Abstract
Background
The sphenopalatine ganglion (SPG) has previously been targeted in trigeminal neuralgia (TN), but its role in this condition has not been established.
Objective
To investigate the safety of injecting onabotulinumtoxinA (BTA) toward the SPG using the MultiGuide® in 10 patients with refractory classical TN, and collect preliminary efficacy data.
Methods
Twenty‐five international units (IU) of BTA were injected toward the SPG in a prospective, open‐label study in 10 patients with refractory classical TN. All patients were recruited and treated on an out‐patient basis at St. Olav's University Hospital in Trondheim (Norway). Primary outcome: adverse events (AEs). Primary efficacy outcome: number of TN attacks at weeks 5‐8 after injection compared to baseline. A treatment responder was predefined as at least 50% reduction in the median number of attacks per day between baseline and weeks 5‐8. Other efficacy outcomes were intensity of attacks (numeric rating scale, 0 to 10) and functional level (1 to 4; 1 best and 4 worst) at weeks 5‐8 after injection compared to baseline. Percentage of the day with concomitant persistent pain was registered at baseline and at weeks 1‐4, 6, 8, and 12 after injection. Patient global impression of change (PGIC) was ascertained at month 3.
Results
For the primary endpoint, we analyzed data for all 10 patients. For efficacy outcomes we analyzed data for 9 patients (1 patient violated protocol). We registered 13 AEs, none of which were serious. The median number of TN attacks during the 4‐week baseline and weeks 5‐8 after injection was 5.5 (range: 1.0‐51.5) and 5 (range: 0‐225.0), respectively (P = .401). Four patients were treatment responders. The median intensity of attacks at baseline and weeks 5‐8 after injection was 6 (range: 3.0‐8.5) and 3 (range: 0.0‐9.0) respectively (P = .024). The median functional level at baseline was 2 (range: 1.0‐3.3) and at month 2, 1 (range 1.0‐4.0; P = .750). Median percentage of the day with concomitant persistent pain was 75% (minimum 37.5%, maximum 100%) at baseline and 18.75% (minimum 0%, maximum 100%) at week 8 (P = .023).
Conclusions
Injection of BTA toward the SPG using the MultiGuide® in patients with TN appears to be safe and well tolerated. This study was negative for the main efficacy endpoint (reduction in the number of attacks from baseline to weeks 5‐8). Further studies examining the role of the SPG in TN are necessary.
Keywords: trigeminal neuralgia, sphenopalatine ganglion, pterygopalatine ganglion, botulinum toxin, sensitization
Abbreviations
- CT
computerized tomography
- MRI
magnetic resonance imaging
- SD
standard deviation
- SPG
sphenopalatine ganglion
- TN
trigeminal neuralgia
Introduction
Classical trigeminal neuralgia (TN) is defined as recurrent paroxysms of unilateral facial pain.1 The etiology of classical TN has been researched extensively, but the exact pathophysiological processes leading to pain are not fully understood. Central to the pathogenesis seems to be a neurovascular contact,2 but there is also evidence of the involvement of central pain mechanisms.3, 4 Patients with TN often have a refractory period and this well‐documented clinical feature suggests a central mechanism.3, 5
In a prospective series of 158 patients with classical TN, 31% had autonomic symptoms.6 These symptoms included conjunctival injection and tearing, rhinorrhea, and nasal congestion.6 These symptoms may reflect activation of cranial parasympathetic efferents from the sphenopalatine ganglion (SPG).7, 8
The SPG may be involved in pain sensitization and it has been suggested that parasympathetic outflow contributes to pain by activating or sensitizing intracranial nociceptors.9 In the same series of 158 patients with TN cited above, it was observed that 78 patients (49%) had concomitant persistent pain.6 Central facilitation of trigeminal nociceptive processing has been described in patients with TN with concomitant persistent facial pain.4
Treatment of TN includes both pharmacological and surgical treatments.10 The role of the SPG in the pathogenesis of TN is not clear and high‐quality randomized controlled trials (RCTs) have not been performed. Studies attempting to block the SPG in TN have been summarized in the literature.11, 12 The overall grade of recommendation for SPG block in TN is grade B.11 In the only RCT conducted in TN attempting to block the SPG, 25 patients were randomized to be treated with either intranasal lidocaine 8% or placebo for second‐division TN.13 The lidocaine group had prompt but temporary analgesia. It should be noted that intranasal injection of drugs has not been proven to achieve blockade of the SPG and that proper blinding of intranasal local anesthetics may not have been achieved.14 Other authors have also targeted the SPG in TN with varying results.15, 16, 17, 18, 19, 20, 21, 22
Parasympathetic fibers synapse in the SPG using acetylcholine as neurotransmitter.7 onabotulinumtoxinA (BTA) blocks the release of acetylcholine and 2 pilot trials have examined the safety of injections of BTA toward the SPG in patients with intractable chronic cluster headache and intractable chronic migraine.23, 24
Given the reports suggesting that the SPG may be a viable therapeutic target for trigeminal pain syndromes, including TN, and that blockade of the SPG with BTA may be an effective intervention, we decided to examine the safety of injections with BTA toward the SPG in patients with classical TN using a new navigation device (the MultiGuide®) and to collect pilot data on efficacy to inform and power future potential RCTs.
