Abstract
Psoriasis is a chronic disease that requires long‐term treatment. Consequently, understanding the safety and tolerability of any potential treatment over time is critical to effective prescribing. The biologic agents currently available for the treatment of psoriasis target a number of different inflammatory cytokines involved in psoriasis disease pathogenesis. The monoclonal antibodies tildrakizumab, guselkumab and risankizumab target the p19 subunit that is specific to interleukin (IL)‐23. This article reviews published data on the safety of these IL‐23p19 inhibitors in patients with psoriasis compared with other currently available biologic therapies. Data from randomized, placebo‐ and active‐controlled phase 3 clinical trials show tildrakizumab, guselkumab and risankizumab to have a favourable risk–benefit profile in patients with moderate to severe psoriasis. No significant safety concerns have been observed for any of these IL‐23p19 inhibitors in the data published to date. The most commonly reported adverse events (AEs) associated with these agents in phase 3 studies were upper respiratory tract infections. No increase was seen in rates of serious infections, malignancies or major adverse cardiovascular events, with no signals suggestive of an elevated risk of opportunistic infections, active tuberculosis or reactivation of latent tuberculosis infection, mucocutaneous Candida infections, triggering or worsening of inflammatory bowel disease, demyelinating disorders or suicidal ideation. Selectively targeting IL‐23p19 may help avoid AEs that have been associated with biologic agents with other mechanisms of action. Data from long‐term extension studies and patient registries will further establish the safety profile of IL‐23p19 inhibitors for the treatment of moderate to severe psoriasis in routine practice.
Introduction
Psoriasis is a chronic T‐cell‐mediated inflammatory skin disease, estimated to affect more than 100 million individuals worldwide, of whom approximately 20% have moderate to severe disease.1, 2 The pathogenesis of psoriasis is complex; however, there is robust evidence that the interleukin (IL)‐23/IL‐17 immune axis is a key driver of psoriatic inflammation.3 Over the past 2 decades, biologic treatment of moderate to severe psoriasis has changed the disease management paradigm. Multiple biologic therapies are now available or in late stages of development (Table 1), targeting different inflammatory cytokines (Fig. 1). These include tumour necrosis factor (TNF) antagonists, IL‐17 inhibitors, an IL‐12/23p40 inhibitor and monoclonal antibodies that target the p19 subunit that is specific to IL‐23.
Table 1.
Biologics for the treatment of moderate to severe psoriasis in adults approved or filed for approval by the United States Food and Drug Administration as of June 2019
| Biologic drug | Therapeutic target | Approval date | Other approved indications/notes |
|---|---|---|---|
| Etanercept | TNF | April 2004 |
Moderate to severe rheumatoid arthritis (November 1998) Moderate to severe polyarticular juvenile idiopathic arthritis (≥2 years) (May 1999) Psoriatic arthritis (January 2002) Ankylosing spondylitis (July 2003) Paediatric (4–17 years) chronic moderate to severe psoriasis (November 2016) |
| Infliximab | TNF | September 2006 |
Crohn's disease (August 1998) Rheumatoid arthritis (November 1999) Ankylosing spondylitis (December 2004) Psoriatic arthritis (May 2005) Ulcerative colitis (September 2005) Paediatric (≥6 years) Crohn's disease (May 2006) Paediatric (≥6 years) ulcerative colitis (September 2011) |
| Adalimumab | TNF | January 2008 |
Rheumatoid arthritis (December 2002) Psoriatic arthritis (October 2005) Ankylosing spondylitis (July 2006) Crohn's disease (February 2007) Polyarticular juvenile idiopathic arthritis (≥2 years) (February 2008) Ulcerative colitis (September 2012) Paediatric (≥6 years) Crohn's disease (September 2014) Moderate to severe hidradenitis suppurativa (≥12 years) (September 2015) Uveitis (≥2 years) (June 2016) |
| Ustekinumab | IL‐12/IL‐23p40 | September 2009 |
Active psoriatic arthritis (September 2013) Moderately to severely active Crohn's disease (September 2016) Adolescent (≥12 years) moderate to severe psoriasis (October 2017) |
| Secukinumab | IL‐17A | January 2015 |
Active psoriatic arthritis (January 2016) Active ankylosing spondylitis (January 2016) |
| Ixekizumab | IL‐17A | March 2016 | Active psoriatic arthritis (December 2017) |
| Brodalumab † | IL‐17A receptor | February 2017 | — |
| Guselkumab | IL‐23p19 | July 2017 | — |
| Tildrakizumab | IL‐23p19 | March 2018 | — |
| Certolizumab pegol | TNF | May 2018 |
Moderate to severe Crohn's disease (April 2008) Moderately to severely active rheumatoid arthritis (May 2009) Active psoriatic arthritis (September 2013) Active ankylosing spondylitis (October 2013) |
| Risankizumab | IL‐23p19 | Filed for approval April 2019 | — |
†Availability restricted through a Risk Evaluation and Mitigation Strategy (REMS) programme in the United States.
IL, interleukin; TNF, tumour necrosis factor.
Figure 1.
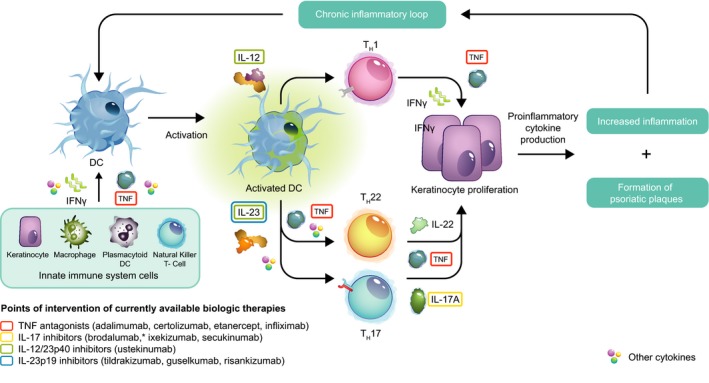
Schematic overview of psoriasis disease pathogenesis highlighting points of intervention of currently available biologic treatment options. *Brodalumab is directed against the IL‐17 receptor, and not IL‐17. DC, dendritic cell; IFN, interferon; IL, interleukin; TH, T helper; TNF, tumour necrosis factor.
