Abstract
The clinical experience with cell replacement therapy for advanced PD has yielded notable successes and failures. A recent autopsy case report of an individual that received implants of fetal dopamine neurons 16 years previously, but at no time experienced clinical benefit despite the best documented survival of grafted neurons and most extensive reinnervation of the striatum, raises sobering issues. With good reason, a great deal of effort in cell replacement science continues to focus on optimizing the cell source and implantation procedure. Here, we describe our preclinical studies in aged rats indicating that despite survival of large numbers of transplanted dopamine neurons and dense reinnervation of the striatum, synaptic connections between graft and host are markedly decreased and behavioral recovery is impaired. This leads us to the hypothesis that the variability in therapeutic response to dopamine neuron grafts may be less about the viability of transplanted neurons and more about the integrity of the aged, dopamine‐depleted striatum and its capacity for repair. Replacement of dopamine innervation only can be fully effective if the correct target is present. © 2019 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Keywords: aging, cell transplantation, dopamine, Parkinson's disease, striatum
The clinical experience with cell replacement therapy for Parkinson's disease (PD) is filled with conflicting results ranging from significant, sustained benefit to no effect, to a noteworthy incidence of graft‐induced side effect.1, 2 Taken together, these findings have resulted in a prudent moratorium in implementing the approach in the clinical setting, with a few recent exceptions.3 In this opinion piece, we will use findings associated with implantation of fetal ventral mesencephalic tissue that contains the developing midbrain dopamine (DA) neurons, in both animals and humans, as the most abundant literature on the topic. We discuss what is known about integration of transplanted cells, drawing attention to the overlooked contribution of the integrity of the target striatum as a limiting factor in therapeutic efficacy.
What We Know
Dopamine Neuron Grafts in Animal Models of Parkinsonism
For more than 35 years, the properties of transplanted fetal DA neurons have been documented in rodent4, 5 and nonhuman primate6, 7 models of parkinsonism. In nearly all cases, cell implants have been studied in the context of grafting to the DA‐depleted striatum generated by the neurotoxic effects of 6‐hydroxydopamine or MPTP. These models reproduce the environment of severe depletion of striatal DA that is a key feature of the motor symptoms of disease, but do not adequately model nonmotor symptoms refractory to DA replacement. Accordingly, cell transplantation studies have focused on motor symptoms of PD. In addition, the majority of studies were conducted using young adult animals as transplant recipients. Aging remains the primary risk factor for PD, and, arguably, studies of neural grafting in young adult animals may have fostered an overly optimistic view of potential therapeutic efficacy in a largely aged human clinical population. These preclinical studies support the concepts that: (1) fetal DA neurons survive the transplantation procedure.4, 5, 6, 7 (2) Grafted DA neurons send axonal projections into the surrounding host striatum.4, 5, 6, 7 (3) Transplanted DA neurons increase levels of DA in the surrounding striatum.8, 9 (4) Grafted DA neurons express some, but not all, of the electrophysiological characteristics of these neurons in the adult brain.10 Differences likely reflect the immaturity of grafted neurons and their ectopic placement. (5) Electron microscopic images indicate that transplanted DA neurons form synaptic connections with the host striatum that are atypical.11, 12, 13 The vast majority of normal striatal DA innervation forms en passant nonsynaptic associations with striatal medium spiny neurons (MSNs) with sparse synaptic profiles that are almost exclusively “symmetric” and on the necks of dendritic spines. Contacts made by grafted DA neurons shift dramatically to many more synaptic contacts with dendritic shafts or the cell soma, with a high percentage of these making “asymmetric” contacts.12 (6) Transplanted DA neurons diminish abnormalities in motor behavior associated with the models.4, 5, 14, 15 In grafted rats, simple asymmetric motor abnormalities, such as rotational behavior, readily are corrected, whereas more complex behaviors requiring fine motor control and decision making are impacted less. In summary, DA neuron grafting in animal models of parkinsonism indicate that transplanted cells survive in sufficient numbers, send axonal projections into the target striatum, synthesize and release DA, and correct many of the sensorimotor abnormalities associated with the models. The biggest disconnect between DA neurons in situ and grafted DA neurons is the structure and organization of their synaptic interactions with striatal MSNs.
