Abstract
Background & Aims
The prevalence of non‐alcoholic fatty liver disease (NAFLD) is increasing, with concomitant high incidence of lipoprotein abnormalities. Cardiovascular disease (CVD) is the main cause of death in subjects with NAFLD and management of dyslipidaemia is pivotal for prevention. We aimed to determine cardiovascular risk and indication for statin therapy in subjects with NAFLD.
Methods
A cross‐sectional analysis of the population‐based Lifelines Cohort Study of 34 240 adult individuals. Subjects with reported use of lipid‐lowering drugs were excluded. Suspected NAFLD was defined as Fatty Liver Index (FLI) ≥60 and advanced hepatic fibrosis as NAFLD fibrosis score (NFS) >0.676. Cardiovascular risk and indication for statin therapy were defined according to the European Society of Cardiology and European Atherosclerosis Society Guideline for the Management of Dyslipidaemias.
Results
FLI ≥ 60 was present in 7067 (20.6%) participants and coincided with increased prevalence of type 2 diabetes mellitus, metabolic syndrome, CVD and impaired renal function (all P < 0.001). 10‐year predicted cardiovascular risk was significantly increased in subjects with elevated FLI and NFS (both P < 0.001). Indication for statin use was significantly increased in subjects with FLI ≥ 60 (31.0% vs 15.6%, P < 0.001) and NFS > 0.676 (73.2% vs 30.6%, P < 0.001). In multivariable analyses, FLI ≥ 60 (OR 1.26, 95%CI: 1.13‐1.41, P < 0.001) and NFS > 0.676 (OR 5.03, 95%CI: 2.76‐9.17, P < 0.001) were independent predictors for indication regarding statin therapy.
Conclusions
Because of increased cardiovascular risk, substantial proportions of subjects with suspected NAFLD and/or fibrosis have an indication for lipid‐lowering treatment and could benefit from statin therapy.
Keywords: cardiovascular risk, dyslipidaemia, fatty liver Index, NAFLD fibrosis score, non‐alcoholic fatty liver disease, statin therapy
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ApoA‐I
apolipoprotein A‐I
- ApoB
apolipoprotein B
- AST
aspartate aminotransferase
- ATC codes
Anatomical Therapeutic Chemical Classification System
- AUC
area under the curve
- BMI
body mass index
- CI
confidence interval
- CRP
C‐reactive protein
- CVD
cardiovascular disease
- EAS
European Atherosclerosis Society
- eGFR
estimated glomerular filtration rate
- ESC
European Society of Cardiology
- FIB‐4
Fibrosis‐4
- FLI
Fatty Liver Index
- GGT
gamma‐glutamyltransferase
- HbA1c
haemoglobin A1c
- HDL
high‐density lipoproteins
- IQR
interquartile ranges
- LDL
low‐density lipoproteins
- MetS
metabolic syndrome
- NAFLD
non‐alcoholic fatty liver disease
- NASH
non‐alcoholic steatohepatitis
- NFS
NAFLD fibrosis score
- OR
odds ratio
- ROC
receiver operating characteristics
- SD
standard deviation
- T2D
type 2 diabetes mellitus
- VLDL
very low‐density lipoproteins
Key points.
Subjects with suspected non‐alcoholic fatty liver disease (NAFLD) and advanced fibrosis have an increased 10‐year predicted cardiovascular risk. Consequently, indication for statin therapy is significantly higher in subjects with suspected NAFLD and advanced fibrosis and these subjects could benefit from lipid‐lowering treatment.
1. INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis in the absence of excessive alcohol use, and is emerging as the most common cause of chronic liver disease as a result of the global obesity epidemic. The spectrum of NAFLD ranges from simple steatosis to non‐alcoholic steatohepatitis (NASH), fibrosis and eventually cirrhosis.1 NAFLD is the hepatic manifestation of the metabolic syndrome (MetS) and coincides with an increased risk for development of type 2 diabetes mellitus (T2D).1, 2
NAFLD coincides with plasma lipoprotein abnormalities, including elevations in apolipoprotein (Apo)B‐containing lipoproteins, an increase in the ratio of ApoB to ApoA‐I, decreased levels of high‐density lipoprotein (HDL) cholesterol and increased levels of low‐density lipoprotein (LDL) cholesterol.3, 4, 5 Enhanced delivery of adipose tissue‐derived fatty acids to the liver provides a central mechanism responsible for hepatic fat accumulation. In turn, increased liver fat content is regarded as the driving force of enhanced production of very low‐density lipoproteins (VLDL) by the liver, resulting in an increased plasma concentration of large VLDL particles and consequently in higher triglycerides.6, 7 Such plasma lipoprotein abnormalities predispose to atherosclerotic cardiovascular disease (CVD)8, 9 with increased intima‐media thickness and carotid plaques indicating higher risk of atherosclerotic CVD in NAFLD.10