Psoriasis is a chronic disease that often requires ongoing treatment. Consequently, the long‐term safety and tolerability of any treatment are critical to effective prescribing. Early biologic agents for the treatment of psoriasis typically induced non‐specific immunosuppression, including agents targeting T‐cell activation and migration (efalizumab and alefacept, both of which have now been withdrawn) and anti‐TNF agents that target a key cytokine involved in most inflammatory and infectious processes in the body.4 TNF antagonists have been associated with increased risk of serious bacterial, viral and fungal infections in patients with psoriasis, including active tuberculosis and reactivation of latent tuberculosis infection.5, 6, 7, 8, 9 The data on malignancy risk with long‐term exposure to TNF antagonists are conflicting, with some studies and recent registry data suggesting a potential increased risk.5, 6, 7, 8, 10 Rare cases of new onset or exacerbation of demyelinating disorders have also been reported during treatment with TNF antagonists.5, 6, 7, 8
As understanding of psoriasis disease mechanisms has advanced, more targeted therapies have become available with the potential for improved safety and tolerability. Three agents targeting the IL‐17 cytokine pathway are available; secukinumab and ixekizumab are specific to IL‐17A, whereas brodalumab is directed against the IL‐17 receptor. IL‐17 inhibitors have shown generally favourable safety profiles in patients with psoriasis.11, 12, 13 However, increased risk of Candida infections, worsening of pre‐existing inflammatory bowel disease and, rarely, new‐onset ulcerative colitis and Crohn's disease have been reported during treatment.11, 12, 13, 14, 15, 16 The observed increase in Candida infections is not unexpected, as IL‐17 is known to play a key role in the host defence against yeast and fungi.17, 18 In terms of inflammatory bowel disease, it is possible that blocking IL‐17 signalling may interfere with a protective function of IL‐17A in the intestine.19 In addition, brodalumab has a warning for suicidal ideation and behaviour, although a causal relationship has not been established,20 and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) programme in the United States.21
Several agents targeting the IL‐23 cytokine pathway are now available. IL‐23 is a heterodimer composed of two subunits: p40, which is shared with IL‐12, and p19.3 Data from long‐term clinical trials and a large safety registry (Psoriasis Longitudinal Assessment and Registry; PSOLAR) have shown the IL‐12/23p40 inhibitor ustekinumab to be well tolerated in patients with psoriasis.22, 23, 24, 25, 26 However, another anti‐IL‐12/23p40 agent, briakinumab, showed signs of a possible increased risk of major cardiovascular adverse events (MACE), infections and malignancies in clinical trials,27, 28 and development was stopped before approval. Additionally, there is evidence that blockade of IL‐12 may be counterproductive in treating patients with psoriasis: mice lacking IL‐12 signalling components develop worse psoriasis than wild‐type animals29 and IL‐12 exhibits protective roles against malignancies30 and infections.31, 32 However, clinical studies of IL‐12/23 inhibitors have not detected signals for these safety events.24, 25 Differences in safety may exist among agents targeting the same cytokine(s), owing to dosing, pharmacokinetics, antibody‐binding sites and affinities.
Selectively targeting IL‐23p19 may avoid adverse events (AEs) that have been associated with biologic agents with other mechanisms of action. Here we review published data on the safety of the IL‐23p19 inhibitors guselkumab, tildrakizumab and risankizumab in patients with psoriasis, focusing on the frequency of AEs that have been associated with other biologic therapies in pivotal randomized, controlled phase 3 clinical trials. An additional IL‐23p19 inhibitor, mirikizumab, is in development, but clinical trial data have not yet been published.
Safety data from clinical trials
Results of phase 1 and 2 studies showed favourable safety and tolerability profiles in adult patients with moderate to severe psoriasis for guselkumab,33, 34, 35, 36 tildrakizumab37, 38 and risankizumab.39, 40 These findings were confirmed by results of randomized, controlled phase 3 clinical trials (Table 2), with no major safety concerns identified for any available IL‐23p19 inhibitor.41, 42, 43, 44, 45 Key exclusion criteria for these studies typically included any malignancy within the past 5 years (except non‐melanoma skin cancer), active or untreated latent tuberculosis and testing positive for human immunodeficiency, hepatitis B virus or hepatitis C virus infection. Women could not be pregnant, and those of child‐bearing potential had to practice abstinence or use medically accepted contraception methods.
Table 2.