Method
Study Design and Participants
A total of 10 patients with classical TN (ICDH‐3 Beta criteria) were recruited and treated between September 2015 and October 2018 at St. Olav's University Hospital, Trondheim, Norway. There was a baseline registration of 1 month previous to injection and the follow up was 3 months. One study month equaled 28 days.
Table 1 shows inclusion and exclusion criteria.
Table 1.
Inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
BTA = botulinum toxin type A; ICHD‐3b = International Classification of Headache Disorders, 3 beta edition; TN = trigeminal neuralgia.
All 10 patients were examined by a neurologist and CT and MRI scans of the sphenopalatine fossa were obtained before injection. Patients had to keep a daily diary 4 weeks prior to and for 3 months after the injection recording adverse events (AEs), number of TN attacks, intensity (using a numeric rating scale [NRS] from 0 to 10) and functional level (“how much of your planned activities for the day did you manage to complete”: 1: all; 2: more than 50%; 3: less than 50%; 4: none). Patients were instructed to count each paroxysm as an attack. The intensity level was recorded as an average of the individual paroxysms through 1 day.
Description of the Procedure
Our research group has developed a novel injection device to perform surgical navigation‐assisted administration of BTA toward the SPG (the MultiGuide®, Fig. 1) ipsilateral to the pain. A single treatment was performed on an awake participant, using local anesthesia, in an outpatient office‐based setting using a percutaneous, infrazygomatic approach using the MultiGuide®, aided by surgical navigation (Brainlab Kick version 1, Brainlab AG, Feldkirchen, Germany). Surgical navigation is a system that tracks and displays the tip of an instrument relative to a pre‐acquired medical image. MultiGuide® enables the use of surgical navigation for high‐precision injections on awake individuals and it enables repeated treatments without acquiring new CT and/or MRI for better radiation hygiene and lower cost. Pre‐treatment planning of CT and MRI was performed with Brainlab iPlan 3.0 (Brainlab AG, Feldkirchen, Germany). The SPG ipsilateral to the pain was localized visually and marked on fused MRI and CT scans. With the patient in a supine position, the skin and deep structures toward the sphenopalatine fossa were anesthetized with 5‐7 ml Marcaine‐Adrenalin (5 mg/ml‐5 µg/ml, AstraZeneca, Oslo, Norway) and a 1‐2mm skin incision was made. Aided by surgical navigation and the MultiGuide®, 25 international units BTA suspended in 0.5 ml isotonic saline was injected toward the SPG ipsilateral to the pain. The estimated duration of the injection is around 3 minutes, and for the whole procedure including navigation system setup 20‐30 minutes. In this study, we used the same injection technique as in pilot trials in intractable chronic cluster headache and intractable chronic migraine.23, 24
Figure 1.
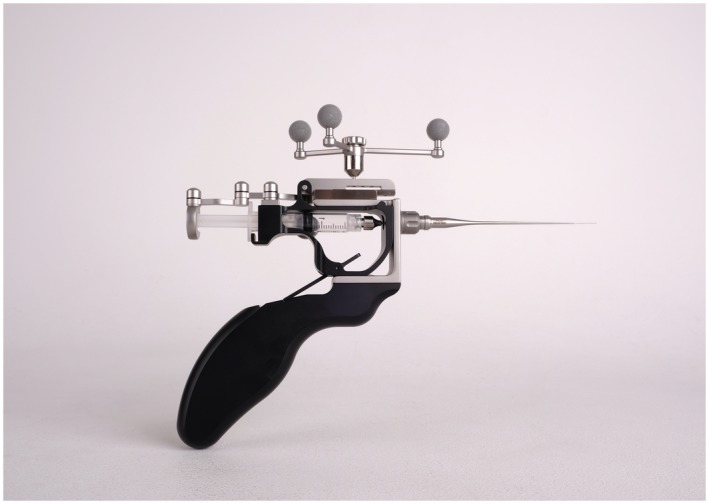
The MultiGuide, a novel injection device to perform surgical navigation‐assisted administration of botulinum toxin toward the sphenopalatine ganglion.
