Summary
Hundreds of nonphotosynthetic mycoheterotrophic plant species cheat the arbuscular mycorrhizal symbiosis. Their patchy local occurrence suggests constraints by biotic and abiotic factors, among which the role of soil chemistry and nutrient status has not been investigated.
Here, we examine the edaphic drivers predicting the local‐scale distribution of mycoheterotrophic plants in two lowland rainforests in South America. We compared soil chemistry and nutrient status in plots where mycoheterotrophic plants were present with those without these plants.
Soil pH, soil nitrate, and the interaction between soil potassium and nitrate concentrations were the best predictors for the occurrence of mycoheterotrophic plants in these tropical rainforests. Mycoheterotrophic plant occurrences decreased with a rise in each of these predictors. This indicates that these plants are associated with low‐fertility patches. Such low‐fertility conditions coincide with conditions that potentially favour a weak mutualism between plants and arbuscular mycorrhizal fungi according to the trade balance model.
Our study points out which soil properties favour the cheating of arbuscular mycorrhizal networks in tropical forests. The patchy occurrence of mycoheterotrophic plants suggests that local soil heterogeneity causes the stability of arbuscular mycorrhizal networks to vary at a very small scale.
Keywords: arbuscular mycorrhizal fungi, cheating, mycoheterotrophy, nitrate, potassium, soil
Introduction
Mycorrhizal symbiosis is one of the most widespread interactions on Earth (van der Heijden et al., 2015). Typically, it is a mutually beneficial interaction in which plants transfer photosynthesised carbon to their mycorrhizal fungal partners, which in turn facilitate the uptake of mineral nutrients from the soil, enhancing plant nutrition (Smith & Read, 2008). Symbiosis is therefore extremely important in soils of low nutrient availability or where the distribution of nutrients is heterogeneous (Cavagnaro et al., 2005). Yet, mycoheterotrophic plants have evolved a strategy in which the carbon flux is reversed from their fungal partners to themselves so that the plants depend exclusively on their mycorrhizal partners to obtain carbohydrates (Leake, 1994). It remains to be investigated whether these plants provide any benefit to their associated fungi, such as vitamins or protection, and therefore reciprocate Alternatively, they may subvert the ‘biological market’ established between plants and mycorrhizal fungi, where plants trade carbohydrates for soil nutrients with their mycorrhizal partners (Selosse & Rousset, 2011). There are over 500 fully mycoheterotrophic plant species, of which about half the total number is associated with arbuscular mycorrhizal (AM) fungi (Merckx, 2013). As these plants require the continuous presence of an established mycorrhizal network to support their carbon demands during the entire life cycle, ultimately relying on the surrounding photosynthetic plants, mycoheterotrophy can be regarded as a mechanism enabling cheating of mycorrhizal symbiosis (van der Heijden & Walder, 2016).
Many species of mycoheterotrophic plants have remarkably widespread distributions, yet at a local scale their distribution is often highly scattered (Cheek & Williams, 1999; Bergman et al., 2006; Merckx et al., 2013; Yamato et al., 2016). The patchy occurrence of these plants suggests that, besides the need to fulfil general global scale requirements, such as appropriate soil water content (Maas et al., 1986; Cheek & Williams, 1999) or shade conditions (Leake, 1994; Cheek & Williams, 1999; Bidartondo et al., 2004) within particular forest types (Gomes et al., 2019), the presence of mycoheterotrophic plants is constrained by particular local‐scale factors. Due to the reliance of mycoheterotrophic plants on mycorrhizal networks, both biotic (interactions with their fungi) and abiotic (soil conditions) factors can potentially contribute to their occurrence at a local scale. Previous studies have shown highly species‐specific interactions between these plants and their fungal partners from a local to a global scale (Yamato et al., 2016; Gomes et al., 2017a; Renny et al., 2017), although the degree of specificity may vary (Courty et al., 2011; Gomes et al., 2017b). This situation could indicate that the occurrence of their fungal associates may determine the distribution of mycoheterotrophic plants (Bougoure et al., 2009; McCormick et al., 2009; Yamato et al., 2016). However, Merckx et al. (2017), suggesting that the distribution of AM fungi does not drive the distribution of highly specialised mycoheterotrophic plants in the genus Thismia, as their specific fungal associates were found to occur beyond the range of the plants’ distribution. Also, for mycoheterotrophic plants associated with ectomycorrhizal fungi, it has been shown that mycoheterotrophs are not present in all instances in which their fungal partners are available (Ogura‐Tsujita & Yukawa, 2008; Waterman et al., 2011; Davis et al., 2015). Hence, the presence of specific fungi alone is probably not sufficient to explain why mycoheterotrophs establish in particular patches of soil and avoid others. Neither is the presence of specific autotrophic green hosts, as AM mycoheterotrophs have been reported to occur in forests dominated by diverse tree species across continents (Merckx et al., 2013 and references therein). Moreover, soil nutrient availability may have an impact on the occurrence of mycoheterotrophic plants by affecting these directly or indirectly via the AM networks upon which these plants rely. Studies that examine which soil characteristics influence the occurrence of mycoheterotrophic plants at a local scale, either directly or indirectly, are lacking. Here, we examined which soil properties are associated with the patchy presence of AM mycoheterotrophic plants at the local scale. We conducted our study in two tropical rainforest regions differing in, among other factors, soil fertility, to assess the generality of the relationships.
