Abstract
Background
Dynamic nasal valve collapse (NVC) is a common factor contributing to nasal obstruction; however, it is often underdiagnosed and untreated. An in‐office, minimally invasive procedure addressing dynamic NVC uses a bioabsorbable implant (Latera) to support the lateral nasal wall. This study aimed to evaluate the safety and effectiveness of the treatment in a randomized controlled trial (RCT) with sham control.
Methods
In this prospective, multicenter, single‐blinded RCT, 137 patients from 10 clinics were randomized into 2 arms: treatment arm (70 patients) and sham control arm (67 patients). Outcome measures were followed through 3 months after the procedure. The primary endpoint was the responder rate (percentage of patients with reduction in clinical severity by ≥1 category or ≥20% reduction in Nasal Obstruction Symptom Evaluation [NOSE] score).
Results
Before the procedure, there were no statistically significant differences in patient demographics and nasal obstruction symptom measures between the 2 arms. Three months after the procedure, responder rate was significantly higher for the treatment arm compared to the control (82.5% vs 54.7%, p = 0.001). Patients in the treatment arm also had a significantly greater decrease in NOSE score (–42.4 ± 23.4 vs –22.7 ± 27.9, p < 0.0001) and significantly lower visual analogue scale (VAS) scores (–39.0 ± 29.7 vs –13.3 ± 30.0, p < 0.0001) than the sham control arm. Seventeen patients reported 19 procedure/implant‐related adverse events, all of which resolved with no clinical sequelae.
Conclusion
Our study shows the safety and effectiveness of the bioabsorbable implant in reducing patients’ nasal obstruction symptoms.
Keywords: evidence‐based medicine, quality of life, disease severity, sham‐control, nasal airway obstruction in‐office procedures
Dynamic nasal valve collapse (NVC) is 1 of the common anatomic factors that contributes to nasal airway obstruction (NAO), an unpleasant condition that impacts patients’ activities such as breathing and sleeping.1 Treatment options have traditionally included nonsurgical medical management (eg, nasal sprays, nasal strips) and surgical procedures, such as functional rhinoplasty with batten grafts, bone‐anchored sutures, or lateral crural strut grafts, to support the lateral nasal wall.2, 3, 4, 5 Nonsurgical medical management does not directly address the weakness in the lateral nasal wall for patients with dynamic NVC. Surgical procedures that strengthen the lateral nasal wall require an operating room and, therefore, are invasive, costly, and time‐consuming. For these reasons, dynamic NVC is often underdiagnosed and left untreated despite its relatively high prevalence.6
A new technique to treat dynamic NVC uses an absorbable nasal implant comprised of a 70:30 blend of poly (L‐lactide) and poly (D‐lactide) to support the upper and lower cartilage inside the lateral nasal wall. The implant can be introduced under local anesthesia in physicians’ office through an endonasal insertion using a delivery tool. The procedure is minimally invasive and the implant can also be placed during a traditional nasal surgery concurrently with other procedures such as septoplasty and/or inferior turbinate reduction.
Prospective, nonrandomized, single‐arm clinical studies have examined the safety and effectiveness of this device by comparing NAO symptoms before and after treatment. San Nicoló et al.7 from Germany published the first clinical study of 30 patients in whom dynamic NVC was a major contributor to NAO symptoms. They showed that the bioabsorbable implant could be placed under local anesthesia in the physicians’ office or under general anesthesia in the operating room, and that it significantly improved NAO symptoms with a low retrieval rate and minimal cosmetic impact.7 A follow‐up study by the same authors showed that the effect of the implant was stable up to 24 months.8 A combined interim analysis of 2 studies examined 101 patients and showed that the implant alone or placed adjunctively with septoplasty and/or turbinate reduction resulted in improvement in NAO symptoms through 6 months.9 In these studies, 80% of the patients responded to the implant and response dynamics showed that the improvement in symptoms stabilized at 3 months after implant placement.
Although these studies have confirmed that the bioabsorbable nasal implant is safe, minimally invasive, and easily performed in either physician's office or operating room setting, they are limited due to their lack of controls and potential confounding factors. To mitigate an expected placebo effect stemming from the procedure, we conducted a prospective, multicenter, single‐blinded, randomized sham‐controlled trial to examine the effectiveness and safety of the bioabsorbable nasal implant for treatment of NAO due to dynamic NVC in a physician's office setting. To our knowledge, this is the first sham‐controlled clinical trial to test a new device for a treatment that directly addresses dynamic NVC.