Outcome and Statistical Analysis
The primary outcome was occurrence of AEs. All medical complications that participants experienced after the injection during the 3‐month follow‐up were evaluated as a possible AE. Information for possible AEs was collected from each telephone consultation (at weeks 1‐4, 6, and 8 after injection), at last visit (month 3 after injection) and in the headache diaries (each day had a free text box for AEs). All health complaints (also those not requiring further medical intervention) were evaluated as a possible AEs and where in doubt, they were coded as AEs. The main efficacy outcome was number of TN attacks at weeks 5‐8 after injection compared to baseline. Efficacy outcomes were measured at weeks 5‐8 (predefined in protocol) since onset of efficacy may require up to 4 weeks and maximal benefit would be expected during month 2 prior to the eventual and usual attenuation of the therapeutic effect of BTA during the 3rd month after injection. A treatment responder was predefined as at least 50% reduction in the median number of attacks per day between baseline and weeks 5‐8. Other efficacy outcomes were intensity of the attacks, functional level at weeks 5‐8 after injection compared to baseline, Patient Global Impression of Change (PGIC) and percentage of the day with concomitant persistent pain.
PGIC was used to record patient's assessment of the change in overall status according to a 7‐point NRS (1: very much improved, 2: much improved, 3: minimally improved, 4: no change, 5: minimally worse, 6: much worse, and 7 very much worse) at month 3 after injection.
Patients were asked to record the percentage of the day with concomitant persistent pain at baseline and weeks 1‐4, 6, 8, and 12 after injection. The percentage of the day with concomitant persistent pain was stratified as 0%, 1 to 24%, 25 to 49%, 50 to 74%, 75 to 99%, and 100%. A Friedman test (non‐parametric analysis for repeated measurements) was performed. A Wilcoxon rank‐sum exact test was used to analyze changes at weeks 1‐4, 6, 8, and 12 after injection compared to baseline.
A scale developed to screen for cranial parasympathetic symptoms (CAPS scale25) was administered at baseline and 3 months after injection.
For the primary endpoint, we analyzed data for all 10 patients. A protocol violator was defined as a participant with less than 60% of diary days registered or change of prophylactic medication during the study. Missing values were estimated using the last observation carried forward methodology. For efficacy outcomes, we analyzed data for 9 patients (one patient was considered a protocol violator due to failure to count the number of attacks and document their intensity).
The study protocol was approved by the regional ethical committee (REK 2015/1193) and the Norwegian Medicines Agency. All participants signed a written informed consent. This trial received the EUDRACT number: 2015‐002643‐33 and was registered at ClinicalTrial.gov (NCT02662972).
SPSS version 25.0 (SPSS Inc, Chicago, IL, USA) was used in the data analyses. For efficacy measures, we used the Wilcoxon signed rank test, and 2‐sided P < .05 was considered statistically significant. A Friedman test for repeated measurements was performed to analyze changes in the percentage of the day with concomitant persistent pain. Results are given as median and range. Means (±SD) were calculated in order to produce comparable results to other studies targeting the SPG using the same technique.23, 24
Since the study is an exploratory safety study, no power calculation was performed prior to study start.
Results
A total of 12 patients were screened. Two patients were considered screening failures during baseline (one due to MRI findings of a brain stem lesion likely causing TN and the other did not feel impacted enough to undergo the study procedure). About 10 patients (3 women and 7 men) completed the study, 1 patient was a protocol violator and efficacy data could not be obtained for this patient. See Table 2 for demographics of the sample.
Table 2.
Demographics of the Sample
| Demographics of the sample | |
|---|---|
| Number of screened patients | 12 |
| Number of included patients | 10 |
| Number of females/males | 3/7 |
| Mean age, years ± SD (range) | 59.4 ± 11.77 (39‐74) |
| Mean years with trigeminal neuralgia ± SD (range) | 8.3 ± 8.6 (1.5‐29) |
| Number of Caucasians | 10/10 |
| Side left/right | 6/4 |
| Hyperesthesia (in the trigeminal territory) | 3/10 |
| Allodynia (in the trigeminal territory) | 2/10 |
| Branches affected | |
| V1 | 7/10 |
| V2 | 10/10 |
| V3 | 9/10 |
| Previous history of stroke | 4/10 |
| Previous history of ischemic heart disease | 2/10 |
| Previous history of hypertension | 3/10 |
| Previous history of depression | 2/10 |
SD = standard deviation; V1 = ophthalmic nerve; V2 = maxillary nerve; V3 = mandibular nerve.
Table 3 summarizes the drugs currently used or previously tried by the 10 patients. The patients had been treated with a mean of 3.3 evidence‐based medications (minimum of 2 and maximum of 6 medications) prior to inclusion in this trial.
Table 3.
Drugs Used by the Participants of the Study
| Drug | Number of Patients (n = 10) | ||
|---|---|---|---|
| Current Use | Previous Use | Not Tried | |
| Carbamazepine | 3 | 5 | 2 |
| Oxcarbazepine | 3 | 2 | 5 |
| Gabapentine | 1 | 9 | 0 |
| Pregabaline | 2 | 3 | 5 |
| Baclofen | 0 | 3 | 7 |
| Lamotrigin | 0 | 1 | 9 |
| Fosphenytoin | 0 | 1 | 9 |
Three patients had previously undergone microvascular decompression, 1 patient had previously tried glycerol rhizolysis of the trigeminal ganglion, and 1 patient had undergone balloon‐compression of the trigeminal ganglion.
