Abstract
Background
Experimental autoimmune myocarditis (EAM) is a common animal model for the investigation of the pathophysiology of myocarditis. Because of diverging findings from previous studies, we performed serial echocardiographic examinations throughout the course of the disease and investigated the dimensions of the murine heart and left ventricular (LV) systolic function.
Materials and Methods
Experimental autoimmune myocarditis was induced in male Balb/c mice by subcutaneous injection of a fragment of the α‐myosin heavy chain (MyHC‐α 614‐629: Ac‐SLKLMATLFSTYASAD). Transthoracic echocardiography was performed on days 0, 7 and 21 in healthy animals and mice with EAM.
Results
Experimental autoimmune myocarditis was associated with a reduction in LV systolic function and an increase in LV internal diameter in diastole (LVIDd) and systole (LVIDs) 7 days postimmunization. After 21 days, EAM led to a significant increase in LV‐thickness (1.3‐fold increase in LV anterior wall diameter in diastole [LVAWDd]), but there was no difference in LV systolic function between immunized animals and healthy controls. LV‐thickness correlated well with the severity of myocarditis in the histopathological examination (LVAWDd: rs = 0.603, P = 0.003, LV anterior wall diameter in systole (LVAWDs): rs = 0.718, P < 0.0001).
Conclusion
Our results indicate that EAM leads to an initial dilatation of the LV that is followed by ventricular “hypertrophy.” On day 21, there was no significant difference in LV systolic function between immunized animals and controls. Furthermore, the ageing of the animals had a major impact on the echocardiographic parameters; therefore, the use of healthy age‐matched controls seems warranted when echocardiography is performed in rodents.
Keywords: Balb/c, echocardiography, experimental autoimmune myocarditis
1. INTRODUCTION
Myocarditis is a serious health issue around the world.1 Although prognosis is excellent for patients with acute myocarditis and preserved left ventricular (LV) systolic function, patients with a decreased LV systolic function at presentation had a 4‐year‐mortality rate of 56% in the Myocarditis Treatment Trial.2 Myocarditis is a common cause for sudden cardiac death (SCD), and it accounts for approximately 12% of unexplained deaths in autopsy studies.1, 3 Moreover, myocarditis can result in a deterioration of LV systolic function that may progress to dilated cardiomyopathy (DCM), which is associated with formidable symptoms and increased mortality.4
In order to investigate the pathophysiologic mechanisms behind this devastating disease, various animal models have been developed and are now frequently used in the scientific field. For example, the disease can be induced in susceptible mouse strains, such as A/J mice or Balb/c mice, by immunization with a fragment of the cardiac myosin heavy chain (MyHC‐α 614‐629) together with a strong adjuvant.5, 6 The majority of the immunized animals then develop a CD4+ cell‐mediated experimental autoimmune myocarditis (EAM) peaking 21 days after the first immunization, despite no apparent clinical symptoms of heart failure.7, 8 In addition to the histopathological examination, the effect of applied therapeutic remedies is then frequently assessed by transthoracic echocardiography (TTE), which depicts the macroscopic and functional sequelae of underlying pathophysiologic processes.9, 10 To improve the feasibility of the examination, TTE is frequently conducted under continuous anaesthesia (eg with isoflurane), which can affect haemodynamics and LV systolic function. Therefore, close monitoring of the depth of anaesthesia is essential to obtain valid and reliable results.11
Since echocardiographic parameters, such as LV systolic function, have been used as outcome parameters for interventional studies in EAM before, and previous studies reported diverging echocardiographic findings in this setting, we performed serial TTE examinations throughout the course of the disease to establish imaging standards and reference values for future examiners.
2. MATERIALS AND METHODS
All animal experiments were approved by the government of the state of Salzburg, Austria (20901‐TVG/108/22‐2018), and conducted in compliance with the Declaration of Helsinki.
2.1. Primary and secondary outcome measures
The primary outcome measure of the study was LV systolic function throughout the course of the disease.
The secondary outcome measures were other structural and functional parameters obtained by TTE, such as LV anterior wall diameter in diastole and systole (LVAWDd, LVAWDs), LV posterior wall diameter in diastole and systole (LVPWDd, LVPWDs), LV internal diameter in diastole and systole (LVIDd, LVIDs) and diastolic dysfunction.
