Abstract
Background
Cluster headache attacks follow a striking circadian rhythm with an intriguing influence of sleep. We aim to investigate differences in sleep quality, chronotype, and the ability to alter individual sleep rhythms in episodic and chronic cluster headache patients vs controls.
Methods
Cluster headache patients and non‐headache controls from the Dutch Leiden University Cluster headache neuro‐Analysis program aged 18 and above completed web‐based questionnaires in a cross‐sectional study.
Results
A total of 478 episodic, 147 chronic cluster headache patients and 367 controls participated. Chronic cluster headache patients had more often early chronotypes than controls, as measured by mid‐sleep phase (P = .021 adjusted B −15.85 minutes CI −29.30; −2.40). Compared to controls, chronic cluster headache participants were less able to alter their sleep rhythms (P < .001 adjusted B −1.65 CI −2.55; 0.74), while episodic cluster headache participants reported more difficulty in coping with reduced sleep (P = .025 adjusted B 0.75 CI 0.09; 1.40). Sleep quality was reduced in both types of cluster headache compared to controls (“poor sleepers”: 71.4% (105/147) in chronic and 48.3% (235/367) in episodic cluster headache vs 25.6% (94/367) in controls; both P < .001; episodic adjusted B −1.71 CI 0.10; 0.32; chronic adjusted B −0.93 CI 0.24; 0.65).
Conclusion
Sleep quality is decreased in both episodic and chronic cluster headache, most likely caused by cluster headache attacks that strike during the night. Episodic cluster headache patients report more difficulty in coping with reduced sleep, while chronic patients are less able to alter their sleep rhythm. Although not directly proven, cluster headache patients will likely benefit from a structured, regular daily schedule.
Keywords: cluster headache, chronotype, circadian timing, münich chronotype questionnaire, sleep
Introduction
Cluster headache is a disabling primary headache disorder,1 associated with a lower quality of life2 and considerable social impact.3 One of the fascinating aspects of cluster headache is its episodic nature. Cluster headache attacks and attack periods often display an intriguing seasonal (autumn, spring) and/or circadian rhythmicity.4, 5, 6 Up to 80% of patients report that attacks strike during (nocturnal) sleep.4
It has been hypothesized that the hypothalamus plays a pivotal role in the episodic nature and autonomic features of cluster headache.7, 8, 9 A PET‐study showed higher activation of the ipsilateral hypothalamus in episodic cluster headache patients during attacks.10 Irregular sleep–wake patterns, decreased total amount and proportion of REM‐sleep and longer REM latencies were reported during, but not outside a cluster headache period.11, 12 These sleep abnormalities might be related to dysfunction of the biological clock, which resides in the suprachiasmatic nucleus of the anterior hypothalamus. Arkink et al have reported an increase in the volume of the anterior hypothalamus in cluster headache patients vs controls, suggesting a structural change in the area of the suprachiasmatic nucleus.13 Together, these findings may point at an association between sleep patterns and the biological clock in the pathophysiology of cluster headache.
Chronotype refers to interindividual differences in how the biological clock is entrained by light and darkness (going to sleep/waking up very early to going to sleep/waking up very late). The capability to cope with less sleep or cope with variation in sleep/wake pattern can also differ. These variations in circadian preference might explain differences in sleep quality in people with and without cluster headache.14 Whether and how these differences influence cluster headache (or vice versa) is unknown. Insight into this matter may improve our understanding of the pathophysiology of this disorder.
In this study, we assessed (1) the distribution of chronotypes, (2) the ability to alter sleep rhythms (ie, the degree of difficulties adjusting sleep rhythm when needed) by measuring amplitude and stability of circadian rhythms, and (3) sleep quality in a large well‐characterized cohort of episodic and chronic cluster headache patients vs controls. Furthermore, we assessed (4) an association between sleep patterns and the occurrence of individual cluster headache attacks.
Methods
Study Design
This explorative cross‐sectional study was conducted as part of the Leiden University Cluster headache neuro‐Analysis program (LUCA).15, 16, 17, 18, 19, 20, 21 The LUCA program was heavily promoted throughout The Netherlands to motivate as many potential cluster headache patients of 18 years and older to participate. In addition, patients attending the Leiden University Medical Center and other headache outpatient departments in the Netherlands were invited as well to participate. The LUCA program includes a validated, web‐based, screening questionnaire for cluster headache based on the ICHD‐II criteria22 with a diagnostic specificity of 0.89.15 All persons who fulfilled the ICHD‐II criteria also fulfilled to the ICHD‐III Beta version for cluster headache.16, 22 All people who screened positive received a second, more extensive web‐based questionnaire.