Primary Outcome (Safety)
Six out of 10 patients experienced AEs, none were serious (Table 4). All AEs were considered to be mild except for 1 patient who experienced diplopia moderately affecting his daily activities. This was assumed to be due to diffusion of BTA through the inferior orbital fissure which clinically produced a moderate paralysis of the inferior rectus muscle with hypertropia in abduction. The symptoms slowly improved and resolved 1 month after conclusion of the study. This patient had a remarkably narrow sphenopalatine fossa. We believe that this anatomical characteristic played an important role on the development of this AE and this will be taken into consideration for further injections in similar patients.
Table 4.
Adverse Events
| Adverse Events | Number of Patients | ||
|---|---|---|---|
| Resolved <4 Weeks | Resolved 4‐12 Weeks | Resolved Within 4 Months After Injection | |
| Pain or swelling | 3 | — | — |
| Jaw problems | 2 | 1 | 1 |
| Nasolabial fold asymmetry | — | — | 2 |
| Diplopia | — | — | 1 |
| Dry eye | — | 1 | — |
| Dysphagia | 1 | — | — |
| Rash | 1 | — | — |
Two patients experienced mild nasolabial fold asymmetry assumed to have been caused by diffusion of botulinum toxin toward the zygomatic muscles. Both patients reported that the slight asymmetry was not bothersome and resolved within 1 month after the study ended.
One patient experienced mild dysphagia (approximately 2 weeks after injection, it was slightly harder to swallow phlegm, but he did not have dysphagia when drinking or eating). This resolved within 1 month after injection.
Three patients had mild pain or swelling at the injection side that resolved in all cases within the first month after injection. Just 1 of the patients had to take additional analgesics on the day of injection.
Four patients reported mild discomfort in the jaw (ipsilateral to the injection side) at maximal gaping. These jaw problems did not interfere with chewing, eating or speaking and did not require further treatment. Symptoms resolved spontaneously within 1 month after injection in 2 patients, after 3 months in 1 patient, and after 4 months in 1 patient.
One patient experienced mild symptoms of dry eye ipsilateral to the injection. These symptoms appeared 5 weeks after injection and resolved 7 weeks after injection and did not require any treatment.
Of the 13 observed AEs, 7 were considered to be secondary to the procedure (pain or swelling at the injection side and jaw problems) and 5 secondary to BTA (nasolabial fold asymmetry, diplopia, dry eye, and dysphagia). One of the patients developed a mild bilateral facial rash during the study that was not thought to be related to the procedure or the experimental drug.
Secondary Outcomes (Efficacy)
For the efficacy outcomes we have analyzed data for 9 patients (excluding the protocol violator with no data available). A 2‐sided Wilcoxon Signed Ranks Test was performed to compare the number of attacks, intensity, and function level at baseline and at weeks 5‐8 after injection (see Table 5). The median number of attacks per day when comparing baseline vs weeks 5‐8 was not statistically significant (P = .401). Four patients were treatment responders with at least 50% reduction in the median number of attacks between baseline and weeks 5‐8. Two patients achieved full remission after the injection (patients 5 and 10 in Table 6).
Table 5.
Number of Attacks per Day, Intensity of Attacks Using a Numeric Rating Scale (NRS 0 to 10) and Functional Level (“How Much of Your Planned Activities for the Day did you Manage to Complete”: 1: all; 2: more than 50%; 3: less than 50%; 4: none)
| Baseline | Weeks 5‐8 | Weeks 5‐8 vs Baseline | ||
|---|---|---|---|---|
| Number of attacks per day | Median (range) | 5.5 (1.0‐51.5) | 5.0 (0.0‐225.0) | P = .401 |
| Mean ± SD | 11.9 ± 15.6 | 28.3 ± 71.8 | ||
| Intensity of attacks | Median (range) | 6.0 (3.0‐8.5) | 3.0 (0.0‐9.0) | P = .024 |
| Mean ± SD | 5.8 ± 2.1 | 3.65 ± 3 | ||
| Functional level | Median (range) | 2.0 (1.0‐3.3) | 1.0 (1.0‐4.0) | P = .750 |
| Mean ± SD | 1.9 ± 0.81 | 2.0 ± 1.12 |
SD = standard deviation.
Table 6.