Dopamine Neuron Grafts in Persons With PD
Whereas the number of studies and variety of endpoints examined are necessarily limited in human studies, the conclusions are similar to those in animal studies.16, 17, 18 Relevant to this discussion, an ultrastructural analysis conducted by Kordower and colleagues16 on grafts in PD patients found that graft‐derived synaptic contacts onto host striatal neurons exhibited atypical contacts similar to those found in animal studies: a shift from primarily dendritic spine contacts of symmetric morphology (suggestive of inhibitory signaling), to contacts with dendritic shafts with asymmetric morphology (suggestive of excitatory signaling). In addition, it has been documented that immature nigrostriatal DA axon terminals (postnatal day 15 in rats), likely similar to grafted neurons, show double labeling for tyrosine hydroxylase (TH) and vesicular glutamate transporter 2, suggesting corelease of dopamine and glutamate.19
Two major differences from animal studies emerged in PD clinical trials. First, 15% to 56% of transplant recipients developed a novel form of aberrant motor behavior classified as graft‐induced dyskinesia (GID).17, 18 This topic is beyond the scope of the present discussion, but is covered in several reviews.20, 21 Second, blinded, controlled clinical trials revealed substantial variability in the clinical response to transplantation. The two trials funded by the National Institutes of Health failed to achieve their primary endpoints, but drew secondary conclusions: (1) Older individuals experienced less benefit than younger individuals17; 2) individuals with more severe disease benefited less than those with milder disease.22 Arguably these features may go hand‐in‐hand and draw attention to a potentially critical role for the aged, severely DA depleted striatum in the therapeutic response to cell therapy.
GAPS & Controversies
Aging Limits the Ability to Repair the Parkinsonian Brain
In 1999, we published a report on fetal DA neuron transplantation in rats, asking the question “Does the chronological age of the transplant recipient, and/or the length of time the striatum was DA‐depleted, impact the viability and function of the transplanted neurons?”23 We found that the age of the host at the time of transplantation (4, 17, and 24 months of age; mean life span for this strain = 24 months), and not duration of DA depletion (1 or 14 months), was the primary determinant of the behavioral response to DA neuron grafting. In this study, we held the number of transplanted cells constant at 120,000, while varying the age of the transplant recipient. Our behavioral readout was rotational behavior in response to administration of amphetamine. As discussed previously, this behavior readily recovers in response to cell transplantation, but is sensitive to graded increases in striatal DA leading to its use in multiple studies. That being said, improvement in this asymmetric behavior is unlikely to translate to significant therapeutic benefit in patients, and, over time, the preclinical toolbox for assessing motor behavior in rodents has increased significantly in sophistication. With this limitation in mind, we found that young adult rats exhibited complete amelioration of this behavior by 3 weeks postgrafting, middle‐aged animals achieved 45% to 55% recovery at 9 weeks postgrafting, and old‐aged rats exhibited a nonsignificant 8% to 21% recovery at 9 weeks (total duration of the experiment). The behavioral benefit produced by DA neuron transplants in animals of varying chronological age corresponded to the relative survival of grafted neurons: approximately 50% for middle‐aged rats and 20% for old‐aged rats as compared to cell survival in young rats. These findings suggested that reduced survival of grafted neurons in the aging brain could be responsible for limited behavioral recovery.
With this hypothesis in mind, our subsequent report examined the capacity of the aged, parkinsonian striatum to support grafted cell survival that would result in behavioral benefit by increasing the number of grafted DA neurons in aged rats.24 Accordingly, young, middle‐aged, and aged parkinsonian rats received grafts with proportionately increasing numbers of embryonic ventral mesencephalic cells to evaluate whether limitations of the graft environment in subjects of advancing age could be offset by increased numbers of grafted neurons. We reasoned that if survival of grafted neurons was the primary factor influencing behavioral recovery in aged hosts, then equal numbers of surviving grafted DA neurons should provide equal behavioral recovery, irrespective of graft recipient age. Thus, young parkinsonian rats were grafted with 1× cells, middle‐aged rats with 2× cells, and aged rats with 5× cells. Grafts were allowed to mature and DA‐responsive behavioral deficits were assessed, including reversal of rotational behavior and, in addition, effects on a more complex repertoire of behavior, levodopa‐induced dyskinesias (LIDs). To our surprise, grafting larger numbers of DA neurons in rats of advancing age did not yield equivalent survival across age groups, but far exceeded our prediction. We found that compared to young rats, the middle‐aged rats showed roughly twice as many surviving grafted DA neurons, and the aged rats almost 5 times as many and twice the graft‐derived neurite outgrowth into the parkinsonian striatum compared to young rats (Fig. 1). In retrospect, this outcome was not entirely unexpected based on our previous finding that increased concentration of grafted cells was associated with increased survival in young rats, presumably related to an autocrine neurotrophic effect of an increased density of grafted DA neurons.25 Despite the extensive TH+ fiber outgrowth and large numbers of grafted DA neurons in middle‐aged and aged rats, behavioral benefit in these older animals showed mixed results. Whereas animals of all age groups now exhibited complete recovery of amphetamine rotational behavior, recovery of behavior associated with LID was delayed compared to the young rats and, for some individual attributes of these more complex behaviors, completely unchanged in aged rats (Fig. 2). We detected a morphological correlate to the aging‐related impairment in graft‐induced behavioral recovery. Using dual‐label immunocytochemistry for TH as a marker of fibers from transplanted cells reinnervating the striatum and synaptopodin as a marker for postsynaptic dendritic spines on host brain striatal MSNs, we found a dramatic decline in the number of contacts associated with grafting in the aged brain. When expressed as the number of apparent synaptic appositions made per surviving grafted DA neuron, both middle‐aged and old‐aged animals displayed a decrease of approximately 75% compared to young hosts, with this morphological index correlating with behavioral improvement (Fig. 3). Taken together, our findings indicate that variable beneficial effects of DA grafts in aging subjects is less about the number of surviving grafted cells and more about the capacity of grafted cells to integrate with the environment of the aged, DA‐depleted striatum.