For this study, all available persons diagnosed with cluster headache were asked to fill out an additional questionnaire on sleep. This questionnaire included the Munich Chronotype Questionnaire, Circadian Type Inventory, the Pittsburgh Sleep Quality Index, and the Hospital Anxiety and Depression Scale.23, 24, 25, 26, 27 For comparison, we included healthy controls who were screened for not having any primary headache like migraine, tension‐type headache, or cluster headache. The healthy controls were recruited as part of the LUCA and Leiden University Medical Center Migraine Neuro‐Analysis programmes (LUMINA).28 They were recruited via public announcements, advertising in lay press and via the research website.
Persons who did not respond to the initial invitation to fill out the sleep questionnaire were reminded twice by email and once by phone. Only persons who completely filled out the sleep questionnaire were included in this study, because the questions were mandatory fields in our digital questionnaire (and therefore there was no missing data). There was thus no participants who did not fully complete the survey.
The study was approved by the local medical ethical committee of the Leiden University Medical Centre and all participants gave written informed consent.
Chronotype, Sleep Time, and Sleep Duration
A Dutch version of the Munich Chronotype Questionnaire was used to assess the chronotype of participants.28 Chronotype is the individual difference in diurnal preference for waking up and falling asleep. The original Munich Chronotype Questionnaire was developed to assign each individual to one of seven defined chronotype groups (extreme early, moderate early, slightly early, normal, slightly late, moderate late, extreme late), calculated using the mid‐sleep phase on free days.24, 25 Chronotype was corrected for individual sleep debt accumulated during the workweek according to Roenneberg et al29 Chronotype is also dependent on age and sex.29
Circadian Rhythm Amplitude and Stability
A Dutch version of the Circadian Type Inventory was used to assess the ability to alter sleep rhythm (ie, the degree of difficulties adjusting sleep rhythm when needed) by measuring the circadian rhythm amplitude and stability in headache participants and controls.28, 30 Rhythm amplitude was assessed via the factor “languid/vigorous.” Languid types find it difficult to overcome drowsiness and feel lethargic following reduced sleep. Rhythm stability was assessed via the factor “flexibility/rigidity of sleeping habits.” Rigid types have a preference to eat and sleep at regular times. A lower “languid/vigorous” score indicated greater ability to manage on less sleep, while a higher “flexibility/rigidity” score indicated greater flexibility in circadian rhythm. Vigorous and flexible types show better circadian adjustment both physiologically and psychologically.30 The revised Circadian Type Inventory questionnaire consisted of 11 items, with 5 answer options.23, 31 In this 11‐item version, 50% of the sample variance was explained by the two factors languid/vigorous and flexibility/rigidity with an internal consistency of 0.72 for languid/vigorous and 0.79 for flexibility/rigidity.23
Sleep Quality
A Dutch version of the Pittsburgh Sleep Quality Index was used to assess sleep quality.28, 32 The Pittsburgh Sleep Quality Index consists of 19 items grouped into 7 subscores. A person with a total Pittsburgh Sleep Quality Index score of ≥5 was defined as a poor sleeper with a sensitivity of 89.6% and a specificity of 86.5%.26
Circadian Timing of Attack Onset
Cluster headache participants were asked to indicate during which part of the day the most and during which part the least attacks tended to appear. For this, 4‐hour timeframes were chosen. Moreover, they were asked to report the effect of sleep duration and sleep onset on the frequency of their cluster headache attacks.
Statistics
No power analysis was performed for this explorative cross‐sectional study. Baseline data were compared using Mann‐Whitney test for ordinal data, and Fisher's Exact test for categorical data.
Separate linear regressions were performed for each of the following six outcomes: (1) sleep duration; (2) sleep onset; (3) mid‐sleep phase; (4) chronotype distribution; (5) Languid Circadian Type Inventory score; and (6) Flexibility Circadian Type Inventory score. All outcomes were checked for normality. In each model, the predictor of interest was headache diagnosis (episodic cluster headache, chronic cluster headache, and controls). The following possible confounders were added (entered) to each of the models: age (years), sex (male/female), years of education, BMI, recreational drug use (yes/no), alcohol use (glasses per week), smoking (packyears), and Hospital Anxiety and Depression Scale (HADS) total score.