Percentages of patients reporting AEs of special interest in pivotal phase 3 studies of biologics selectively targeting IL‐23p1941, 42, 43, 44, 45, 46
| IL‐23p19 inhibitor | Guselkumab | Tildrakizumab | Risankizumab | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | VOYAGE 1 & 2 | NAVIGATE† | reSURFACE 1 & 2 | UltIMMa‐1 & UltIMMa‐2 | ||||||||
| Intervention |
GUS 100 mg |
ADA 40 mg |
PBO |
GUS 100 mg |
UST 45/90 mg |
TIL 200 mg |
TIL 100 mg |
ETN‡
50 mg |
PBO |
RIS 150 mg |
UST 45/90 mg |
PBO |
| Dosing schedule | ||||||||||||
| Initial | Wk 0, 4 | Wk 0,§ 1 | Wk 0, 4 | Wk 16, 20 | Wk 0, 4 | Wk 0, 4 | Wk 0, 4 | biw | Wk 0, 4 | Wk 0, 4 | Wk 0, 4 | Wk 0, 4 |
| Maintenance | q8w | q2w | q8w | q8w | q12w | q12w | q12w | qw | q12w | q12w | q12w | q12w |
| N | 823 | 581 | 422 | 622 | 616 | 313 | 310 | 598 | 199 | 200 | ||
| Safety, % | Wk 0–16 | Wk 0–16 | Wk 0–16 | Wk 0–12 | Wk 0–12 | Wk 0–12 | Wk 0–12 | Wk 0–16 | Wk 0–16 | Wk 0–16 | ||
| AE | 48–52 | 48–51 | 45–49 | 42–49 | 44–47 | 54 | 48–55 | 46–50 | 50–54 | 46–51 | ||
| Discontinued owing to AE | 1 | 1–2 | 1 | 1–2 | 0–1 | 2 | 1 | 0.3–0.7 | 0–2 | 1–4 | ||
| SAE | 2 | 2 | 1–2 | 2–3 | 1–2 | 2 | 1–3 | 2 | 3–8 | 1–3 | ||
| Infection | 22–26 | 23–26 | 19–25 | NR | NR | NR | NR | 19–25 | 20 | 9–17 | ||
| Serious infection | 0–0.2 | 0.6–0.8 | 0–0.4 | 0.3 | 0–0.3 | 0 | 0‐0.6 | 0.3–1 | 1–3 | 0 | ||
| Malignancies | 0 | 0 | 0 | 0–0.3 | 0–0.3 | 0.3 | 0 | 0.3 | 0 | 0–1 | ||
| MACE | 0–0.3 | 0.3–0.4 | 0 | 0 | 0–0.3 | 0 | 0 | 0 | 0 | 0 | ||
| N | 823 | 581 | 135 | 133 | 597 | 594 | 289 | 588 | 193 | |||
| Long‐term safety, % | Wk 0–48¶ | Wk 0–48¶ | Wk 16–60 | Wk 16–60 | Wk 12–28 | Wk 12–28 | Wk 12–28 | Wk 16–52 | Wk 16–52 | |||
| AE | 58–74 | 63–75 | 64 | 56 | 40–45 | 44–46 | 57 | 56–61 | 67–75 | |||
| Discontinued owing to AE | 2–3 | 2–4 | 2 | 2 | 0.3–1 | 0.3 | 1 | 0–0.7 | 0–2 | |||
| SAE | 4–5 | 4–5 | 7 | 5 | 2 | 2–3 | 5 | 5 | 4 | |||
| Infection | 31–52 | 35–50 | 42 | 35 | NR | NR | NR | 35–38 | 41–49 | |||
| Serious infection | 0.6 | 0.9–1 | 0.7 | 0 | 0.3–0.7 | 0.3–0.7 | 1 | 0.7 | 0–1 | |||
| Malignancies | 0.2–0.6 | 0 | 1 | 0 | 0–0.7 | 0–0.3 | 1 | 0–0.3 | 0–1 | |||
| MACE | 0.2–0.3 | 0.3–0.4 | 1 | 0.8 | 0 | 0 | 0 | 0–0.7 | 0 | |||
†Randomized patients only. ‡reSURFACE 2 only. §Initial dose of 80 mg at Wk 0. ¶Weeks 0–28 in VOYAGE 2.
ADA, adalimumab; AE, adverse event; biw, twice per week; ETN, etanercept; GUS, guselkumab; IL, interleukin; MACE, major cardiovascular adverse event; N, number of patients included; NR, not reported; PBO, placebo; RIS, risankizumab; SAE, serious adverse event; TIL, tildrakizumab; UST ustekinumab; q2w, every 2 weeks; q8w, every 8 weeks; q12w, every 12 weeks; qw, every week; Wk, week.
No head‐to‐head studies have compared the different IL‐23p19 inhibitors, and care must be taken when comparing data among studies because of differing study designs, patient populations and study lengths.
Guselkumab
Results of three pivotal phase 3 trials of guselkumab in adult patients with moderate to severe psoriasis have been reported: VOYAGE 1 (NCT02207231), VOYAGE 2 (NCT02207244) and NAVIGATE (NCT02203032).41, 42, 43 In VOYAGE 1 (n = 837) and VOYAGE 2 (n = 922), patients were randomized to guselkumab (100 mg at Weeks 0 and 4, then every 8 weeks), adalimumab (80 mg at Week 0, 40 mg at Week 1, then 40 mg every 2 weeks) or placebo (Weeks 0, 4 and 12, then guselkumab at Weeks 16 and 20, then every 8 weeks).41, 42 Rates of AEs, serious AEs (SAEs) and infections were comparable across groups in both studies over the 48 weeks of follow‐up (Table 2). The most common AEs in all groups were nasopharyngitis, headache and upper respiratory infection, and few patients (≤4%) discontinued treatment owing to AEs. Incidence rates of serious infections and candidiasis were low and comparable between groups in both studies, and no AEs of inflammatory bowel disease were reported. Rates of malignancies and MACE were low in all groups (<1% over the study period). A single suicide attempt was reported in a patient taking adalimumab in VOYAGE 1.41 In VOYAGE 2, greater improvements in anxiety and depression scores were seen in patients treated with guselkumab than in those who received adalimumab or placebo.46
The NAVIGATE trial assessed guselkumab in adult patients with an inadequate response to ustekinumab.43 All patients (n = 871) initially received open‐label ustekinumab (45 or 90 mg at Weeks 0 and 4). At Week 16, 268 patients with an inadequate response were randomized to guselkumab (100 mg at Weeks 16 and 20, then every 8 weeks) or remain on ustekinumab. No safety concerns were identified in patients transitioning from ustekinumab to guselkumab, despite use of a guselkumab loading dose. The overall incidence of AEs was slightly higher in the guselkumab group than the ustekinumab group (64.4% vs. 55.6%) owing to a higher incidence of musculoskeletal and connective tissue disorders (e.g. back pain and psoriatic arthropathy), general disorders and mild injection‐site reactions. Overall, infections were the most common AE, occurring at a similar rate in both groups (41.5% for guselkumab and 35.3% for ustekinumab). Few patients discontinued treatment owing to AEs [3 (2.2%) in the guselkumab group and 2 (1.5%) in the ustekinumab group]. Rates of serious infection, malignancies and MACE were low (all ≤1.5% in both groups). No opportunistic infections, cases of active tuberculosis or AEs of Crohn's disease were reported.
Guselkumab was also well tolerated in a 52‐week, phase 3, placebo‐controlled study in 192 adult Japanese patients with moderate to severe plaque psoriasis (NCT02325219), with no safety concerns identified in this population.47 Again, nasopharyngitis was the most commonly reported AE in all groups.