Figure 1.
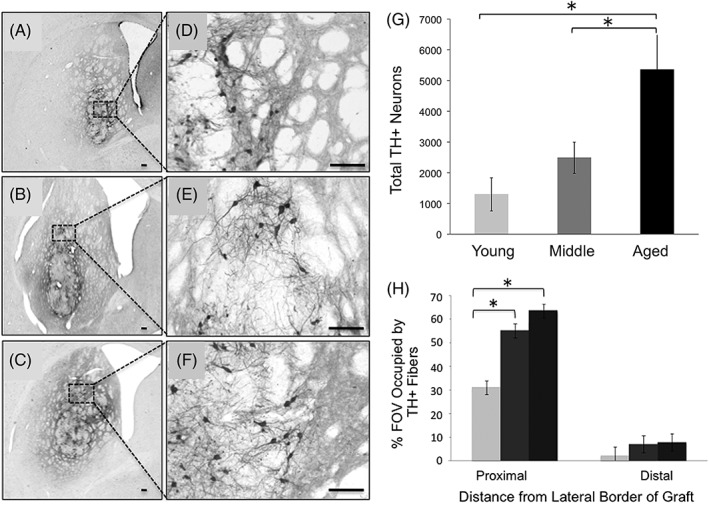
The aged brain is capable of supporting survival of large numbers of grafted DA neurons and extensive graft‐derived neurite outgrowth. (A,D) DA graft in young rat; (B,E) DA graft in middle‐aged rat; and (C,F) DA graft in aged rat. (G) There are significantly more grafted TH+/DA neurons in the aged brain, (H) with significantly more TH+ fibers in the middle‐aged and aged striatum proximal to the graft compared to young striatum. Proximal: 0–850 μm from the graft border; distal: 850–1,700 μm from the graft border. Asterisks denote significant differences (P < 0.05) for indicated comparison. Images modified from Collier and colleagues.24
Figure 2.
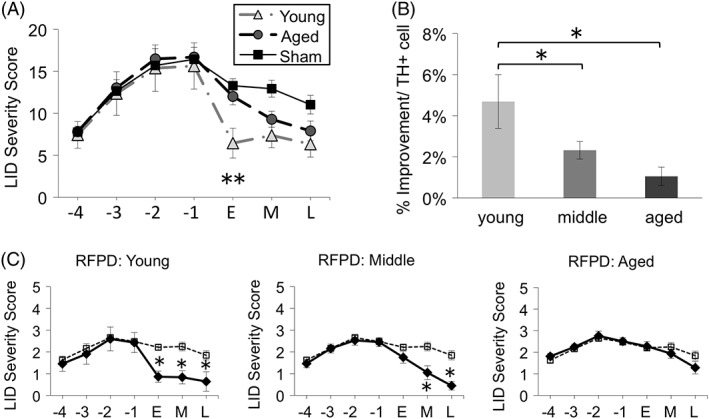
Behavioral impact of DA grafts decreases with increasing age of the host. DA grafts decrease severity of LID (y‐axis; A,B). (A) Although the aged brain is capable of supporting survival of large numbers of grafted DA neurons and extensive reinnervation of the striatum (see Fig. 1), improvement in LID behaviors is delayed and/or inferior in middle‐aged and aged parkinsonian rats compared to young. (B) Behavioral improvement on a per grafted DA cell basis (%Improvement/TH+ cell) is significantly less in middle‐aged and aged rats compared to young. (C) This is most evident when individual attributes of LID behaviors are examined. Shown here is the hyperkinetic and dystonic profile referred to as right forepaw dyskinesia (RFPD). Solid line indicates grafted subjects, dashed line indicates sham‐grafted subjects. x‐axis for (A) and (C): “‐4, ‐3, ‐2, ‐1” are pregrafting time points (in weeks) where l‐dopa priming occurred; “E” early time point = 2 to 4 weeks postgrafting; “M” middle time point = 6 to 8 weeks postgrafting; and “L” late time point =10 to 11 weeks postgrafting. In (A) and (B), asterisk (“*”) denotes significant differences (p < 0.05) at indicated time point for sham versus graft groups; in (C) asterisk (“*”) denotes significant differences indicated. Images modified from Collier and colleagues.24
Figure 3.
