‐36v2 scores for the hATTR patient sample relative to age‐ and gender‐matched general population norms. hATTR, hereditary ATTR amyloidosis; MCS, Mental Component Summary; PCS, Physical Component Summary. Error bars represent standard errors of means. P < 0.05 for all comparisons. All differences exceeded minimally important difference (MID) thresholds except for mental health and MCS. Magnitude of effect sizes (Cohen's d‐values) for differences were as follows: physical functioning (d = 0.90); role‐physical (0.74); bodily pain (0.37); general health (0.53); vitality (0.33); social functioning (0.43); role‐emotional (0.35); mental health (0.17); PCS (0.82); and MCS (0.11).
Burden of hATTR Amyloidosis Relative to Condition‐Specific Benchmarks
Comparisons between the NEURO‐TTR samples’ baseline SF‐36v2 scores and several of the condition‐specific benchmarks showed relative burden of hATTR amyloidosis on patients’ physical functioning (Fig. 2). Patients with hATTR amyloidosis showed clinically meaningful deficits (i.e., exceed group‐level MID threshold values) on physical functioning relative to CD (d = 0.28), DN (d = 0.27), and IBS (d = 0.73) benchmarks (P < 0.001 for all), although not with CHF or MS (both d < 0.10). Correspondingly, differences between hATTR amyloidosis patients’ PCS scores and those from these 3 benchmark comparators as well as MS also exceeded MID thresholds (>2 points for all differences). The NEURO‐TTR sample reported less burden on global mental well‐being relative to each of the condition‐specific benchmarks (Fig. 3; P < 0.005; Cohen's d = 0.33–0.64 for MCS) with the exception of IBS (P = 0.33; Cohen's d = 0.08 for MCS).
Figure 2.
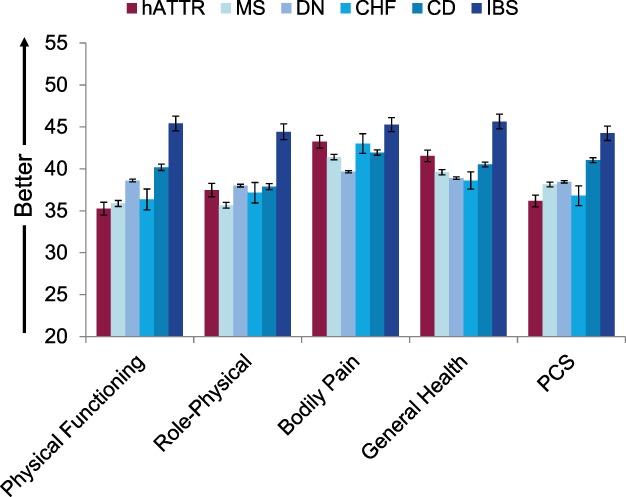
Mean SF‐36v2 physical health scores for the hATTR patient sample relative to age‐ and gender‐matched chronic condition benchmarks. CD, Crohn's disease; CHF, congestive heart failure; DN, diabetic neuropathy; hATTR, hereditary ATTR amyloidosis; IBS, irritable bowel syndrome; MS, multiple sclerosis; PCS, Physical Component Summary. Error bars represent standard errors of means.
Figure 3.
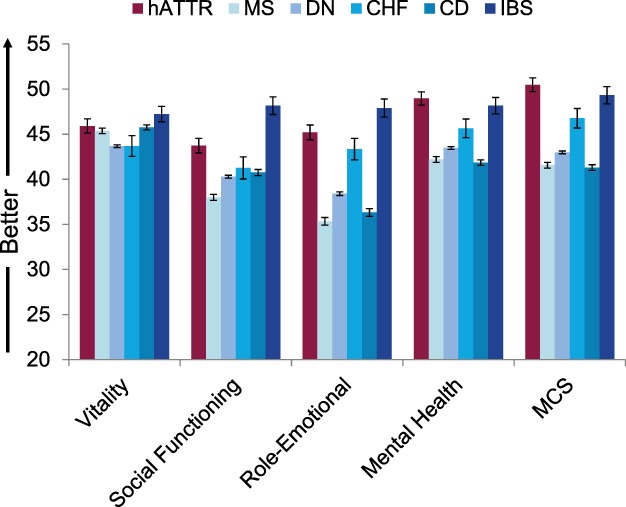
Mean SF‐36v2 psychological and social health scores for the hATTR patient sample relative to age‐ and gender‐matched chronic condition benchmarks. CD, Crohn's disease; CHF, congestive heart failure; DN, diabetic neuropathy; hATTR, hereditary ATTR amyloidosis; IBS, irritable bowel syndrome; MCS, Mental Component Summary; MS, multiple sclerosis. Error bars represent standard errors of mean.