Hereby, we wanted to establish reference values for 6‐8 weeks old Balb/c mice with and without EAM under anaesthesia with 2%‐3% isoflurane to facilitate future research in these animals.
2.2. Induction of experimental autoimmune myocarditis
Experimental autoimmune myocarditis was induced in 6‐8 weeks old male Balb/c mice by subcutaneous injection of a fragment of the α‐myosin heavy chain (MyHC‐α 614‐629: Ac‐SLKLMATLFSTYASAD, purity: 95.04%, CASLO Laboratories) on day 0 and day 7, as previously published5, 7 (Figure 1), in 12 animals. 10 animals served as controls.
Figure 1.
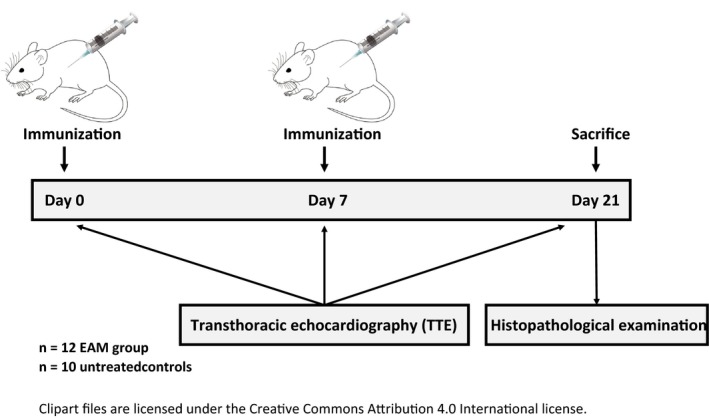
Timeline of the study
2.3. Anaesthesia and transthoracic echocardiography
Echocardiography was performed under continuous anaesthesia with 2%‐3% isoflurane using the SomnoSuite small animal anaesthesia system (Kent Scientific Corporation, USA) on days 0, 7 and 21 (Figure 1). The body temperature of the animals was regulated at 37 ± 0.5°C using a heating pad and a rectal probe. Heart rate and oxygen saturation were monitored with pulse oximetry, and depth of anaesthesia was controlled regularly by assessing the toe‐withdrawal reflex and the heart rate (the target HR was 400 ± 50 bpm).
Transthoracic echocardiography was conducted using an Alpinion E‐cube i7 (Alpinion Medical Systems Co. Ltd., Seoul, South Korea) with a IO8‐17T high‐frequency linear probe (15 MHz, Alpinion Medical Systems). Standard echo views (parasternal long axis view [PLAX], parasternal short axis view [PSAX] and apical four‐chamber view [4‐CV]) were performed as previously published.12, 13, 14 All measurements were taken from M‐mode images in the parasternal short axis view (PSAX) at the level of the papillary muscles (Figure 2). Three repeated measurements were taken of all investigated parameters.
Figure 2.
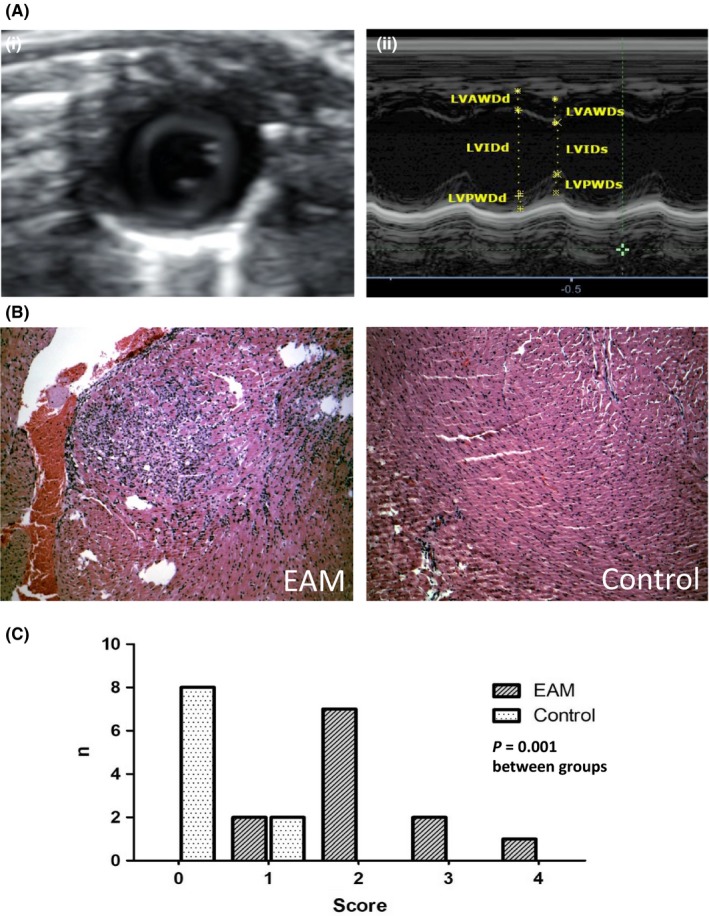
A, (i) Parasternal short axis view (PSAX) of the LV at the level of the papillary muscles. (ii) M‐mode image of the PSAX with the respective measurements; LVAWDd, LV anterior wall diameter in diastole; LVAWDs, LV anterior wall diameter in systole; LVPWDd, LV posterior wall diameter in diastole; LVPWDs, LV posterior wall diameter in systole; LVIDd, LV internal diameter in diastole; LVIDs, LV internal diameter in systole. B, Animals with EAM showed significant inflammatory lesions in the histopathological examination; haematoxylin and eosin staining of mouse myocardium, magnification of 10×. C, Histogram of the myocarditis score in both groups; 0: no inflammatory infiltrates; 1: small foci of inflammatory cells between myocytes; 2: larger foci of >100 inflammatory cells; 3: < 10% of a cross‐section involved; 4: >30% of a cross‐section involved
For the evaluation of LV systolic function (ejection fraction [EF]), the LV end‐diastolic volume (LVEDV) and the LV end‐systolic volume (LVESV) were calculated as follows15:
The LV myocardial mass was calculated as follows15:
For the evaluation of diastolic dysfunction, the transmitral inflow pattern was measured in an apical four‐chamber view (4‐CV) using pulsed wave (pw)‐doppler sonography. Normal LV filling was defined as E/A: 1‐2, diastolic dysfunction grade 1 as E/A: <1, diastolic dysfunction >2 as E/A: >2.14
2.4. Preparation of paraffin sections and histopathological examination
Animals were sacrificed 21 days after EAM induction. Immediately after sacrifice, hearts were excised and cut along the short axis of the LV at a mid‐ventricular level. After fixation in 4% formaldehyde overnight, the tissue was prepared for paraffin sections and stained with haematoxylin and eosine.
The acquired sections were analysed on a Leica DM2000 microscope (Leica Biosystems, Nussloch, Germany) at a magnification of 1.6×, 10×, 20× and 40×. The presence of myocarditis was graded according to a semi‐quantitative scale as previously published (0: no inflammatory infiltrates; 1: small foci of inflammatory cells between myocytes; 2: larger foci of >100 inflammatory cells; 3: < 10% of a cross‐section involved; 4:> 30% of a cross‐section involved).7, 16
Measurements of the cross‐sectional area (CSA) of cardiomyocytes were performed in representative areas of the histological sections and not directly in the regions of the inflammatory infiltrate. Image analysis was conducted using ImageJ software (ImageJ, Wayne Rasband).
2.5. Statistical analyses
Statistical analyses were performed using SPSS (version 24.0, IBM Corp., Armonk, NY, USA) and GraphPad Prism (GraphPad Software, San Diego, CA, USA). Due to the sample size, continuous variables were expressed as median and interquartile range (25%‐75%‐quartile) and a Mann‐Whitney U test was used to compare medians between the groups. A Wilcoxon signed‐rank test was applied for the comparison of paired observations, and a Fisher´s exact test was applied for the comparison of categorical data. In order to further explore the association between the severity of myocarditis and echocardiographic parameters, correlation analysis was performed (using Spearman's correlation coefficient). A two‐sided P‐value < 0.05 was considered to be statistically significant.
3. RESULTS
In total, we investigated 22 6‐8 weeks old male Balb/c mice in this study. Of these, 12 animals constituted the EAM group and 10 the control group. No animal died before the sacrifice on day 21.
3.1. Results from the histopathological examination
The presence of myocarditis was confirmed by significantly more prominent inflammatory infiltrates in animals from the EAM group (semi‐quantitative scale: EAM 2.17 ± 0.83 vs controls 0.2 ± 0.42, P = 0.001, Figure 2).
