Abstract
Several radionuclides of the transition metal manganese are known and accessible. Three of them, 51Mn, 52mMn, and 52gMn, are positron emitters that are potentially interesting for positron emission tomography (PET) applications and, thus, have caught the interest of the radiochemical/radiopharmaceutical and nuclear medicine communities. This mini‐review provides an overview of the production routes and physical properties of these radionuclides. For medical imaging, the focus is on the longer‐living 52gMn and its application for the radiolabelling of molecules and other entities exhibiting long biological half‐lives, the imaging of manganese‐dependent biological processes, and the development of bimodal PET/magnetic resonance imaging (MRI) probes in combination with paramagnetic natMn as a contrast agent.
Keywords: cell labelling, immunoPET, manganese‐52g, PET/MRI, radiolabelled liposomes
1. INTRODUCTION
Radioactive nuclides have been used in nuclear medicine for the assessment of functional processes since about a century.1 However, to be suitable for in vivo imaging applications, a radionuclide needs to meet specific physical demands: Its decay radiation should be in an energy range sufficiently high to escape a patient's body in detectable amounts but also low enough to allow an efficient measurement by available detectors. Suitable γ energies for this purpose are usually in the range of 50 to 600 keV for single photon emission computed tomography (SPECT) and 511 keV for positron emission tomography (PET). Furthermore, the physical half‐life (t1/2) of the nuclide needs to be on the one hand long enough for a work‐up procedure, processing and suitable for the time scale needed to track the biological process of interest, but on the other hand short enough to result in an acceptable radiation burden for the investigated subject. Last but not least, practical radionuclides need to be accessible.
Since these requirements are not easily fulfilled, only a relatively small number of radionuclides have made their way into clinical practice. For scintigraphy and SPECT applications, nuclides like indium‐111 (111In, t1/2 = 2.8 d), iodine‐123 (123I, t1/2 = 13.2 h), and technetium‐99m (99mTc, t1/2 = 6.0 h) are nowadays routinely used, with 99mTc being the working horse of nuclear medicine. For PET applications, an imaging technique based on the detection of annihilation radiation of positrons (β+), mainly short‐living nuclides produced by proton bombardment of appropriate targets in cyclotrons, are applied. Here, fluorine‐18 (18F, t1/2 = 109.7 min) has become the most common nuclide because of its accessibility and excellent physical properties. Other important PET nuclides in this context are carbon‐11 (11C, t1/2 = 20.4 min), nitrogen‐13 (13N, t1/2 = 10.0 min), and oxygen‐15 (15O, t1/2 = 2.0 min). Furthermore, gallium‐68 (68Ga, t1/2 = 67.6 min) has increasingly found applications as a PET radiometal because of the introduction of 68Ga generators and the establishment of somatostatin‐ and prostate‐specific membrane antigen (PSMA) tracers in the clinic.2 However, all these PET nuclides have a t1/2 of 2 to 110 minutes. Therefore, they are not suitable to track compounds in vivo that exhibit a slow pharmacokinetic (several hours to days), such as antibodies, cells, nanoparticles, and liposomes.
The number of applications of antibodies radiolabelled with a PET radionuclide (immunoPET) has significantly increased over the last decade.3 Consequently, PET nuclides with a longer t1/2 like zirconium‐89 (89Zr, t1/2 = 78.4 h) or copper‐64 (64Cu, t1/2 = 12.7 h) have been investigated to match an antibody's biological half‐life.4 As of recently, the PET isotope manganese‐52g (52gMn) has been proposed as a suitable candidate for the combination with antibodies and proteins. It can be readily produced with a standard 16‐MeV cyclotron and has the potential to be used in bimodal PET/magnetic resonance (MR) systems as traceable nuclide in combination with a paramagnetic contrast agent based on nonradioactive manganese at the same time.
In this review, we give an overview of medically relevant β+ emitting manganese isotopes and their utility for applications in PET and PET/MR imaging to address questions of medical relevance. Although publications about the preclinical use of [51Mn]MnCl2 are available, we mainly focus on the longer‐living 52gMn compounds and their applications.
2. RELEVANT PET RADIONUCLIDES OF MANGANESE FOR MEDICAL IMAGING
2.1. Physical properties
Table 1 provides an overview of all neutron‐deficient manganese isotopes with a t1/2 useful for medical applications (>1 min). Manganese‐53 (53Mn) and ‐54 (54Mn) are excluded from the discussion below because of their overall poor physical decay characteristics, such as negligible β+ intensity and unfavourably long t1/2. However, they have to be considered as possible side products/contaminants during the production of other manganese isotopes.
Table 1.
Positron emission tomography (PET) isotopes of manganese and their physical properties
| Isotope | ß+, % | Eßmax, keV | Iγ, keVa | Half‐life |
|---|---|---|---|---|
| 51Mn | 97 | 2185.27 | – | 46.2 min |
| 52mMn | 97 | 2633.36 | 1434.07 (98.3%) | 21.1 min |
| 52gMn | 29 | 575 | 744.23 (90.0%); 935.54 (94.5%); 1434.07 (100%) | 5.6 d |
| 53Mn | 0/EC | – | X‐rays only | 3.74 × 106 y |
| 54Mn | 5.7 × 10−7 | 1377 | 834.85 (99.9%) | 312.2 d |
Most abundant (intensity > 10%).
51Mn has a favourable β+ branching fraction and a t1/2 (t1/2 = 46 min) comparable with that of 68Ga (t1/2 = 68 min), which is suitable for the imaging of fast biological processes. However, the short t1/2 of 51Mn leads to constraints regarding target separation and radiolabelling chemistry. The β+ energy is relatively high (Eßmax = 2.19 MeV) in comparison with the “standard” PET nuclide 18F (Eßmax = 0.6 MeV), which leads to an unfavourably long penetration range of the β+s in tissue and, thus, a deteriorated spatial resolution in the PET images.5
52mMn has a shorter t1/2 than 51Mn, comparable with that of the widely used 11C (t1/2 = 20 min), which renders its radiochemical handling even more challenging. Furthermore, the β+ energy of 52mMn (2633 keV) is higher than that of 51Mn. This results in a mean ß+ range of 5.3 mm in tissue6 and, thus, in a correspondingly lower resolution of PET images. Additionally, 52mMn decays partially via internal conversion to its ground state 52gMn, leading to an increasing contamination with the longer‐living 52gMn over time. A further drawback of this isotope with regard to medical applications is the presence of an additional prompt γ with relatively high energy (Table 1). Together with the β+ branching fraction of 93%, the arising dose rates and radiation protection concerns are unfavourable.