Burden of hATTR Amyloidosis on Physical Functioning Relative to General Population and Chronic Condition Benchmarks
The percentage of patients indicating they were “Limited a lot” for each SF‐36v2 physical functioning item from the NEURO‐TTR sample and all benchmark samples are reported in Table 2. The percentage of patients in the NEURO‐TTR sample selecting the “Limited a lot” option was substantially greater than that of the general population for all physical functioning items, with the exception of “bathing/dressing.” Patients in the NEURO‐TTR sample were more likely to endorse “Limited a lot” when compared with patients in the other chronic condition samples on 6 of 10 items, including the ability to engage in vigorous activities (such as running, lifting heavy objects, or participating in strenuous sports), ability to engage in moderate activities (such as moving a table, pushing a vacuum cleaner, bowling, or playing golf), lifting/carrying groceries, climbing several flights of stairs, bending/kneeling/stooping, and walking more than 1 mile.
Table 2.
Percentage of patients selecting the “Limited a lot” option for SF‐36v2 physical functioning items.
| General population (n = 4,040) | hATTR (n = 172) | CHF (n = 141) | IBS (n = 321) | CD (n = 2,059) | DN (n = 5,682) | MS (n = 1,901) | ||
|---|---|---|---|---|---|---|---|---|
| Age [mean (SD)] | 50.9 (17.3) | 59.2 (13.0) | 61.7 (15.7) | 53.5 (16.7) | 42.7 (15.0) | 57.7 (13.9) | 45.1 (14.7) | |
| Male [n (%)] | 1,995 (49.4) | 118 (68.6) | 79 (56.0) | 109 (34.0) | 1178 (57.2) | 3,288 (57.9) | 893 (47.0) | |
| Item | Content | |||||||
| PF01 | Engage in vigorous activities | 26% | 80% | 67% | 42% | 51% | 68% | 61% |
| PF02 | Engage in moderate activities | 9% | 43% | 27% | 18% | 25% | 28% | 32% |
| PF03 | Lifting/carrying groceries | 6% | 34% | 19% | 12% | 22% | 21% | 27% |
| PF04 | Climbing several flights of stairs | 14% | 58% | 47% | 24% | 27% | 44% | 41% |
| PF05 | Climbing one flight of stairs | 6% | 26% | 23% | 11% | 23% | 25% | 30% |
| PF06 | Bending/kneeling/stooping | 11% | 39% | 30% | 18% | 24% | 33% | 29% |
| PF07 | Walking >1 mile | 17% | 57% | 50% | 27% | 31% | 50% | 44% |
| PF08 | Walking several hundred yards | 9% | 36% | 36% | 15% | 26% | 33% | 34% |
| PF09 | Walking 100 yards | 6% | 16% | 23% | 9% | 23% | 24% | 30% |
| PF10 | Bathing/dressing | 2% | 7% | 7% | 2% | 18% | 9% | 18% |
CD, Crohn's disease; CHF, congestive heart failure; DN, diabetic neuropathy; hATTR, hereditary ATTR amyloidosis; IBS, irritable bowel syndrome; MS, multiple sclerosis; PF, physical functioning.
Burden of hATTR Amyloidosis on Patient Subgroups Relative to the General Population
The magnitude of burden relative to general population norms was assessed between key subgroups of patients in the NEURO‐TTR sample (see Figs. [Link], [Link], [Link] online). Relative to the general population, patients in the NEURO‐TTR sample with early onset of symptoms (beginning at or before 50 years old) reported slightly larger deficits in QOL than patients whose symptoms first occurred later in life, with the largest differences in bodily pain and perception of general health (see Fig. S1 online).