Materials and Methods
Study area
Mycoheterotrophic plants are ephemeral, and their flowering periods are quite short (Leake, 1994). This situation indicates that there is probably a temporal and seasonal variation in the factors that drive the occurrence of these plants. This study took place at the beginning of the wet season, and coincided with the time of year when mycoheterotrophs have been recorded to flower (Cheek & Williams, 1999), reflecting the most favourable conditions to find them in the field. We sampled two forest regions in Colombia, where mycoheterotrophic plant species are known to occur. We spent 5 d sampling in each region. The first region consisted of wet tropical lowland forest on terra firme, part of the Amazon rainforest near Leticia (‘Amazon’; 4°00′30″S 70°06′12″W). The second region consisted of wet tropical coastal forest on terra firme, part of the Chocó rainforest, near Buenaventura (‘coast’; 3°55′24″N 77°18′56″W).
Large‐scale patterns of soil properties do not necessarily reflect the high heterogeneous profiles of soil at a local scale, therefore we opted for a paired plot sampling strategy – in each of the regions – where a ‘positive’ plot with mycoheterotrophic plants was simultaneously selected alongside with a nearby ‘negative’ plot without visible mycoheterotrophic plants. Because mycoheterotrophic plants without flowers can be overseen due to their small habit and lack of vegetative structures, it is possible that these plants were still present in the negative plots, therefore we carefully searched for these by removing the leaf litter in the ‘negative’ plots. Through this design we were able to identify the effects of specific local differences in soil properties on the patchy occurrences of mycoheterotrophy, within the presumable large‐scale variation in soil parameters among regions. Furthermore, it is possible that particular autotrophic plant species have a certain impact in shaping soil properties; this could also contribute to differences between plots. In this case, the impact of functional plant types is reflected in the soil properties, including their potential associations to particular AM fungi, which are accounted for by using the paired design of our study.
We established 16 pairs of plots of 4 × 4 m in the two forests that represented the spatial extent to which populations of mycoheterotrophic plants often occur, based on > 10 yr of personal observations in the field (by VSFT Merckx, and personal communication with P. Maas), and previous studies (Gomes et al., 2017b). We delimited five pairs of plots in the Amazon and 11 on the coast. Positive and negative plots were 5–10 m apart, this distance was close enough to avoid a change in substrate conditions once mycoheterotrophic plants were no longer observed. Pairs of plots were separated by at least 30 m to ensure sufficient distance between paired plots. The number of mycoheterotrophic plants in the positive plots varied between 1 and 22 individuals, and we found up to six species per plot (Supporting Information Table S1). Within each plot, we randomly collected six soil cores, and combined them into a 250 g composite sample per plot. The soil in both regions had clay texture. Soil cores were taken in the shallow top layer of the soil (0–5 cm depth) because we were interested in the chemical properties and nutrient abundance in the soil layer where the roots of the mycoheterotrophic plants are found. Big stones and roots were removed from the samples. The soil was homogenised and preserved on ice immediately after collection and during transportation to the laboratory for further processing.
While we recognise that biotic interactions including the abundance of associated fungi can play an important role on plant's occurrence patterns (Chagnon & Bradley, 2013), methods to quantify biomass of particular AM taxa while excluding others – to target the preferred AM fungi associated with mycoheterotrophs – are currently unavailable. Furthermore, previous studies have shown that the mere presence of the preferred AM fungi does not guarantee the presence of a mycoheterotroph (Ogura‐Tsujita & Yukawa, 2008; Waterman et al., 2011; Davis et al., 2015; Merckx et al., 2017). Therefore, we did not include biotic interactions in our analysis.
Soil chemistry and nutrient analyses
Soil chemical and nutrient properties were assessed for all 32 plots. Each sample was analysed for soil pH. Total amounts of nitrogen (Ntot) and phosphorus (Ptot) were estimated using the Kjeldahl method (Bremner, 1960). The available nitrogen (NH4 + and NO3 −) in the soil was determined using spectrophotometry and 1 N potassium chloride (Maynard & Kalra, 1993). The available phosphorus (Pav) was extracted using a Bray II solution (Murphy & Riley, 1962). Exchangeable bases (Na, K, Ca and Mg) were measured using the ammonium acetate method (Hanway & Heidel, 1952) and determined using atomic absorption spectrometry. The available micronutrients (Cu, Zn, Mn and Fe), available boron (B), sulfur (S), aluminium (Al), cation exchange capacity (CEC), and soil moisture content were determined according to Carter & Gregorich (2008). Organic matter (OM) content in the soil was determined according to Walkley & Black, 1934. All analyses were performed using the Centro Internacional de Agricultura Tropical in Colombia. Total soil C and N (on air‐dried soil), and abundance of δ13C and δ15N were analysed at UC Davis (University of California, Davis). To evaluate the influence of nutrient stoichiometry on soil processes, we calculated the N : P, N : K, C : P and C : N ratios.
Data analysis
We tested for differences in overall soil composition among positive and negative plots across both regions – that might affect the relationships to the local patchy occurrence of mycohetereotrophic plants – using a one‐way permutational multivariate analysis of variance (perMANOVA with 999 permutations). We tested for homogeneity of dispersion among groups before performing the perMANOVA and confirmed the assumption of homogeneous dispersion among regions (P = 0.753), and between negative and positive plots within the Amazon (P = 0.198) and the coast (P = 0.873). We visualised these differences through principal component analysis (PCA).