Patients and methods
Study design
This was a prospective, multicenter, single‐blinded, randomized controlled trial (clinicaltrials.gov NCT 03400787). The aim of the trial was to compare the outcomes for patients with severe to extreme Nasal Obstruction Symptom Evaluation (NOSE) scores10, 11, 12 treated with a bioabsorbable implant comprised of a 70:30 poly (L‐lactide) and poly (D‐lactide) (Latera, Stryker ENT, Plymouth, MN) with those treated with a sham control procedure. The Institutional Review Board for each study center provided initial approval and annual review for the clinical trial protocol. Each patient provided written informed consent before enrollment.
Patients were enrolled at the time of consent. Upon enrollment, baseline data were collected including demographic information, general medical history, nasal medical history including risk factors, NAO breathing assessment using a visual analogue scale (VAS), NOSE score, nasal exam including assessment of the septum and turbinates, and modified Cottle maneuver.13, 14 Women of childbearing potential also had a pregnancy test.
Procedures were performed in the physician's office. Randomization, using an interactive Web response system, occurred after local anesthesia was administered. The randomization method was developed using a SAS program (SAS Institute, Inc., Cary, NC). The randomization was defined as stratifying by site and randomly using block sizes of 4 and 6. Patients in the active treatment arm received the implant, delivered using a cannula inserted into the nasal lateral wall.7 Patients in the sham control arm had an identical cannula inserted into the nasal lateral wall but received no implant. Treatment assignment was blinded to the patients.
Follow‐up visits took place at 7 days, 30 days, and 3 months after procedure. During each follow‐up visit, internal and external nasal exams were performed, as well as collection of NOSE scores, VAS scores for NAO breathing assessment, and adverse event assessment. Physical examinations included an evaluation of nasal skin and mucosa appearance, and the presence of any implant extrusions, fractures, or migrations. In order to reduce potential bias from the investigator, an electronic system was used by the patients to record the questionnaire responses. This allowed for the patient to complete the questionnaires privately without the investigator/treating physician present.
Enrollment
Enrollment occurred between December 2017 and September 2018 at 10 clinics across the United States. Eligible patients were at least 18 years of age, seeking treatment for NAO due to dynamic bilateral nasal wall insufficiency (confirmed by positive modified Cottle maneuver). In addition, patients had NOSE scores ≥55 (severe, extreme) and had failed to benefit from at least 4 weeks of medical management based on local standard of care (eg, nasal steroids or antihistamines), as evidenced by lack of efficacy or tolerability. Eligible patients had appropriate nasal and facial anatomy to receive the implant and were willing to undergo an in‐office procedure to receive the implant. Appropriate facial anatomy can include several features. One is whether there is sufficient nasal cartilage for the implant to support, as the device is indicated for the support of lateral wall cartilage. Patients who have had multiple reduction rhinoplasties may not have enough cartilage. Second, it is necessary to have a stable and reasonably wide nasal bone base to stabilize the implant. An overreduced or aggressively osteotomized and narrow nasal bones would make a particular patient a poor candidate.
Patients were ineligible if they had any of the following: (1) functional endoscopic sinus surgery (FESS), sinuplasty, septoplasty, inferior turbinate reduction (ITR), or rhinoplasty within the past 6 months; (2) pathology other than lateral wall insufficiency (LWI) as the primary contributor to NAO; (3) planning to have other rhinoplasty procedures or use external dilators within 24 months after the index procedure; (4) required or were anticipated to require other concurrent nasal procedures outside of the index procedure within 12 months after the procedure; or (5) severe obstructive sleep apnea and were unable to refrain from continuous positive airway pressure for up to 2 weeks after procedure.
Statistical analysis
Baseline characteristics were compared across the 2 study arms using the t test for continuous variables and the chi‐square test or Fisher's exact test for categorical variables. Endpoint analyses were completed on all patients who completed 3‐month follow‐up and did not violate any major protocol requirements for analysis (eg, unblinded to study arm). NOSE scores were converted to a 100‐point scale by multiplying the total score by 5.10, 11 NOSE score severity was classified according to the system reported by Lipan and Most12: mild (5 to 25 points), moderate (30 to 50 points), severe (55 to75 points), or extreme (80 to 100 points). VAS scores were used to capture patients’ perception of their ability to breathe through the nose with 0 indicating no difficulty and 100 indicating maximum imaginable difficulty.