Relative to the general population, patients in the NEURO‐TTR sample with stage 2 ambulatory disability reported markedly more QOL burden than patients with stage 1 ambulatory disability (see Fig. S2 online), with differences on the majority of domains.
When compared with the general population, patients in the NEURO‐TTR sample with cardiomyopathy reported substantially more physical QOL burden than patients without cardiomyopathy (see Fig. S3 online), particularly for physical functioning, role‐physical, general health, and vitality domains.
DISCUSSION
Previous studies10, 11 have documented that patients with hATTR amyloidosis experience a burden on QOL, particularly with regard to physically oriented QOL (e.g., the PCS on the SF‐12v2), whereas other studies have shown a burden on neuropathy‐related QOL, which increases with symptom duration.12 The purpose of the current analysis was to provide a context for interpreting the magnitude of burden of hATTR amyloidosis on both generic and neuropathy‐related QOL.
The analysis of neuropathy‐related QOL found that the burden experienced by patients with hATTR amyloidosis is nearly equivalent to that of patients with type 2 diabetes who have DN accompanied by a history of ulceration, gangrene, or amputation. Our analysis also showed a substantial burden on physical aspects of QOL for patients with hATTR amyloidosis relative to the general population, particularly for physical functioning, role‐physical, and perception of general health. The burden on physical functioning for patients with hATTR amyloidosis was worse than that of patients with CD, DN, IBS, and is comparable to that of patients with MS and CHF.
The largest deficit for patients with hATTR amyloidosis with symptoms of polyneuropathy was observed in physical functioning. This is consistent with the types of clinical manifestations experienced by these patients, which includes a length‐dependent sensory and motor neuropathy that starts in the hands and feet and moves proximally, eventually leading to a wheelchair or bedbound state. The cohort of patients with hATTR amyloidosis enrolled in the NEURO‐TTR trial and analyzed here included early‐ to mid‐stage patients (67% stage 1, 33% stage 2); stage 3 patients (wheelchair or bed‐bound) were excluded from enrolling in the NEURO‐TTR trial.
Examination of responses from patients with hATTR amyloidosis to individual items from the physical functioning domain of the SF‐36v2 provides a more concrete interpretation of difficulties faced by patients with early‐ to mid‐stage hATTR amyloidosis. The proportion of patients reporting severe impairment in physical activities ranged from 7% who were limited a lot in bathing and dressing themselves to 80% who were limited a lot in vigorous activities (e.g., running, lifting heavy objects, participating in strenuous sports). Results from the item‐level analyses indicate that more patients with hATTR amyloidosis than patients with CHF, IBS, CD, DN, or MS had severe difficulty in engaging in vigorous activities, moderate activities, lifting/carrying groceries, climbing several flights of stairs, bending/kneeling/stooping, and walking more than 1 mile. For other aspects of physical functioning, such as climbing one flight of stairs or walking several hundred yards, patients with hATTR amyloidosis showed similar burdens as those with CHF, DN, and MS.
A previous study using the SF‐12v2, which is a subset of 12 items from the SF‐36v2 and is scored similarly, showed substantial burden for patients with ATTR amyloidosis on physical‐oriented QOL (PCS = 33.6) but not on mental‐oriented QOL (MCS = 47.1).10 Our findings with respect to summary component measures are quite consistent: the NEURO‐TTR sample showed considerable burden on physically oriented QOL (PCS = 36.2), with seemingly no burden on mental‐oriented QOL (MCS = 50.5). However, by examining the domains of the SF‐36v2, we get a more precise understanding of the actual mental burden experienced by this patient population. In particular, in the NEURO‐TTR sample we can see that there is clinically meaningful burden on several scales that contribute positively to the scoring of MCS, including vitality (mean = 45.9), social functioning (mean = 43.7), and role‐emotional (mean = 45.2) scales, which would not be evident from examining the MCS on its own. This distinction points to the importance of going beyond total and summary scores of QOL measures and looking at scores on particular domains to get a richer profile of patients’ experiences with the disease.