Tildrakizumab
Week 28 data from two phase 3 trials of tildrakizumab have been reported: reSURFACE 1 (NCT01722331) and reSURFACE 2 (NCT01729754).44 A total of 772 adult patients were randomized (2 : 2 : 1) to tildrakizumab 200 mg, tildrakizumab 100 mg or placebo in reSURFACE 1; in reSURFACE 2, 1090 patients were randomized (2 : 2 : 2 : 1) to tildrakizumab 200 mg, tildrakizumab 100 mg, etanercept or placebo. In both studies, AEs and SAEs were reported at similar rates across all treatment groups, and discontinuations due to AEs were infrequent (Table 2). Nasopharyngitis was the most common AE in both studies. Candida skin infections and oral candidiasis were infrequent, and no cases of new onset or worsening of inflammatory bowel disease were reported. Incidences of serious infections, malignancies and MACE were low and similar across groups (≤1% in any group). One patient died in reSURFACE 2 in the tildrakizumab 100‐mg group; the patient had alcoholic cardiomyopathy and steatohepatitis, and adjudication was unable to determine the cause of death.
These results were confirmed by a pooled analysis of data from three randomized controlled trials [a phase 2b study (NCT01225731) and reSURFACE 1 and 2], which showed that long‐term treatment (>64 weeks) with tildrakizumab was well tolerated in patients with moderate to severe psoriasis.48 Data were included for 2081 adult patients treated with tildrakizumab 200 mg, tildrakizumab 100 mg, etanercept or placebo. Over the full‐trial period, exposure‐adjusted rates of AEs and SAEs with tildrakizumab were lower than or comparable with etanercept and placebo (Table 3). Rates of discontinuation due to AEs were lower in the tildrakizumab groups {both 2.2 per 100 patient‐years [95% confidence interval (CI) 1.3–3.3]} than etanercept [5.9 per 100 patient‐years (95% CI 2.7–11.0)] and were comparable with the placebo group [2.3 per 100 patient‐years (95% CI 0.7–5.3)]. No AEs of new onset or exacerbation of pre‐existing inflammatory bowel disease were reported. Candida skin infections and oral candidiasis were infrequent, with exposure‐adjusted rates per 100 patient‐years of 0.7, 0.2, 0 and 0 in the tildrakizumab 200‐mg, tildrakizumab 100‐mg, placebo and etanercept groups, respectively. Rates of severe infections, malignancies and MACE were low and similar across groups and compared favourably with those reported in similar pooled analyses of safety data for other classes of biologic agents.11, 12, 24, 49 No AEs of multiple sclerosis or suicides were reported. One suicide attempt was reported in a patient receiving tildrakizumab 200 mg and antipsychotic therapy for a known history of schizophrenia; this was considered unrelated to treatment by the investigator.
Table 3.
Incidence of AEs of special interest from long‐term follow‐up studies, pooled analyses and patient registries. Data are presented as rates per 100 PY11, 12, 24, 48, 49
| Biologic therapy | N | PY | AE | SAE | Infections | Severe/serious infections | Malignancies | NMSC | MACE | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Tildrakizumab 200 mg | 1041 | 929 | 79.3 | 7.2 | 52.6 | 1.6 | 1.2 | 0.9 | 0.3 | 48 |
| Tildrakizumab 100 mg | 1083 | 998 | 77.0 | 5.8 | 48.9 | 1.1 | 1.7 | 1.1 | 0.4 | |
| Etanercept 50 mg | 313 | 153 | 148.6 | 13.0 | 86.0 | 2.0 | 2.6 | 1.3 | 0 | |
| Placebo | 588 | 219 | 153.5 | 6.4 | 79.5 | 0.9 | 0.9 | 0.9 | 0.5 | |
| Ustekinumab 45 mg | 1319 | 3766 | 242.6 | 7.0 | 89.8 | 1.0 | 1.2 | 0.6 | 0.6 | 24 |
| Ustekinumab 90 mg | 2001 | 5232 | 225.3 | 7.2 | 84.1 | 1.2 | 1.1 | 0.4 | 0.4 | |
| Secukinumab pooled | 3430 | 2725 | 252.9 | 7.8 | 91.4 | 1.5 | 1.0 | 0.5 | 0.4 | 11 |
| Secukinumab 300 mg | 1410 | 1178 | 236.1 | 7.4 | 91.1 | 1.4 | 0.8 | 0.4 | 0.4 | |
| Secukinumab 150 mg | 1395 | 1142 | 239.9 | 6.8 | 85.3 | 1.1 | 1.0 | 0.6 | 0.4 | |
| Etanercept | 323 | 294 | 243.4 | 7.0 | 93.7 | 1.4 | 0.7 | 0 | 0.3 | |
| Ixekizumab 80 mg† | 4209 | 6480 | NR | NR | 39.2 | 1.3 | 0.9 | 0.4 | 0.6 | 12 |
| Ixekizumab 80 mg‡ | 1463 | 337 | 250.5 | 8.3 | 113.2 | 2.1 | 0.9 | 0.6 | 0.3 | |
| Etanercept‡ | 739 | 169 | 235.8 | 8.3 | 93.9 | 1.8 | 0.6 | 0 | 0.6 | |
| Placebo‡ | 360 | 83 | 192.3 | 8.4 | 89.0 | 2.4 | 0 | 0 | 1.2 | |
| Adalimumab | 6051 | 23 660 | 21.8 | 4.4 | NR | 1.0 | 1.0 | NR | <0.1§ | 49 |
†All treatment periods. ‡Placebo‐controlled treatment period (Weeks 0–12). §Congestive heart failure only.
AE, adverse event; MACE, major cardiovascular adverse event; N, number of patients included; NMSC, non‐melanoma skin cancer; NR, not reported; PY, patient‐years; SAE, serious adverse event.