In contrast to the two Mn isotopes discussed above, 52gMn has a convenient long t1/2 (5.6 d), which is advantageous for target separations and chemical handling of the radionuclide. In addition, its t1/2 is well suited for the investigation of slow biological processes, eg, the pharmacokinetics of antibodies. 52gMn decays with a branching fraction for β+ of 29%, which is significantly lower than that of the previously mentioned Mn isotopes (Table 1). The β+ are emitted with a low maximum energy of Eßmax = 0.6 MeV, which is among the lowest energies of all β+ emitting nuclides. This results in a comparatively low tissue penetration range of the β+s and, thus, better resolution of PET images. The main disadvantage of 52gMn is the occurrence of three prompt γ rays with high energy and intensity (Table 1). These γ rays would significantly contribute to the radiation burden of patients and personnel of nuclear medicine departments. Furthermore, the prompt gammas cause erroneous signals in the PET detectors, which necessitates the implementation of prompt gamma correction techniques when using 52gMn for PET imaging.7
2.2. Nuclide production
In theory, a large number of nuclear reactions can lead to the formation of each of the different manganese isotopes discussed in this review, including possible side products such as 53Mn and 54Mn (see IAEAs EXFOR database).8 However, many of the reported production routes have no practical relevance, and a comprehensive discussion of all possibilities is beyond the scope of this review. Instead, we focus on the most efficient ones, which includes the irradiation of solid chromium targets.
The production of 51Mn was discussed by Klein et al including a survey of potential nuclear reactions as well as a short summary of previous results by other groups.9 They concluded that the 50Cr(d,n)51Mn reaction previously investigated by Cogneau et al10 and the natCr(p,x)51Mn reactions are the most promising candidates for this task. The 50Cr(d,n)51Mn reaction is based on the irradiation of isotopically enriched 50Cr with 14 → 3 MeV deuterons, resulting in the desired isotope in good yields (Table 2). The alternative reaction natCr(p,xn)51Mn utilizes the high abundance of 52Cr (83.8%) in natural chromium and uses the 52Cr(p,2n)51Mn reaction. However, even if conducted with an optimized proton energy window, the coformation of the long‐living 54Mn and 52mMn as well as 52gMn by competing (p,n) reactions cannot be avoided. It should be mentioned that the formation of 54Mn can be circumvented by using highly enriched 52Cr as target material. The potential formation of long‐living 53Mn when using enriched 52Cr targets has not been investigated so far.
Table 2.
Positron emission tomography (PET) isotopes of manganese and their production routes
One of the most promising routes for the production of 52gMn and 52mMn is represented by the irradiation of suitable chromium targets with 16 → 8 MeV protons (Table 2). Several studies demonstrated the feasibility of 52Mn production by the natCr(p,xn)52Mn reaction using 16 MeV cyclotrons.12, 13, 14 Another study presents cross‐sectional data for target beams up to 20 MeV.15 Because of similar energy thresholds, 52mMn is always coproduced with 52gMn when chromium targets are irradiated with protons. An isomerically pure production of these nuclides is therefore not possible by this approach. However, for the production of 52gMn for imaging applications, this is not an issue. Because of the significant difference in t1/2 of 52gMn (21.1 min) and 52mMn (5.6 d), the contamination can be removed by just simply letting the 52mMn decay. The same applies also to potentially coproduced 51Mn. The only reported relevant impurity of 52gMn mentioned in the literature is 54Mn, which is produced in minor amounts from 54Cr by a (p,n) reaction. Information about the potential long‐living impurity 53Mn is scarcely discussed albeit considerable cross sections for the 53Cr(p,n)53Mn reaction have been published.16 However, the formation of both long‐living impurities (53Mn and 54Mn) can be avoided by irradiation of highly enriched 52Cr and should therefore not impose any restriction for potential clinical applications of 52gMn.
Alternatively to the production of Mn isotopes via proton irradiation, 52mMn is available in high isotopic purity via a 52Fe/52mMn generator (t1/2(52Fe) = 8.28 h).17 However, because of the poor accessibility of the mother nuclide 52Fe, this approach has not been fully explored yet.
2.3. Target separation
To separate 52gMn isotopes from the chromium target material, several chromatographic ion exchange methods have been published.18, 19, 20 In most of them, the chromium target disc is first dissolved in an acidic medium and flushed over an ion exchange column. The radiomanganese is retained while chromium is washed out of the column. In a next step, the radiomanganese is eluted from the column with a different solvent composition. In the case of insufficient removal of chromium, the procedure has to be repeated. Different ion exchange resins, solvents, and solvent mixtures have been evaluated. For example, a recently published method required multiple subsequent column purifications resulting in a 52gMn recovery below 70%.21 Thus, there is still room for future improvements. A further development is the combination of chemical and chromatographic separation techniques, resulting in higher purity of the desired 52gMn.22 The different purification conditions are summarized in a review by Chaple et al,18 although none of them can be considered yet as “perfect” in terms of simplicity and recovery of the radionuclide.
It should be noted that, unlike in case of 52gMn, these elaborate and lengthy separation techniques are not adequate to isolate the short‐living manganese PET isotopes 52mMn and 51Mn, if produced via a solid chromium target. The development of a suitable liquid target enabling fast separation by solid phase extraction technology might provide a solution (a similar discussion is ongoing regarding the cyclotron production of 68Ga).23
In summary, the transition metal manganese offers three isotopes interesting for potential PET applications. Two of those, namely, 51Mn and 52mMn, are short living and thus, have the potential for the imaging of fast biological processes. In comparison, 51Mn is the better candidate because of its favourable decay characteristics (longer t1/2, practically no additional γ‐rays), which are comparable with those of 68Ga. 52gMn, on the other hand, has a suitable half‐life for the PET imaging of slow biological processes including applications in immunoPET. However, the presence of several high‐energy γ rays with high intensity necessitates the use of suitable corrections in PET imaging and is demanding with regard to radiation protection.
3. MANGANESE‐52G IN PRECLINICAL RESEARCH
3.1. Manganese complexes—coordination behaviour and paramagnetism
Manganese is a first‐row transition metal and homologue of technetium and rhenium. A broad range of coordination compounds of manganese are known, most of them with the metal in the oxidation state +II and coordination numbers of 6 or 7.24 As a “hard” Lewis acid, the most stable manganese complexes are obtained with ligands coordinating via oxygen and nitrogen atoms. For example, manganese (II) forms stable complexes with DOTA 1 and DO3A (logKML = 19.89 and 19.30, respectively).25, 26, 27 Such complexes can be obtained at room temperature under mild reaction conditions and within short reaction times.25 Other studied chelators for the complexation of Mn (II) are EDTA 3 and its derivatives such as CDTA 4 (Figure 1).28
Figure 1.