As expected, physical burden for patients with hATTR amyloidosis varied as a function of key clinical indicators. The burden on bodily pain and perception of general health was greater for patients with early onset of symptoms than those with late onset. Patients with stage 2 ambulatory disability and those with cardiomyopathy showed considerably more burden on physical functioning, role‐physical, and perception of general health domains, as well as on global physical well‐being, than did patients in stage 1 and without cardiomyopathy, respectively. These findings show that progression of the disease, such as greater buildup of TTR amyloids in the peripheral nervous system and cardiac tissue, is associated with an increasing burden on the QOL of patients with hATTR amyloidosis.
The rarity of hATTR amyloidosis and the variability of its clinical manifestations make early diagnosis difficult.8, 20 Further, available treatments for this disease (liver transplantation or pharmacotherapy) may limit production of new amyloid fibrils, but may not able to remove existing fibril deposits from the tissue, meaning the pathology and clinical symptoms are typically nonreversible. Thus, to minimize burden of disease, early diagnosis and treatment of the disease is essential, so that treatment can preserve QOL in patients while QOL is still viable.
There are several limitations of our study. One limitation is that the clinical trial with patients with hATTR amyloidosis excluded those who were already nonambulatory or had advanced neurological disability. Thus, the degree of burden is likely underestimated in the current sample. Caution should therefore be used when generalizing these findings to other hATTR samples, particularly to those with a higher percentage of patients in advanced stages of the disease.
A second limitation to generalizability is the heterogeneity of TTR genotypes in the hATTR sample. Approximately half of the patients had the Val30Met mutation. The remaining subjects had 1 of 26 different TTR mutations, with frequencies ranging from 1 (0.6%) to 22 (12.8%) of the subjects. The analyses did not examine the impact of genotype on disease burden, and therefore these findings may not generalize to samples of patients with different mutations.
Another limitation rests on the different geographical and cultural backgrounds of the populations for which we are making comparisons. It is possible that, due to cultural differences, responses on the Norfolk QOL‐DN may differ between the DN participants in the Eastern European country of Romania (as in the study by Veresiu et al.15) and patients enrolled in the NEURO‐TTR study who lived in 10 countries (Argentina, Brazil, France, Germany, Italy, New Zealand, Portugal, Spain, UK, and the USA) with potentially very different cultures. Further, all benchmark samples for the comparisons of SF‐36v2 scores included only subjects from the USA. The impact of heterogeneity of cultural backgrounds for each of the samples being compared on our findings cannot be assessed.
Another limitation is that there is a mismatch between hATTR, a progressive and seemingly irreversible disorder, and treatable conditions for which QOL burden can be alleviated after therapeutic interventions. Thus, interpreting findings when comparing burden across these different disease types is not straightforward, and these results should be interpreted with caution.
In conclusion, our analysis has shown a substantial burden on neuropathy‐related QOL and on physical aspects of generic QOL for patients with hATTR amyloidosis, particularly with regard to physical functioning, role limitations due to physical health problems, and perception of general health. The finding that physical burden was especially profound in patients with a greater progression of disease points to the importance of early diagnosis and treatment of this disease.
Portions of this study were presented at the Academy of Managed Care Pharmacy Annual Meeting, April 2018, Boston, Massachusetts, and at the annual meeting of the International Society for Pharmacoeconomics and Outcomes Research, May 2018, Baltimore, Maryland.
Ethical Publication Statement: We (the authors) confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1
Figure S2
Figure S3
Supplementary Table 1 Baseline Characteristics of hATTR Patient Samples
Funding: Akcea Therapeutics.
Conflicts of Interest: M.P. is an employee of and owns stock in Akcea Therapeutics. S.G. was an employee of and owned stock in Akcea Therapeutics when this research was conducted. A.Y., A.L., A.S.K., and M.K.W. are employees of Optum, which received payment from Akcea Therapeutics to conduct these analyses and develop this article. M.A.G. has received consulting fees from Alnylam, Ionis, and Prothena. N.D. has participated on the cardiology advisory boards for Ionis, Akcea Therapeutics, and Pfizer and has been on the speakers’ bureau for Akcea Therapeutics and Pfizer. L.O. has received speaking and consulting fees from Akcea Therapeutics, Pfizer, and Alnylam. E.J.A. has received consulting fees from Akcea Therapeutics. The remaining authors have no conflicts of interest to disclose. Akcea Therapeutics developed and is marketing a drug, inotersen (Tegsedi) for treating hereditary transthyretin (hATTR) amyloidosis using antisense oligonucleotide technology.