The primary endpoint was the responder rate at 3 months after the index procedure. Responders were defined as patients who had at least 1 NOSE class improvement or a NOSE score reduction of at least 20% from baseline. The primary hypothesis was that the responder rate for the implant treatment is superior to the responder rate for the sham treatment (control). A 1‐sided binomial test of proportions was used to compare responder rates between study arms with a value of p < 0.025 considered statistically significant.
Secondary endpoints included the frequency of procedure‐related adverse events at index procedure and all follow‐up visits, and the change in NOSE and VAS scores from baseline to all follow‐up visits. Two‐sided t tests were used to compare these endpoints between study arms, with a value of p < 0.05 deemed statistically significant.
Statistical analyses were performed by an independent statistician (Syntactx Technologies, New York, NY) using SAS version 9.4.
Results
A total of 137 patients were enrolled in the study and randomized, with 70 patients randomized to the treatment arm and 67 patients randomized to the sham control arm (Fig. 1). One patient randomized to the sham control arm was inadvertently treated with the implant. This patient was analyzed with the treatment arm, resulting in a total of 71 patients analyzed in the treatment arm and 66 patients analyzed in the sham control arm. Two patients in the sham control arm exited prior to the 3‐month follow‐up. Eight patients in the treatment arm were excluded from the analysis due to unblinding prior to the 3‐month follow‐up (6) and protocol deviations (2). Thus, there were 127 patients included in the final analysis (63 treatment, 64 sham control) followed through 3 months after procedure.
Figure 1.
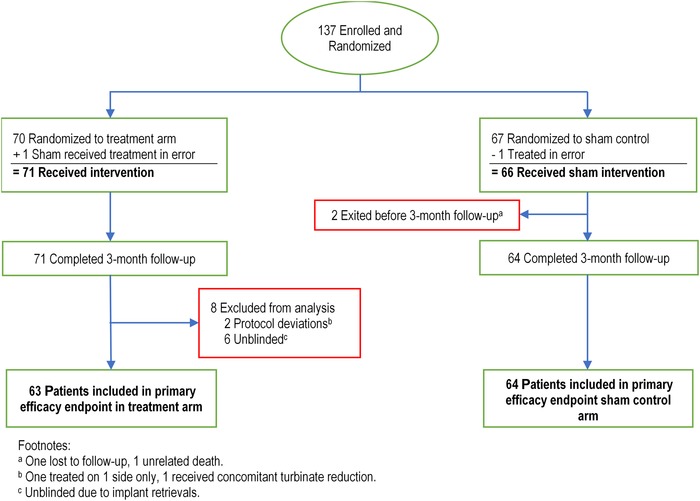
Enrollment, randomization, and follow‐up of patients randomized to treatment arm vs sham control arm.
Demographics and relevant clinical history for the 2 study arms are described in Table 1.
Table 1.
Patient baseline demographics and characteristics*
| Characteristic | Treatment arm (N = 63) | Sham control arm (N = 64) | p a |
|---|---|---|---|
| Age (years) | 50.9 ± 14.2 | 51.3 ± 13.5 | 0.888 |
| Sex (male) | 26/63 (41.3) | 24/64 (37.5) | 0.665 |
| BMI (kg/m2) | 28.6 ± 6.8 | 28.3 ± 5.5 | 0.790 |
| Race | 0.073 | ||
| White | 58/63 (92.1) | 51/64 (79.7) | |
| Black or African American | 0/63 (0.0) | 1/64 (1.6) | |
| Asian | 2/63 (3.2) | 5/64 (7.8) | |
| Native Hawaiian or Pacific Islander | 0/63 (0.0) | 0/64 (0.0) | |
| American Indian or Alaskan Native | 1/63 (1.6) | 1/64 (1.6) | |
| Other | 2/63 (3.2) | 4/64 (6.3) | |
| Not available | 0/63 (0.0) | 2/64 (3.1) | |
| Medical history | |||
| Surgical history | 34/63 (54.0) | 42/64 (65.6) | 0.182 |
| Allergic rhinitis | 22/63 (34.9) | 30/64 (46.9) | 0.172 |
| Sinus disease | 13/63 (20.6) | 18/64 (28.1) | 0.328 |
| Obstructive sleep apnea | 16/63 (25.4) | 17/64 (26.6) | 0.881 |
| Nonsurgical medical management | 63/63 (100.0) | 64/64 (100.0) | 1.000 |
| Mechanical nasal treatments | 54/63 (85.7) | 54/64 (84.4) | 0.833 |
| Scores | |||
| Baseline NOSE score | 77.4 ± 13.1 | 77.7 ± 15.1 | 0.888 |
| Baseline VAS score | 76.6 ± 12.9 | 71.2 ± 15.8 | 0.038 |
*Results are presented as mean ± SD or n/N (%).