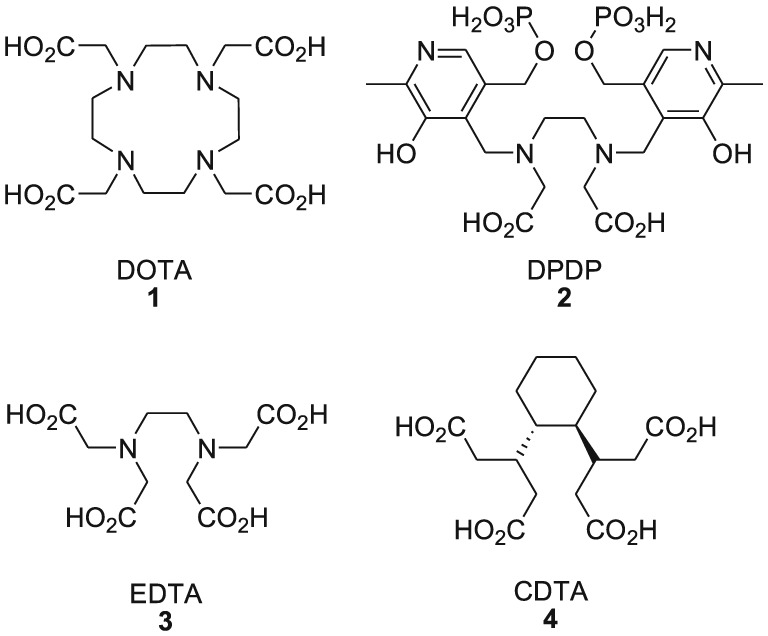
Examples of important chelating agents for manganese (II)
Mn (II) in its octahedral high spin complexes owns five unpaired electrons, which results in a high paramagnetic moment. For this reason, manganese (II) complexes have been investigated as possible magnetic resonance imaging (MRI) contrast agents. For example, the dipyridoxyl diphosphate (DPDP) complex of manganese (II) (Teslascan, Figure 2) was used in the clinic for the diagnosis of liver lesions. However, the compound has meanwhile been withdrawn from the market because of its insufficient stability in vivo.29
Figure 2.
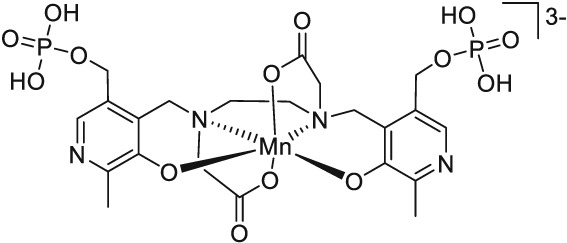
Mn‐DPDP (Teslascan), a manganese‐based T1 contrast agent
3.2. Biological pathways of MnCl 2 in vivo and neuronal connectivity imaging
Manganese is an essential element for all beings, for example, participating in numerous enzymatic processes as a cofactor.30 In the oxidation states +II and +III, it is transported in the blood bound to serum proteins31 before it accumulates in organs and tissue or is excreted.32 Each human body contains roughly 12 mg of manganese mostly stored in the bones, liver, and kidney.33 Notably, it is also known to cross the blood‐brain‐barrier.34 In higher doses, free manganese ions are known to cause a neurological disorder condition called manganism with psychiatric and, in later stages, Parkinson‐like symptoms.35
With regard to potential medical applications of manganese compounds, the pharmacokinetic profile of intravenously injected [52gMn]MnCl2 was investigated in mice.36 The highest accumulation of the radiometal was found in the liver, kidney, salivary glands, thyroid, and pancreas, whereas only low uptake of radioactivity was observed in the bones. These data may help to predict the fate of Mn (II) ions in case they get released from radiopharmaceuticals in vivo. Interestingly, the biodistribution pattern changed when [52gMn]MnCl2 was given by inhalation as a saline aerosol (eg, decreased uptake in the bones).36
To study the uptake and retention of manganese with regard to possible neuroimaging techniques, manganese‐enhanced magnetic imaging (MEMRI) as well as PET imaging was utilized by Brunnquell et al.37 For this purpose, different doses of nonradioactive MnCl2 were administered intravenously to rats. The uptake in different parts of the brain was studied using quantitative MEMRI at different time points postinjection (24 h to 14 d). Brain uptake studies with nca and ca [52gMn]MnCl2 were performed via gamma counting of resected brain areas. However, the authors concluded that the brain uptake of [52gMn]Mn2+ with intact blood‐brain barrier was too low for neuroimaging applications, especially when using the carrier added radiotracer. In addition, the authors stated that [52gMn]MnCl2, although not suitable in this case, still holds promise as a radiotracer for specific uptake in the salivary glands and pancreas. Another publication evaluated 52gMn for neuroimaging by stereotactic injection into the rat brain.38 The neuronal pathways between rat brain regions could be imaged successfully. Through application of different doses of nca [52gMn]MnCl2 (30 kBq‐170 MBq), the radiotoxicity was also studied, revealing that a low dose of 20 kBq is sufficient for imaging, while no histological and behavioural noxious effects occurred.
Saar et al studied the biodistribution of 51Mn and 52gMn in different organs.39 Further, they investigated the neuronal olfactory pathway in monkeys and rodents using nasally administrated 51Mn and 52gMn. It was shown that an administration of [52gMn]MnCl2 allows for a tracing of neuronal connections and that 52gMn is able to enter excitable cells in a similar manner as nonradioactive manganese ions in MEMRI.
3.3. Manganese‐52g for immunoPET, cell labelling, and ß‐cell mass monitoring
The use of radiolabelled antibodies for immunoPET has become an important tool in nuclear oncology.3, 40 Radiolabelled antibodies offer the advantage of high affinity and specificity. On the other hand, antibodies exhibit slow pharmacokinetics and the time until satisfying tumour uptake and/or tumour‐to‐background ratios are reached is often too long for other short‐living PET radionuclides such as 68Ga, 18F, or even 64Cu. Therefore, the longer‐living 89Zr (t1/2 = 78.4 h) is currently predominantly used for the labelling of clinically relevant antibodies.40 However, 52gMn also offers a suitable t1/2 for immunoPET applications while displaying a lower β+ energy and a higher β+ intensity than 89Zr (Table 3). Thus, 52gMn may represent a promising, alternative radiometal for applications in immunoPET.41
Table 3.
Nuclides used for radiolabelling of antibodies and their physical properties
| Nuclide | ß+, % | Eßmax, keV | Iγ, keVa | Half‐life, h |
|---|---|---|---|---|
| 64Cu | 17.9 | 653 | – | 12.7 |
| 89Zr | 22.8 | 902 | 909 (99.0%) | 78.4 |
| 52gMn | 29 | 575 | 744.23 (90.0%); 935.54 (94.5%); 1434.07 (100%) | 134.4 |
Most abundant (intensity > 10%).