There was no statistically significant difference in the cross‐sectional area (CSA) of cardiomyocytes between animals with EAM and healthy controls (EAM 444 µm2 vs control 439 µm2, P = 0.869, Figure S1).
3.2. Echocardiographic findings
At day 0, the median ejection fraction (EF) of animals in the EAM group was 74.6% (IQR = 66.02‐81.8), the median LVIDd was 3.0 mm (IQR = 2.8‐3.3) and the median LV mass was 92.3 mg (IQR = 81.9‐142.9). There was no statistically significant difference in EF or LV mass between animals in the EAM group and the control group prior to immunization, but interestingly, controls had a slightly larger LVIDd at day 0 (Table 1).
Table 1.
Echocardiographic findings in both groups at day 0
| DAY 0 | EAM (n = 12) | Control (n = 10) | P‐value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| LVIDd (mm) | 3.0 | 2.8‐3.3 | 3.3 | 3.1‐3.6 | 0.011 |
| LVIDs (mm) | 1.8 | 1.4‐2.0 | 2.0 | 1.9‐2.2 | 0.050 |
| EF (%) | 74.6 | 66.0‐81.8 | 73.0 | 66.5‐77.3 | 0.382 |
| LV‐mass (mg) | 92.3 | 81.9‐142.9 | 98.3 | 82.9‐138.0 | 0.862 |
| (n = 9) | % | (n = 7) | % | ||
|---|---|---|---|---|---|
| Diastolic dysf.: 0 | 4 | 44.44 | 5 | 71.25 | 0.351 |
| 1 | 2 | 22.22 | 0 | 0 | |
| >2 | 3 | 33.33 | 2 | 28.57 |
Throughout the study, the LV systolic function decreased significantly (EAM: median EF 65.2% at day 7 and median EF 62.3% at day 21, P < 0.0001), whereas the LVIDd, the LV internal diameter in systole (LVIDs) and the LV mass increased significantly in both investigated groups (Figure 3 and Table 2).
Figure 3.
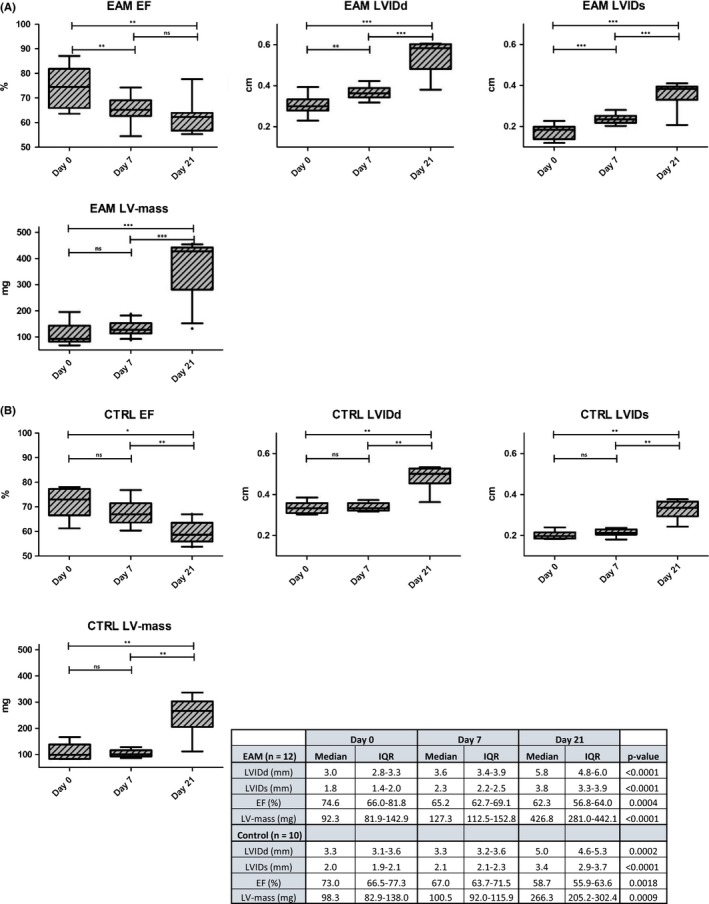
Echocardiographic findings in animals from the (A) EAM group and the (B) control group throughout the study; EF = ejection fraction
Table 2.