Although most patients with NAFLD are not at risk of dying from liver disease, they have increased risk of early morbidity and mortality because of CVD, which is the main cause of death in NAFLD.1, 9 Furthermore, cardiovascular and all‐cause morbidity and mortality increases exponentially with increasing fibrosis stage in NAFLD.11, 12 Consequently, prevention and treatment of dyslipidaemia are especially important in subjects with NAFLD. The main therapeutic aim in the treatment of dyslipidaemias is LDL cholesterol‐lowering treatment with 3‐hydroxy 3‐methylglutaryl‐coenzyme A reductase inhibitors (statins).13 However, restraint has been shown in the prescription of statins because of concerns about increased risk of hepatotoxicity.14, 15 Nevertheless, statin treatment appears to be safe in NAFLD patients with elevated liver enzymes.16, 17, 18, 19 In addition, experimental animal models with chronic liver injury have shown that statins have an anti‐inflammatory and anti‐fibrotic effect and decrease complications in NAFLD.20, 21, 22, 23 Also in humans, statin use may improve disease progression, reduce cardiovascular morbidity14, 16, 17 and decrease complications of chronic liver disease.24
There are only a few small studies, which assess the utilization of statin therapy in patients with NAFLD and dyslipidaemia. Three small studies described appropriate prescription of statin therapy in only 44%‐71% of NAFLD patients with dyslipidaemias and these patients were less likely than patients without NAFLD to receive appropriate statin care.25, 26, 27 Furthermore, NAFLD was an independent factor in the lack of statin prescription in subjects with indication for lipid‐lowering treatment.27
Given the pivotal role of lipoprotein abnormalities in the development of NAFLD and consequent CVD, with high morbidity and mortality, the importance of dyslipidaemia treatment in subjects with NAFLD is evident. Therefore, we sought to determine the cardiovascular risk as well as the proportion of subjects with indication for statin therapy in subjects with suspected NAFLD in a large population‐based cohort study.
2. MATERIALS AND METHODS
2.1. Study population
The study is a cross‐sectional analysis of the population‐based Lifelines Cohort Study including a total of 167 729 persons from the northern part of the Netherlands.28, 29 All participants provided written informed consent. The medical ethics committee of the University of Groningen, the Netherlands, approved the study conforming to the Declaration of Helsinki.28, 29
Only subjects with data required to calculate Fatty Liver Index (FLI),30 used as a proxy of NAFLD (described below), and cardiovascular risk scores were included. Exclusion criteria were participants <18 years, non‐fasting participants at time of blood collection, immigrants, participants with self‐reported excessive alcohol use (>1 drink in women and >2 drinks in men per day), those previous diagnosed with hepatitis or cirrhosis as well as all participants using lipid‐lowering drugs. The use of lipid‐lowering drugs (statins, fibrates, bile acid sequestrates, nicotinic acid and derivatives, combination drugs of lipid‐modifying agents and other lipid‐lowering drugs) was determined on description of individual drug use by the Anatomical Therapeutic Chemical Classification System (ATC codes).
2.2. Measurements and definitions
Data were collected in the Lifelines Cohort Study between 2006 and 2013. Questionnaires were collected, anthropometry and blood pressure were measured and biomaterial (blood) was collected at the Lifelines research sites. A standardized protocol was used to obtain blood pressure and anthropometric measurements (height, weight, waist circumference and Body Mass Index [BMI]).3, 4, 28, 29
Venous blood samples were processed for laboratory measurements with standardized laboratory measurements and quality assessment control at the Department of Laboratory Medicine of the University Medical Center Groningen, the Netherlands.28, 29 High sensitivity C‐reactive protein (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma‐glutamyltransferase (GGT), alkaline phosphatase (ALP), albumin (measured with a BCG albumin assay kit for colorimetric testing on a Roche Modular P chemistry analyzer), haemoglobin A1c (HbA1c), glucose, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, ApoB and ApoA‐I were measured as previously described.3, 4, 28, 29 Non‐HDL cholesterol was calculated by subtracting HDL cholesterol from the total cholesterol level.