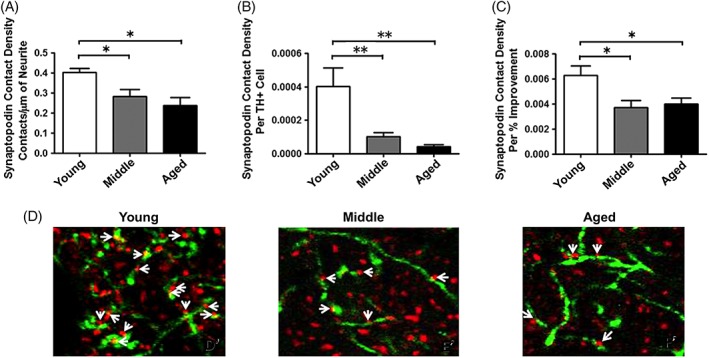
There are fewer putative synaptic contacts formed between graft‐derived TH+ neurites and synaptopodin (SP)+ dendritic spines of host MSNs in rats of more advanced age compared to young rats. SP is an actin‐binding protein located in the spine neck of MSNs. We used dual‐label fluorescence immunohistochemistry, using antibodies against TH and synaptopodin, and confocal microscopy to estimate the degree to which grafted cells in rats of varying ages were making putative synaptic connection with MSNs. Despite 5× more grafted TH+ neurons, there are significantly fewer TH/SP appositions in the aged, parkinsonian brain (A,B,D), and these appositions correlate with the degree of behavioral improvement (C). (D) TH+ neurites = green; SP+ dendritic spines = red. The asterisk (“*”) denotes P < 0.05 for the indicated comparisons. Image modified from Collier and colleagues.24 [Color figure can be viewed at wileyonlinelibrary.com]
This preclinical data are reminiscent of the autopsy results reported in a clinical case report of a PD individual that received fetal DA neuron grafts 16 years preceding death.26
This report provided postmortem histology showing the largest number of surviving DA neurons and the densest, most widespread, graft‐derived reinnervation following a transplant procedure reported to date. Despite this, there was no clinical recovery at any time over the 16 years after grafting. The question of why viable, healthy‐appearing DA neurons that seem to provide significant replacement of DA terminals throughout the surrounding striatum failed to provide motor benefit in this individual remains unanswered. However, the observations suggest that the lack of clinical benefit likely was not attriubuted to problems with the graft, per se, but rather to host‐associated pathology such as that described here that influence the ability of grafted DA neurons to remodel or restructure the parkinsonian striatum.
Striatal Neuron Architecture in PD and Aging
Our recent data suggest that inferior behavioral recovery in aged parkinsonian rats is associated with inferior integration between graft and host24 (Fig. 3). A likely contributing factor is the documented decrease in dendritic spine density on striatal MSNs associated with PD,27 DA depletion,28, 29 and aging itself.29 Dendritic spines are the structural element upon which cortical glutamate and nigral DA afferent signaling are integrated, a process that is necessary for normal motor behavior. Whereas spine loss related to DA depletion can be prevented pharmacologically with CaV1.3 calcium channel antagonists,28, 30 recent data from our laboratory show that aging‐related spine loss is not compensated for through this mechanism31 (Fig. 4). These data reveal that the aged “normal” striatum has the same density of dendritic spines as the young DA‐depleted striatum. Striatal DA depletion in aged rats further reduces the density of spines. Treatment with the CaV1.3 calcium channel antagonist, nimodipine, restores spine density related to DA depletion in both young and aged animals, but has no impact on spine loss specifically associated with aging itself.31 As discussed previously, studies have demonstrated that loss of dendritic spines in the DA‐depleted striatum is associated with abnormal synaptic appositions between grafted DA neurons and host MSNs11, 12, 13 and that preventing spine loss associated with DA depletion can enhance graft function.30 This leads us to the hypothesis that the variability in therapeutic response to DA neuron grafts may be less about the viability of transplanted neurons, and more about the integrity of the aged, DA‐depleted striatum. It is reasonable to suggest that replacement of DA innervation may only be fully effective if the correct structural targets are present.