Value of p from Fisher's exact test for dichotomous variables; Cochran–Mantel–Haenszel (CMH) for categorical variables; 2‐sample t test for continuous variables.
BMI = body mass index; NOSE = Nasal Obstruction Symptom Evaluation; SD = standard deviation; VAS = visual analogue scale.
Primary endpoint
Figure 2 shows the results of the primary endpoint of the study, the comparison of responder rates between the randomization arms at 3 months. The responder rate is significantly higher for the treatment arm (82.5%, 52/63) compared to the sham control arm (54.7%, 35/64), demonstrating the treatment arm is superior to the sham control arm (p = 0.001).
Figure 2.
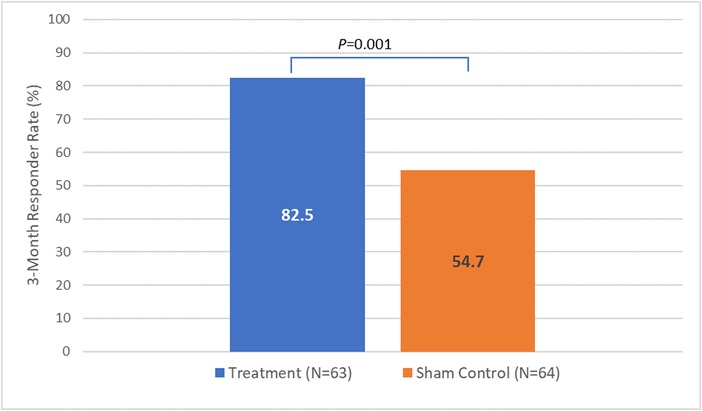
Primary endpoint: comparison between study arms for 3‐month responder rate. Value of p is based on a 1‐sided binomial test of proportions comparing responder rate between study arms with p < 0.025 indicating statistical significance. Implant treatment is superior to sham control.
Secondary endpoints
A total of 19 procedure‐related or implant‐related adverse events were reported in 17 patients. These events included implant retrievals (6), pain (4), foreign body sensation (3), localized swelling (2), inflammation (1), skin puncture (1), and vasovagal response (2). The investigators confirmed the implant retrievals were intranasal and not due to adverse physiologic tissue rejection. The implant retrieval rate was 4% (6/142). All events were observed in the treatment arm and resolved with no clinical sequelae.
We examined nasal obstruction symptoms measured by NOSE score for the 2 study arms over 3 months after treatment. Before treatment (baseline), both study arms had similar NOSE scores (Table 1). At 3 months after treatment, the treatment arm had a significantly greater reduction in the mean NOSE score compared with the sham arm (3 months; –42.4 ± 23.4 vs –22.7 ± 27.9, p < 0.0001) (Table 2).
Table 2.
Change in NOSE scores from baseline to follow‐up by study arm*
| Treatment arm | Sham control arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time point | N | Baseline NOSE score | Follow‐up NOSE score | Mean change | N | Baseline NOSE score | Follow‐up NOSE score | Mean change | p a |
| 1 month | 61 | 77.5 ± 12.9 | 40.9 ± 21.0 | −36.6 ± 24.8 | 60 | 77.4 ± 15.0 | 45.6 ± 24.2 | −31.8 ± 25.5 | 0.295 |
| 3 months | 63 | 77.4 ± 13.1 | 35.0 ± 22.6 | −42.4 ± 23.4 | 64 | 77.7 ± 15.1 | 55.0 ± 25.2 | −22.7 ± 27.9 | <0.0001 |
*Results are presented as mean ± SD.
Value of p from 2‐sided, 2‐sample Student t test for differences in the mean change between randomized arms.
NOSE = Nasal Obstruction Symptom Evaluation; SD = standard deviation.