In order to categorize subjects with a high probability for the diagnosis of NAFLD, the FLI was used. FLI was calculated according to the formula published by Bedogni et al.30 FLI = (e0.953*log (triglycerides)+0.139*BMI+0.718*log (GGT)+0.053*waist circumference–15.745)/(1+e0.953*log (triglycerides)+0.139*BMI +0.718*log(GGT) +0.053*waist circumference–15.745) × 100. An optimal cut‐off value for the FLI of 60 was reported with an accuracy of 0.84, a sensitivity of 61% and a specificity of 86% for detecting NAFLD as determined by ultrasonography.30 A FLI ≥ 60 was thus used as a proxy of NAFLD. The 2016 EASL‐EASD‐EASO NAFLD guideline recommends that for larger scale screening studies, serum biomarkers are the preferred diagnostic tool with the FLI currently considered to be one of the best validated steatosis scores.31 To identify NAFLD patients with suspected advanced fibrosis, the NAFLD fibrosis score (NFS) was used in subjects with FLI ≥ 60. To calculate the NAFLD fibrosis score (NFS), the formula published by Angulo et al was used.32 NFS = −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/presence of diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet count (x109/L) − 6.6 × albumin (g/dL). By applying a cut‐off score >0.676, the presence of advanced fibrosis in NAFLD could be diagnosed with a sensitivity of 43%, specificity of 96% and positive predictive value of 82%.32 The NFS is currently considered to be one of the best validated biomarkers to diagnose fibrosis among NAFLD subjects.31, 33, 34 As alternative fibrosis marker, we used the Fibrosis‐4 (FIB‐4) score as published by Vallet‐Pichard et al,35 where FIB‐4 = (age [years] × AST [U/L])/(platelet [109/L] × √ALT [U/L]). A low cut‐off of ≤1.30 was used to diagnose the absence of advanced fibrosis in NAFLD with a sensitivity of 74%, specificity of 71% and positive predictive value of 43% and a high cut‐off of ≥2.67 was used to diagnose the presence of advanced fibrosis in NAFLD with a sensitivity of 33%, specificity of 98% and positive predictive value of 80%.33, 36
The diagnosis of T2D was confirmed when a subject had either self‐reported on T2D, used glucose‐lowering medication, had a fasting glucose ≥7.0 mmol/L or a HbA1c ≥47.5 mmol/mol (6.5%). Impaired fasting glucose was defined as fasting glucose ≥6.1 mmol/L. MetS was defined by the revised diagnostic criteria from the American Heart Association by the National Cholesterol Education Program Adult Treatment Panel III.37
CVD was established by the 2016 Guidelines for the Management of Dyslipidaemias by the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS); defined by the presence of a self‐reported history of having myocardial ischemia, aortic aneurysm, narrowing of the carotid arteries or history of angioplasty, bypass surgery, transient ischaemic accident or stroke.13 Estimated 10‐year cardiovascular risk (based on age, sex, systolic blood pressure, total cholesterol level and smoking), estimated 10‐year cardiovascular risk groups (based on 10‐year cardiovascular risk, presence of CVD, T2D, chronic kidney disease and markedly elevated total cholesterol or blood pressure) and indication for statin treatment (based on estimated 10‐year cardiovascular risk groups and LDL cholesterol level) were also defined according to the ESC/EAS Guideline for the Management of Dyslipidaemias.13 Impaired renal function was defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, calculated using the Modification of Diet in Renal Disease Study Equation.
2.3. Statistical analysis
Statistical analyses were performed with SPSS software (version 25.0, IBM Corporation, Armonk, NY). Data are expressed using means with standard deviations (SD), medians with interquartile ranges (IQR) and in numbers with percentages. Normality of distribution was assessed and checked for skewness using the Kolmogorov‐Smirnov goodness‐of‐fit test. Between‐group differences were tested using Student t test for normally distributed variables, Mann‐Whitney U test for non‐normally distributed or skewed variables and chi‐square test for categorical variables. Multivariable logistic regression analysis was performed to identify the independent associations of indication for statin therapy. Results are presented by odds ratio (OR) with 95% confidence intervals (CI). Forest plots were used to present risk‐adjusted algorithms of FLI and NFS as independent predictor of indication for statin therapy. Receiver operating characteristic (ROC) curve analyses with area under the curve (AUC) were performed to evaluate the accuracy of FLI and NFS as independent predictors of indication for statin therapy. Two‐sided P ‐values of < 0.05 was considered statistically significant.
3. RESULTS
From the Lifelines Cohort Study, 57 272 participants were older than 18 years and had available biomedical data. After applying exclusion criteria, the final study group consisted of 34 240 participants (Figure 1). Our study group was predominantly female (62.9%) with a mean age of 43 years.
Figure 1.
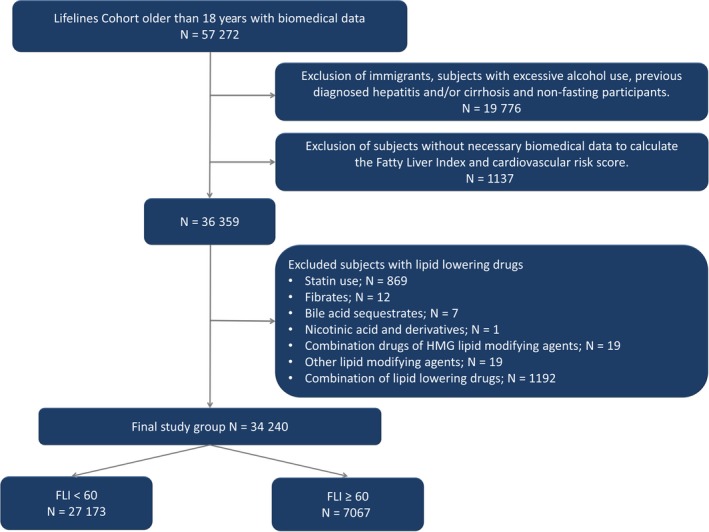
Flow chart of the study population
Suspected NAFLD, defined as FLI ≥ 60, was present in 7067 (20.6%) subjects. Table 1 shows the clinical and laboratory characteristics in subjects with and without suspected NAFLD. Subjects with a FLI ≥ 60 were more likely to be classified with T2D, MetS, history of CVD and impaired renal function (all P < 0.001). As expected, the proportion of subjects with a NFS > 0.676, as proxy of advanced fibrosis, was also higher in subjects with suspected NAFLD (1.0% vs 0.3%, P < 0.001). Characteristics from 869 excluded subjects using statins are shown in Table S1.