Figure 4.
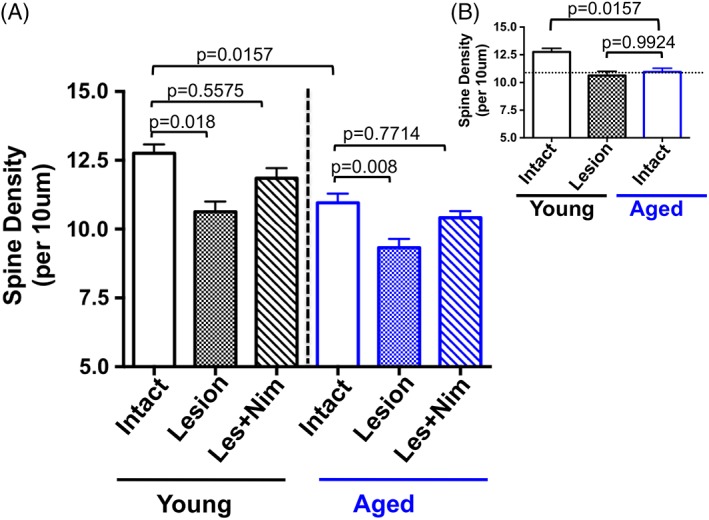
Dendritic spine dynamics in young and aged rats. (A,B) Young rats (3 months) have significantly more spines in the intact striatum compared to aged rats (20 months). There is no difference in spine number with DA depletion (“Lesion”) in young versus aged rats (P = 0.2289; not shown on graph). DA‐depletion–related spine loss is reversible with chronic CaV1.3 calcium channel antagonism with nimodipine in both young and aged rats. (B) The aged “normal” striatum has the same density of dendritic spines as the young parkinsonian striatum. EXP DETAILS: Young and aged rats were unilaterally lesioned with 6‐OHDA. One cohort from each age group received vehicle pellets and a second cohort received slow‐release subcutaneous pellets containing the CaV1.3 calcium channel inhibitor, nimodipine, per Soderstrom and colleagues.30 Pellets were implanted 10 days after lesion, a time when striatal MSNs show significant dendritic spine loss. Rats were sacrificed 3 weeks after pellet implantation for spine density analysis with Golgi impregnation techniques. Image modified from Mercado and colleagues.31 [Color figure can be viewed at wileyonlinelibrary.com]
Future Prospects
Taken together, clinical and preclinical evidence support the case for early intervention, a concept generally accepted by specialists in the field. For example, reaching the threshold of reduced striatal DA transporter scan signal (DaT scan), suggestive of probable PD, may represent a window of time in which DA depletion has not reached a level that produces MSN spine loss and aberrant remodeling of circuitry: a more optimal environment for connectivity of transplanted cells. Inhibition of CaV1.3 channels by administration of clinically available dihydropyridine drugs during this time interval may further slow, and potentially reverse, morphological changes of MSNs associated with ongoing DA depletion. A strong positive response to l‐dopa may be important given that intrastriatal grafts of DA neurons are not expected to address DA‐resistant symptoms. However, until factors are identified that render some individuals resistant to the benefit, or susceptible to GID side effects, cell therapy likely will remain a choice of last resort for the foreseeable future.
In addition to addressing global factors, such as aging or degree of DA depletion, in this era of personalized medicine it will be pertinent to consider that a subpopulation of individuals that show suboptimal responses to grafting may have genotype specific factors that should be considered when selecting appropriate therapeutic interventions. One such example is the gene encoding brain‐derived neurotrophic factor (BDNF). BDNF is a member of the neurotrophin family of secreted growth factors with well‐established activity in promoting dendritic spine density, synaptic plasticity, and neuronal survival. In the striatum, BDNF is known to play an important role in spine dynamics and actin remodeling of MSNs, sculpting the structure and function of synapses.32 Striatal BDNF is primarily derived from cortical afferents that release BDNF in an activity‐dependent manner. Upon release, the interaction of BDNF with its high‐affinity receptor, tropomyosin receptor kinase B (TrkB), located on striatal MSNs, initiates signaling pathways involved in spine remodeling and synapse dynamics. Preclinical studies show that: (1) in nonhuman primates, aging is associated with a significant decline in striatal BDNF protein33; (2) in rats, nigrostriatal lesions are associated with a compensatory increase in striatal BDNF protein in young animals, but this compensation fails in aged animals34; and (3) in rats, intrastriatal infusion of BDNF in conjunction with fetal DA neuron transplants significantly increases graft‐derived reinnervation of the striatum and enhances recovery of motor deficits.35 In PD, postmortem analysis reveals a significant decrease in BDNF mRNA in remaining SN neurons36 and serum levels of BDNF correlate with the severity of motor symptoms.37