Given the heterogeneity in soil properties at the regional scale, average concentrations of nutrients in the soil in positive and negative plots can disguise the true effect of specific soil properties on the local selection of mycoheterotrophic occurrences. Our paired plot design allows a detailed local‐scale analysis, focusing on local differences. Therefore, we calculated the difference in the soil parameter values within each pair of negative and positive plots, which hereafter we refer to as delta (∆). A negative delta indicated that a specific parameter was lower in plots where mycoheterotrophic plants were absent, and a positive delta indicated that the parameter was lower in the plot where these plants were present. We tested whether there were significant differences across all deltas of the soil properties among regions using perMANOVA (homogeneity of dispersion: P = 0.713). We examined whether the delta of individual soil properties varied across regions using ANOVAs with ‘Region’ as factor, using the general linear hypothesis test (glht) function of R package lsmeans. Accounting for region in the analysis, allows assessing whether soil properties associated to the presence of mycoheterotrophic plants differ between regions. These delta values do not represent the actual concentrations under which plants are influenced, but they allow us to better quantify the effects of local‐scale variations in soil nutrients. Furthermore, we examined whether the actual values of the soil properties varied between positive and negative plots within each region using ANOVAs with subsequent Tukey's Honest Significant Difference test for correction of the P‐values.
To assess which combination of soil properties was most strongly related to the distribution of mycoheterotrophic plants, we selected all soil properties that were significantly different between positive and negative plots. Using the actual measurements from the soil properties in each positive and negative plot, each soil property was standardised to mean = 0 and SD = 1 to avoid scaling variance issues due to different measurement scales. With the soil properties, we built generalised mixed‐effects models (GLMMs) with ‘region’ and ‘plot’ as random effect terms to account for the overall soil properties differences among regions, and our paired plots design, respectively. To better understand the drivers for both the occurrence and abundance of mycoheterotrophic plants, we built two models with the presence/absence, and the density of mycoheterotrophic plants, respectively, as dependent variable in each of the models.
Model selection was performed for each model separately by adding terms, including interactions between variables, and selecting the terms that gave the greatest improvement to the model likelihood, as assessed using the lowest Bayesian Information Criteria (BIC; Aho et al., 2014). The variables included in the final model were retained if they were significant, and had a variance inflation factor (VIF) < 4 (Zuur et al., 2010) and showed a Pearson correlation with all other modelled predictors < |0.70| (Dormann et al., 2013). The coefficient of determination (R 2) of the GLMMs was calculated based on Nakagawa & Schielzeth (2013).
All analyses were performed in R 3.4.1 (R Core Team, 2016), using the packages ‘lme4’, ‘multcomp’, ‘r2glmm’, ‘vegan’, ‘lsmeans’.
Results
Soil characteristics
We obtained 21 soil parameters from the soil analyses (Table S2). Overall, soil characteristics were significantly different between the two regions (F = 28.338, R 2 = 0.49, P = 0.001; Fig. S1). Across all soil parameters, there was no general significant difference between positive and negative plots in the Amazon (F = 0.738, R 2 = 0.08, P = 0.627; Fig. 1a), but there was a difference in the coast (F = 3.079, R 2 = 0.13, P = 0.044; Fig. 1b). In addition, across all deltas of soil parameters there was a tendency for more positive delta values in the Amazon than in the coast (F = 0.266, R 2 = 0.14, P = 0.051; Fig. 2). When considering each soil property individually, in the Amazon region, the availability of NO3, Pav, CEC and pH was significantly lower (Table S2) in the positive plots compared to the respective negative plots. These are different soil properties than those varying most within the Amazon region, being OM, soil moisture content, Ntot, 15N, B and Zn (Fig. 1a). This result indicates that the variation in soil properties at the regional scale in the Amazon was different from the variation in soil properties at the local scale. At the coast, positive plots had significantly higher availability of OM, soil moisture content, CEC, Ntot, and positive ions such as K, Ca, Mg, B, Zn; and lower availability of 15N (Table S2), which corresponded to the same soil properties that had more variation within this region (Fig. 1b). This indicates that the variation in soil properties at the local scale is similar to those at the regional scale in the coast. At the local scale, the deltas of soil showed different trends within each region, suggesting that the local selection of mycoheterotrophic plants by soil properties, leading to the local patchy distribution of mycoheterotrophic plants, differs among the regions (Fig. 2; Table S3). We selected those soil properties that were significantly different between positive and negative plots in both regions (Table S2) to build the generalised mixed‐effects models.
Figure 1.
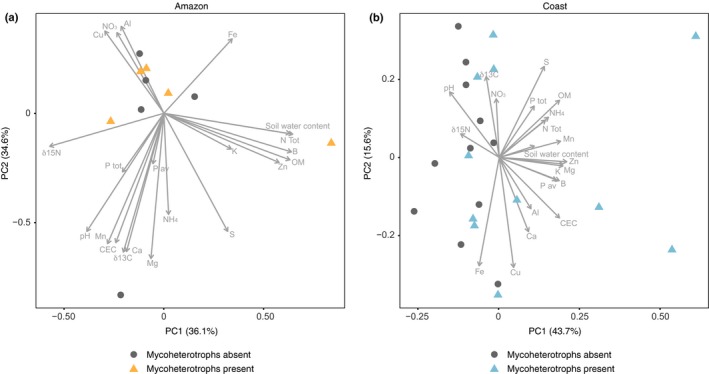
Principal component (PC) analysis of the soil properties in the positive plots (triangles) and negative plots (circles) present in (a) the Amazon and (b) the coast. Length of the arrows represents the relative importance of individual properties in explaining the overall pattern.