Echocardiographic findings in both groups at day 7 and day 21
| DAY 7 | EAM (n = 12) | Control (n = 10) | P‐value | DAY 21 | EAM (n = 12) | Control (n = 10) | P‐value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | ||||
| LVIDd (mm) | 3.6 | 3.4‐3.9 | 3.3 | 3.2‐3.6 | 0.028 | LVIDd (mm) | 5.8 | 4.8‐6.0 | 5.0 | 4.6‐5.3 | 0.0515 |
| LVIDs (mm) | 2.3 | 2.2‐2.5 | 2.1 | 2.1‐2.3 | 0.041 | LVIDs (mm) | 3.8 | 3.3‐3.9 | 3.4 | 2.9‐3.7 | 0.0443 |
| EF (%) | 65.2 | 62.7‐69.1 | 67.0 | 63.7‐71.5 | 0.508 | EF (%) | 62.3 | 56.8‐64.0 | 58.7 | 55.9‐63.6 | 0.4483 |
| LV‐mass (mg) | 127.3 | 112.5‐152.8 | 100.4 | 92.0‐115.8 | 0.012 | LV‐mass (mg) | 426.8 | 281.0‐442.1 | 266.3 | 205.2‐302.4 | 0.0161 |
| (n = 11) | % | (n = 8) | % | (n = 11) | % | (n = 10) | % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diastolic dysf.: 0 | 4 | 36.36 | 3 | 37.5 | 0.943 | Diastolic dysf.: 0 | 5 | 45.45 | 4 | 40 | 0.969 |
| 1 | 5 | 45.45 | 4 | 50 | 1 | 2 | 18.18 | 2 | 20 | ||
| >2 | 2 | 18.18 | 1 | 12.5 | >2 | 4 | 36.36 | 4 | 40 |
In contrast to the controls, we observed a statistically significant change in LVIDd (median 3.0 mm vs 3.6 mm, P = 0.0068), LVIDs (median 1.8 mm vs 2.3 mm, P = 0.0005), EF (median 74.6% vs 65.2%, P = 0.0034) and fractional shortening (FS; median 42.7% vs 35.2%, P = 0.0093) in mice with EAM between day 0 and day 7 (Figure 3).
Consequently, animals in the EAM group had a significantly larger LVIDd (median 3.6 mm vs 3.3 mm, P = 0.028) and LVIDs (median 2.3 mm vs 2.1 mm, P = 0.041) and the LV mass was significantly higher (median 127.3 mg vs 100.4 mg, P = 0.012) than in the control group at day 7. Moreover, LV systolic function was lower in animals from the EAM group, yet this did not reach statistical significance (median EF 67% in controls vs 65.2% in EAM, P = 0.508, Table 2).
At day 21, there was a significant difference in LV‐thickness (LV anterior diameter in diastole [LVAWDd] median in EAM 0.76 mm (IQR = 0.65‐0.82) vs control 0.6 mm (IQR = 0.52‐0.65), P = 0.0017, 1.3 fold change; LVAWDs: median in EAM 1.2 mm (IQR = 1.1‐1.3) vs control 1.0 mm (IQR = 0.8‐1.02), P = 0.0044), LV mass (median in EAM 426.8 mg (IQR = 281.0‐442.1) vs control 266.3 mg (IQR = 205.2‐302.4), P = 0.0161) and LVIDs, but there was no statistically significant difference in LVIDd or EF between the two investigated groups (Table 2). Similarly to LVAWDs, we observed a larger LV posterior diameter in systole (LVPWDs) in mice with EAM, yet, this finding remained statistically insignificant.
Furthermore, we found a significant correlation of LV‐thickness on day 21 with the extent of myocarditis in the histopathological examination (LVAWDd rs = 0.603, P = 0.003; LVAWDs rs = 0.718, P < 0.0001; LVPWDs rs = 0.553, P = 0.0076, Table 3). Notably, there was no significant correlation between systolic LV function, FS or posterior wall thickening (PWT) and the extent of myocarditis. Moreover, there was no statistically significant difference in diastolic dysfunction between animals in the EAM group and animals in the control group at neither of the three echo‐examinations.
Table 3.