Baseline VAS scores were comparable between arms (Table 1). At 3 months after treatment, the treatment arm had a significantly greater reduction in the mean VAS score than the sham control arm (–39.0 ± 29.7 vs –13.3 ± 30.0, p < 0.0001) (Table 3).
Table 3.
Change in VAS scores from baseline to follow‐up by study arm*
| Treatment arm | Sham control arm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time point | N | Baseline VAS score | Follow‐up VAS score | Mean change | N | Baseline VAS score | Follow‐up VAS score | Mean change | p a |
| 1 month | 61 | 76.6 ± 13.1 | 45.6 ± 29.3 | –30.9 ± 29.9 | 60 | 70.9 ± 16.2 | 49.6 ± 30.5 | –21.3 ± 33.3 | 0.096 |
| 3 months | 63 | 76.6 ± 12.9 | 37.6 ± 29.5 | –39.0 ± 29.7 | 64 | 71.2 ± 15.8 | 57.9 ± 26.6 | –13.3 ± 30.0 | <0.0001 |
*Results are presented as mean ± SD.
Value of p from 2‐sided, 2‐sample Student t test for differences in the mean change between randomized arms.
SD = standard deviation; VAS = visual analogue scale.
Discussion
This study shows that in‐office treatment of NAO patients using the bioabsorbable nasal implant provides statistically significant improvements in NAO symptoms that are superior to the sham control group. At 3 months after treatment, patients treated with the implant had significantly reduced nasal obstruction symptoms (as measured by NOSE scores and NOSE severity categories) and better perception of their ability to breathe through the nose (VAS scores) than the sham control patients. It is also notable that the treatment arm exhibited improvement from 1 to 3 months, whereas the sham control arm showed worsening outcomes, suggesting that over time there is an increasing benefit of the implant over the sham. Altogether, these results show a clear effect of the minimally invasive nasal implant in improving the NAO symptoms and strengthening of the lateral nasal wall at 3‐month follow‐up for patients with dynamic NVC. Being minimally invasive, this treatment strategy has the potential to reduce costs associated with anesthesia and operating room (OR) facility, pain, and postoperative recovery time for patients with the appropriate indications.
A randomized, placebo‐controlled or sham‐controlled clinical trial is considered the gold standard to most accurately determine the actual effect of an intervention. However, these trials are relatively uncommon in surgical research.15 Not only are RCTs of surgical interventions more difficult and expensive to conduct, but there are also ethical concerns that performing an invasive sham surgery that has no potential therapeutic benefit for research participants does not minimize the risk of harm.16 However, a systematic review of RCTs with placebo (sham) control arms reported that more than one‐half (51%, 27/53) of the published trials had similar results between the treatment and control arms,15 which emphasizes the benefit of evaluating surgical interventions using RCT with placebo (sham) control. The common nasal surgeries used to treat NAO include septoplasty, inferior turbinate reduction, and functional rhinoplasty, most of which are invasive and often require an OR setting. Consistent with the trend in general surgical research, RCT with placebo (sham) control is not common and only less invasive technologies for inferior turbinate reduction have been assessed in RCT with sham control.17, 18 Our study is the first randomized, sham‐controlled trial evaluating a minimally invasive nasal implant to address dynamic NVC, which contributes the highest level of evidence. In addition, utilizing a 16gauge needle in the sham arm did not introduce additional harm or complications to the patients, thereby providing a safe and effective control.
The implant procedure showed significantly more improvement in NAO scores than the sham procedure. We observed a mean reduction of –22.7 in NOSE scores in the sham group and 54.7% were responders at 3 months, which is at the high end of a typical placebo response for medical devices (40‐60%).19 We propose a few factors that may have contributed to the placebo response in the sham control arm. The primary factor comes from patients’ expectations about a new treatment, together with the care and attention provided by study staff. Second, outcome measures are mainly patient‐reported (NOSE and VAS scores), which have been shown to augment the placebo effect when a treatment had an optimistic presentation.20 Third, patients in the sham arm had a cannula inserted into the nasal lateral wall, possibly resulting in temporary mechanical support due to minor scar tissue.