Risankizumab
Two 52‐week, phase 3 trials of risankizumab vs. ustekinumab have been reported, with no unexpected safety findings: UltIMMa‐1 (NCT02684370) and UltIMMa‐2 (NCT02684357).45 In both studies, adult patients were randomized (3 : 1 : 1) to receive risankizumab 150 mg, ustekinumab 45 mg or 90 mg or placebo (506 in UltIMMa‐1 and 491 in UltIMMa‐2). Rates of AEs were similar across all groups during both the placebo‐controlled period (Weeks 0–16) and long‐term follow‐up (Weeks 16–52) (Table 2). Rates of discontinuation due to AEs were low and similar across all groups during both study periods. In both studies, infections were more frequently reported in patients receiving risankizumab or ustekinumab compared with placebo, and the most common AE was upper respiratory tract infection. Rates of serious infections were low (≤1% for risankizumab and placebo, ≤3% for ustekinumab). No clinically relevant opportunistic infections were reported, and there were no reports of Candida infections or new onset/worsening of inflammatory bowel disease. Incidences of malignancies and MACE were low (all ≤1%). No demyelinating disorders or suicide were reported. In UltIMMa‐2, one patient died in the risankizumab group of an unknown cause.
Other safety data
Data from head‐to‐head comparator trials of IL‐23p19 inhibitors vs. IL‐17 or IL‐12/23p40 inhibitors are starting to emerge. Risankizumab was compared with ustekinumab in a 48‐week phase 2 trial (NCT02054481)40 and in the phase 3 UltIMMAa‐1 and UltIMMAa‐2 studies, in which broadly similar safety profiles were reported for both treatments.45 Ustekinumab was compared with guselkumab in the NAVIGATE trial (described above),43 in which more AEs occurred in the guselkumab group (64%) than the ustekinumab group (56%). Further studies comparing treatments are ongoing or awaiting publication, including guselkumab vs. secukinumab (phase 2, NCT03553823; phase 3, NCT03090100) and vs. ixekizumab (phase 4, NCT03573323) and risankizumab vs. secukinumab (phase 3, NCT03478787). In addition, three recent meta‐analyses have assessed the safety of IL‐23p19 inhibitors compared with other classes of biologic agents used for the treatment of psoriasis.50, 51, 52 To avoid risk of confounding, these meta‐analyses exclusively used data from the placebo‐controlled treatment periods of randomized controlled trials (typically 12–16 weeks). Sbidian and colleagues50 conducted a network meta‐analysis of 109 studies to compare the efficacy and safety of conventional systemic agents, small molecules and biologic agents in 39 882 patients with moderate to severe psoriasis. They found no significant differences between tildrakizumab, guselkumab, ustekinumab, etanercept, infliximab, adalimumab and certolizumab verses placebo in terms of the risk of SAEs, serious infections, malignancies or MACE.50
The second meta‐analysis assessed the efficacy and safety of IL‐23p19, IL‐12/23p40 and IL‐17 inhibitors in moderate to severe plaque psoriasis and included data from 24 trials involving 15 388 patients.51 Safety outcomes included the number of patients with at least one AE, with at least one SAE and withdrawing from therapy because of an AE at 12–16 weeks. No differences in any safety outcomes were reported for tildrakizumab 100 mg or 200 mg compared with placebo. A slight, but statistically significant, increased risk in the number of AEs compared with placebo was seen for ustekinumab 45 mg, secukinumab 150 mg or 300 mg and brodalumab 140 or 210 mg. The number of withdrawals due to AEs was significantly higher for ixekizumab 80 mg vs. placebo irrespective of dosing frequency (every 2 or 4 weeks). Analysis of the other safety endpoints (number of SAEs and AEs) was not performed for ixekizumab owing to lack of available data. An increased frequency of Candida infections was seen with the IL‐17 inhibitors secukinumab, ixekizumab and brodalumab, consistent with previous reports.11, 12, 13
The third meta‐analysis was undertaken to compare the safety of biologics targeting IL‐17 and IL‐23 in the treatment of moderate to severe plaque psoriasis and found anti‐IL‐23 agents were associated with a lower risk of AEs.52 Data from the placebo‐controlled periods of 21 randomized clinical trials of secukinumab, brodalumab, ixekizumab, ustekinumab, guselkumab and tildrakizumab were included, involving 14 935 patients (11 100 of whom were treated with a biologic and 3835 with placebo). Tildrakizumab 200 mg was associated with the lowest risk of AEs [relative risk (RR) 0.88 (95% CI 0.78–0.99); P = 0.04 vs. placebo], and ixekizumab (160 mg at Week 0, then 80 mg every 4 weeks) was associated with the highest risk [RR 1.27 (95% CI 1.16–1.39); P < 0.00001 vs. placebo]. No statistically significant differences in SAEs were seen for any agent vs. placebo. The risk of AEs was significantly higher for biologics targeting IL‐17 [RR 1.18 (95% CI 1.12–1.24); P < 0.00001 vs. placebo] compared with IL‐23 [RR 0.97 (95% CI 0.91–1.04); P = 0.44 vs. placebo]. However, the risk of SAEs was similar in the IL‐17 and IL‐23 groups [RR 0.98 (95% CI 0.66–1.47); P = 0.94, and RR 1.13 (95% CI 0.69–1.86); P = 0.63, respectively, vs. placebo].