A logistic regression model was used for the outcome of the Pittsburgh Sleep Quality Index scores (good vs bad sleeper) again with headache diagnosis as the predictor of interest. The same variables/confounders were added as in the linear regression models above.
The analyses were revised during the peer review process. Besides adjusting our for age (years) and sex (male/female), we also adjusted all regression models for years of education, BMI, recreational drug use (yes/no), alcohol use (glasses per week), smoking (packyears), and Hospital Anxiety and Depression Scale (HADS) total score (as described above). There were no post hoc analyses except for these revised analyses.
For all statistical hypotheses two‐tailed testing was used. Due to the explorative nature of this study, no corrections for multiple comparisons were done. All data analyses were performed using SPSS 23.0 (SPSS Inc., IBM, USA), with the statistical threshold at P < .05.
Results
A total of 804 cluster headache participants and 408 non‐headache controls were eligible in the LUCA program in April 2015 and were invited. The response rate was 78.9% (634/804) in the cluster headache group and 90.0% (367/408) in the control group. Cluster headache had been diagnosed by a physician in 95.6% (606/634) according to the participants. Non‐responder analysis among cluster headache patients revealed that they had a lower age of onset compared to responders (Supplementary Table 1).
Clinical Characteristics
Compared to controls, episodic and chronic cluster headache participants more often were male, older, lower educated, shift‐working, smoking, users of recreational drugs with a higher BMI and more often had higher scores on the Hospital Anxiety and Depression Scale. Chronic cluster headache participants more often were without work (Table 1).
Table 1.
Characteristics of Study Population
| Chronic Cluster Headache‡ | Chronic vs Controls | Episodic Cluster Headache‡ | Episodic vs Controls | Controls | |
|---|---|---|---|---|---|
| N = 147 | P value | N = 487 | P value | N = 367 | |
| Age (years), mean ± SD | 48.5 ± 11.3 | .001 | 50.4 ± 12.1 | <.001* | 43.9 ± 15.5 |
| Male, N (%) | 109 (74.1) | <.001* | 364 (74.7) | <.001* | 161 (43.9) |
| Years of education, mean ± SD | 12.3 ± 3.0 | <.001* | 13.1 ± 3.3 | <.001* | 14.1 ± 3.6 |
| BMI‡, mean ± SD | 25.6 ± 4.3 | <.001* | 25.3 ± 3.6 | <.001* | 24.0 ± 4.0 |
| Recreational drugs use, N (%) | 20 (13.6) | <.001* | 42 (8.6) | .005* | 14 (3.8) |
| Smoking (pack years), mean ± SD | 17.9 ± 14.9 | <.001* | 18.0 ± 16.6 | <.001* | 5.6 ± 10.3 |
| Alcohol (glasses per week), mean ± SD | 4.9 ± 8.0 | <.001* | 7.4 ± 8.6 | .547 | 6.5 ± 6.6 |
| HADS‡ total score, mean ± SD | 14.4 ± 9.1 | <.001* | 10.0 ± 7.1 | <.001* | 6.4 ± 5.5 |
| Shiftwork ever, N (%) | 52 (35.4) | .053 | 165 (33.9) | .020* | 97 (26.4) |
| Shiftwork last week, N (%) | 7 (4.8) | .814 | 35 (7.2) | .756 | 21 (5.7) |
| Not working, N (%) | 62 (42.2) | <.001* | 51 (10.5) | .489 | 33 (9.0) |
| Retired, N (%) | 10 (6.8) | .188 | 74 (15.2) | .084 | 40 (10.9) |
| Inside a cluster headache period, N (%) | N/A | 45 (9.2) | N/A |
P < .05 (Mann‐Whitney test for ordinal data and Fisher's Exact test for nominal data), no correction for multiple testing.
According to ICHD‐III beta version criteria.1
BMI = body mass index; HADS = hospital anxiety and depression scale; N/A = not applicable.