Figure 2.
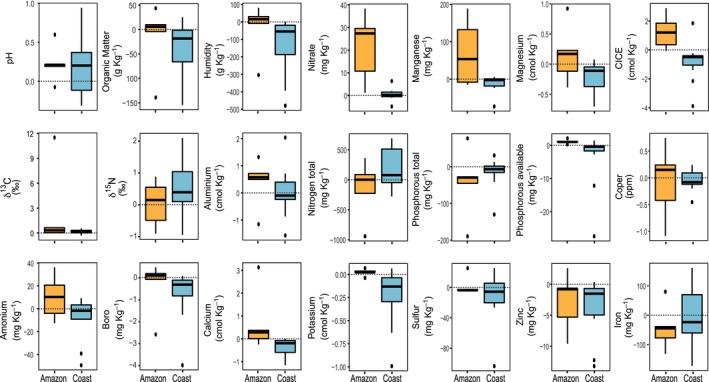
Box and whisker plots representing the variation of soil properties between the negative and positive plots in the Amazon (yellow) and the coast (blue). Positive values indicate higher availability of a soil property in the negative plots, while negative values indicate a higher availability in the positive plots.
Model selection
The best model for the occurrence of mycoheterotrophic plants showed a significant effect of NO3, pH and the interaction between NO3 and K (GLMM: R 2 = 0.64, BIC = 43.8; Table 1). The nature of the interaction between NO3 and K is explained by NO3 varying while K is constant in the Amazon, and of K varying while NO3 is constant in the coast among negative and positive plots (Fig. 3a; Table S3). The second best model showed a significant effect of soil moisture content and pH and the interaction between soil moisture content and OM (GLMM: R 2 = 0.54, BIC = 49.7; Table 1). OM was highly correlated with K (Pearson correlation: R 2 = 0.67) and Zn (Pearson correlation: R 2 = 0.72), and K correlated with Zn (Pearson correlation: R 2 = 0.74), not allowing to separate their impacts. Therefore, these parameters were not included in the same model.
Table 1.
Outcomes of the Generalised Linear Mixed Effect modelling aimed to explain the occurrence of mycoheterotrophic plants
| Model | Terms | Coefficient | SE | z value | P‐value |
|---|---|---|---|---|---|
| 1 | Intercept | 1.038 | 0.710 | 1.463 | 0.144 |
| NO3 | −3.432 | 1.347 | −2.548 | 0.011 | |
| pH | −1.405 | 0.820 | −1.713 | 0.087 | |
| NO3 : K | −11.165 | 4.264 | −2.618 | 0.009 | |
| 2 | Intercept | −0.474 | 0.614 | −0.772 | 0.440 |
| Soil moisture content | 5.052 | 2.377 | 2.126 | 0.034 | |
| pH | −2.157 | 1.137 | −1.897 | 0.058 | |
| pH : OM | 4.538 | 2.226 | 2.038 | 0.042 |
Model 1 is the best model; 2 is the best alternative model (ΔBIC = 5.9).
Figure 3.
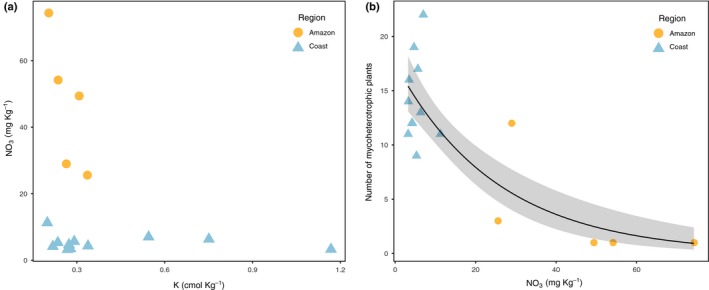
Relationships between (a) the actual concentration of NO 3 vs K when in positive plots, showing the nature of interaction between the two nutrients. When K is the lowest, NO 3 varies, and when NO 3 is available in the lowest concentrations, K varies; and (b) the number of mycoheterotrophic plants observed in the positive 4 × 4 m plots and the concentration of NO 3. The solid line represents the observed trend with the 95% confidence interval (grey area).
When density of mycoheterotrophic plants was evaluated instead of occurrence, we obtained two undistinguishable best models. The first model showed a significant effect of soil moisture content, pH and the interaction between NO3 and K (GLMM: R 2 = 0.11, BIC = 247.1; Table 2). The other best model showed a significant effect of soil moisture content and pH and the interaction between pH and OM (GLMM: R 2 = 0.11, BIC = 244.9; Table 2).
Table 2.
Outcomes of the Generalised Linear Mixed Effect modelling aimed to explain the density of mycoheterotrophic plants
| Model | Terms | Coefficient | SE | z value | P‐value |
|---|---|---|---|---|---|
| 1 | Intercept | −0.538 | 1.240 | −0.434 | 0.665 |
| Soil moisture content | 2.440 | 0.974 | 2.505 | 0.012 | |
| pH | −1.377 | 0.417 | −3.306 | 0.001 | |
| NO3 : K | −2.634 | 1.131 | −2.330 | 0.020 | |
| 2 | Intercept | 0.674 | 0.69 | 0.996 | 0.334 |
| Soil moisture content | 3.023 | 0.746 | 4.050 | 5.12e−05 | |
| pH | −1.976 | 0.391 | −5.056 | 4.29e−07 | |
| pH : OM | 1.108 | 0.360 | 3.080 | 0.002 |
Models 1 and 2 are not significantly different (ΔBIC = 2.2).