Table 1.
Clinical and biochemical characteristics in 27 173 subjects with a Fatty Liver Index (FLI) < 60 and in 7067 subjects with a FLI ≥ 60
|
FLI < 60 N = 27 173 (79.4%) |
FLI ≥ 60 N = 7067 (20.6%) |
P‐value | |
|---|---|---|---|
| Baseline characteristics | |||
| Sex: men/women, n (%) | 8783 (32.3)/18 390 (67.7) | 3927 (55.6)/3140 (44.4) | <0.001 |
| Age (y), mean ± SD | 42.5 ± 12.0 | 46.4 ± 10.9 | <0.001 |
| BMI (kg/m2), median (IQR) | 24.5 (22.7‐26.5) | 30.6 (28.5‐33.7) | <0.001 |
| BMI | |||
| Normal; ≤25 kg/m2, n (%) | 15 718 (57.8) | 116 (1.6) | <0.001 |
| Overweight; 25‐30 kg/m2, n (%) | 10 399 (38.3) | 2701 (38.2) | 0.939 |
| Obese; ≥30 kg/m2, n (%) | 1056 (3.9) | 4250 (60.1) | <0.001 |
| Waist circumference (cm) | |||
| Men, median (IQR) | 91 (86‐95) | 105 (100‐110) | <0.001 |
| Women, median (IQR) | 84 (77‐90) | 105 (100‐111) | <0.001 |
| Smoking, n (%) | 4952 (18.4) | 1449 (20.7) | <0.001 |
| Blood tests | |||
| CRP (mg/L), median (IQR) | 1.0 (0.5‐2.2) | 2.2 (1.1‐5.0) | <0.001 |
| ALT (U/L), median (IQR) | 18 (13‐24) | 28 (20‐40) | <0.001 |
| AST (U/L), median (IQR) | 22 (19‐26) | 25 (21‐30) | <0.001 |
| GGT (U/L), median (IQR) | 18 (14‐25) | 33 (24‐48) | <0.001 |
| ALP (U/L), mean ± SD | 60 ± 17 | 71 ± 21 | <0.001 |
| Albumin (g/L), mean ± SD | 45.0 ± 0.2 | 44.7 ± 0.2 | <0.001 |
| HbA1c (mmol/mol), mean ± SD | 36.9 ± 4.0 | 39.2 ± 6.0 | <0.001 |
| HbA1c (%), mean ± SD | 5.5 ± 0.4 | 5.7 ± 0.5 | <0.001 |
| Fasting glucose (mmol/L), median (IQR) | 4.8 (4.5‐5.1) | 5.2 (4.9‐5.6) | <0.001 |
| Total cholesterol (mmol/L), mean ± SD | 5.0 ± 0.9 | 5.5 ± 1.0 | <0.001 |
| HDL cholesterol (mmol/L), mean ± SD | 1.5 ± 0.4 | 1.2 ± 0.3 | <0.001 |
| LDL cholesterol (mmol/L), mean ± SD | 3.1 ± 0.9 | 3.6 ± 0.9 | <0.001 |
| Non‐HDL cholesterol (mmol/L), median (IQR) | 3.4 (2.8‐4.1) | 4.3 (3.6‐4.9) | <0.001 |
| Triglycerides (mmol/L), median (IQR) | 0.9 (0.7‐1.1) | 1.6 (1.2‐2.2) | <0.001 |
| ApoB (g/L), mean ± SD | 0.9 ± 0.1 | 1.0 ± 0.2 | <0.001 |
| ApoA‐I (g/L), mean ± SD | 1.5 ± 0.3 | 1.3 ± 0.2 | <0.001 |
| Comorbidities | |||
| Type 2 diabetes mellitus, n (%) | 233 (0.9) | 407 (5.8) | <0.001 |
| Metabolic syndrome, n (%) | 1296 (4.8) | 3504 (49.7) | <0.001 |
| Abdominal obesity, n (%) | 6600 (24.3) | 5741 (81.2) | <0.001 |
| Hyperglycaemia, n (%) | 1822 (6.7) | 1911 (27.1) | <0.001 |
| Hypertension, n (%) | 8111 (29.9) | 4222 (59.8) | <0.001 |
| Elevated TG, n (%) | 1620 (6.0) | 3085 (43.7) | <0.001 |
| Low HDL cholesterol, n (%) | 4235 (15.6) | 3181 (45.0) | <0.001 |
| Cardiovascular disease, n (%) | 2044 (7.5) | 616 (8.7) | 0.001 |
| Impaired renal function, n (%)) | 2248 (8.3) | 1341 (19.0) | <0.001 |
| Fibrosis, NFS > 0.676, n (%) | 83 (0.3) | 71 (1.0) | <0.001 |
Data are given in number with percentages (%), mean ± standard deviations (SD) or median with interquartile ranges (IQR). For comparison between two groups, Student t test (for normally distributed variables) and Mann‐Whitney U test were used for skewed continuous variables and for binary variables chi‐square test were used. Non‐HDL cholesterol was calculated as cholesterol—high‐density lipoprotein cholesterol. Metabolic syndrome was defined according to NCEP ATPIII criteria. Cardiovascular disease was defined as having myocardial ischaemia, aortic aneurysm, narrowing of the carotid arteries or history of angioplasty, bypass surgery, transient ischaemic accident or stroke (2016 ESC/EAS Guidelines for the Management of Dyslipidaemias). Impaired renal function was defined as estimated glomerular filtration rate (<60 mL/min/1.73 m2). FLI = (e0.953*log(triglycerides) +0.139*BMI +0.718*log (GGT)+0.053*waist circumference‐15.745)/(1+e0.953*log(triglycerides)+0.139*BMI +0.718*log(GGT)+0.053*waist circumference‐15.745) × 100. NAFLD fibrosis score = −1.675 + 0.037 × age (y) + 0.094 × BMI (kg/m2) + 1.13 x impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio–0.013 × platelet (x109/L) – 0.66 × albumin (g/dL).