The first and so far only in vivo study with a 52gMn‐labelled antibody was published in 2015 by Graves et al.21 For this study, the chelator DOTA was conjugated to the AT1‐targeting antibody TRC105 and tested in radiolabelled form for the PET imaging of a breast cancer xenograft mouse model. The chelator DOTA allowed for the 52gMn‐labelling at room temperature (see above), which is a necessary requirement to avoid denaturation of the protein during radiolabelling. Despite a slower blood clearance, the in vivo biodistribution and PET imaging yielded comparable results to a similar 89Zr‐labelled antibody conjugate (Figure 3). The research group used 52gMn instead of the more common 89Zr to demonstrate the opportunity of PET imaging at late time points postinjection of the radiotracer and the possibility for triple coincidence PET measurements,42 a new imaging technology for which 52gMn has suitable physical properties.
Figure 3.
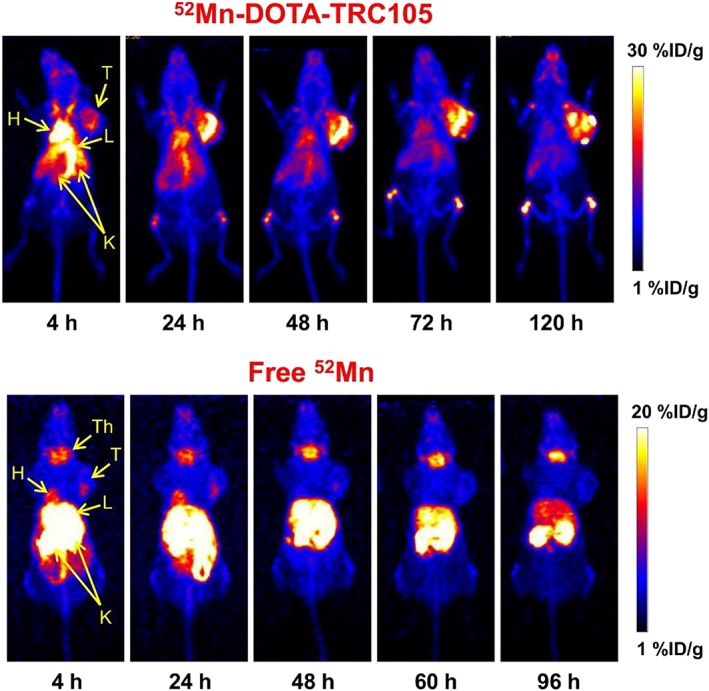
Serial maximum intensity projection (MIP) positron emission tomography (PET) images of mice injected with 52Mn‐DOTA‐TRC105 and 52MnCl2. Significant thyroid accumulation in the 52MnCl2 images contrasting the lack of uptake in the 52Mn‐DOTA‐TRC105 images indicates highly stable DOTA chelation of 52Mn2+ even at late time points. H, heart; K, kidneys; L, liver; T, tumour; Th, thyroid. Reprinted with permission from Graves et al21
Another potential use for longer‐living radiometals in nuclear medicine is the labelling and tracking of cells and liposomes in vivo. In 2018, Gawne et al demonstrated the suitability of [52gMn]Mn (oxinate)2 to label different cell types as well as DOXIL/CAEXYL liposomes.43 It was shown that the efficiency of the radiolabelling was comparable with analogous 89Zr‐labelling, although the method was limited by cell efflux of [52gMn]Mn2+. In vivo studies of 52gMn‐labelled liposomes in mice showed sufficient stability of the conjugate for up to 24 hours as long as the compound remained in the bloodstream. After the liposomes entered cells and tissues, uptake of radioactivity in the kidneys, salivary glands, and pancreas was detected, indicating decomposition and release of free [52gMn]Mn2+ ions. The authors concluded that their method was not suitable for in vivo tracking of cells but could serve as a model to study the biological fate of 52gMn once delivered inside of cells in vivo.
A different approach towards radiolabelled liposomes using 52gMn was published by Jensen et al.44 In this comparative study, 52gMn(II) and 64Cu(II) were prepared as their DOTA chelates and used for both internal loading and surface labelling of liposomes. In vivo biodistribution studies with the 52gMn‐labelled liposome preparations revealed that liposomes with internal 52gMn‐loading had a longer plasma half‐life than their surface labelled counterparts. The authors concluded that the reduced blood plasma half‐live of the surface modified liposomes could result from insufficient in vivo stability of the radiometal‐DOTA chelates.44
Mn+2 was shown to be taken up significantly by the pancreas31 (see also above), possibly by mimicking Ca2+ in pancreatic metabolic pathways. This feature was used by Hernandez et al to monitor ß‐cell mass with [52gMn]MnCl2 by ex vivo and in vivo imaging of ß‐cell metabolism in type 1 and type 2 diabetes mouse models.45 Previous work on this topic using MRI and nonradioactive manganese ions as contrast agents showed that the utility of this method was limited by the toxicity of free Mn2+ ions. Using the radiotracer [52gMn]MnCl2 and PET imaging solved the toxicity issues. It was shown that the uptake of [52gMn]Mn2+ strongly depends on the activity of ß‐cell voltage‐dependent Ca2+ channels and that the uptake of the radiometal correlates with Ca2+ uptake. The authors concluded further that because of the rapid uptake mechanisms, the use of the shorter‐living 51Mn might be a good alternative. This was later confirmed by a subsequent study using [51Mn]MnCl2.46
3.4. Relaxivity and stability—manganese for PET/MRI
In the past years, complexes of paramagnetic Mn (II) have been discussed as potential alternatives to the well‐established, but in some cases, disputed gadolinium‐based contrast agents (eg, Gadopentetat‐Dimeglumine, Magnevist).47, 48 For details on Mn‐based MRI contrast agents, the reader is referred to an excellent review on the topic.49 In addition, the availability of PET nuclides of manganese offers the possibility of isotopically radiolabelled manganese MR contrast agents for use in bimodal PET/MR imaging. Hybrid imaging by PET/MRI has lately received considerable attention in the nuclear medicine and radiology because it combines the high sensitivity PET with the high resolution of MRI.50 The concept is attractive, however, hampered by the different sensitivities of the modalities: PET allows for the application of very low concentrations of the radioactive substance (10−9‐10−12M) for achieving excellent contrast. In comparison, paramagnetic contrast agents for MRI are applied in millimolar concentrations (one dose of Gd‐DTPA, Gadovist: 0.1 mmol/kg body weight).51 The contradicting requirements of the two modalities for contrast agents can be met by using mixtures of a PET tracers with the respective nonradioactive analogous MRI contrast agent (carrier added radiotracers). Because the PET tracer (52gMn) and the MR contrast agent (natMn) are structurally identical, they exhibit equal biological properties.52 Employment of such mixtures enables, eg, the quantification of the MR contrast agent in areas of low uptake by PET. Furthermore, the concept is particularly attractive for applications to “smart MR contrast agents”.53, 54
The ability of a coordination compound to enhance longitudinal MR contrast is described by the value “relaxivity” r1. The relaxivity of paramagnetic coordination compounds is influenced not only by the number of unpaired electrons of the central metal ion but also by its internal rotation, size, and most importantly, magnetic influence on directly bound and surrounding water molecules.55
Since a water molecule as an additional ligand inside the coordination sphere of the metal is required for contrast enhancement, Mn (II) complexes with hexa‐ or lower dentate ligand systems and at least one inner sphere water molecule are potential candidates for MRI contrast agents.60 The tendency of the chelator DOTA 1 to form octadentate complexes with manganese of excellent stability (see above) has its downside in this context: Because of the lack of an inner sphere water molecule in [natMn (DOTA)], there is no MR contrast (Table 4). Therefore, complexes of Mn (II) with DOTA 1 (and DO3A)28 have good properties for PET imaging but are not suitable for PET/MR applications.