The number of mycoheterotrophic plants decreased with the increasing difference in concentration of ΔNO3 or NO3, corresponding to an increase in total concentration of NO3 (Pearson correlation between ΔNO3 and NO3: R 2 = 0.77; Fig. 3b).
Discussion
In this study, we compared soil characteristics of paired plots with and without flowering mycoheterotrophic plants to infer local‐scale drivers that influenced the occurrence and abundance of plant cheaters in the AM symbiosis in tropical rainforests. The Amazon and coast sites had overall different soil properties, leading to distinct soil properties that affected the presence of mycoheterotrophic plants at the regional scale. These regional differences in soil properties and associated regional preferences of mycoheterotrophs do not necessarily reflect local‐scale preferences. When the datasets of the Amazon and coast regions were analysed together, to better understand the local‐scale drivers for the occurrence of mycoheterotrophic plants, we found that the strongest local edaphic predictors of mycoheterotrophic species occurrences involved the interaction between NO3 and K, and the individual effects of NO3 and pH. Concerning the density of mycoheterotrophic plants individuals, we found that soil moisture content and pH together with an interaction between either NO3 and K, or pH and OM, best explained the densities found in the sampled plots. The model predicting the density of mycoheterotrophic plants explained less variance than the model predicting their occurrence, suggesting that other predictors, not accounted in the present study, may be important to determine the density of these plants. It also showed that soil moisture content appeared to influence mycoheterotrophic plants’ occurrence, but to a lesser extent than soil fertility at the local scale overall the two regions in this study. Soil moisture has been hypothesised to be the main limiting factor to the occurrence of these plants at the global scale, due to their sensitivity to desiccation (Leake, 1994; Klooster & Culley, 2009), but our study showed that mycoheterotrophic plants had a stronger selection for other soil conditions at the local scale. Due to the high correlation between K and OM and K and Zn, the effect of K in both models may also partly resemble effects through OM and/or Zn. Consistently the same interacting terms, between NO3 and K, were selected in models for both the occurrence and density of mycoheterotrophic plants.
The interaction between soil NO3 and K is well known to mediate crop responses to fertilisation. Crop response to added nitrogen fertilisers decreases when the exchangeable potassium content of a soil is below an optimal level, because plants deficient in potassium content are not able to produce proteins despite an abundance of available nitrogen (Ranade‐Malvi, 2011). In addition, several studies have shown a negative impact of nitrogen addition in agriculture systems on the AM symbiosis performance (Kabir et al., 1998; Galvez et al., 2001; Oehl et al., 2004), because an increased availability of nitrogen to plant roots can lead to a reduced allocation of carbon to their AM fungal partners, which can in turn induce phosphorus deficiency due to carbon limitation to the fungi (Olsson et al., 2005). Moreover, excessive amounts of N reduce the plant uptake of P, K and other micronutrients (Ranade‐Malvi, 2011). Nutrient stoichiometry in soils has been shown to be crucial in determining the relative availability of nutrients for plant uptake and the stability of the AM symbiosis (Johnson, 2010; Khan et al., 2015). The importance of NO3 : K stoichiometry revealed using our models suggested that for the occurrence and density of mycoheterotrophic plants, nutrient stoichiometry matters. Next to nutrient stoichiometry, pH was also an important predictor. The pH strongly influences the availability of nutrients in the soil, which in turn also impacts the efficiency of nutrient uptake by plants (Rippy et al., 2004), and by the AM fungi directly (see for example Ouzounidou et al., 2015), or indirectly through other associated microbes such as bacteria (Svenningsen et al., 2018).
Our study showed that the impacts of NO3 and K on mycoheterotrophic plant occurrence depend on soil fertility. At conditions of heterogeneous high fertility patches and high soil moisture in the Amazon, mycoheterotrophic plants tend to occur in patches with low NO3, probably to avoid high fertility conditions, while there is no selection for K. In the coast, where fertility is lower than in the Amazon, there is no selection for NO3 but occurrence of mycoheterotrophs tends to be driven by potassium availability instead. The effect of potassium in the coast may reflect a preference for high OM in this region. potassium and OM were correlated, and OM was significantly higher in positive plots. This implies that habitat conditions at the regional level determined the extent to which particular local drivers are important. While there seems to be a consistent avoidance of higher fertility patches, also reflected in the lower density of plants found with increasing NO3 availability (Fig. 3b), it remains unclear how K influences the distribution of these plants. A possible explanation for the overall increased availability and patchiness of K in the coast is the effect of salt spray from the sea that is close by. The uptake of K by photosynthetic plants is enhanced by the association with AM fungi, which require a minimum availability of K in the soil for the stability of the AM symbiosis (Khan et al., 2015).