Abbreviations: ApoA‐I, apolipoprotein A‐I; ApoB, apolipoprotein B; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C‐reactive protein; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; FLI, Fatty Liver Index; GGT, gamma‐glutamyltransferase; HbA1c, haemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NAFLD, non‐alcoholic fatty liver disease; NFS, NAFLD fibrosis score.
Estimated 10‐year predicted cardiovascular risk was higher in subjects with a FLI ≥ 60 compared with subjects with a FLI < 60 (Table 2); very high cardiovascular risk (13.9% vs 8.4%), high cardiovascular risk (18.5% vs 8.5%), medium cardiovascular risk (16.9% vs 9.9%) and low cardiovascular risk (50.8% vs 73.2%), all P < 0.001. Consequently, subjects with a FLI ≥ 60 had an approximately two times higher need for drug intervention strategy (statin therapy) based on CVD risk prediction and their LDL cholesterol level (31.0% vs 15.6%, P < 0.001). The proportion of subjects with a FLI ≥ 60 in need for statin therapy increased with higher LDL cholesterol levels and CVD risk prediction category as can be seen in Table 3.
Table 2.
Cardiovascular risk according to ESC/EAS dyslipidaemia guideline in 27 173 subjects with a Fatty Liver Index (FLI) < 60 and in 7067 subjects with a FLI ≥ 60
|
FLI < 60 N = 27 173 (79.4%) |
FLI ≥ 60 N = 7067 (20.6%) |
P‐value | |
|---|---|---|---|
| Estimated 10‐y predicted CVD risk | |||
| Low risk, n (%) | 19 882 (73.2) | 3587 (50.8) | < 0.001 |
| Medium risk, n (%) | 2683 (9.9) | 1195 (16.9) | < 0.001 |
| High risk, n (%) | 2313 (8.5) | 1305 (18.5) | < 0.001 |
| Very high risk, n (%) | 2295 (8.4) | 980 (13.9) | < 0.001 |
| Need for drug intervention strategy as function of CVD risk and LDL cholesterol level | |||
| No intervention, n (%) | 19 743 (72.7) | 3482 (49.3) | < 0.001 |
| Lifestyle intervention (if uncontrolled drug consideration), n (%) | 3198 (11.8) | 1395 (19.7) | < 0.001 |
| Drug intervention (statin) with concomitant lifestyle intervention, n (%) | 4232 (15.6) | 2190 (31.0) | < 0.001 |
| Primary treatment LDL cholesterol target | |||
| High LDL cholesterol (≥1.8 mmol/L) in very high‐risk subjects, n (%) | 2199 (8.1) | 966 (13.7) | < 0.001 |
| High LDL cholesterol (≥2.6 mmol/L) in high‐risk subjects, n (%) | 2033 (7.5) | 1224 (17.3) | < 0.001 |
| High LDL cholesterol (≥3.0 mmol/L) in low‐ to moderate‐risk subjects, n (%) | 11 555 (42.5) | 3557 (50.3) | < 0.001 |
| Secondary treatment non‐HDL cholesterol target | |||
| High non‐HDL cholesterol (≥2.6 mmol/L) in very high‐risk subjects, n (%) | 1867 (6.9) | 952 (13.5) | < 0.001 |
| High non‐HDL cholesterol (≥3.4 mmol/L) in high‐risk subjects, n (%) | 1582 (5.8) | 1160 (16.4) | < 0.001 |
| High non‐HDL cholesterol (≥3.8 mmol/L) in moderate‐risk subjects, n (%) | 1680 (6.2) | 948 (13.4) | < 0.001 |
| Secondary treatment ApoB target | |||
| High ApoB lipoprotein (≥80 mg/dL) in very high‐risk subjects, n (%) | 119 (0.5) | 57 (0.9) | < 0.001 |
| High ApoB lipoprotein (≥100 mg/dL) in high‐risk subjects, n (%) | 24 (0.1) | 17 (0.3) | < 0.001 |
Data are given in number with percentages (%). CVD risk and indication for intervention strategies were based on the 2016 ESC/EAS Guideline for the Management of Dyslipidaemias. FLI = (e0.953*log(triglycerides) +0.139*BMI +0.718*log (GGT)+0.053*waist circumference‐15.745)/(1+e0.953*log(triglycerides)+0.139*BMI +0.718*log(GGT)+0.053*waist circumference‐15.745) × 100.