Chronotype
Participants with episodic cluster headache more often classified themselves as late chronotypes compared to both participants with chronic cluster headache and controls (proportion classified as: episodic 40%; P = .041 adjusted B 0.13; CI 0.01; 0.25, chronic 29.9%; P = .503 adjusted B −0.06 CI −0.23;0.11, controls 28.9%). Chronotype distribution as measured by mid‐sleep phase showed that chronic cluster headache participants more often had an earlier chronotype than controls (relatively P = .834 adjusted B 1.03 minutes CI −8.58; 10.65 minutes for episodic and P = .021 adjusted B −15.85 minutes CI −29.30; −2.40 minutes for chronic; Fig. 1). Sleep duration did not differ between both episodic and chronic cluster headache vs controls (relatively P = .097 adjusted B −9.22 minutes CI −20.11; 1.67 minutes for episodic and P = .369 adjusted B −6.97 minutes CI −22.20; 8.26 minutes for chronic). There was no difference in sleep onset between both episodic or chronic cluster headache patients and controls (relatively P = .725 adjusted B −1.73 minutes CI −11.37; 7.91 minutes for episodic and P = .363 adjusted B −6.24 minutes CI −19.72; 7.23 minutes for chronic). There were no differences between episodic cluster headache participants in vs outside a cluster headache period (mid‐sleep phase: P = .661 adjusted B 4.26 minutes CI −14.8; 23.330; sleep duration: P = .778 adjusted B −3.22 minutes CI −25.66; 19.23 minutes; sleep onset: P = .075 adjusted B 18.01 minutes CI −1.82; 37.85 minutes).
Figure 1.
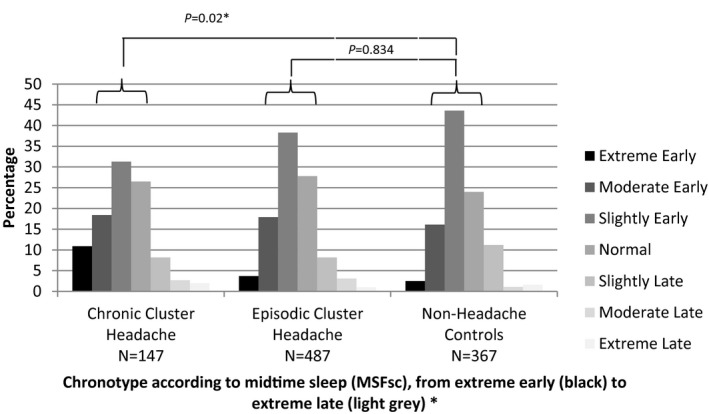
The distribution of chronotypes using mid‐sleep phase. Notes: The objective distribution of chronotypes, calculated using mid‐sleep phase, in episodic cluster headache, chronic cluster headache, and controls according to the Munich Chronotype Questionnaire. P values based upon a linear regression model corrected for age, sex, years of education, BMI, recreational drug use, alcohol use, smoking, and HADS total score. *P < .05.
Circadian Rhythm Amplitude and Stability
Episodic cluster headache participants were more often languid types compared to controls, which was not the case in chronic cluster headache participants (episodic P = .025 adjusted B 0.75 CI 0.09; 1.40; chronic P = .632 adjusted B 0.22 CI −0.69; 1.14). Chronic cluster headache participants more often were rigid types than controls (chronic P < .001 adjusted B −1.65 CI −2.55; −0.74; episodic: P = .515 adjusted B −0.22 CI −0.86; 0.43) (Fig. 2). Episodic cluster headache participants in a period did not differ from those outside a cluster headache period (languid P = .379 adjusted B −0.58; CI −1.89; 0.72; rigid P = .873 adjusted B 0.11 CI −1.21; 1.42).
Figure 2.
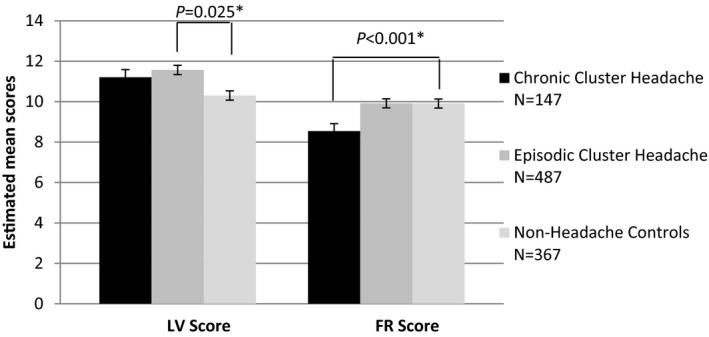
Circadian Type Inventory scores episodic cluster headache, chronic cluster headache and controls, corrected for age and gender. Notes: Estimated Mean LV score (languid/vigorous score) and FR score (flexibility/rigidity score). Significant differences in languid/vigorous score are shown in the figure. P values based upon a linear regression model corrected for age, sex, years of education, BMI, recreational drug use, alcohol use, smoking, and HADS total score. *P < .05.