Studies of IL‐23p19 inhibitors in other indications, including ankylosing spondylitis, psoriatic arthritis and Crohn's disease, have also not identified any new or unexpected safety concerns.53, 54, 55, 56, 57
Unmet needs
Real‐world data concerning the safety of IL‐23p19 inhibitors in routine practice settings are not yet available. Registry data are important for identifying safety signals arising during wider and longer term clinical use, which may not have been recognized during clinical trials. Registry data may also provide reassurance regarding real‐world safety and tolerability by failing to confirm spurious signals occasionally seen in trials. For example, although three possible MACE were recorded in a phase 2 ustekinumab study,58 no further signal was identified in long‐term follow‐up or registry data.24, 25, 26
Although a significant proportion of patients with psoriasis are women of child‐bearing potential, only limited data are available concerning the safety of biologic agents during pregnancy. No reports of pregnancies during treatment with an IL‐23p19 inhibitor in clinical trials have been published, although preliminary data from 13 pregnancies during treatment with tildrakizumab reported no anomalies.59 Similarly, data from pregnant women with psoriasis treated with ustekinumab have not identified any safety concerns.60, 61 It is generally recommended that live vaccines should not be given to infants exposed to biologic agents in utero for at least 6 months after birth, because of a theoretical concern about the response to vaccination. Although there are no published data following exposure to IL‐23p19 inhibitors, the Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes (PIANO) registry found use of other biologic therapies by pregnant women with inflammatory bowel disease did not affect infant response to Haemophilus influenzae B or tetanus vaccines.62 In adult patients, long‐term (≥3 years) treatment with ustekinumab was not found to compromise immune response to pneumococcal or tetanus vaccines, but there are no data on vaccine response during treatment with IL‐23p19 inhibitors.63 However, it is recommended that patients receive all age‐appropriate immunizations prior to initiating treatment, and patients should not receive live vaccines while on therapy with ustekinumab, guselkumab or tildrakizumab.64, 65, 66
Biologic therapies are increasingly used for the treatment of moderate to severe psoriasis in children.67 Data on the safety of IL‐23p19 inhibitors in paediatric patients are not yet available, although a study of guselkumab in adolescents is ongoing (PROTOSTAR; NCT03451851). Ustekinumab has been approved for the treatment of moderate to severe plaque psoriasis in adolescents aged 12 years or older; the safety profile is similar to that in adults, with nasopharyngitis the most common AE and no unexpected AEs.68
Conclusions
Available data show anti‐IL‐23p19 agents have an acceptable safety profile in adults with moderate to severe psoriasis. The most commonly reported AEs during treatment with IL‐23p19 inhibitors in phase 3 studies were upper respiratory tract infections. Rates of serious infections, malignancies or MACE were extremely low and comparable to those seen with placebo or active comparators. Importantly, few patients discontinued treatment with IL‐23p19 inhibitors owing to AEs during clinical trials. Selectively targeting IL‐23p19 appears to avoid AEs that have been associated with biologic agents with other mechanisms of action, with no signals for elevated risk of opportunistic infections, tuberculosis, mucocutaneous Candida infections, de novo onset or potential exacerbation of inflammatory bowel disease or demyelinating disorders observed to date. Data from long‐term extension studies and patient registries are needed to fully establish the safety profile of these agents for the treatment of moderate to severe psoriasis in routine practice.
Conflicts of interest
JJC has received research/grant support from AbbVie, Amgen, Boehringer Ingelheim, Janssen, Lilly, MC2 Therapeutics, Merck & Co., Novartis, Pfizer, Regeneron, Sandoz, Sanofi, Sun Pharmaceuticals, UCB, Verrica Pharmaceuticals; has served as consultant for AbbVie, Amgen, Celgene, Dermira, Lilly, Novartis, Sun Pharmaceuticals, UCB; has worked on speakers bureau for AbbVie, Janssen, Lilly, Novartis, Regeneron, Sanofi, and UCB. RBW has received research grants from AbbVie, Almirall, Amgen, Celgene, Janssen, Lilly, Leo, Novartis, Pfizer & UCB; has received consulting fees from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen, Leo, Lilly, Novartis, Pfizer, Sanofi, Xenoport and UCB. JCC has received research support from AbbVie, Amgen, Celgene, Eli Lilly, Foamix, Galderma, Janssen, Leo Pharma, Menlo Therapeutics, Merck, Novartis, Pfizer, Regeneron, UCB; has served as a consultant for AbbVie, Celgene, Eli Lilly, Regeneron; has worked on speakers bureau for AbbVie, Celgene, Eli Lilly, and Regeneron.
Funding sources
Medical writing and editorial assistance in the development of this manuscript were provided by JK Associates, Inc., part of the Fishawack Group of Companies, and funded by Sun Pharmaceutical Industries, Inc.
References
- 1. World Health Organization . Global Report on Psoriasis. World Health Organization, Geneva, Switzerland, 2016. [Google Scholar]
- 2. Helmick CG, Lee‐Han H, Hirsch SC, Baird TL, Bartlett CL. Prevalence of psoriasis among adults in the U.S.: 2003‐2006 and 2009‐2010 National Health and Nutrition examination surveys. Am J Prev Med 2014; 47: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Girolomoni G, Strohal R, Puig L et al The role of IL‐23 and the IL‐23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ronholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci 2017; 18: E2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amgen . ENBREL® (etanercept). Highlights of prescribing information. November 2017. URL https://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/enbrel/enbrel_pi.pdf (last accessed: 10 October 2018).
- 6. AbbVie . HUMIRA® (adalimumab). Highlights of prescribing information. August 2018. URL https://www.rxabbvie.com/pdf/humira.pdf (last accessed: 10 October 2018).
- 7. Janssen Biotech . REMICADE® (infliximab). Highlights of prescribing information. June 2018. URL http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/REMICADE-pi.pdf (last accessed: 10 October 2018).
- 8. UCB I . CIMZIA® (certolizumab pegol). Highlights of prescribing information. June 2018. URL https://www.cimzia.com/sites/default/files/docs/CIMZIA_full_prescribing_information.pdf (last accessed 10 October 2018).
- 9. Kalb RE, Fiorentino DF, Lebwohl MG et al Risk of serious infection with biologic and systemic treatment of psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol 2015; 151: 961–969. [DOI] [PubMed] [Google Scholar]
- 10. Fiorentino D, Ho V, Lebwohl MG et al Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol 2017; 77: 845–854.e845. [DOI] [PubMed] [Google Scholar]
- 11. van de Kerkhof PC, Griffiths CE, Reich K et al Secukinumab long‐term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol 2016; 75: 83–98.e84. [DOI] [PubMed] [Google Scholar]
- 12. Strober B, Leonardi C, Papp KA et al Short‐ and long‐term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol 2017; 76: 432–440.e417. [DOI] [PubMed] [Google Scholar]
- 13. Farahnik B, Beroukhim K, Abrouk M et al Brodalumab for the treatment of psoriasis: a review of phase III trials. Dermatol Ther (Heidelb) 2016; 6: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hueber W, Sands BE, Lewitzky S et al Secukinumab, a human anti‐IL‐17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double‐blind placebo‐controlled trial. Gut 2012; 61: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lebwohl M, Strober B, Menter A et al Phase 3 Studies Comparing Brodalumab with Ustekinumab in Psoriasis. N Engl J Med 2015; 373: 1318–1328. [DOI] [PubMed] [Google Scholar]
- 16. Targan SR, Feagan B, Vermeire S et al A randomized, double‐blind, placebo‐controlled phase 2 study of brodalumab in patients with moderate‐to‐severe Crohn's disease. Am J Gastroenterol 2016; 111: 1599–1607. [DOI] [PubMed] [Google Scholar]
- 17. Conti HR, Shen F, Nayyar N et al Th17 cells and IL‐17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 2009; 206: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Puel A, Cypowyj S, Bustamante J et al Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin‐17 immunity. Science 2011; 332: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Connor W Jr, Kamanaka M, Booth CJ et al A protective function for interleukin 17A in T cell‐mediated intestinal inflammation. Nat Immunol 2009; 10: 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lebwohl MG, Papp KA, Marangell LB et al Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol 2018; 78: 81–89.e85. [DOI] [PubMed] [Google Scholar]
- 21. Valeant Pharmaceuticals . SILIQ™ (brodalumab). Highlights of prescribing information. February 2017. URL https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf (last accessed: 10 October 2018).