Abbreviations: ApoB, apolipoprotein B; CVD, cardiovascular disease; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; FLI, Fatty Liver Index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 3.
Intervention strategies in 27 173 subjects with a Fatty Liver Index (FLI) < 60 and in 7067 subjects with a FLI ≥ 60 as function of total cardiovascular risk and low‐density lipoprotein cholesterol level
| Total CV risk (SCORE) | LDL cholesterol levels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1.8 mmol/L | 1.8 to < 2.6 mmol/L | 2.6 to < 4.0 mmol/L | 4.0 to < 4.9 mmol/L | ≥4.9 mmol/L | ||||||
| FLI < 60 | FLI ≥ 60 | FLI < 60 | FLI ≥ 60 | FLI < 60 | FLI ≥ 60 | FLI < 60 | FLI ≥ 60 | FLI < 60 | FLI ≥ 60 | |
| <1%, or low risk | 870 (94.5) | 51 (5.5) | 5,600 (92.0) | 485 (8.0) | 11,242 (83.9) | 2,163 (16.1) | 1,836 (72.1) | 709 (27.9) | 334 (65.1) | 179 (34.9) |
| ≥1 to < 5%, or medium risk | 15 (71.4) | 6 (28.6) | 155 (72.8) | 58 (27.2) | 1,540 (71.6) | 612 (28.4) | 779 (66.9) | 385 (33.1) | 194 (59.1) | 134 (40.9) |
| ≥5 to < 10%, or high risk | 25 (71.4) | 10 (28.6) | 255 (78.2) | 71 (21.8) | 1,371 (66.6) | 688 (33.4) | 482 (54.5) | 403 (45.5) | 180 (57.5) | 133 (42.5) |
| ≥10%, or very high risk | 96 (87.3) | 14 (12.7) | 498 (83.8) | 96 (16.2) | 1,316 (70.3) | 556 (29.7) | 311 (56.1) | 243 (43.9) | 74 (51.0) | 71 (49.0) |
Table is based on Table 5 from the 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Data are given in numbers with percentages (%). Green cells represent no lipid intervention; yellow cells represent lifestyle intervention and if uncontrolled LDL cholesterol levels consideration of drug intervention; red cells represent drug intervention with concomitant lifestyle intervention. FLI = (e0.953*log(triglycerides) +0.139*BMI +0.718*log (GGT)+0.053*waist circumference‐15.745)/(1+e0.953*log(triglycerides)+0.139*BMI +0.718*log(GGT)+0.053*waist circumference‐15.745) × 100.
In subjects with suspected NAFLD and a NFS > 0.676 vs subjects with suspected NAFLD and a NFS ≤ 0.676, essentially similar differences in CVD risk and LDL cholesterol treatment intervention strategies were found (Table 4). Estimated 10‐year predicted total very high cardiovascular risk was approximately four times higher in subjects with a NFS > 0.676 compared to subjects with a NFS ≤ 0.676 (63.4% vs 13.4%, P < 0.001). Consequently, subjects with a NFS > 0.676 had an approximately 2.5 times higher indication for statin therapy (73.2% vs 30.6%, P < 0.001) and proportion of subjects with a NFS > 0.676 in need for statin therapy increased with higher LDL cholesterol levels and CVD risk prediction category (Table 5). In alternative analyses using a FIB‐4 score ≥ 2.67 instead of a NFS > 0.676, essentially similar results were found (Table S2).
Table 4.