Interestingly, available phosphorus did not relate to local mycoheterotrophic plants’ occurrences in this study, even though we find a trend for plants to select for lower N : P ratios in the positive plots compared with the negative plots (Table S2). This result strongly contrasted to the impacts of phosphorus on plant and microbial communities at regional scales: available phosphorus – together with soil moisture – has been suggested to be the strongest environmental predictor of plant species distributions in tropical forests (Condit et al., 2013); and phosphorus is considered to be the main limiting element in tropical forests for microbial processes, including mycorrhizal fungi (Camenzind et al., 2017). Moreover, AM fungal diversity is known to be lower when phosphorus availability is very high (Gosling et al., 2013). At regional scales, phosphorus has also been shown to play an important role in determining plant and fungal distributions, including those of mycoheterotrophic plants (Sheldrake et al., 2017). In a natural fertility gradient across a 65 km forest in Panama, Sheldrake et al. (2017) tested the impact of nutrient availability on the occurrence and density of two mycoheterotrophic species of the genus Voyria, at the regional scale. Their results suggested that the occurrence of these plants is limited by high phosphorus concentration in the soil, which was further supported by the outcomes of their nutrient addition experiment. In our study, at the local scale, phosphorus did not significantly vary between the positive and negative plots. This finding suggested that there is not a direct selection for particular concentrations of phosphorus at the selected local ecological scale. Instead, our results highlighted that nutrient stoichiometry (as exemplified by the NO3 : K interaction) rather than the actual concentration of any nutrient, including phosphorus, drives the local occurrence and density of mycoheterotrophic plants. Hence, our study stressed the fact that regional patterns can be different from local drivers.
Also, the trade balance model (Johnson, 2010) that aims to explain the stability of symbiotic outcomes between plants and AM fungi, stresses the critical role of stoichiometry of soil nutrients compared to the actual abundances of nutrients. Within this framework, a stable relationship is expected when the trade of C‐for‐P is advantageous for both plants and AM fungi. When this stable relationship occurs at high N : P ratios, both AM fungi and plants have easy access to N. Therefore, high N : P ratios may stimulate plants to invest more C to their AM fungi, which in turn provides plants with increased access to P in the soil, resulting in a strong mutualism (Johnson et al., 2003). By contrast, at low N : P ratios, plants and AM fungi compete for N, originating less C available in the system, leading to a less balanced or weaker mutualism (Johnson et al., 1997). Despite the known importance of P in determining the general occurrence and distribution of plants (Condit et al., 2013) and AM fungi (Camenzind et al., 2017), and of the N : P ratio for the stability of AM networks (Johnson, 2010), the N : K ratio and not the N : P ratio appeared to be a more relevant predictor for the local distribution of mycoheterotrophic plants. In our study, mycoheterotrophic plants preferred patches with lower N : K ratios by selecting for high K at high fertility conditions, whereas at low‐fertility conditions, they preferred patches with general lower nutrients availability and lower N and P – and to a lesser extent K – but not sufficiently to significantly affect the effect of N : K and K : P ratios (Table S2). In our study, mycoheterotrophic plants seemed to avoid high fertility patches where fungi are prone to parasitise autotrophic plants (high N, high P or K), as suggested by lower N : P or N : K ratios. Following the trade balance model (Johnson, 2010), we hypothesised that mycoheterotrophic plants avoid patches with conditions that coincide with those favouring a strong mutualism between plants and AM fungi (high N, low P or K), preferring conditions that were similar to when the mutualism is weak (low N, low P or K). In conditions leading to commensalism between plants and AM fungi (low N, high P or K), cheating is less likely to occur, as both partners are exchanging limited resources, and therefore it is theoretically more difficult for mycoheterotrophic plants to obtain carbon from these fungi. We believe that this hypothesis deserves further testing.
In conclusion, our study highlighted the scale‐dependent effect of environmental drivers and complemented the current knowledge on the ecological edaphic preferences of mycoheterotrophic plants. Leake (1994) has suggested high soil moisture and deep shaded forests as the preferred habitats for the distribution of mycoheterotrophic plants at the global scale. Sheldrake et al. (2017) revealed that phosphorus concentrations limit the occurrence of mycoheterotrophic plants along gradients at the regional scale. Finally, the present study unravelled the importance of nutrient stoichiometry in the soil to explain the patchy occurrence pattern of these plants at the very local scale. Our findings also showed a negative response of the abundance of mycoheterotrophs individuals to an increase in N availability. Furthermore, our results provided empirical support to pose the hypothesis that mycoheterotrophic plants seem to avoid conditions that could favour a strong AM mutualism (high N, low P or K), according to the trade balance model (Johnson, 2010).
Author contributions
SIFG, PMvB, VSFTM and NAS designed the study. SIFG and VSFTM conducted fieldwork. SIFG conducted the analyses and wrote the first draft of the manuscript. All authors contributed to discussion and to earlier versions of the manuscript.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Principal component analysis of the soil properties in the Amazon and the coast.
Table S1 Mycoheterotrophic plant species present in the study plots.
Table S2 Overall soil parameters in the negative and positive plots within the Amazon and coast regions.
Table S3 Variation of the soil parameters measured in the plots within the Amazon and coast calculated by the difference between negative and positive plots (deltas).
Acknowledgements
The authors thank the local guides from the ‘comunidad Maloca Los Yaguas’ in the Amazon and Julian Perdomo for assistance in the field. SIFG and NAS are supported by a VIDI grant 016.161.318 issued by the Netherlands Organization of Scientific Research. The field work was financially supported by a KNAW (Royal Netherlands Academy of Arts and Sciences) grant awarded to SIFG. We thank the valuable comments of three anonymous reviewers and the editor who helped improve the quality of the manuscript.