Sleep Quality
Compared to controls, chronic and episodic cluster headache participants reported higher scores on all subgroups of the Pittsburgh Sleep Quality Index, which reflects lower sleep quality (Supplementary Table 2). Controls (25.6%; N = 94/367) were less often “poor sleepers” compared to both episodic (48.3% poor sleepers; N = 235/367 P < .001 adjusted B −1.71 CI 0.10; 0.32) and chronic cluster headache participants (71.4% poor sleepers; N = 105/147 P < .001 adjusted B −0.93 CI 0.24; 0.65). Episodic cluster headache participants in a cluster headache period showed a lower sleep quality compared to those outside (inside a period: N = 34/45 vs outside a cluster headache period: N = 201/442; P = .001 adjusted B 1.41 CI 1.80; 9.25).
Circadian Timing of Attack Onset
A predictable circadian pattern of attacks was reported in 76.6% (373/487) of episodic and 57.8% (85/147) of chronic cluster headache participants. Attacks occurred most often in episodic (53.6% 200/373) and in chronic cluster headache (51.8% 44/85) between 00:00 and 04:00 AM and least often (4.6% N = 17/373 in episodic and 1.2% N = 1/85 in chronic cluster headache) between 12:00 and 16:00 PM (Fig. 3).
Figure 3.
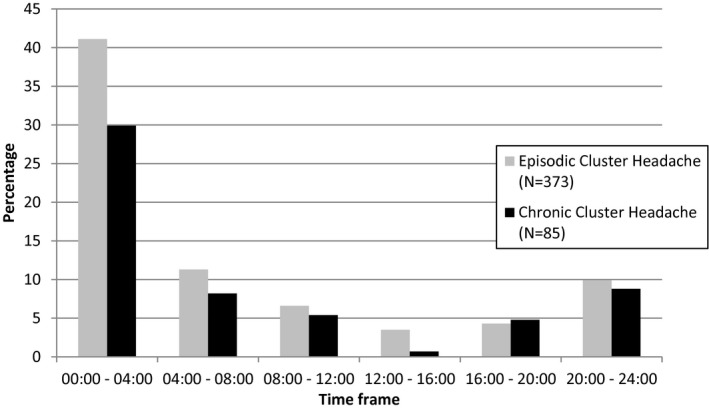
4‐hour timeframes of cluster headache attack occurrence.
Sleep Pattern
In 26.7% (130/486) episodic and 42.9% (63/147) chronic cluster headache participants, an association between the sleep pattern and the probability of developing a cluster headache attack was reported. Lack of sleep (63.1% 82/130 in episodic and 66.7% 42/63 in chronic cluster headache) and troubled sleep (55.4% 72/130 in episodic and 66.7% 42/63 in chronic cluster headache) were most commonly reported. Too much sleep or waking up late increased the chance of developing an attack the next day according to about 50% of the participants (too much sleep: in episodic cluster headache 53.1% 69/130; in chronic cluster headache 52.4% 33/63; waking up late: in episodic cluster headache 54.6% 71/130; in chronic cluster headache 46.0% 29/63). Waking up early was generally reported to have no influence on the development of attacks (Fig. 4).
Figure 4.
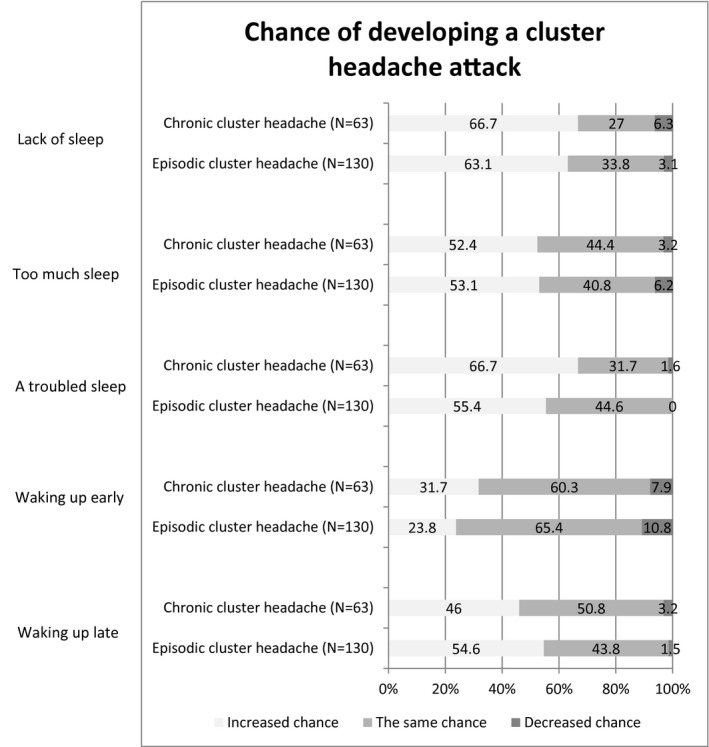
Reported influence of sleep on developing a cluster headache attack.