- 22. Lebwohl M, Leonardi C, Griffiths CE et al Long‐term safety experience of ustekinumab in patients with moderate‐to‐severe psoriasis (Part I of II): results from analyses of general safety parameters from pooled Phase 2 and 3 clinical trials. J Am Acad Dermatol 2012; 66: 731–741. [DOI] [PubMed] [Google Scholar]
- 23. Gordon KB, Papp KA, Langley RG et al Long‐term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol 2012; 66: 742–751. [DOI] [PubMed] [Google Scholar]
- 24. Papp KA, Griffiths CE, Gordon K et al Long‐term safety of ustekinumab in patients with moderate‐to‐severe psoriasis: final results from 5 years of follow‐up. Br J Dermatol 2013; 168: 844–854. [DOI] [PubMed] [Google Scholar]
- 25. Papp K, Gottlieb AB, Naldi L et al Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol 2015; 14: 706–714. [PubMed] [Google Scholar]
- 26. Bissonnette R, Kerdel F, Naldi L et al Evaluation of risk of major adverse cardiovascular events with biologic therapy in patients with psoriasis. J Drugs Dermatol 2017; 16: 1002–1013. [PubMed] [Google Scholar]
- 27. Gordon KB, Langley RG, Gottlieb AB et al A phase III, randomized, controlled trial of the fully human IL‐12/23 mAb briakinumab in moderate‐to‐severe psoriasis. J Invest Dermatol 2012; 132: 304–314. [DOI] [PubMed] [Google Scholar]
- 28. Langley RG, Papp K, Gottlieb AB et al Safety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open‐label extension trial of the interleukin‐12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis. J Eur Acad Dermatol Venereol 2013; 27: 1252–1261. [DOI] [PubMed] [Google Scholar]
- 29. Kulig P, Musiol S, Freiberger SN et al IL‐12 protects from psoriasiform skin inflammation. Nat Commun 2016; 7: 13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cao X, Leonard K, Collins LI et al Interleukin 12 stimulates IFN‐gamma‐mediated inhibition of tumor‐induced regulatory T‐cell proliferation and enhances tumor clearance. Cancer Res 2009; 69: 8700–8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garcia K, Sun Z, Mattson E, Li L, Smyth K, Xiao Z. IL‐12 is required for mTOR regulation of memory CTLs during viral infection. Genes Immun 2014; 15: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haraguchi S, Day NK, Nelson RP Jr et al Interleukin 12 deficiency associated with recurrent infections. Proc Natl Acad Sci USA 1998; 95: 13125–13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhuang Y, Calderon C, Marciniak SJ Jr et al First‐in‐human study to assess guselkumab (anti‐IL‐23 mAb) pharmacokinetics/safety in healthy subjects and patients with moderate‐to‐severe psoriasis. Eur J Clin Pharmacol 2016; 72: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 34. Sofen H, Smith S, Matheson RT et al Guselkumab (an IL‐23‐specific mAb) demonstrates clinical and molecular response in patients with moderate‐to‐severe psoriasis. J Allergy Clin Immunol 2014; 133: 1032–1040. [DOI] [PubMed] [Google Scholar]
- 35. Nemoto O, Hirose K, Shibata S, Li K, Kubo H. Safety and efficacy of guselkumab in Japanese patients with moderate‐to‐severe plaque psoriasis: a randomized, placebo‐controlled, ascending‐dose study. Br J Dermatol 2018; 178: 689–696. [DOI] [PubMed] [Google Scholar]
- 36. Gordon KB, Duffin KC, Bissonnette R et al A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med 2015; 373: 136–144. [DOI] [PubMed] [Google Scholar]
- 37. Kopp T, Riedl E, Bangert C et al Clinical improvement in psoriasis with specific targeting of interleukin‐23. Nature 2015; 521: 222–226. [DOI] [PubMed] [Google Scholar]
- 38. Papp K, Thaci D, Reich K et al Tildrakizumab (MK‐3222), an anti‐interleukin‐23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo‐controlled trial. Br J Dermatol 2015; 173: 930–939. [DOI] [PubMed] [Google Scholar]
- 39. Krueger JG, Ferris LK, Menter A et al Anti‐IL‐23A mAb BI 655066 for treatment of moderate‐to‐severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single‐rising‐dose, randomized, double‐blind, placebo‐controlled trial. J Allergy Clin Immunol 2015; 136: 116–124.e117. [DOI] [PubMed] [Google Scholar]
- 40. Papp KA, Blauvelt A, Bukhalo M et al Risankizumab versus ustekinumab for moderate‐to‐severe plaque psoriasis. N Engl J Med 2017; 376: 1551–1560. [DOI] [PubMed] [Google Scholar]
- 41. Blauvelt A, Papp KA, Griffiths CE et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76: 405–417. [DOI] [PubMed] [Google Scholar]
- 42. Reich K, Armstrong AW, Foley P et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76: 418–431. [DOI] [PubMed] [Google Scholar]
- 43. Langley RG, Tsai TF, Flavin S et al Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double‐blind, phase 3 NAVIGATE trial. Br J Dermatol 2018; 178: 114–123. [DOI] [PubMed] [Google Scholar]
- 44. Reich K, Papp KA, Blauvelt A et al Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017; 390: 276–288. [DOI] [PubMed] [Google Scholar]
- 45. Gordon KB, Strober B, Lebwohl M et al Efficacy and safety of risankizumab in moderate‐to‐severe plaque psoriasis (UltIMMa‐1 and UltIMMa‐2): results from two double‐blind, randomised, placebo‐controlled and ustekinumab‐controlled phase 3 trials. Lancet 2018; 392: 650–661. [DOI] [PubMed] [Google Scholar]