Table 4.
Contrast agents based on Gd and Mn and their physicochemical properties
| Contrast Agent | Longitudinal Relaxivity r1, 37°C, 20 MHz [mmol−1s−1] | Stability Constant logKML a | Ref |
|---|---|---|---|
| Gd‐DOTA (Dotarem) | 3.83 | 24.7 | 56, 57, 58 |
| Gd‐DTPA (Magnevist) | 4.02 | 22.5 | 25 |
| Mn‐DPDP (Teslascan) | 2.80 | 11.6 | 59 |
| Mn‐CDTA | 2.75 | 14.3 | 28 |
| Mn‐PyC3A | 2.10 | 14.1 | 60 |
| Mn‐DOTA | – | 19.9 | 60 |
| Mn‐DO3A | 1.30 | 19.4 | 27 |
KML = Equilibrium stability constant: [ML]/[M]*[L] with [ML] complex concentration; [M] metal ion concentration; [L] ligand concentration, in equilibrium.
While the combination of Mn (II) with DOTA 1 provides stable complexes but no contrast enhancement, the use of other chelators (eg, EDTA 3) results in complexes with appropriate relaxivity but insufficient stability. Therefore, the quest for new chelators that fulfil both requirements is still ongoing. The best compromise between sufficient stability and contrast enhancement described so far was achieved with chelators on the basis of the 1,2‐trans‐cyclohexyldiaminocarboxylate (CDTA 4) scaffold, for example, PyC3A 5 (Figure 4 with structure 4‐6).56 Gale et al reported in 2015 that the Mn (II) complex of PyC3A proved to be of sufficient thermodynamic stability (logKMnL = 14.14) and suitable for contrast‐enhanced MR angiography.60 This successful proof‐of‐concept study represents an important step towards the development of new alternatives of Gd‐based MR contrast agents.
Figure 4.
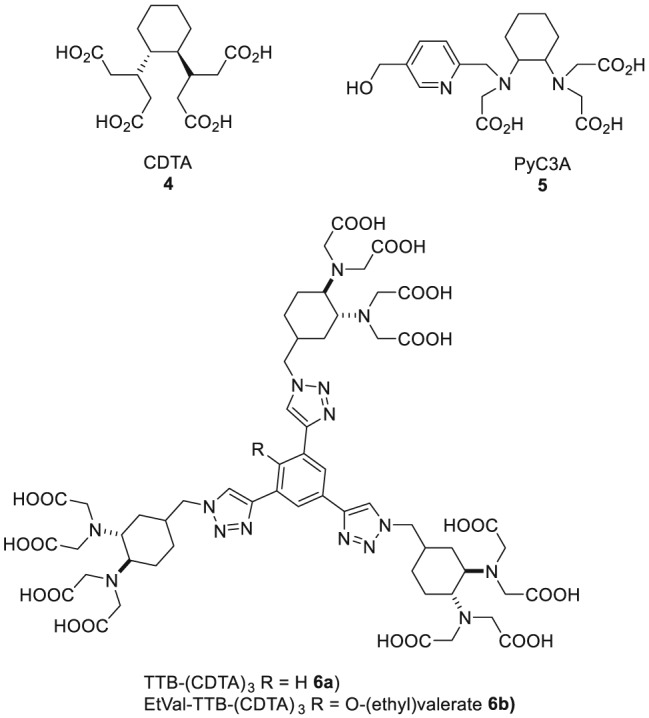
Successful chelating agents for Mn based on CDTA 4 for magnetic resonance imaging (MRI) using manganese
In 2016, Vanasschen et al published the first isotopically labelled bimodal PET/MRI agent based on mixtures of radioactive and nonradioactive manganese.61 The stability of the investigated [52g/55Mn]Mn‐CDTA complex in human blood serum (HBS) was studied, as well as its radiolabelling reaction using nca and ca [52g/55Mn]Mn+2. The radiolabelling could be performed successfully (radiochemical yield > 99%) at room temperature within 30 minutes. The stability study in HBS showed a dissociation half‐life of [52g/55Mn]Mn‐CDTA of 12 hours with most of the released [52gMn]Mn2+ being bound to larger serum proteins.61
Inspired by earlier work of Zhu et al,62 a rigid dendrimeric scaffold with three isotopically 52g/55Mn‐labelled complexes has been recently reported.63 The investigated contrast agent natMn‐Tris‐CDTA‐1,3,5‐tris‐triazolobenzene [natMn][Mn3(TTB‐(CDTA)3] 6a exhibited a dramatically increased overall T1 relaxivity in comparison with monomeric Mn‐CDTA, obviously the result of the presence of multiple paramagnetic centres. Also, the relaxivity of each paramagnetic centre could be increased by 144% because of the rigidity and restricted internal rotation of the molecule in comparison with Mn‐CDTA.28, 63 Labelled with isotopic mixtures of natMn and 52gMn, a bimodal PET/MR contrast agent based on manganese with high relaxivity was obtained. Through functionalization of the chelator, a bifunctionalized chelating agent ((EtVal‐TTB‐(CDTA)3 6b was synthesized to be used for further derivatization and potentially for bioconjugations.
It should be noted that other approaches for the development of PET/MRI imaging agents have also been reported. For example, Notni et al combined [68/69Ga]Ga and natGd in a scaffold containing the chelators TRAP and DOTA52; Frullano et al studied natGd‐DOTA complexes with a pendant 18F atom as PET reporter.53 In addition, iron particles spiked with radionuclides have been studied by different groups as potential PET/MRI imaging agents.64
4. SUMMARY AND CONCLUSION
There are three isotopes of manganese that are interesting for PET applications. The most promising among them is 52gMn due to its low β+ energy and suitable t1/2. 52Mn can be readily produced using a small 16‐MeV cyclotron and separated from the target material by effective but time‐consuming ion exchange chromatography. For clinical applications, 52gMn with a half‐life of 5.5 days is an interesting candidate for the radiolabelling of molecules with slow pharmacokinetics, for example, antibodies for immunoPET. In this context, 52gMn outperforms the current standard for immunoPET 89Zr not only in terms of t1/2 but also in terms of β+ energy, the latter resulting in better resolution of PET images for 52gMn. On the other hand, a drawback of 52gMn in comparison with 89Zr is the occurrence of high‐energy γs that will increase the dose rates for the patients, as well as the lack of commercial sources, which currently restricts its use in radiopharmaceutical research.