In addition to alterations of BDNF related to aging and PD, there is a compelling genetic source of altered BDNF signaling that has not received consideration as a potential risk factor for impaired functional benefit in the subpopulation of PD patients that fail to show clinical improvement despite an abundance of surviving grafted DA neurons. Specifically, a common single‐nucleotide polymorphism (SNP) in the gene that encodes BDNF is present in the human population (rs6265; Val66Met).38 In the SNP, there is a methionine (Met) substitution for valine (Val) at codon 66 (Val66Met). The Met allele of the Bdnf SNP rs6265 has a prevalence of 40.6% in the general population (Major/Minor or Val/Met = 35.4%, Minor/Minor or Met/Met = 5.2%, allelic frequency assuming Hardy‐Weinberg). Both the heterozygous major allele (Val/Met) and homozygous minor allele (Met/Met) of the Bdnf SNP results in decreased activity‐dependent release of BDNF by disruption of packaging into secretory vesicles, whereas constitutive levels of BDNF remain unaffected.38 The majority of BDNF in the adult brain is released from neurons by the regulated, activity‐dependent secretory pathway; therefore, the impact of the Bdnf SNP rs6265 leads to a significant decrease in available extracellular BDNF in approximately 40% of the human population. We have recently documented that this SNP diminishes the therapeutic efficacy of oral l‐dopa in two distinct cohorts of PD patients39 and posit that this genetic risk factor may also contribute to variability in clinical response to grafting. In combination with other clinical assessments, genotyping for this and/or other SNPs could provide an accessible means to provide additional information that may prove useful in predicting response to therapy, including cell transplantation.
Conclusions
The rationale for use of cell therapy as a therapeutic approach in PD remains strong and essentially unchanged during the 30+ years it has been studied: To the extent that degeneration of a specific population of nerve cells is responsible for features of a clinical phenotype, replacement of that population of cells should provide therapeutic benefit. In fact, cell replacement offers the only approach currently available with potential to treat motor symptoms of advanced PD in which the neural elements potentially preserved by neuroprotective therapies are irreversibly absent. We now know that loss of striatal DA innervation occurs early in PD and is virtually absent within 5 years of diagnosis.40 The debate surrounding the issue of why cell transplantation does not provide benefit for every PD individual treated with the procedure often revolves around two issues. First, whether the cell transplanted is the “best cell.” But, arguably at present, the fetal DA neuron is superior to any alternative in maintaining phenotype and innervating the DA‐depleted striatum. Second, whether the details of the transplantation procedure are “performed correctly”: tissue implanted as pieces, noodles, suspensions, number of donors, with and without immunosuppression and for how long, unilateral or bilateral implantation simultaneously, or delayed. It can be argued that none of these factors is make‐or‐break for success. While identifying the optimal cell for grafting and the procedure for implantation continue to be important goals, we suggest equal consideration be paid to the other side of the equation: the target. It is unreasonable to expect that a striatum structurally altered by aging, DA depletion, and genetic factors that may predispose to impaired plasticity, perhaps irreversibly, can support appropriate integration of new DA input, uniformly producing therapeutic benefit. The contributions of the target provide a less‐explored opportunity for development of approaches to optimize the chances that the individual that receives cell replacement therapy also is the individual that benefits from cell replacement therapy.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
T.J.C.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
C.S.: 1A, 1B, 1C, 2C, 3B
N.M.M.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
K.S.‐C.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Financial Disclosures
T.J.C. = NINDS: NS110398, NS094460; Michigan State University: Gibby & Friends vs Parky award, Edwin Brophy endowment. C.E.S. = NINDS: NS090107, NS098079; Grant reviewer honorarium from Michael J Fox Foundation, NIH, Weston Brain Institute. N.M.M. = MSU Graduate Stipend; K.S.C. = NINDS: NS090107, NS098079, NS110398; Michael J Fox Foundation Target Advancement Program grant; NIH grant reviewer honorarium.
Funding agencies: Edwin Brophy Endowment at Michigan State University (T.J.C.), NS09107 (K.S.‐C.), Parkinson's Foundation (K.S.‐C.).
T.J.C. and K.S.‐C. contributed equally to this work.