Correlation analysis between the extent of myocarditis (graded by semi‐quantitative myocarditis severity scale) and the echocardiographic findings on day 21
| DAY 21 | LVIDd | LVIDs | LVPWDd | LVPWDs | LVAWDd | LVAWDs | LV mass | E/A | PWT | FS | EF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs | 0.3158 | 0.1698 | 0.2868 | 0.553 | 0.603 | 0.718 | 0.4076 | 0.1450 | 0.3958 | 0.3451 | 0.3195 |
| P‐value | 0.1521 | 0.4498 | 0.1955 | 0.0076 | 0.0030 | 0.0001 | 0.0596 | 0.5305 | 0.0682 | 0.1157 | 0.1471 |
Abbreviation: rs = correlation coefficient.
4. DISCUSSION
Experimental autoimmune myocarditis in susceptible mouse strains is amongst the most commonly used animal models for the investigation of the pathophysiologic mechanisms behind myocarditis and its progress to DCM. In this study, we performed serial echocardiographic examinations of Balb/c mice with EAM, because previous studies reported diverging findings regarding LV systolic function in immunized animals. Hereby, we wanted to establish reference values for future examiners who use TTE in this animal model.
Interestingly, we found that EAM leads to a significant change in the LV internal diameter in diastole (LVIDd), the LV internal diameter in systole (LVIDs) and LV systolic function in the first week after the immunization, whereas an increase in LV‐thickness (1.3‐fold increase in LV anterior wall diameter in diastole [LVAWDd]) seems to be the predominating pathology 3 weeks postimmunization. These results indicate an initial dilatation of the left ventricle that is followed by ventricular “hypertrophy.” Since we did not find a difference in the cross‐sectional area (CSA) of cardiomyocytes between animals with EAM and healthy controls, the increase in LV‐thickness most likely results from inflammatory oedema or changes in the extracellular matrix.17, 18, 19 This assumption is strengthened by the significant correlation between LV‐thickness and the extent of myocarditis in the histopathological examination.
Our results from day 21 are well in line with the results of a recent study by Pistulli et al, who found that EAM is associated with LV‐hypertrophy and diastolic dysfunction, but not with a deterioration in LV systolic function, when immunized animals are compared to healthy age‐matched controls.20 Interestingly, various previous studies reported little or no decrease in LV systolic function in EAM,21, 22 whereas others found a significant systolic dysfunction.23, 24
In humans, myocarditis can result in a variety of echocardiographic findings including decreased LV systolic function, LV‐hypertrophy, diastolic dysfunction or regional wall motion abnormalities.3, 25, 26 Interestingly, Felker et al found significant differences between the clinical syndromes of fulminant and acute myocarditis that portray different courses of the same disease. According to the authors, patients who suffer from acute myocarditis tend to show LV dilatation and a significantly reduced LV systolic function at the time of presentation, whereas patients with the fulminant course of the disease present with LV‐hypertrophy and a mildly reduced systolic function.27 Considering the diverging echocardiographic findings from previous studies, we speculate that there might be similar “syndromes” in mice with EAM that portray different courses of the autoimmune disease. Interestingly, the authors of the studies reported that little or no decrease in LV systolic function in EAM (Pistulli et al and Miyawaki et al20, 22) used the same amino acid sequence (MyHC‐α 614‐629: Ac‐SLKLMATLFSTYASAD) for the immunization of the animals that was used in our study. Perhaps the amino acid sequence of the peptide has an influence on the underlying pathophysiologic processes, and hence on the course of the disease, which is further displayed in the findings upon TTE.10 This hypothesis could be investigated in future studies by comparing the effects of different peptides that can be used for the induction of EAM.6, 28
Although we found a significant decrease in LV systolic function in animals with EAM throughout the study, there was also a significant decline in LV systolic function in animals from the control group, which was accompanied by an increase in LVIDd, LVIDs and LV mass. We speculate that the aforementioned changes in the control group can be attributed to the growth and ageing of the animals during the 3 weeks of investigation. These findings highlight the necessity of using age‐matched controls when echocardiographic examinations are performed in rodents. Hence, the sole use of the trend of LV systolic function throughout EAM, without the use of a proper control group, could lead to erroneous interpretations of the acquired results. For upcoming studies, we herein provide detailed echocardiographic measurements for healthy male 6‐8 weeks old Balb/c mice under anaesthesia with 2%‐3% isoflurane that can be used as a reference in the future.