Median Number of Attacks per day and Median Intensity of Attacks at Baseline and at Weeks 5‐8 for Each Participant
| Patient | Number of Attacks per Day, Median (Range) | Intensity of Attacks, Median (Range) | Surgical Interventions Tried | |||
|---|---|---|---|---|---|---|
| Baseline | Weeks 5‐8 | Baseline | Weeks 5‐8 | Current Prophylactic Medication | ||
| 1 | 5.5 (3‐10) | 5.0 (3‐8) | 6.0 (3‐9) | 5.0 (4‐8) | Carbamazepine | — |
| 2 | 52.5 (16‐90) | 225.0 (25‐420) | 8.0 (6‐9) | 9.0 (7‐9) | Gabapentine | — |
| 3 | 3.0 (2‐4) | 2.0 (2‐4) | 3.0 (3‐5) | 3.0 (2‐4) | Pregabaline | — |
| 4 | 2.5 (0‐5) | 5.0 (4‐10) | 3.5 (0‐10) | 2.0 (2‐4) | Carbamazepine | Microvascular decompression |
| Glycerol rhizolysis of the trigeminal ganglion | ||||||
| 5 | 16.0 (8‐40) | 0.0 (0‐0) | 8.5 (8‐9.5) | 0.0 (0‐0) | Paracetamol/codein | — |
| 6 | 13.5 (0‐18) | 6 (6‐6) | 7.0 (0‐8) | 6.0 (6‐6) | Oxcarbazepine | — |
| 7 | 15.0 (2‐25) | 6.5 (0‐113) | 8.5 (6.5‐10) | 6.3 (0‐10) | Pregabaline | Microvascular decompression |
| Balloon‐compression of the trigeminal ganglion | ||||||
| 8 | 1.0 (1‐1) | 1.0 (0‐5) | 4.0 (1‐8) | 1.5 (0‐5) | Oxcarbazepine | — |
| 9 | † | † | † | † | Carbamazepine | Microvascular decompression |
| 10 | 2.0 (0‐4) | 0.0 (0‐0) | 4.0 (0‐8) | 0.0 (0‐0) | Oxcarbazepine | — |
Patient number 9 was none compliant with the headache diary and was considered a protocol violator.
Table 6 shows the median number of attacks and median intensity of attacks at baseline and at weeks 5‐8 for each participant.
One can observe that the mean, but not the median, number of attacks per day at weeks 5‐8 was increased (Table 5). This was due to an outlier (patient 2 in Table 6), who had a worsening of his TN.
The median intensity of attacks was significantly reduced from baseline (median 6, range 3.0‐8.5) vs weeks 5‐8 (median 3, range 0.0‐9.0; P = .024).
The median function level when comparing baseline vs weeks 5‐8 was not statistically significant (P = .750).
All patients but 1 had a CAPS scale of 0 (no autonomic parasympathetic symptoms) both at baseline and at month 3 after injection. One patient had 1 point in the CAPS scale before injection due to mild conjunctival injection during attacks (he did not have lacrimation or other symptoms). His CAPS score 3 months after injection was 0. This patient was a responder and went into full remission after treatment.
All patients had persistent concomitant pain at baseline with a median percentage of the day with concomitant persistent pain of 75% (minimum 37.5%, maximum 100%). The Friedman test for repetitive measurements was statistically significant (P = .031) indicating reduction in concomitant persistent pain after injection. Concomitant persistent pain at weeks 2 and 8 were significantly lower than at baseline (P = .027 and P = .023, respectively). These inferences were not statistically significant after proper adjustment for multiplicity. The median percentage of the day with concomitant persistent pain at week 8 was 18.75% (minimum 0%, maximum 100%).
One patient had a PGIC of “very much improved,” 2 patients “much improved,” 2 patients “minimally improved,” 2 “no change,” 3 “minimally worse (none “much worse” or “very much worse”) after injection.
The pain inflicted upon the patient during the injection was reported on an NRS from 0 to 10 immediately after injection. The mean pain reported was 2 (range 0‐2). One out of 10 patients had to use additional analgesics on the day of the injection. Eight out of 10 patients in this study would recommend this treatment to other patients with TN.
Discussion
In this paper, we have shown that injections of BTA toward the SPG in patients with TN, using a new navigation tool (the MultiGuide®), is safe. No serious AEs were reported in these 10 patients. All AEs remitted at the latest 4 months after the treatment as one would expect with BTA. The main efficacy outcome in this study was negative with median of 5.5 attacks per day in the baseline period vs 5.0 (P = .401) in weeks 5‐8. Four patients were treatment responders with at least 50% reduction in the median number of attacks between baseline and weeks 5‐8. Two patients had a complete remission after the injection and experienced a recurrence of attacks 1 month after the study end.