References
- Aho K, Derryberry D, Peterson T. 2014. Model selection for ecologists: the worldviews of AIC and BIC. Ecology 95: 631–636. [DOI] [PubMed] [Google Scholar]
- Bergman E, Ackerman JD, Thompson J, Zimmerman JK. 2006. Land‐use history affects the distribution of the saprophytic orchid Wullschlaegelia calcarata in Puerto Rico's tabonuco forest. Biotropica 38: 492–499. [Google Scholar]
- Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ. 2004. Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proceedings Biological Sciences/The Royal Society 271: 1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougoure J, Ludwig M, Brundrett M, Grierson P. 2009. Identity and specificity of the fungi forming mycorrhizas with the rare mycoheterotrophic orchid Rhizanthella gardneri . Mycological Research 113: 1097–1106. [DOI] [PubMed] [Google Scholar]
- Bremner JM. 1960. Determination of nitrogen in soil by the Kjeldahl method. Journal of Agricultural Science 55: 11–33. [Google Scholar]
- Camenzind T, Hättenschwiler S, Treseder KK, Lehmann A, Rillig MC. 2017. Nutrient limitation of soil microbial processes in tropical forests. Ecological Monographs 88: 4–21. [Google Scholar]
- Carter MR, Gregorich E, eds. 2008. Soil sampling and methods of analysis, 2nd edn Boca Raton: CRC Press, Taylor & Francis Group. [Google Scholar]
- Cavagnaro TR, Smith FA, Smith SE, Jakobsen I. 2005. Functional diversity in arbuscular mycorrhizas: exploitation of soil patches with different phosphate enrichment differs among fungal species. Plant, Cell & Environment 28: 642–650. [Google Scholar]
- Chagnon PL, Bradley RL. 2013. Evidence that soil nutrient stoichiometry controls the competitive abilities of arbuscular mycorrhizal vs. root‐borne non‐mycorrhizal fungi. Fungal Ecology 6: 557–560. [Google Scholar]
- Cheek M, Williams S. 1999. A review of african saprophytic flowering plants In: Timberlake J, Kativu S, eds. African plants: biodiversity, taxonomy and uses. Kew: Royal Botanic Garden, 39–49. [Google Scholar]
- Condit R, Engelbrecht BMJ, Pino D, Perez R, Turner BL. 2013. Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proceedings of the National Academy of Sciences, USA 110: 5064–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courty P‐E, Walder F, Boller T, Ineichen K, Wiemken A, Rousteau A, Selosse M‐A. 2011. Carbon and nitrogen metabolism in mycorrhizal networks and mycoheterotrophic plants of tropical forests: a stable isotope analysis. Plant Physiology 156: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Phillips RD, Wright M, Linde CC, Dixon KW. 2015. Continent‐wide distribution in mycorrhizal fungi: implications for the biogeography of specialized orchids. Annals of Botany 116: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ et al 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 027–046. [Google Scholar]
- Galvez L, Douds DD, Drinkwater LE, Wagoner P. 2001. Effect of tillage and farming system upon VAM fungus populations and mycorrhizas and nutrient uptake of maize. Plant and Soil 228: 299–308. [Google Scholar]
- Gomes SIF, Aguirre‐Gutiérrez J, Bidartondo MI, Merckx VSFT. 2017a. Arbuscular mycorrhizal interactions of mycoheterotrophic Thismia are more specialized than in autotrophic plants. New Phytologist 213: 1418–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes SIF, Merckx VSFT, Saavedra S. 2017b. Fungal‐host diversity among mycoheterotrophic plants increases proportionally to their fungal‐host overlap. Ecology and Evolution 7: 3623–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes SIF, van Bodegom P, Merckx VSFT, Soudzilovskaia NA. 2019. Global distribution of mycoheterotrophic plants. Global Ecology and Biogeography doi: http://10.1111/geb.12920 [Google Scholar]
- Gosling P, Mead A, Proctor M, Hammond JP, Bending GD. 2013. Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytologist 198: 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanway JJ, Heidel H. 1952. Soil analyses methods as used in Iowa state college soil testing laboratory. Iowa Agriculture 57: 1–31. [Google Scholar]
- van der Heijden MG, Martin FM, Selosse M-A, Sanders IR. 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist 205: 1406–1423. [DOI] [PubMed] [Google Scholar]
- van der Heijden MGA, Walder F. 2016. Reply to ‘Misconceptions on the application of biological market theory to the mycorrhizal symbiosis’. Nature Plants 2: 16062. [DOI] [PubMed] [Google Scholar]
- Johnson NC. 2010. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytologist 185: 631–647. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Graham JH, Smith FA. 1997. Functioning of mycorrhizal associations along the mutualism‐parasitism continuum. New Phytologist 135: 575–586. [Google Scholar]
- Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB. 2003. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84: 1895–1908. [Google Scholar]
- Kabir Z, O'Halloran IP, Fyles JW, Hamel C. 1998. Dynamics of the mycorrhizal symbiosis of corn (Zea mays L.): effects of host physiology, tillage practice and fertilization on spatial distribution of extra‐radical mycorrhizal hyphae in the field. Agriculture, Ecosystems and Environment 68: 151–163. [Google Scholar]
- Khan MH, Meghvansi MK, Gupta R, Veer V. 2015. Elemental stoichiometry indicates predominant influence of potassium and phosphorus limitation on arbuscular mycorrhizal symbiosis in acidic soil at high altitude. Journal of Plant Physiology 189: 105–112. [DOI] [PubMed] [Google Scholar]