Because natMn (II) is paramagnetic and PET isotopes of the metal are available, isotopically radiolabelled manganese‐based PET/MRI contrast agents are within reach. The main challenge is the identification of suitable chelators, which provide manganese complexes of sufficient stability and relaxivity. Different approaches towards the development of such manganese complexes have been reported, but issues regarding the in vivo stability remain to be addressed.
In summary, manganese is a versatile transition metal with available isotopes well suited for MR, PET, and PET/MR imaging. Because of the currently limited availability, only a relatively small number of publications describe the production and use of manganese PET radionuclides. However, this may change in the future as in particular 52gMn has high potential to become a new emerging radiometal for PET and PET/MR applications in nuclear medicine.
ACKNOWLEDGEMENT
This work was supported by the Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (grant SNSF 205321_157216).
Biographies
Marie Brandt studied Chemistry at the Universities of Bonn and Cologne, Germany. She received her PhD in Radiochemistry from the Institute of Neuroscience and Medicine INM‐5: Nuclear Chemistry at Forschungszentrum Jülich, Germany in 2017. In fall 2017, she joined the LBI Applied Diagnostics / Department of Biomedical Imaging and Image Guided Therapy, Division of Nuclear Medicine at the Medical University of Vienna, Austria as postdoctoral fellow in Prof. Mindt's program line.

Jens Cardinale studied Chemistry at the University of Cologne, Germany. He received his PhD in Radiochemistry from the Institute of Neuroscience and Medicine INM‐5: Nuclear Chemistry at Forschungszentrum Jülich, Germany in 2012. From 2013 – 2017, he joined the research group of Prof. Kopka at German Cancer Research Center in Heidelberg, Germany. In spring 2017, he became Senior Scientist in Prof. Mindt's group at the LBI Applied Diagnostics / Departmentof Biomedical Imaging and Image Guided Therapy, Division of Nuclear Medicine at the Medical University of Vienna, Austria.

Ivo Rausch has studied technical Physics at the UT Vienna and holds a PhD in Medical Physics. Today, he is a post doc researcher in the Quantitative Imaging and Medical Physics group at the Center of Medical Physics and Biomedical Engineering, Medical University of Vienna. His main research interests relate to quantitative hybrid imaging in nuclear medicine with a focus on PET/CT and PET/MRI imaging.

Thomas L. Mindt is the Head of the Program Line “Imaging Biomarkers“ at the Ludwig Boltzmann Institute Applied Diagnostics. He obtained a chemical engineering degree from the University of Applied Sciences (Switzerland) and Ph.D. in organic chemistry from Brown University (USA). Upon return to Europe,he worked as a senior scientist in Radiopharmaceutical Sciences at the ETH in Zurich (Switzerland). In 2009, he accepted a call of the University of Basel (Switzerland) as an Assistant Professor in Radiopharmaceutical Chemistry and in 2015, he was promoted to Honorary Professor in the same discipline. In 2016, he moved to Vienna as a co‐founder of the new Ludwig Boltzmann Institute. Since 2018 he is a private lecturer (P.D.) at the Faculty of Chemistry of the University of Vienna.

Brandt M, Cardinale J, Rausch I, Mindt TL. Manganese in PET imaging: Opportunities and challenges. J Label Compd Radiopharm. 2019;62:541‐551. 10.1002/jlcr.3754
REFERENCES
- 1. Blumgart HL, Yens OC. Studies on the velocity of blood flow: I. The method utilized. J Clin Invest. 1926;4:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brandt M, Cardinale J, Aulsebrook ML, Gasser G, Mindt TL. An overview on PET radiochemistry: part 2‐radiometals. J Nucl Med. 2018;59:1500‐1506. [DOI] [PubMed] [Google Scholar]
- 3. van de Watering FCJ, Rijpkema M, Perk L, Brinkmann U, Oyen WJG, Boerman OC. Zirconium‐89 labeled antibodies: a new tool for molecular imaging in cancer patients. Biomed Res Int. 2014;2014:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson CJ, Ferdani R. Copper‐64 radiopharmaceuticals for PET imaging of cancer: advances in preclinical and clinical research. Cancer Biother Radiopharm. 2009;24(4):379‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchez‐Crespo A, Andreo P, Larsson SA. Positron flight in human tissues and its influence on PET spatial resolution. EJNMMI. 2004;31:44‐51. [DOI] [PubMed] [Google Scholar]
- 6. Jodal L, Le Loirec C, Champion C. Positron range in PET imaging: non‐conventional isotopes. Phys Med Biol. 2014;59(23):7419‐7434. [DOI] [PubMed] [Google Scholar]
- 7. Martin CC, Christian BT, Satter MR, Nickerson LDH, Nickles RJ. Quantitative PET with positron emitters that emit prompt gamma rays. IEEE Trans Med Imaging. 1995;14(4):681‐687. [DOI] [PubMed] [Google Scholar]
- 8.https://www‐nds.iaea.org/exfor/ (26.02.2019)
- 9. Klein ATJ, Rösch F, Qaim SM. Investigation of 50Cr(d,n)51Mn and natCr(p,x)51Mn processes with respect to the production of the positron emitter 51Mn. Radiochim Acta. 2000;88:253–264. [Google Scholar]
- 10. Cogneau M, Gilly L, Cara J. Absolute cross sections and excitation functions for deuteron induced reactions on chromium between 2 and 12 MeV. Nucl Phys. 1966;79(1):203‐208. [Google Scholar]
- 11. Klein ATJ, Rösch F, Coenen HH, Qaim SM. Labelling of manganese‐based magnetic resonance imaging (MRI) contrast agents with the positron emitter 51Mn, as exemplified by manganese‐tetraphenyl‐porphin‐sulfonate (MnTPPS4). Radiochim Acta. 2002;90:167‐177. [DOI] [PubMed] [Google Scholar]
- 12. Wooten AL, Lewis BC, Lapi SE. Cross‐sections for (p,x) reactions on natural chromium for the production of (52,52m,54)Mn radioisotopes. Appl Radiat Isot. 2014;96:154‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Topping GJ, Schaffer P, Hoehr C, Ruth TJ, Sossi V. Manganese‐52 positron emission tomography tracer characterization and initial results in phantoms and in vivo. Med Phys. 2013;40(4):042502. [DOI] [PubMed] [Google Scholar]