Despite the improvement in the sham control group, we found that a significantly larger proportion of implant‐treated patients experienced a statistically significant improvement. The improvement in NAO symptoms, measured by the mean NOSE score reduction in the treatment arm, is similar to what has been reported in surgical studies in the operating room setting. A recent meta‐analysis of functional rhinoplasty studies reported a 50‐point (95% confidence interval [CI], 45 to 54) mean NOSE score reduction 3 to 6 months after treatment.21 Similarly, another recent meta‐analysis that focused on lateral nasal wall repair surgical studies reported a 45.0‐point (95% CI, 42.2 to 47.8) mean NOSE score reduction ≤3 months after treatment.22 In this study, the mean reduction in NOSE score at 3 months after treatment for the treatment arm was –42.4 points, representing a similar effect size as functional rhinoplasty. In contrast, the sham control arm had a –22.7 reduction in mean NOSE score, which is below the range of what is reported in the meta‐analyses of functional rhinoplasty studies. This comparison shows that for patients with dynamic NVC, even being blinded to the treatment, an in‐office procedure with a bioabsorbable implant can achieve NAO symptom relief comparable to functional rhinoplasty and surgeries aimed at repairing the nasal lateral wall.
The implant retrieval rate (4%) in the treatment arm is similar to that seen in a previous study.7 Contrary to the external extrusion events observed for more invasive procedures involving permanent, nonabsorbable alloplastic implants,23, 24 the implant retrievals in this study were intranasal and not due to an adverse physiologic tissue rejection. Tissue rejection was ruled out because the investigators did not see tissue inflammation. Additionally, all of the retrievals were unilateral, thereby providing further evidence the retrievals were not due to tissue rejection. The investigators hypothesize the retrievals could be due to improper placement or unknown manipulation of the nose. All adverse events resolved with no clinical sequelae. Our study confirmed the previous findings that the nasal implant is safe for NAO patients.
There are a few limitations of this study. This study reports short‐term follow‐up data up to 3 months only. However, previous studies of the bioabsorbable implant have shown that patients’ response to treatment stabilized at 3 months and were consistent with data observed at 12‐month, 18‐month, and 24‐month follow‐up. Furthermore, this is a single‐blinded study in which all patients were blinded but physicians were aware of the assignment, which may have introduced risk of bias. Our study design mitigated this risk by using patient‐centered outcomes (NOSE and VAS scores) as the study endpoints.
Conclusion
Our study provides the highest level of evidence demonstrating the safety and effectiveness of an in‐office, minimally invasive procedure for patients in whom dynamic NVC is a main contributor to their NAO. The nasal implant significantly improves patients’ NAO symptoms when compared with a sham, demonstrating the implant is superior. The minimal invasiveness of this treatment strategy may help reduce cost, pain, and postoperative recovery time for patients with the appropriate indications.
Acknowledgments
We thank the following physicians for participating in this study: Nora Perkins, MD, Jordon Pritikin, MD, Manish Wani, MD, Douglas Liepert, MD, Cooper Scurry, MD, Jose Barrera, MD, and Steven Davis, MD. We thank the DSMB committee consisting of John Delgaudio, MD, Lisa Grunebaum, MD, and Gavin Setzen, MD, for their review of interim data for safety. We also thank Vaishali Suraj, Stryker ENT, for assistance with manuscript writing and preparation.
How to Cite this Article: Stolovitzky P, Senior B, Ow RA, Mehendale N, Bikhazi N, Sidle DM. Assessment of bioabsorbable implant treatment for nasal valve collapse compared to a sham group: a randomized control trial. Int Forum Allergy Rhinol. 2019;9:850–856.
Funding sources for the study: Stryker ENT.
Potential conflict of interest: None provided.
Public clinical trial registration: http://clinicaltrials.gov/show/NCT03400787. Latera RCT ‐ Latera® Absorbable Nasal Implant vs. Sham Control for Lateral Nasal Valve Collapse.