Relevant conflicts of interest/financial disclosures: Nothing to report.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1. Barker RA, Drouin‐Oullet J, Parmar M. Cell‐based therapies for Parkinson's disease—past insights and future potential. Nat Rev Neurol 2015;11:492–503. [DOI] [PubMed] [Google Scholar]
- 2. Lindvall O. Developing dopaminergic cell therapy for Parkinson's disease—give up or move forward? Mov Disord 2013;28:268–273. [DOI] [PubMed] [Google Scholar]
- 3. Stoker TB, Blair NF, Barker RA. Neural grafting for Parkinson's disease: challenges and prospects. Neural Regen Res 2017;12:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bjorklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res 1979;177:555–560. [DOI] [PubMed] [Google Scholar]
- 5. Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 1979;204:643–647. [DOI] [PubMed] [Google Scholar]
- 6. Bakay RA, Fiandaca MS, Barrow DL, Schiff A, Collins DC. Preliminary report on the use of fetal tissue transplantation to correct MPTP‐induced Parkinson‐like syndrome in primates. Appl Neurophysiol 1985;48:358–361. [DOI] [PubMed] [Google Scholar]
- 7. Redmond DE, Sladek JR, Jr. , Roth RH, Collier TJ, Elsworth JD, Deutch AY, Haber S. Fetal neuronal grafts in monkeys given methylphenyltetrahydropyridine. Lancet 1986;1:1125–1127. [DOI] [PubMed] [Google Scholar]
- 8. Zetterstrom T, Brundin P, Gage FH, et al. In vivo measurement of spontaneous release and metabolism of dopamine from intrastriatal nigral grafts using intracerebral dialysis. Brain Res 1986;362:344–349. [DOI] [PubMed] [Google Scholar]
- 9. Elsworth JD, Sladek JR, Jr. , Taylor JR, Collier TJ, Redmond DE, Jr. , Roth RH. Early gestational mesencephalon grafts, but not later gestational mesencephalon, cerebellum or sham grafts, increase dopamine in caudate nucleus of MPTP‐treated monkeys. Neuroscience 1996;72:477–484. [DOI] [PubMed] [Google Scholar]
- 10. Sørensen, AT , Thompson L, Kirik D, Bjorklund A, Lindvall O, Kokaia M. Functional properties and synaptic integration of genetically labelled dopaminergic neurons in intrastriatal grafts. Eur J Neurosci 2005;21:2793–2799. [DOI] [PubMed] [Google Scholar]
- 11. Freund TF, Bolam JP, Bjorklund A, Stenevi U, Dunnett SB, Powell JF, Smith AD. Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: a tyrosine hydroxylase immunocytochemical study. J Neurosci 1985;5:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soderstrom KE, Meredith G, Freeman TB, et al. The synaptic impact of the host immune response in a parkinsonian allograft rat model: influence on graft‐derived aberrant behaviors. Neurobiol Dis 2008;32:229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leranth C, Sladek JR, Jr. , Roth RH, Redmond DE, Jr . Efferent synaptic connections of dopaminergic neurons grafted into the caudate nucleus of experimentally induced parkinsonian monkeys are different from those of control animals. Exp Brain Res 1998;123:323–333. [DOI] [PubMed] [Google Scholar]
- 14. Mandel RJ, Brundin P, Bjorklund A. Importance of graft placement and task complexity for transplant‐induced recovery of simple and complex sensorimotor deficits in dopamine denervated rats. Eur J Neurosci 1990;2:888–894. [DOI] [PubMed] [Google Scholar]
- 15. Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr. , Collier TJ, Redmond DE, Jr . Grafting of fetal substantia nigra to striatum reverses behavioral deficits induced by MPTP in primates: comparison with other types of grafts and controls. Exp Brain Res 1991;85:335–348. [DOI] [PubMed] [Google Scholar]
- 16. Kordower JH, Rosenstein JM, Collier TJ, et al. Functional fetal nigral grafts in a patient with Parkinson's disease: chemoanatomic, ultrastructural, and metabolic studies. J Comp Neurol 1996;370:203–230. [DOI] [PubMed] [Google Scholar]
- 17. Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 2001;344:710–719. [DOI] [PubMed] [Google Scholar]
- 18. Olanow CW, Goetz CG, Kordower JH, et al Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double‐blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 2003;54:403–414. [DOI] [PubMed] [Google Scholar]
- 19. El Mestikawy S, Wallen‐Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co‐release to vesibular synergy: vesicular glutamate transporters. Nat Rev Neurosci 2011;12:204–216. [DOI] [PubMed] [Google Scholar]
- 20. Lane EL, Winkler C. L‐DOPA‐ and graft‐induced dyskinesia following transplantation. Prog Brain Res 2012;200:143–168. [DOI] [PubMed] [Google Scholar]