Patients with TN who do not have a satisfactory response to pharmacological treatment are often referred for surgical treatment. Quality evidence for efficacy of most neurosurgical procedures for TN has been reported to be very low because of the poor quality of the trials.26 TN incidence increases with age27 and affects a population group with high prevalence of comorbidities. Surgical interventions for TN have been reported to be highly effective but they also have a high risk for permanent and severe AEs. Up to 10% of patients undergoing a microvascular compression experience severe perioperative complications.28 Taha et al have published a review on several percutaneous techniques used in TN and the prevalence of side effects observed in different studies28, 29). Newer publications examining these techniques have found similar complication rates.30 Tuleasca et al have summarized the effects of repeat Gamma Knife treatment for TN and found that between 11 and 80% of the patients develop trigeminal hypesthesia.31 The main concern of the percutaneous techniques are the feared risk of anesthesia of the cornea and anesthesia dolorosa, in addition to high risk of hypoesthesia. These complications are typically permanent while the effect of the treatment is temporary. The risk for side effects in a non‐negligible percentage of patients undergoing surgical procedures and the increasing prevalence with age underlines the need for novel, minimally invasive and well tolerated approaches. The AEs of the technique used in this study appears to offer a favorable AE profile with mostly mild and transient AEs and with no severe AEs reported. These results are similar to earlier reports by our group in 2 other pilot trials.23, 24
The role of the SPG in TN has not been established but several authors have tried to target this structure.11, 12, 13, 15, 16, 17, 18, 19, 20, 21, 22, 32 This study was negative for the main efficacy endpoint (reduction of the number of attacks), but there are several aspects to consider. Four of the 9 subjects were treatment responders. Two patients had a complete remission starting the day following the injection. This remission was sustained for at least 1 month after completion of the study. None of these 2 patients had experienced a similar spontaneous remission previously to the study treatment. In addition, a statistically significant reduction in the intensity of the attacks and concomitant persistent pain was observed. Concomitant persistent pain in patients with TN has not been properly studied nor regarded as an endpoint in clinical trials in TN. ICHD‐3 describes a subgroup of classical TN with concomitant continuous or near‐continuous pain between attacks in the affected trigeminal distribution.1 Concomitant persistent pain has been reported in up to 49% of patients with TN.6 The 2 patients who went into full remission also experienced a complete disappearance of their concomitant persistent pain.
Central pain mechanisms have been invoked in the pathophysiology of TN.3, 4 Lesions induced in the spinal trigeminal nucleus, but not in the trigeminal ganglion, of cats or rats produce a marked overreaction to tactile stimulation of the face and the occurrence of spontaneous paroxysms of pain also suggesting a central involvement.3 The refractory period observed in most patients with TN suggests involvement of the central nervous system.3, 5 In patients with TN and concomitant continuous or near‐continuous facial pain, central facilitation of trigeminal nociceptive processing, most likely at a supraspinal level, has been demonstrated. This may be an underlying mechanism for development of continuous facial pain due to overactivation of central sensory transmission.4 The mechanism by which concomitant persistent pain was reduced in this study could either relate to a role of the SPG in pain sensitization,9 placebo effect, or regression to the mean. Blockade of the SPG may produce a reduction in parasympathetic outflow and thus reduced the activation/sensitization of the intracranial nociceptors and central nociceptive neurons in the spinal trigeminal nucleus, which could theoretically reduce the intensity of attacks and concomitant persistent pain, but not the number of attacks, as observed in this pilot trial. In a series of 158 prospective patients with TN, 48 patients (31%) had autonomic symptoms, the recorded symptoms included conjunctival injection/tearing (22%) and running/clogged nose (16%).6 All patients but 1 had a CAPS scale of 0 (no autonomic parasympathetic symptoms) during the baseline phase in the present study. The single patient whose CAPS score was reduced to zero 3 months after injection also experienced complete remission of pain. The presence of cranial parasympathetic symptoms may be a marker that predicts response and the low prevalence of patients with cranial parasympathetic symptoms in this study population may have negatively affected the efficacy outcomes.
Limitations of the Study
This was a small open‐label study. The placebo response in a previous study were patients with TN were randomized to a multi‐point injection (between the epidermis and dermis) of either 25 IU of BTA, 75 IU of BTA or placebo was 32.1%.33 The reduction of intensity in attacks and reduction in the percentage of the day with concomitant persistent pain observed in this study might have been due to placebo effect. It has also been documented that regression to the mean and periods of remission may bias the results in uncontrolled studies.34
Conclusion
Injection of botulinum toxin toward the SPG using the novel MultiGuide® system in patients with TN appears to have an acceptable adverse event profile as has been shown in other studies using the same technique.23, 24
The main efficacy endpoint in this study (reduction in number of attacks) was negative, but a significant reduction in the intensity of the attacks and concomitant persistent pain was observed. There were 4 patients with at least 50% reduction in the median number of attacks between baseline and weeks 5‐8, and 2 patients experienced complete remission of pain after the injection.
This study does not give any indication for effect in reducing the number of TN attacks after injection of 25 IU of BTA toward the SPG. Further studies examining the role of the SPG as a therapeutic target for TN are necessary.
Clinical Implications
The injection of onabotulinum toxin A toward the SPG in TN appears to be safe.