References
- 1. Barrett DM, Casanueva FJ, Cook TA. Management of the nasal valve. Facial Plast Surg Clin North Am. 2016;24:219‐234. [DOI] [PubMed] [Google Scholar]
- 2. Sheen JH. Spreader graft: a method of reconstructing the roof of the middle nasal vault following rhinoplasty. Plast Reconstr Surg. 1984;73:230‐239. [PubMed] [Google Scholar]
- 3. Goode RL. Surgery of the incompetent nasal valve. Laryngoscope. 1985;95:546‐555. [DOI] [PubMed] [Google Scholar]
- 4. Most SP. Anterior septal reconstruction: outcomes after a modified extracorporeal septoplasty technique. Arch Facial Plast Surg. 2006;8:202‐207. [DOI] [PubMed] [Google Scholar]
- 5. Surowitz J, Lee MK, Most SP. Anterior septal reconstruction for treatment of severe caudal septal deviation: clinical severity and outcomes. Otolaryngol Head Neck Surg. 2015;153:27‐33. [DOI] [PubMed] [Google Scholar]
- 6. Clark DW, Del Signore AG, Raithatha R, Senior BA. Nasal airway obstruction: prevalence and anatomic contributors. Ear Nose Throat J. 2018;97:173‐176. [DOI] [PubMed] [Google Scholar]
- 7. San Nicoló M, Stelter K, Sadick H, Bas M, Berghaus A. Absorbable implant to treat nasal valve collapse. Facial Plast Surg. 2017;33:233‐240. [DOI] [PubMed] [Google Scholar]
- 8. San Nicoló M, Stelter K, Sadick H, Bas M, Berghaus A. A 2‐year follow‐up study of an absorbable implant to treat nasal valve collapse. Facial Plast Surg. 2018;34:545‐550. [DOI] [PubMed] [Google Scholar]
- 9. Stolovitzky P, Sidle DM, Ow RA, Nachlas NE, Most SP. A prospective study for treatment of nasal valve collapse due to lateral wall insufficiency: outcomes using a bioabsorbable implant. Laryngoscope. 2018;128:2483‐2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130:157‐163. [DOI] [PubMed] [Google Scholar]
- 11. Stewart MG, Smith TL, Weaver EM, et al. Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg. 2004;130:283‐290. [DOI] [PubMed] [Google Scholar]
- 12. Lipan MJ, Most SP. Development of a severity classification system for subjective nasal obstruction. JAMA Facial Plast Surg. 2013;15:358‐361. [DOI] [PubMed] [Google Scholar]
- 13. Rhee JS, Weaver EM, Park SS, et al. Clinical consensus statement: diagnosis and management of nasal valve compromise. Otolaryngol Head Neck Surg. 2010;143:48‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fung E, Hong P, Moore C, Taylor SM. The effectiveness of modified Cottle maneuver in predicting outcomes in functional rhinoplasty. Plast Surg Int. 2014;2014:618313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wartolowska K, Judge A, Hopewell S, et al. Use of placebo controls in the evaluation of surgery: systematic review. BMJ. 2014;348:g3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macklin R. The ethical problems with sham surgery in clinical research. N Engl J Med. 1999;341:992‐996. [DOI] [PubMed] [Google Scholar]
- 17. Powell NB, Zonato AI, Weaver EM, et al. Radiofrequency treatment of turbinate hypertrophy in subjects using continuous positive airway pressure: a randomized, double‐blind, placebo‐controlled clinical pilot trial. Laryngoscope. 2001;111:1783‐1790. [DOI] [PubMed] [Google Scholar]
- 18. Nease CJ, Krempl GA. Radiofrequency treatment of turbinate hypertrophy: a randomized, blinded, placebo‐controlled clinical trial. Otolaryngol Head Neck Surg. 2004;130:291‐299. [DOI] [PubMed] [Google Scholar]
- 19. Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53:786‐792. [DOI] [PubMed] [Google Scholar]
- 20. Wise RA, Bartlett SJ, Brown ED, et al. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol. 2009;124:436‐444.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Floyd EM, Ho S, Patel P, Rosenfeld RM, Gordin E. Systematic review and meta‐analysis of studies evaluating functional rhinoplasty outcomes with the NOSE Score. Otolaryngol Head Neck Surg. 2017;156:809‐815. [DOI] [PubMed] [Google Scholar]
- 22. Kandathil CK, Spataro EA, Laimi K, Moubayed SP, Most SP, Saltychev M. Repair of the lateral nasal wall in nasal airway obstruction: a systematic review and meta‐analysis. JAMA Facial Plast Surg. 2018;20:307‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramakrishnan JB, Danner CJ, Yee SW. The use of porous polyethylene implants to correct nasal valve collapse. Otolaryngol Head Neck Surg. 2007;136:357‐361. [DOI] [PubMed] [Google Scholar]
- 24. Winkler AA, Soler ZM, Leong PL, Murphy A, Wang TD, Cook TA. Complications associated with alloplastic implants in rhinoplasty. Arch Facial Plast Surg. 2012;14:437‐441. [DOI] [PubMed] [Google Scholar]