Cardiovascular risk according to ESC/EAS dyslipidaemia guideline in 6969 subjects with suspected NAFLD and a NAFLD fibrosis score (NFS) ≤ 0.676 and in 71 subjects with suspected NAFLD and a NFS > 0.676
|
NFS ≤ 0.676 N = 6969 (99.0%) |
NFS > 0.676 N = 71 (1.0%) |
P‐value | |
|---|---|---|---|
| Estimated 10‐y predicted CVD risk | |||
| Low risk, n (%) | 3562 (51.1) | 10 (14.1) | <0.001 |
| Medium risk, n (%) | 1182 (17.0) | 7 (9.9) | 0.112 |
| High risk, n (%) | 1291 (18.5) | 9 (12.7) | 0.206 |
| Very high risk, n (%) | 934 (13.4) | 45 (63.4) | <0.001 |
| Need for drug intervention strategy as function of CVD risk and LDL cholesterol level | |||
| No intervention, n (%) | 3455 (49.6) | 12 (16.9) | <0.001 |
| Lifestyle intervention (if uncontrolled drug consideration), n (%) | 1382 (19.8) | 7 (9.9) | 0.036 |
| Drug intervention (statin) with concomitant lifestyle intervention, n (%) | 2132 (30.6) | 52 (73.2) | <0.001 |
| Primary treatment LDL cholesterol target | |||
| High LDL cholesterol (≥1.8 mmol/L) in very high‐risk subjects, n (%) | 920 (13.2) | 45 (63.4) | <0.001 |
| High LDL cholesterol (≥2.6 mmol/L) in high‐risk subjects, n (%) | 1212 (17.4) | 7 (9.9) | 0.095 |
| High LDL cholesterol (≥3.0 mmol/L) in low‐ to moderate‐risk subjects, n (%) | 3531 (50.7) | 11 (15.5) | <0.001 |
| Secondary treatment non‐HDL cholesterol target | |||
| High non‐HDL cholesterol (≥2.6 mmol/L) in very high‐risk subjects, n (%) | 908 (13.0) | 43 (60.6) | <0.001 |
| High non‐HDL cholesterol (≥3.4 mmol/L) in high‐risk subjects, n (%) | 1148 (16.5) | 7 (9.9) | 0.134 |
| High non‐HDL cholesterol (≥3.8 mmol/L) in moderate‐risk subjects, n (%) | 938 (13.5) | 4 (5.6) | 0.054 |
| Secondary treatment ApoB target | |||
| High ApoB lipoprotein (≥80 mg/dL) in very high‐risk subjects, n (%) | 57 (0.9) | 0 (0) | 1.000 |
| High ApoB lipoprotein (≥100 mg/dL) in high‐risk subjects, n (%) | 17 (0.3) | 0 (0) | 1.000 |
Data are given in number with percentages (%). CVD risk and indication for intervention strategies were based on the 2016 ESC/EAS Guideline for the Management of Dyslipidaemias. NAFLD fibrosis score was calculated in 7040 subjects with FLI ≥ 60. NAFLD fibrosis score = −1.675 + 0.037*age (y) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio – 0.013 × platelet (×109/L) – 0.66 × albumin (g/dL).
Abbreviations: ApoB, apolipoprotein B; CVD, cardiovascular disease; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NFS, NAFLD fibrosis score.
Table 5.
Intervention strategies in 6969 subjects with suspected NAFLD and a NAFLD fibrosis score (NFS) ≤ 0.676 and in 71 subjects with suspected NAFLD and a NFS > 0.676 as function of total cardiovascular risk and low‐density lipoprotein cholesterol level
| Total CV risk (SCORE) | LDL cholesterol levels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1.8 mmol/L | 1.8 to < 2.6 mmol/L | 2.6 to < 4.0 mmol/L | 4.0 to < 4.9 mmol/L | ≥4.9 mmol/L | ||||||
| NFS ≤ 0.676 | NFS > 0.676 | NFS ≤ 0.676 | NFS > 0.676 | NFS ≤ 0.676 | NFS > 0.676 | NFS ≤ 0.676 | NFS > 0.676 | NFS ≤ 0.676 | NFS > 0.676 | |
| <1%, or low risk | 50 (98.0) | 1 (2.0) | 480 (99.6) | 2 (0.4) | 2,149 (99.8) | 4 (0.2) | 705 (99.7) | 2 (0.3) | 178 (99.4) | 1 (0.6) |
| ≥1 to < 5%, or medium risk | 6 (100) | 0 (0) | 55 (94.8) | 3 (5.2) | 609 (99.8) | 1 (0.2) | 378 (99.2) | 3 (0.8) | 134 (100.0) | 0 (0) |
| ≥5 to < 10%, or high risk | 10 (100) | 0 (0) | 69 (97.2) | 2 (2.8) | 685 (99.6) | 3 (0.4) | 398 (99.7) | 1 (0.3) | 129 (97.7) | 3 (2.3) |
| ≥10%, or very high risk | 14 (100) | 0 (0) | 88 (91.7) | 8 (8.3) | 533 (95.9) | 23 (4.1) | 230 (95.0) | 12 (5.0) | 69 (97.2) | 2 (2.8) |
Table is based on Table 5 from the 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Data are given in numbers with percentages (%). Green cells represent no lipid intervention; yellow cells represent lifestyle intervention and if uncontrolled LDL‐cholesterol levels consideration of drug intervention; red cells represent drug intervention with concomitant lifestyle intervention. NAFLD fibrosis score was calculated in 7040 subjects with FLI ≥ 60. NAFLD fibrosis score = −1.675 + 0.037 × age (y) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio–0.013 × platelet (×109/L) – 0.66 × albumin (g/dL).