- 21. Steece‐Collier K, Rademacher DJ, Soderstrom K. Anatomy of graft‐induced dyskinesias: circuit remodeling in the parkinsonian striatum. Basal Ganglia 2012;2:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piccini P, Pavese N, Hagell P, et al. Factors affecting the clinical outcome after neural transplantation in Parkinson's disease. Brain 2005;128:2977–2986. [DOI] [PubMed] [Google Scholar]
- 23. Collier TJ, Sortwell CE, Daley BF. Diminished viability, growth, and behavioral efficacy of fetal dopamine neuron grafts in aging rats with long‐term dopamine depletion: an argument for neurotrophic supplementation. J Neurosci 1999;19:5563–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Collier TJ, O'Malley J, Rademacher DJ, et al. Interrogating the aged striatum: robust survival of grafted dopamine neurons in aging rats produces inferior behavioral recovery and evidence of impaired integration. Neurobiol Dis 2015;77:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Terpstra BT, Collier TJ, Marchionini DM, Levine ND, Paumier KL, Sortwell CE. Increased cell suspension concentration augments the survival rate of grafted tyrosine hydroxylase immunoreactive neurons. J Neurosci Meth 2007;166:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kordower JH, Goetz CG, Chu Y, et al. Robust graft survival and normalized dopaminergic innervation do not obligate recovery in a Parkinson disease patient. Ann Neurol 2017;81:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson's disease. Brain Res 1988;455:148–152. [DOI] [PubMed] [Google Scholar]
- 28. Fieblinger T, Graves SM, Sebel LE, et al. Cell type‐specific plasticity of striatal projection neurons in parkinsonism and L‐DOPA‐induced dyskinesia. Nat Commun 2014;5:5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6‐hydroxydopamine lesions and with age. Brain Res 1989;503:334–338. [DOI] [PubMed] [Google Scholar]
- 30. Soderstrom KE, O'Malley JA, Levine ND, Sortwell CE, Collier TJ, Steece‐Collier K. Impact of dendritic spine preservation in medium spiny neurons on dopamine graft efficacy and the expression of dyskinesias in parkinsonian rats. Eur J Neurosci 2010;31:478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mercado NM, Collier TJ, Sortwell CE, Steece‐Collier K. BDNF in the aged brain: translational implications for Parkinson's disease. Austin Neurol Neurosci 2017;2:1021. [PMC free article] [PubMed] [Google Scholar]
- 32. Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology 2014;76:628–638. [DOI] [PubMed] [Google Scholar]
- 33. Collier TJ, Dung Ling Z, Carvey PM, Fletcher‐Turner A, Yurek DM, Sladek JR, Jr. , Kordower JH. Striatal trophic factor activity in aging monkeys with uynilateral MPTP‐induced parkinsonism. Exp Neurol 2005;191(Suppl 1):S60–S67. [DOI] [PubMed] [Google Scholar]
- 34. Yurek DM, Fletcher‐Turner A. Lesion‐induced increase of BDNF is greater in the striatum of young versus old rat brain. Exp Neurol 2000;161:392–396. [DOI] [PubMed] [Google Scholar]
- 35. Yurek DM, Lu W, Hipkens S, Wiegand SJ. BDNF enhances the functional reinnervation of the striatum by grafted fetal dopamine neurons. Exp Neurol 1996;137:105–118. [DOI] [PubMed] [Google Scholar]
- 36. Howells DW, Porritt MJ, Wong JY Batchelor PD, Kalnins R, Hughes AF, Donnan GA. Reduced BDNF mRNA expression in the Parkinson's disease substantia nigra. Exp Neurol 2000;166:127–135. [DOI] [PubMed] [Google Scholar]
- 37. Scalzo P, Kümmer A, Bretas TL, Cardoso F, Teixeira AK. Serum levels of brain‐derived neurotrophic factor correlate with motor impairment in Parkinson's disease. J Neurol 2010;257:540–545. [DOI] [PubMed] [Google Scholar]
- 38. Egan MF, Kojima M, Callicott JH, et al. The BDNF vall66met polymorphism affects activity‐dependent secretion of BDNF and human memory and hippocampal function. Cell 2003;112:257–269. [DOI] [PubMed] [Google Scholar]
- 39. Sortwell C, Auinger P, Goudreau J, et al. Specific Bdnf variants are associated with suboptimal response to levodopa but not to other dopaminergic medications or deep brain stimulation in Parkinson's disease [abstract]. Mov Disord 2017;32(Suppl 2). https://www.mdsabstracts.org/abstract/specific‐bdnf‐variants‐are‐associated‐with‐suboptimal‐response‐to‐levodopa‐but‐not‐to‐other‐dopaminergic‐medications‐or‐deep‐brain‐stimulation‐in‐parkinsons‐disease/. Accessed June 5, 2019. [Google Scholar]
- 40. Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain 2013;136:2419–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]