This study does not give any indication for effect in reducing the number of TN attacks after injection of 25 IU of BTA toward the SPG.
There were 4 patients with at least 50% reduction in the median number of attacks between baseline and weeks 5‐8, and 2 patients had complete remission of pain after the injection.
Authors' Contributions
JC, DB, and ET had the original idea for the manuscript. JC, DB and ET analyzed the data. JC reviewed the literature for the introduction and discussion and drafted the manuscript. DB, DD, MM, KJ, and ET: assistance for drafting the manuscript and revision of the text. All authors read and approved the final manuscript.
Conflict of Interest: Dr. Bratbak is co‐inventor of the device used to perform the treatment (patent pending) and may benefit financially from the commercialization of the device. Dr. Tronvik may benefit financially of a commercialization of a proposed treatment targeting the SPG and the intervention device used to perform the treatment through future possible intellectual properties. Within the last 12 months, Dr. Dodick reports personal fees from Amgen, Alder, Allergan, Autonomic Technologies, Biohaven, Eli Lilly, eNeura, Foresight Capital, Neurolief, Zosano, WL Gore, Vedanta Associates, Promius Pharma, Nocira, Novartis, Electrocore, Teva, Ipsen, Impel, Satsuma, Theranica. Compensation for activities related to data safety monitoring committee from Axsome. Compensation related to CME content development: Healthlogix, Medicom Worldwide, Medlogix Communications, MedNet, Miller Medical Communications, PeerView Operation Services America, Web MD/Medscape, American Academy of Neurology, American Headache Society, PeerView Institute for Medical Education, Chameleon Communications, Academy for Continued Healthcare Learning, Universal Meeting Management, Haymarket Medical Education, Global Scientific Communications, UpToDate, Meeting LogiX. Royalties from editorial or book publishing: Oxford University Press, Cambridge University Press, Wiley Blackwell, Sage, Wolters Kluwer Health. Consulting use agreement through employer: NeuroAssessment Systems, Myndshft. Equity (stock options): Aural Analytics, Healint, Theranica, Second Opinion/Mobile Health, Epien, Ontologics. Board of Directors position: King‐Devick Technologies, Epien, Ontologics. Dr. Crespi, Dr. Jamtøy and Dr. Matharu have nothing to disclose.
Funding: This work was supported by a grant given by NTNU (Norwegian University of Science and Technology) and “The Liaison Committee for Education, Research and Innovation in Central Norway” (Samarbeidsorganet); grant number 46056923.
References
- 1. Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 2. Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L. Significance of neurovascular contact in classical trigeminal neuralgia. Brain. 2015;138(Pt 2):311‐319. [DOI] [PubMed] [Google Scholar]
- 3. Fromm GH, Terrence CF, Maroon JC. Trigeminal neuralgia current concepts regarding etiology and pathogenesis. Arch Neurol. 1984;41:1204‐1207. [DOI] [PubMed] [Google Scholar]
- 4. Obermann M, Yoon MS, Ese D, et al. Impaired trigeminal nociceptive processing in patients with trigeminal neuralgia. Neurology. 2007;69:835‐841. [DOI] [PubMed] [Google Scholar]
- 5. Kugelberg E, Lindblom U. The mechanism of the pain in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1959;22:36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Trigeminal neuralgia–A prospective systematic study of clinical characteristics in 158 patients. Headache. 2014;54:1574‐1582. [DOI] [PubMed] [Google Scholar]
- 7. Robbins MS, Robertson CE, Kaplan E, et al. The sphenopalatine ganglion: Anatomy, pathophysiology, and therapeutic targeting in headache. Headache. 2016;56:240‐258. [DOI] [PubMed] [Google Scholar]
- 8. Goadsby PJ. Sphenopalatine (pterygopalatine) ganglion stimulation and cluster headache: New hope for ye who enter here. Cephalalgia. 2013;33:813‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yarnitsky D, Goor‐Aryeh I, Bajwa ZH, et al. 2003 Wolff Award: Possible parasympathetic contributions to peripheral and central sensitization during migraine. Headache. 2003;43:704‐714. [DOI] [PubMed] [Google Scholar]
- 10. Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia – Diagnosis and treatment. Cephalalgia. 2017;37:648‐657. [DOI] [PubMed] [Google Scholar]