Discussion
In this large, well‐characterized cluster headache and control population with a high response rate, we assessed the distribution of chronotypes, the ability to alter sleep rhythms and sleep quality. Furthermore, we assessed if there was a subjective association between sleep patterns and the occurrence of individual cluster headache attacks. Episodic cluster headache patients described themselves more often as having a late chronotype. However, this was not confirmed when assessing their chronotype by their mid‐sleep phase. In contrast, chronic cluster headache patients more often had an early chronotype compared to non‐headache controls. Furthermore, chronic cluster headache patients were less able to alter their sleep rhythm; while on the other hand, episodic cluster headache patients experience more difficulty in coping with reduced sleep. To our knowledge, these aspects of sleep rhythmicity were not assessed before in cluster headache. Sleep quality was decreased in both episodic and chronic cluster headache, probably in association with their predictable nocturnal headache attacks. Changes in sleep patterns were associated with headache attacks in nearly one third of the population studied.
Although episodic cluster headache patients more often classified themselves as “late” chronotypes, no actual difference with controls was found in chronotype distribution as measured by mid‐sleep time. In two smaller studies, chronotype was measured before with similar findings in episodic cluster headache.4, 33 In another cohort, chronic cluster headache patients classified themselves more often as morning persons compared to episodic cluster headache patients.6 We here report similar findings in the mid‐sleep phase: chronic cluster headache patients more often had an early chronotype, suggesting an association between chronotype and the more severe form of cluster headache.
In our study cluster headache patients who reported an association between the occurrences of headache attacks an sleep, most often reported that these attacks occurred within 1–2 hours after falling asleep. Unfortunately, we were not able to analyze whether this is related to chronotype, because of the 4‐hour timeframes we used in our questionnaire. Barloese et al reported indeed an association between the occurrence of the first nocturnal attack and chronotype in cluster headache patients (the “morning type” being earliest [00:50], the “neither type” later [01:02], and the “evening type” later still [02:11]).34
Sleep quality was affected in episodic and even more in chronic cluster headache patients. Since chronic cluster headache is the most severe of the phenotypes, the difference in sleep quality and rhythm between chronic and episodic cluster headache are most likely associated with the number of nights with cluster headache attacks, but other contributing factors cannot be excluded. The first option would, however, suggest that the direction of the association is that cluster headache influences sleep quality, pattern, and rhythm (and not vice versa). However, this causality cannot be directly proven by our data. Lund et al studied the association between cluster headache and sleep using polysomnography. They concluded that disturbed sleep is rather due to a continuing or slowly recovering disturbance of sleep outside the cluster headache episode than due to a transient process associated with the cluster headache attacks themselves.35 Although the exact causality remains unclear, association between cluster headache and sleep seems robust and remains intriguing.
The most common sleep‐related triggers in our study were a lack of sleep and troubled sleep (ie, moving and turning a lot). Nocturnal sleep per se and a lack of sleep have been reported as triggers before.4, 36 It is telling that 42% of another cluster headache population even consciously changed their sleep habits in order to prevent headache attacks.3
In our population, shift work, which disturbs sleep rhythms, was associated with episodic cluster headache, which is in line with previous reports.33, 36 As chronic cluster headache participants showed greater difficulty in adjusting their sleep rhythm and episodic cluster headache patients experience more difficulties in coping with less sleep, we wonder whether shift work might indeed trigger cluster headache in people that are already prone to develop this disorder.
The strength of this study are the large sample size, a well‐defined cohort and, an overall highly motivated group as was reflected by the high response rate. Limitations include that we have no data regarding sleep patterns before the development of cluster headache. Also, participants of the LUCA program needed to have (at least once) online access to register for participation, which could have introduced a bias.