The copyright line for this article was changed on 23 August 2019 after original online publication.
REFERENCES
- 1. Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med 1997;337:898–909. [DOI] [PubMed] [Google Scholar]
- 2. Shin SC, Robinson‐Papp J. Amyloid neuropathies. Mt Sinai J Med 2012;79:733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blancas‐Mejía LM, Ramirez‐Alvarado M. Systemic amyloidoses. Annu Rev Biochem 2013;82:745–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ando Y, Coelho T, Berk JL, Cruz MW, Ericzon BG, Ikeda S, et al Guideline of transthyretin‐related hereditary amyloidosis for clinicians. Orphanet J Rare Dis 2013;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hammarström P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science 2003;299:713–716. [DOI] [PubMed] [Google Scholar]
- 6. Hawkins PN, Ando Y, Dispenzeri A, Gonzalez‐Duarte A, Adams D, Suhr OB. Evolving landscape in the management of transthyretin amyloidosis. Ann Med 2015;47:625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, et al Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol 2016;68:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gertz MA. Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges. Am J Manag Care 2017;23(suppl):S107–S112. [PubMed] [Google Scholar]
- 9. Gertz MA, Benson MD, Dyck PJ, Grogan M, Coelho T, Cruz M, et al Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol 2015;66:2451–2466. [DOI] [PubMed] [Google Scholar]
- 10. Stewart M, Shaffer S, Murphy B, Loftus J, Alvir J, Cicchetti M, et al Characterizing the high disease burden of transthyretin amyloidosis for patients and caregivers. Neurol Ther 2018;7:349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inês M, Coelho T, Conceição I, Ferreira LN, Carvalho M, Costa J. Transthyretin familial amyloid polyneuropathy impact on health‐related quality of life. Orphanet J Rare Dis 2015;10:O28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coelho T, Vinik A, Vinik EJ, Tripp T, Packman J, Grogan DR. Clinical measures in transthyretin familial amyloid polyneuropathy. Muscle Nerve 2017;55:323–332. [DOI] [PubMed] [Google Scholar]
- 13. Vinik EJ, Vinik AI, Paulson JF, Merkies IS, Packman J, Grogan DR. Norfolk QOL‐DN: validation of a patient reported outcome measure in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst 2014;19:104–114. [DOI] [PubMed] [Google Scholar]
- 14.Coutinho P, da Silva AM, Lima JL, Barbosa AR, Glenner GG, e Costa PP, de Freitas AF, et al Forty years of experience with type 1 amyloid neuropathy. Review of 483 cases In: Glenner G, Costa P, de Freitas A, editors. Amyloid and amyloidosis. Amsterdam: Excerpta Medica; 1980. p 88–98. [Google Scholar]
- 15. Veresiu AI, Bondor CI, Florea B, Vinik EJ, Vinik AI, Gâvan NA. Detection of undisclosed neuropathy and assessment of its impact on quality of life: a survey in 25,000 Romanian patients with diabetes. J Diabetes Complications 2015;29:644–649. [DOI] [PubMed] [Google Scholar]
- 16. Maruish ME. User's manual for the SF‐36v2 Health Survey, 3rd ed. Lincoln, RI: QualityMetric, Inc.; 2011. [Google Scholar]
- 17. Vinik EJ, Hayes RP, Oglesby A, Bastyr E, Barlow P, Ford‐Molvik AL, et al The development and validation of the Norfolk QOL‐DN, a new measure of patients' perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther 2005;7:497–508. [DOI] [PubMed] [Google Scholar]
- 18. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–415. [DOI] [PubMed] [Google Scholar]
- 19. Cohen J. Statistical power analysis for the behavioral sciences. Abington, UK: Routledge; 1988. [Google Scholar]
- 20. Cortese A, Vegezzi E, Lozza A, Alfonsi E, Montini A, Moglia A, et al Diagnostic challenges in hereditary transthyretin amyloidosis with polyneuropathy: avoiding misdiagnosis of a treatable hereditary neuropathy. J Neurol Neurosurg Psychiatry 2017;88:457–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Supplementary Table 1 Baseline Characteristics of hATTR Patient Samples