- 11. Ho KWD, Przkora R, Kumar S. Sphenopalatine ganglion: Block, radiofrequency ablation and neurostimulation – A systematic review. J Headache Pain. 2017;18:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piagkou M, Demesticha T, Troupis T, et al. The pterygopalatine ganglion and its role in various pain syndromes: From anatomy to clinical practice. Pain Pract. 2012;12:399‐412. [DOI] [PubMed] [Google Scholar]
- 13. Kanai A, Suzuki A, Kobayashi M, Hoka S. Intranasal lidocaine 8% spray for second‐division trigeminal neuralgia. Br J Anaesth. 2006;97:559‐563. [DOI] [PubMed] [Google Scholar]
- 14. Crespi J, Bratbak D, Dodick D, et al. Measurement and implications of the distance between the sphenopalatine ganglion and nasal mucosa: A neuroimaging study. J Headache Pain. 2018;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manahan AP, Malesker MA, Malone PM. Sphenopalatine ganglion block relieves symptoms of trigeminal neuralgia: A case report. Nebr Med J. 1996;81:306‐309. [PubMed] [Google Scholar]
- 16. Gregoire A, Clair C, Delabrousse E, Aubry R, Boulahdour Z, Kastler B. CT guided neurolysis of the sphenopalatine ganglion for management of refractory trigeminal neuralgia (article in French). J Radiol. 2002;83(9 Pt 1):1082‐1084. [PubMed] [Google Scholar]
- 17. Candido KD, Massey ST, Sauer R, Darabad RR, Knezevic NN. A novel revision to the classical transnasal topical sphenopalatine ganglion block for the treatment of headache and facial pain. Pain Physician. 2013;16:E769‐E778. [PubMed] [Google Scholar]
- 18. Day M. Sphenopalatine ganglion analgesia. Curr Rev Pain. 1999;3:342‐347. [DOI] [PubMed] [Google Scholar]
- 19. Guo J, Kang X, Zhang S. Treatment of primary trigeminal neuralgia with acupuncture at the sphenopalatine ganglion. J Tradit Chin Med. 1995;15:31‐33. [PubMed] [Google Scholar]
- 20. Shuster MA, Isaev VM, Rechitskii VI, Agafonov BV. [Treatment of trigeminal neuralgia, ganglioneuritis of the pterygopalatine ganglion and other types of prosopalgias by helium‐neon laser irradiation of the pterygopalatine ganglion]. Zh Nevropatol Psikhiatr Im S S Korsakova. 1988;88:96‐98. [PubMed] [Google Scholar]
- 21. Gersdorff M. [Surgery of the sphenopalatine ganglion in facial pain]. Acta Otorhinolaryngol Belg. 1981;35:56‐62. [PubMed] [Google Scholar]
- 22. Oomen KP, van Wijck AJ, Hordijk GJ, de Ru JA. Effects of radiofrequency thermocoagulation of the sphenopalatine ganglion on headache and facial pain: Correlation with diagnosis. J Orofac Pain. 2012;26:59‐64. [PubMed] [Google Scholar]
- 23. Bratbak DF, Nordgard S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache. Cephalalgia. 2016;36:503‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bratbak DF, Nordgard S, Stovner LJ, et al. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic migraine. Cephalalgia. 2017;37:356‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riesco N, Perez‐Alvarez AI, Verano L, et al. Prevalence of cranial autonomic parasympathetic symptoms in chronic migraine: Usefulness of a new scale. Cephalalgia. 2016;36:346‐350. [DOI] [PubMed] [Google Scholar]
- 26. Zakrzewska JM, Akram H. Neurosurgical interventions for the treatment of classical trigeminal neuralgia. Cochrane Database Syst Rev. 2011;CD007312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol. 1990;27:89‐95. [DOI] [PubMed] [Google Scholar]
- 28. Taha JM, Tew JM. Comparison of surgical treatments for trigeminal neuralgia: Reevaluation of radiofrequency rhizotomy. Neurosurgery. 1996;38:865‐871. [DOI] [PubMed] [Google Scholar]
- 29. Hufschmidt A, Lücking CH, Rauer S, Glocker FX. Neurologie Compact, 7th ed Stuttgart: Thieme; 2017. [Google Scholar]
- 30. Cheng JS, Lim DA, Chang EF, Barbaro NM. A review of percutaneous treatments for trigeminal neuralgia. Neurosurgery. 2014;10(Suppl. 1):25‐33; discussion 33. [DOI] [PubMed] [Google Scholar]
- 31. Tuleasca C, Carron R, Resseguier N, et al. Repeat Gamma Knife surgery for recurrent trigeminal neuralgia: Long‐term outcomes and systematic review. J Neurosurg. 2014;121(Suppl):210‐221. [DOI] [PubMed] [Google Scholar]
- 32. Saberski L, Ahmad M, Wiske P. Sphenopalatine ganglion block for treatment of sinus arrest in postherpetic neuralgia. Headache. 1999;39:42‐44. [DOI] [PubMed] [Google Scholar]
- 33. Zhang H, Lian Y, Ma Y, et al. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: Observation of therapeutic effect from a randomized, double‐blind, placebo‐controlled trial. J Headache Pain. 2014;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diener HC, Schorn CF, Bingel U, Dodick DW. The importance of placebo in headache research. Cephalalgia. 2008;28:1003‐1011. [DOI] [PubMed] [Google Scholar]