In this large study, we found differences in sleep patterns between cluster headache participants and controls. We would like to suggest that the cluster headache attacks themselves are responsible for these sleep differences. However, the exact association between cluster headache and sleep remains unknown. Although not directly proven, it seems very likely that cluster headache patients will benefit from a structured, regular daily schedule.
Statement of Authorship
Category 1
(a) Conception and Design
Ilse F. de Coo, Willebrordus P. J. van Oosterhout, Michel D. Ferrari, Rolf Fronczek
(b) Acquisition of Data
Ilse F. de Coo, Willebrordus P. J. van Oosterhout, Leopoldine A. Wilbrink
(c) Analysis and Interpretation of Data
Ilse F. de Coo, Erik W. van Zwet, Rolf Fronczek
Category 2
(a) Drafting the Manuscript
Ilse F. de Coo
(b) Revising It for Intellectual Content
Willebrordus P. J. van Oosterhout, Leopoldine A. Wilbrink, Erik W. van Zwet, Michel D. Ferrari, Rolf Fronczek
Category 3
(a) Final Approval of the Completed Manuscript
Ilse F. de Coo, Willebrordus P. J. van Oosterhout, Leopoldine A. Wilbrink, Erik W. van Zwet, Michel D. Ferrari, Rolf Fronczek
Supporting information
Acknowledgment
We like to thank Joost Haan for his contribution.
Conflict of Interest: I. F. de Coo reports support for conference visits from Electrocore. W. P. J. van Oosterhout reports support for conference visits from Menarini and Allergan. M. D. Ferrari reports grants and consultancy or industry support from Medtronic, Amgen, Lilly and Electrocore, and independent support from NWO, ZonMW, NIH, European Community, Dutch Heart Foundation, and Fonds Nuts Ohra (to M. D. Ferrari and L. A. Wilbrink). This study was supported by grants from the Netherlands Organisation for Scientific Research (NWO) and the European Union's Seventh Framework programme (2007‐2013) under grant agreement no. 602633 to M. D. Ferrari. L. A. Wilbrink reports no further conflicts of interest. E. W. van Zwet reports no conflicts of interest. Rolf Fronczek received grant support from the Dutch Brain Foundation and the Innovation Fund of Dutch Healthcare Insurers.
References
- 1. Headache Classification Committee of the International Headache Society . The International Classification of Headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 2. D'Amico D, Rigamonti A, Solari A, et al. Health‐related quality of life in patients with cluster headache during active periods. Cephalalgia. 2002;22:818‐821. [DOI] [PubMed] [Google Scholar]
- 3. Jensen RM, Lyngberg A, Jensen RH. Burden of cluster headache. Cephalalgia. 2007;27:535‐541. [DOI] [PubMed] [Google Scholar]
- 4. Barloese M, Lund N, Petersen A, et al. Sleep and chronobiology in cluster headache. Cephalalgia. 2015;35:969‐978. [DOI] [PubMed] [Google Scholar]
- 5. Rozen TD, Fishman RS. Cluster headache in the United States of America: Demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache. 2012;52:99‐113. [DOI] [PubMed] [Google Scholar]
- 6. Steinberg A, Fourier C, Ran C, et al. Cluster headache – Clinical pattern and a new severity scale in a Swedish cohort. Cephalalgia. 2018;38:1286‐1295. [DOI] [PubMed] [Google Scholar]
- 7. Stillman M, Spears R. Endocrinology of cluster headache: Potential for therapeutic manipulation. Curr Pain Headache Rep. 2008;12:138‐144. [DOI] [PubMed] [Google Scholar]
- 8. Barloese M, Brinth L, Mehlsen J, et al. Blunted autonomic response in cluster headache patients. Cephalalgia. 2015;35:1269‐1277. [DOI] [PubMed] [Google Scholar]
- 9. Holland PR. Headache and sleep: Shared pathophysiological mechanisms. Cephalalgia. 2014;34:725‐744. [DOI] [PubMed] [Google Scholar]
- 10. May A, Bahra A, Büchel C, et al. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology. 2000;55:1328‐1335. [DOI] [PubMed] [Google Scholar]
- 11. Della MG, Vollono C, Rubino M, et al. A sleep study in cluster headache. Cephalalgia. 2006;26:290‐294. [DOI] [PubMed] [Google Scholar]