- 14. Buchholz M, Spahn I, Scholten B, Coenen HH. Cross‐section measurements for the formation of manganese‐52 and its isolation with a non‐hazardous eluent. Radiochim Acta. 2013;101:491‐499. [Google Scholar]
- 15. El Sayed R, Massicano AVF, Queern SL, Loveless CS, Lapi SE. Manganese‐52 production cross‐section measurements via irradiation of natural chromium targets up to 20 MeV. Appl Radiat Isot. 2019;147:165‐170. [DOI] [PubMed] [Google Scholar]
- 16.a Johnson CH, Galonsky A, Ulrich JP. Proton strength functions from (p,n) cross sections. Phys Rev. 1958;209:1243‐1254. [Google Scholar]; b Gardner HJ, Mitchell LW, Anderson MR, Sargood DG. Cross section measurements for the reaction 53Cr(p,g)54Mn, 53Cr(p,n)53Mn and 53Cr(p,p′)53Cr. Aust J Phys. 1981;34(1):25‐34. [Google Scholar]; c Pan H, Zhao Y, Li J, Han Y. Calculation and analysis for p+50,52,53,54,natCr reactions. Ann Nucl Energy. 2014;63:446‐460. [Google Scholar]
- 17. Atcher RW, Friedman AM, Huizenga JR, Rayudu GV, Silverstein EA, Turner DA. Manganese‐52m, a new short‐lived, generator‐produced radionuclide: a potential tracer for positron tomography. J Nucl Med. 1980;6:565‐569. [PubMed] [Google Scholar]
- 18. Chaple IF, Lapi SE. Production and use of first row transition metal PET radionuclides, 43,44Sc, 52Mn and 45Ti. J Nucl Med. 2018;59(11):1655‐1659. [DOI] [PubMed] [Google Scholar]
- 19. Fonslet J, Tietze S, Jensen AI, Graves SA, Severin GW. Optimized procedures for manganese‐52: production, separation and radiolabeling. Appl Radiat Isot. 2017;121:38‐43. [DOI] [PubMed] [Google Scholar]
- 20. Buchholz M, Spahn I, Coenen Heinz H. Optimized separation procedure for production of no‐carrier‐added radiomanganese for positron emission tomography. Radiochim Acta. 2015;103:893‐899. [Google Scholar]
- 21. Graves SA, Hernandez R, Fonslet J, et al. Novel preparation methods of 52Mn for immunoPET imaging. Bioconjug Chem. 2015;26(10):2118‐2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barrett KE, Aluicio‐Sarduy E, Olson AP, et al. Radiochemical isolation method for the production of 52gMn from natCr for accelerator targets. Appl Radiat Isot. 2019;146:99‐103. [DOI] [PubMed] [Google Scholar]
- 23. Riga S, Cicoria G, Pancaldi D, et al. Production of Ga‐68 with a general electric PETtrace cyclotron by liquid target. Eur J Med Phys. 2018;55:116‐126. [DOI] [PubMed] [Google Scholar]
- 24. Holleman AF, Wiberg E, Wiberg N. Lehrbuch der Anorganischen Chemie. Berlin: Walter de Gruyter; 2007:1609‐1613p. [Google Scholar]
- 25. Bianchi A, Calabi L, Giorgi C, et al. Thermodynamic and structural aspects of manganese (II) complexes with polyaminopolycarboxylic ligands based upon 1,4,7,10‐tetraazacyclododecane (cyclen). Crystal structure of dimeric [MnL]2·2CH3OH containing the new ligand 1,4,7,10‐tetraazacyclododecane‐1,4‐diacetate. J Chem Soc Dalton Trans. 2001;917‐922. [Google Scholar]
- 26. Rolla EA, Platas‐Iglesias C, Botta M, Tei L, Helm L. 1H and 17O NMR relaxometric and computational study on Mn (II) complexes. Inorg Chem. 2013;52(6):3268‐3279. [DOI] [PubMed] [Google Scholar]
- 27. Takács A, Napolitano R, Purgel M, et al. Solution structures, stabilities, kinetics, and dynamics of DO3A and DO3A–sulphonamide complexes. Inorg Chem. 2014;53(6):2858‐2872. [DOI] [PubMed] [Google Scholar]
- 28. Kalman F, Tircso G. Kinetic inertness of the Mn2+ complexes formed with AAZTA and some open chain EDTA derivatives. Inorg Chem. 2014;51:1165‐1167. [DOI] [PubMed] [Google Scholar]
- 29. Teslascan (mangafodipir) . Withdrawal of the marketing authorisation in the European Union EMA/486286/2012. Amsterdam, Netherlands: European Medicines Agency. [Google Scholar]
- 30.a) Averill BA, Eldredge P. General chemistry: principles, patterns, and applications. Published online: Flat World; 2011. [Google Scholar]; b). Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Met Ions Life Sci. 2013;13:199‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.a) Gibbons RA, Dixon SN, Hallis K, Russell AM, Sansom BF, Symonds HW. Manganese metabolism in cows and goats. Biochim Biophys Acta. 1976;444:1‐10. [DOI] [PubMed] [Google Scholar]; b). Scheuhammer AM, Cherian MG. Binding of manganese in human and rat plasma. Biochim Biophys Acta. 1985;840:163‐169. [DOI] [PubMed] [Google Scholar]
- 32. Wolf GL, Burnett KR, Goldstein EJ, Joseph PM. Contrast agents for magnetic resonance imaging In: Kressel H, ed. Magnetic Resonance Annual. New York: Raven; 1985:231. [PubMed] [Google Scholar]
- 33. Emsley JM. Nature's Building Blocks: An A‐Z Guide to the Elements. Oxford, UK: Oxford University Press; 2001:249‐253. [Google Scholar]
- 34. Yokel RA. Manganese flux across the blood‐brain barrier. Neuromolecular Med. 2009;11(4):297‐310. [DOI] [PubMed] [Google Scholar]
- 35. Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Met Ions Life Sci. 2013;13:199‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wooten AL, Aweda TA, Lewis BC, Gross RB, Lapi SE. Biodistribution and PET imaging of pharmacokinetics of manganese in mice using manganese‐52. PLoS ONE. 2017;12(3):e0174351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brunnquell CL, Hernandez R, Graves SA, et al. Uptake and retention of manganese contrast agents for PET and MRI in the rodent brain. Contrast Media Mol Imaging. 2016;11(5):371‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Napieczynska H, Severin GW, Fonslet J, et al. Imaging neuronal pathways with 52Mn PET: toxicity evaluation in rats. Neuroimage. 2017;158:112‐125. [DOI] [PubMed] [Google Scholar]