Concluding, LV systolic function appears to be an unsatisfactory outcome parameter for the evaluation of the effect of applied therapeutic remedies in EAM, because there is no significant difference at day 21 between animals with EAM and healthy age‐matched controls. In contrast, evaluating LV‐thickness upon TTE could be a suitable additional parameter to the histopathological examination in this implication. Moreover, the use of age‐matched controls is necessary for a valid interpretation of echocardiographic findings in rodents.
5. CONCLUSION
Experimental autoimmune myocarditis (EAM) leads to a significant change in LVIDd, LVIDs and LV systolic function in the first week after the immunization, whereas ventricular “hypertrophy” seems to be the predominating pathology 3 weeks postimmunization. Interestingly, EAM did not result in a significant difference in LV systolic function 3 weeks postimmunization when immunized animals were compared to healthy controls. Of note, we found significant changes in LV systolic function, LV mass and the dimensions of the heart in animals from the control group throughout the study—findings that can be attributed to the growth of the animals. These results highlight the necessity of age‐matched controls when echocardiographic examinations are performed in rodents.
6. LIMITATIONS
A limitation of our study is the fact that TTE was performed under anaesthesia with isoflurane. Isoflurane has an impact on hemodynamics and LV systolic function, which can lead to erroneous measurements, especially when the depth of anaesthesia is insufficiently monitored. Since the majority of published studies regarding TTE in mice with EAM was conducted under anaesthesia with isoflurane, and we aimed at setting reference values for this examination, we chose to use isoflurane in our study as well. Examiners should consider the possibility of performing TTE in trained conscious mice to exclude this possible impact.
Another limitation of our study is the low number of investigated animals, which can be explained by ethical issues associated with animal experiments. We plan to conduct further studies in order to confirm and strengthen our present findings.
Lastly, the study protocol was nonrandomized and TTE examinations were not blinded. Although TTE was performed according to published recommendations and after a standard operating procedure (SOP), a bias can therefore not be excluded definitely.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests regarding the publication of this paper.
Supporting information
ACKNOWLEDGEMENT
This study was supported by the PMU‐FFF research fund (Paracelsus Medical University, R‐17/04/099‐MIR).
Mirna M, Paar V, Kraus T, et al. Autoimmune myocarditis is not associated with left ventricular systolic dysfunction. Eur J Clin Invest. 2019;49:e13132 10.1111/eci.13132
REFERENCES
- 1. Cooper LT, Keren A, Sliwa K, Matsumori A, Mensah GA. The global burden of myocarditis: part 1: A systematic literature review for the global burden of diseases, injuries, and risk factors 2010 study. Glob Heart. 2014;9(1):121‐129. [DOI] [PubMed] [Google Scholar]
- 2. Mason JW, O'Connell JB, Herskowitz A, et al. A Clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med. 1995;333(5):269‐275. [DOI] [PubMed] [Google Scholar]
- 3. Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis. 2010;52(4):274‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D’Ambrosio A, Patti G, Manzoli A, et al. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart. 2001;85(5):499‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Myers JM, Fairweather D, Huber SA, Cunningham MW, Myocarditis V. Autoimmune myocarditis, valvulitis, and cardiomyopathy. Curr Protoc Immunol. 2013;101(1):15.14.1‐15.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pummerer CL, Luze K, Grässl G, et al. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97(9):2057‐2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoetzenecker K, Zimmermann M, Hoetzenecker W, et al. Mononuclear cell secretome protects from experimental autoimmune myocarditis. Eur Heart J. 2015;36(11):676‐685a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith SC, Allen PM. Myosin‐induced acute myocarditis is a T cell‐mediated disease. J Immunol. 1991;147(7):2141‐2147. [PubMed] [Google Scholar]