- 12. Barloese MC, Jennum PJ, Lund NT, et al. Sleep in cluster headache – Beyond a temporal rapid eye movement relationship? Eur J Neurol. 2015;22:656‐e40. [DOI] [PubMed] [Google Scholar]
- 13. Arkink EB, Schmitz N, Schoonman GG, et al. The anterior hypothalamus in cluster headache. Cephalalgia. 2017;37:1039‐1050. [DOI] [PubMed] [Google Scholar]
- 14. Juda M, Vetter C, Roenneberg T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift‐workers. J Biol Rhythms. 2013;28:141‐151. [DOI] [PubMed] [Google Scholar]
- 15. Wilbrink LA, Weller CM, Cheung C, et al. Stepwise web‐based questionnaires for diagnosing cluster headache: LUCA and QATCH. Cephalalgia. 2013;33:924‐931. [DOI] [PubMed] [Google Scholar]
- 16. de Coo IF, Wilbrink LA, Haan J, et al. Evaluation of the new ICHD‐III beta cluster headache criteria. Cephalalgia. 2016;36:547‐551. [DOI] [PubMed] [Google Scholar]
- 17. de Coo IF, Wilbrink LA, Ie GD, et al. Aura in cluster headache: A cross‐sectional study. Headache. 2018;58:1203‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Coo IF, Naber WC, Wilbrink LA, et al. Increased use of illicit drugs in a Dutch cluster headache population. Cephalalgia. 2019;39:626‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilbrink LA, Cheung C, Weller C, et al. Aura‐related symptoms associated with cluster headache: Outcomes of a LUCA substudy. Ned Tijdschr Geneeskd. 2013;157:5306. [PubMed] [Google Scholar]
- 20. Wilbrink LA, Louter MA, Teernstra OPM, et al. Allodynia in cluster headache. Pain. 2017;158:1113‐1117. [DOI] [PubMed] [Google Scholar]
- 21. Louter MA, Wilbrink LA, Haan J, et al. Cluster headache and depression. Neurology. 2016;87:1899‐1906. [DOI] [PubMed] [Google Scholar]
- 22. Headache Classification Committee of the International Headache Society . The International Classification of Headache disorders. Cephalalgia. 2004;24(Suppl. 1):9‐160. [DOI] [PubMed] [Google Scholar]
- 23. Di Milia L, Smith PA, Folkard S. Refining the pyschometric properties of the circadian type inventory. Personality Individ Differ. 2004;36:1953‐1964. [Google Scholar]
- 24. Roenneberg T, Wirz‐Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80‐90. [DOI] [PubMed] [Google Scholar]
- 25. Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Curr Biol. 2007;17:44‐45. [DOI] [PubMed] [Google Scholar]
- 26. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193‐213. [DOI] [PubMed] [Google Scholar]
- 27. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361‐370. [DOI] [PubMed] [Google Scholar]
- 28. van Oosterhout W, van Someren E, Schoonman GG, et al. Chronotypes and circadian timing in migraine. Cephalalgia. 2018;38:617‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429‐438. [DOI] [PubMed] [Google Scholar]
- 30. Folkard S, Monk TH, Lobban MC. Towards a predictive test of adjustment to shift work. Ergonomics. 1979;22:79‐91. [DOI] [PubMed] [Google Scholar]
- 31. Di Milia L, Smith PA, Folkard S. A validation of the revised circadian type inventory in a working sample. Personality Individ Differ. 2005;39:1293‐1305. [Google Scholar]
- 32. van Oosterhout WP, van Someren EJ, Louter MA, et al. Restless legs syndrome in migraine patients: Prevalence and severity. Eur J Neurol. 2016;23:1110‐1116. [DOI] [PubMed] [Google Scholar]
- 33. Ofte HK, Tronvik E, Alstadhaug KB. Lack of association between cluster headache and PER3 clock gene polymorphism. J Headache Pain. 2015;17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barloese M, Haddock B, Lund NT, et al. Chronorisk in cluster headache: A tool for individualised therapy? Cephalalgia. 2018;38:2058‐2067. [DOI] [PubMed] [Google Scholar]
- 35. Lund NLT, Snoer AH, Petersen AS, et al. Disturbed sleep in cluster headache is not the result of transient processes associated with the cluster period. Eur J Neurol. 2019;26:290‐298. [DOI] [PubMed] [Google Scholar]
- 36. Ofte HK, Berg DH, Bekkelund SI, et al. Insomnia and periodicity of headache in an arctic cluster headache population. Headache. 2013;53:1602‐1612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