- 39. Saar G, Millo CM, Szajek LP, Bacon J, Herscovitch P, Koretsky AP. Anatomy, functionality, and neuronal connectivity with manganese radiotracers for positron emission tomography. Mol Imaging Biol. 2018;20(4):562‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heskamp S, Raavé R, Boerman O, Rijpkema M, Goncalves V, Denat F. 89Zr‐immuno‐positron emission tomography in oncology: state‐of‐the‐art 89Zr radiochemistry. Bioconjug Chem. 2017;28(9):2211‐2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alucio‐Sarduy E, Ellison PE, Barnhart TE, Cai W, Nickles RJ, Engle JW. PET radiometals for antibody imaging. J Labelled Cmpd Radiopharm. 2018;9:636‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cal‐González J, Lage E, Herranz E, et al. Simulation of triple coincidence in PET. Phys Med Biol. 2015;60(1):117‐136. [DOI] [PubMed] [Google Scholar]
- 43. Gawne P, Man F, Fonslet J, et al. Manganese‐52: applications in cell radiolabelling and liposomal nanomedicine PET imaging using oxine (8‐hydroxyquinoline) as an ionophore. Dalton Trans. 2018;47(28):9283‐9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jensen AI, Severin GW, Hansen AE, et al. Remote‐loading of liposomes with manganese‐52 and in vivo evaluation of the stabilities of 52Mn‐DOTA and 64Cu‐DOTA using radiolabelled liposomes and PET imaging. J Control Release. 2018;269:100‐109. [DOI] [PubMed] [Google Scholar]
- 45. Hernandez R, Graves SA, Gregg T, et al. Radiomanganese PET detects changes in functional b‐cell mass in mouse models of diabetes. Diabetes. 2017;66(8):2163‐2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Graves SA, Hernandez R, Valdovinos HF, et al. Preparation and in vivo characterization of 51MnCl2 as PET tracer of Ca2+ channel‐mediated transport. Sci Rep. 2017;7(1):3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guo BJ, Yang ZL, Zhang LJ. Gadolinium deposition in brain: current scientific evidence and future perspectives. Front Mol Neurosci. 2018;11:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drahos B, Lukes I, Tóth E. Manganese (II) complexes as potential contrast agents for MRI. Eur J Inorg Chem. 2012;2012(12):1975‐1986. [Google Scholar]
- 49. Merbach A, Helm L, Tóth E. The Chemistry of Contrast Media in Magnetic Resonance Imaging. Chichester: John Wiley & Sons; 2013. [Google Scholar]
- 50. Rausch I, Quick HH, Cal‐Gonzalez J, Sattler B, Boellaard R, Beyer T. Technical and instrumentational foundations of PET/MRI. Eur J Radiol. 2017;94:A3‐A13. [DOI] [PubMed] [Google Scholar]
- 51. GADOVIST Product Monograph (PDF) . Bayer Inc. August 12, 2016. Retrieved November 11, 2016.
- 52. Notni J, Simecek J, Hermann P, Wester HJ. Convenient synthesis of 68Ga‐labeled gadolinium (III) complexes: towards bimodal responsive probes for functional imaging with PET/MRI. Chem A Eur J. 2013;19:14718‐14722. [DOI] [PubMed] [Google Scholar]
- 53. Frullano L, Catana C, Benner T, Sherry AD, Caravan P. Bimodal MR‐PET agent for quantitative pH imaging. Angew Chem Int Ed. 2010;49(13):2382‐2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rosales RTM. Potential clinical applications of bimodal PET‐MRI or SPECT‐MRI agents. J Label Compd Radiopharm. 2014;57(4):298‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.For more detailed information on MRI contrast agents, we refer to the literature:a)Merbach A, Helm L, Tóth E. The Chemistry of Contrast Media in Magnetic Resonance Imaging. Chichester: John Wiley & Sons; 2013. [Google Scholar]; b) Micskei K, Helm L, Brücher E, Merbach A. Oxygen‐17 NMR study of water exchange on gadolinium polyaminopolyacetates [Gd(DTPA)(H2O)]2‐ and [Gd(DOTA)(H2O)]‐ related to NMR imaging. Inorg Chem. 1993;32(18):3844‐3850. [Google Scholar]; c) Bloembergen N. Proton Relaxation Times in Paramagnetic Solutions. J Chem Phys. 1957;27(2):572‐573. [Google Scholar]; d) Solomon I. Relaxation Processes in a System of Two Spins. Phys Rev. 1955;99(2):559‐565. [Google Scholar]
- 56. Gale EM, Atanasova IP, Blasi F, Ay I, Caravan P. A manganese alternative to gadolinium for MRI contrast. J Am Chem Soc. 2015;137(49):15548‐15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Powell DH, Dhubhghaill OMN, Pubanz D, et al. Structural and dynamic parameters obtained from 17O NMR, EPR, and NMRD studies of monomeric and dimeric Gd3+ complexes of interest in magnetic resonance imaging: an integrated and theoretically self‐consistent approach1. J Am Chem Soc. 1996;118(39):9333‐9346. [Google Scholar]
- 58. Clarke ET, Martell AE. Stabilities of trivalent metal ion complexes of the tetraacetate derivatives of 12‐, 13‐ and 14‐membered tetraazamacrocycles. Inorg Chim Acta. 1991;190(1):37‐46. [Google Scholar]
- 59. Rocklage SM, Cacheris WP, Quay SC, Hahn E, Raymond KN. Manganese (II) N,N′‐dipyridoxylethylenediamine‐N,iV′‐diacetate 5,5/‐bis (phosphate). Synthesis and characterization of a paramagnetic chelate for magnetic resonance imaging enhancement. InorgChem. 1989;28:477‐485. [Google Scholar]
- 60. Gale EM, Wey HY, Ramsay I, Yen YF, Sosnovik DE, Caravan P. A Manganese‐based alternative to gadolinium: contrast‐enhanced mr angiography, excretion, pharmacokinetics, and metabolism. Radiology. 2018;286:865‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vanasschen C, Brandt M, Ermert J, Coenen HH. Radiolabelling with isotopic mixtures of 52g/55Mn(II) as a straight route to stable manganese complexes for bimodal PET/MR imaging. Dalton Trans. 2016;45(4):1315‐1321. [DOI] [PubMed] [Google Scholar]
- 62. Zhu J, Gale EM, Atanasova I, Rietz TA, Caravan P. Hexameric Mn (II) dendrimer as MRI contrast agent. Chemistry. 2014;20(44):14507‐14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brandt MR, Vanasschen C, Ermert J, Coenen HH, Neumaier B. Mn‐labelled CDTA‐based trimeric complexes as novel bimodal PET/MR probes with high relaxivity. Dalton Trans. 2019;48(9):3003‐3008. [DOI] [PubMed] [Google Scholar]
- 64. Garcia J, Tang T, Louie AY. Nanoparticle‐based multimodal PET/MRI probes. Nanomedicine. 2015;10(8):1343‐1359. [DOI] [PubMed] [Google Scholar]