- 46. Gordon KB, Armstrong AW, Han C et al Anxiety and depression in patients with moderate‐to‐severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the Phase 3 VOYAGE 2 study. J Eur Acad Dermatol Venereol 2018; 32: 1940–1949. [DOI] [PubMed] [Google Scholar]
- 47. Ohtsuki M, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, an anti‐interleukin‐23 monoclonal antibody, for the treatment of moderate to severe plaque‐type psoriasis in Japanese patients: efficacy and safety results from a phase 3, randomized, double‐blind, placebo‐controlled study. J Dermatol 2018; 45: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blauvelt A, Reich K, Papp KA et al Safety of tildrakizumab for moderate‐to‐severe plaque psoriasis: pooled analysis of three randomized controlled trials. Br J Dermatol 2018; 179: 615–622. [DOI] [PubMed] [Google Scholar]
- 49. Menter A, Thaci D, Wu JJ et al Long‐term safety and effectiveness of adalimumab for moderate to severe psoriasis: results from 7‐year interim analysis of the ESPRIT registry. Dermatol Ther (Heidelb) 2017; 7: 365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sbidian E, Chaimani A, Garcia‐Doval I et al Systemic pharmacological treatments for chronic plaque psoriasis: a network meta‐analysis. Cochrane Database Syst Rev 2017; 12: CD011535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bilal J, Berlinberg A, Bhattacharjee S, Trost J, Riaz IB, Kurtzman DJB. A systematic review and meta‐analysis of the efficacy and safety of the interleukin (IL)‐12/23 and IL‐17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis. J Dermatolog Treat 2018; 29: 569–578. [DOI] [PubMed] [Google Scholar]
- 52. Cui L, Chen R, Subedi S et al Efficacy and safety of biologics targeting IL‐17 and IL‐23 in the treatment of moderate‐to‐severe plaque psoriasis: a systematic review and meta‐analysis of randomized controlled trials. Int Immunopharmacol 2018; 62: 46–58. [DOI] [PubMed] [Google Scholar]
- 53. Smolen JS, Agarwal SK, Ilivanova E et al A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann Rheum Dis 2017; 76: 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baeten D, Ostergaard M, Wei JC et al Risankizumab, an IL‐23 inhibitor, for ankylosing spondylitis: results of a randomised, double‐blind, placebo‐controlled, proof‐of‐concept, dose‐finding phase 2 study. Ann Rheum Dis 2018; 77: 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deodhar A, Gottlieb AB, Boehncke WH et al Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double‐blind, placebo‐controlled, phase 2 study. Lancet 2018; 391: 2213–2224. [DOI] [PubMed] [Google Scholar]
- 56. Feagan BG, Panes J, Ferrante M et al Risankizumab in patients with moderate to severe Crohn's disease: an open‐label extension study. Lancet Gastroenterol Hepatol 2018; 3: 671–680. [DOI] [PubMed] [Google Scholar]
- 57. Terui T, Kobayashi S, Okubo Y, Murakami M, Hirose K, Kubo H. Efficacy and safety of guselkumab, an anti‐interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol 2018; 154: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krueger GG, Langley RG, Leonardi C et al A human interleukin‐12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 2007; 356: 580–592. [DOI] [PubMed] [Google Scholar]
- 59. Haycraft K, Kimball AB, Brunner M et al Outcomes of pregnancies from tildrakizumab phase 1–3 clinical development program Paper presented at: Dermatology Education Foundation Essential Resource Meeting 2018 NP/PA CME Conference 2018; Las Vegas, NV, USA.
- 60. Galluzzo M, D'Adamio S, Bianchi L, Talamonti M. Psoriasis in pregnancy: case series and literature review of data concerning exposure during pregnancy to ustekinumab. J Dermatolog Treat 2019; 30: 40–44. [DOI] [PubMed] [Google Scholar]
- 61. Watson N, Wu K, Farr P, Reynolds NJ, Hampton PJ. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol 2019; 180: 195–196. [DOI] [PubMed] [Google Scholar]
- 62. Beaulieu DB, Ananthakrishnan AN, Martin C, Cohen RD, Kane SV, Mahadevan U. Use of biologic therapy by pregnant women with inflammatory bowel disease does not affect infant response to vaccines. Clin Gastroenterol Hepatol 2018; 16: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brodmerkel C, Wadman E, Langley RG et al Immune response to pneumococcus and tetanus toxoid in patients with moderate‐to‐severe psoriasis following long‐term ustekinumab use. J Drugs Dermatol 2013; 12: 1122–1129. [PubMed] [Google Scholar]
- 64. Janssen Biotech . STELARA® (ustekinumab). Highlights of prescribing information. October 2017. URL http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/STELARA-pi.pdf (last accessed: 25 October 2018).
- 65. Janssen Biotech . TREMFYA® (guselkumab). Highlights of prescribing information. October 2017. URL http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TREMFYA-pi.pdf (last accessed: 25 October 2018).
- 66. Sun Pharmaceutical Industries . ILUMYA™ (tildrakizumab‐asmn). Highlights of prescribing information. August 2018. URL https://www.ilumyapro.com/assets/pdf/Sun_Pharma_ILUMYA_US_Prescribing_Information.pdf (last accessed: 25 October 2018).
- 67. Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg 2018; 37: 167–172. [DOI] [PubMed] [Google Scholar]
- 68. Landells I, Marano C, Hsu MC et al Ustekinumab in adolescent patients age 12 to 17 years with moderate‐to‐severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol 2015; 73: 594–603. [DOI] [PubMed] [Google Scholar]