- Klooster MR, Culley TM. 2009. Comparative analysis of the reproductive ecology of Monotropa and Monotropsis: two mycoheterotrophic genera in the Monotropoideae (Ericaceae). American Journal of Botany 96: 1337–1347. [DOI] [PubMed] [Google Scholar]
- Leake JR. 1994. The biology of myco‐heterotrophic (‘saprophytic’) plants. New Phytologist 127: 171–216. [DOI] [PubMed] [Google Scholar]
- Maas PJM, Maas-van de Kamer H, Benthem J, Snelders HC, Rübsamen T. 1986. Burmanniaceae. Flora Neotropica 42: 1–189. [Google Scholar]
- Maynard DG, Kalra YP. 1993. Nitrate and exchangeable ammonium nitrogen. Soil Sampling and Methods of Analysis 25–38. [Google Scholar]
- McCormick MK, Whigham DF, O'Neill JP, Becker JJ, Sarah W, Rasmussen HN, Bruns And TD, Taylor DL. 2009. Abundance and distribution of Corallorhiza odontorhiza reflect variations in climate and ectomycorrhizae. Ecological Monographs 79: 619–635. [Google Scholar]
- Merckx VSFT. 2013. Mycoheterotrophy: the biology of plants living on fungi. New York, NY, USA: Springer. [Google Scholar]
- Merckx VSFT, Gomes SIF, Wapstra M, Hunt C, Steenbeeke G, Mennes CB, Walsh N, Smissen R, Hsieh TH, Smets EF et al 2017. The biogeographical history of the interaction between mycoheterotrophic Thismia (Thismiaceae) plants and mycorrhizal Rhizophagus (Glomeraceae) fungi. Journal of Biogeography 44: 1869–1879. [Google Scholar]
- Merckx VSFT, Smets EF, Specht CD. 2013. Biogeography and conservation In: Merckx VSFT, eds. Mycoheterotrophy: the biology of plants living on fungi. New York, NY, USA: Springer, 103–156. [Google Scholar]
- Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27: 31–36. [Google Scholar]
- Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods in Ecology and Evolution 4: 133–142. [Google Scholar]
- Oehl F, Sieverding E, Mäder P, Dubois D, Ineichen K, Boller T, Wiemken A. 2004. Impact of long‐term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 138: 574–583. [DOI] [PubMed] [Google Scholar]
- Ogura-Tsujita Y, Yukawa T. 2008. High mycorrhizal specificity in a widespread mycoheterotrophic plant, Eulophia zollingeri (Orchidaceae). American Journal of Botany 95: 93–97. [DOI] [PubMed] [Google Scholar]
- Olsson PA, Burleigh SH, van Aarle IM. 2005. The influence of external nitrogen on carbon allocation to Glomus intraradices in monoxenic arbuscular mycorrhiza. New Phytologist 168: 677–686. [DOI] [PubMed] [Google Scholar]
- Ouzounidou G, Skiada V, Papadopoulou KK, Stamatis N, Kavvadias V, Eleftheriadis E, Gaitis F. 2015. Effects of soil pH and Arbuscular mycorrhiza (AM) inoculation on growth and chemical composition of chia (Salvia hispanica L.) leaves. Revista Brasileira de Botanica 38: 487–495. [Google Scholar]
- R Core Team . 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Ranade‐Malvi U. 2011. Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka Journal of Agricultural Sciences 24: 106–109. [Google Scholar]
- Renny M, Acosta MC, Cofré N, Domínguez LS, Bidartondo MI, Sérsic AN. 2017. Genetic diversity patterns of arbuscular mycorrhizal fungi associated with the mycoheterotroph Arachnitis uniflora Phil. (Corsiaceae). Annals of Botany 119: 1279–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippy JFM, Peet MM, Louws FJ, Nelson PV, Orr DB, Sorensen KA. 2004. Plant development and harvest yields of greenhouse tomatoes in six organic growing systems. HortScience 39: 223–229. [Google Scholar]
- Selosse MA, Rousset F. 2011. The plant‐fungal marketplace. Science 333: 828–829. [DOI] [PubMed] [Google Scholar]
- Sheldrake M, Rosenstock NP, Revillini D, Olsson PA, Wright SJ, Turner BL. 2017. A phosphorus threshold for mycoheterotrophic plants in tropical forests. Proceedings of the Royal Society B: Biological Sciences 284: 20162093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis. Cambridge, San Diego, CA, USA: Academic Press. [Google Scholar]
- Svenningsen NB, Watts‐Williams SJ, Joner EJ, Battini F, Efthymiou A, Cruz‐Paredes C, Nybroe O, Jakobsen I. 2018. Suppression of the activity of arbuscular mycorrhizal fungi by the soil microbiota. ISME Journal 12: 1296–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley A, Black IA. 1934. An examination of the Degtjareff method for determining organic carbon in soils: effect of variation in digestion conditions and of inorganic soil constituents. Soil Science 63: 251–263. [Google Scholar]
- Waterman RJ, Bidartondo MI, Stofberg J, Combs JK, Gebauer G, Savolainen V, Barraclough TG, Pauw A. 2011. The effects of above‐ and belowground mutualisms on orchid speciation and coexistence. American Naturalist 177: E54–E68. [DOI] [PubMed] [Google Scholar]
- Yamato M, Takahashi H, Shimono A, Kusakabe R, Yukawa T. 2016. Distribution of Petrosavia sakuraii (Petrosaviaceae), a rare mycoheterotrophic plant, may be determined by the abundance of its mycobionts. Mycorrhiza 26: 417–427. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Principal component analysis of the soil properties in the Amazon and the coast.
Table S1 Mycoheterotrophic plant species present in the study plots.
Table S2 Overall soil parameters in the negative and positive plots within the Amazon and coast regions.
Table S3 Variation of the soil parameters measured in the plots within the Amazon and coast calculated by the difference between negative and positive plots (deltas).