- 9. Massilamany C, Khalilzad‐Sharghi V, Gangaplara A, Steffen D, Othman SF, Reddy J. Noninvasive assessment of cardiac abnormalities in experimental autoimmune myocarditis by magnetic resonance microscopy imaging in the mouse. J Vis Exp. 2014;88:e51654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peter AK, Bradford WH, Dalton ND, et al. Increased echogenicity and radiodense foci on echocardiogram and MicroCT in murine myocarditis. PLoS ONE. 2016;11(8):e0159971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phoon C, Turnbull DH. Cardiovascular Imaging in Mice. Curr Protoc Mouse Biol. 2016;6(1):15‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baudouy D, Michiels J‐F, Vukolic A, Wagner K‐D, Wagner N. Echocardiographic and Histological Examination of Cardiac Morphology in the Mouse. J Vis Exp. 2017;(128):e55843, 10.3791/55843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Respress JL, Wehrens X. Transthoracic echocardiography in mice. J Vis Exp. 2010;28(39). 10.3791/1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao S, Ho D, Vatner DE, Vatner SF. Echocardiography in Mice. Curr Protoc Mouse Biol. 2011;1:71–83. 10.1002/9780470942390.mo100130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stypmann J, Engelen MA, Troatz C, Rothenburger M, Eckardt L, Tiemann K. Echocardiographic assessment of global left ventricular function in mice. Lab Anim. 2009;43(2):127‐137. [DOI] [PubMed] [Google Scholar]
- 16. Eriksson U, Kurrer MO, Schmitz N, et al. Interleukin‐6‐deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107(2):320‐325. [DOI] [PubMed] [Google Scholar]
- 17. Papageorgiou A‐P, Heymans S. Interactions between the extracellular matrix and inflammation during viral myocarditis. Immunobiology. 2012;217(5):503‐510. [DOI] [PubMed] [Google Scholar]
- 18. Escher F, Tschöepe C, Lassner D, Schultheiss H‐P. Myocarditis and inflammatory cardiomyopathy: from diagnosis to treatment. Turk Kardiyol Dern Arsivi‐Archives Turkish Soc Cardiol. 2015;43(8):739‐748. [DOI] [PubMed] [Google Scholar]
- 19. Calabrese F, Thiene G. Myocarditis and inflammatory cardiomyopathy: microbiological and molecular biological aspects. Cardiovasc Res. 2003;60(1):11‐25. [DOI] [PubMed] [Google Scholar]
- 20. Pistulli R, Quitter F, Andreas E, et al. Intravital microscopy ‐ a novel tool in characterizing congestive heart failure in experimental autoimmune myocarditis. Clin Hemorheol Microcirc. 2015;63(2):153‐162. [DOI] [PubMed] [Google Scholar]
- 21. Maisel A, Cesario D, Baird S, Rehman J, Haghighi P, Carter S. Experimental autoimmune myocarditis produced by adoptive transfer of splenocytes after myocardial infarction. Circ Res. 1998;82(4):458‐463. [DOI] [PubMed] [Google Scholar]
- 22. Miyawaki A, Mitsuhara Y, Orimoto A, et al. Moesin is activated in cardiomyocytes in experimental autoimmune myocarditis and mediates cytoskeletal reorganization with protrusion formation. Am J Physiol Circ Physiol. 2016;311(2):H476‐H486. [DOI] [PubMed] [Google Scholar]
- 23. Zhang S, Liu X, Sun C, et al. Apigenin attenuates experimental autoimmune myocarditis by modulating th1/th2 cytokine balance in mice. Inflammation. 2016;39(2):678‐686. [DOI] [PubMed] [Google Scholar]
- 24. Hu F, Yan L, Lu S, et al. Effects of 1, 25‐dihydroxyvitamin d3 on experimental autoimmune myocarditis in mice. Cell Physiol Biochem. 2016;38(6):2219‐2229. [DOI] [PubMed] [Google Scholar]
- 25. Bami K, Haddad T, Dick A, Dennie C, Dwivedi G. Noninvasive imaging in acute myocarditis. Curr Opin Cardiol. 2016;31(2):217‐223. [DOI] [PubMed] [Google Scholar]
- 26. Angelini A, Calzolari V, Calabrese F, et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart. 2000;84(3):245‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Felker GM, Boehmer JP, Hruban RH, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36(1):227‐232. [DOI] [PubMed] [Google Scholar]
- 28. Wegmann KW, Zhao W, Griffin AC, Hickey WF. Identification of myocarditogenic peptides derived from cardiac myosin capable of inducing experimental allergic myocarditis in the Lewis rat. The utility of a class II binding motif in selecting self‐reactive peptides. J Immunol. 1994;153(2):892‐900. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