Multivariable logistic regression analyses were subsequently performed in order to establish the extent to which statin therapy was independently associated with a FLI ≥ 60 (Table 6, Figure 2A). After adjustment for age, sex, current smoking, presence of MetS and impaired renal function, indication for statin treatment was positively associated with a FLI ≥ 60 (Table 6, Model 1, OR 1.38, 95% CI: 1.23‐1.53). This positive association was also demonstrated after additional adjustment for individual MetS components (Table 6, Model 2, OR 1.26, 95% CI: 1.13‐1.41). In subjects with suspected NAFLD and elevated NFS (Table 6, Figure 2B), even stronger positive associations of indication for statin therapy with NFS > 0.676 were found (Model 3; OR 5.89, 95% CI: 3.26‐10.63 and Model 4; OR 5.03, 95% CI: 2.76‐9.17).
Table 6.
Multivariable binary logistic regression analyses demonstrating the positive association of indication for statin therapy with an elevated Fatty Liver Index (FLI) in 34 240 subjects and the association of indication for statin therapy with the NAFLD fibrosis score (NFS) in subjects with suspected NAFLD in 7067 subjects
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Age | 1.03 (1.03‐1.04) | <0.001 | 1.03 (1.03‐1.03) | <0.001 | 1.04 (1.04‐1.05) | <0.001 | 1.04 (1.03‐1.05) | <0.001 |
| Sex (male vs female) | 1.06 (0.97‐1.15) | 0.189 | 1.16 (1.06‐1.27) | 0.001 | 0.99 (0.85‐1.15) | 0.993 | 1.01 (0.85‐1.19) | 0.951 |
| FLI ≥ 60 vs < 60 | 1.38 (1.23‐1.53) | <0.001 | 1.26 (1.13‐1.41) | <0.001 | ||||
| NFS > 0.676 vs ≤ 0.676 | 5.89 (3.26‐10.63) | <0.001 | 5.03 (2.76‐9.17) | <0.001 | ||||
| Current smoking (yes/no) | 1.37 (1.25‐1.49) | <0.001 | 1.39 (1.27‐1.52) | <0.001 | 1.43 (1.20‐1.69) | <0.001 | 1.46 (1.23‐1.74) | <0.001 |
| MetS (yes/no) | 1.72 (1.55‐1.91) | <0.001 | 1.69 (1.46‐1.95) | <0.001 | ||||
| Abdominal obesity (yes/no) | 1.04 (0.95‐1.15) | 0.358 | 1.22 (0.98‐1.54) | 0.080 | ||||
| Hyperglycaemia (yes/no) | 2.46 (2.22‐2.71) | <0.001 | 2.52 (2.17‐2.93) | <0.001 | ||||
| Hypertension (yes/no) | 1.22 (1.12‐1.32) | <0.001 | 1.17 (1.01‐1.37) | 0.038 | ||||
| Elevated triglycerides (yes/no) | 1.23 (1.10‐1.37) | <0.001 | 1.20 (1.03‐1.40) | 0.019 | ||||
| Low HDL cholesterol (yes/no) | 1.04 (0.95‐1.14) | 0.392 | 1.12 (0.96‐1.30) | 0.150 | ||||
| Impaired renal function (yes/no) | 82.84 (72.10‐95.16) | <0.001 | 88.98 (77.35‐102.37) | <0.001 | 108.11 (81.39‐143.60) | <0.001 | 121.82 (91.33‐162.48) | <0.001 |
Data are given in odds ratios with 95% confidence intervals. Indication for statin therapy was based on the 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Impaired renal function was defined as estimated glomerular filtration rate (<60 mL/min/1.73 m2). FLI = (e0.953*log(triglycerides) +0.139*BMI +0.718*log (GGT)+0.053*waist circumference‐15.745)/(1+e0.953*log(triglycerides)+0.139*BMI +0.718*log(GGT)+0.053*waist circumference‐15.745) × 100. NAFLD fibrosis score was calculated in 7040 subjects with FLI ≥ 60. NAFLD fibrosis score = −1.675 + 0.037 × age (y) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio – 0.013 × platelet (×109/L) – 0.66 × albumin (g/dL).
Abbreviations: CI, confidence interval; FLI, Fatty Liver Index; HDL, high‐density lipoproteins; MetS, metabolic syndrome; NFS, NAFLD fibrosis score; OR, odds ratio.
Model 1: adjusted for age, sex, Fatty Liver Index, current smoking, metabolic syndrome and impaired renal function.
Model 2: adjusted for age, sex, Fatty Liver Index, current smoking, individual metabolic syndrome criteria (abdominal obesity, hyperglycaemia, hypertension, elevated triglycerides and low HDL cholesterol) and impaired renal function.
Model 3: adjusted for age, sex, NAFLD fibrosis sore, current smoking, metabolic syndrome and impaired renal function.
Model 4: adjusted for age, sex, NAFLD fibrosis sore, current smoking, individual metabolic syndrome criteria (abdominal obesity, hyperglycaemia, hypertension, elevated triglycerides and low HDL cholesterol) and impaired renal function.
Figure 2.
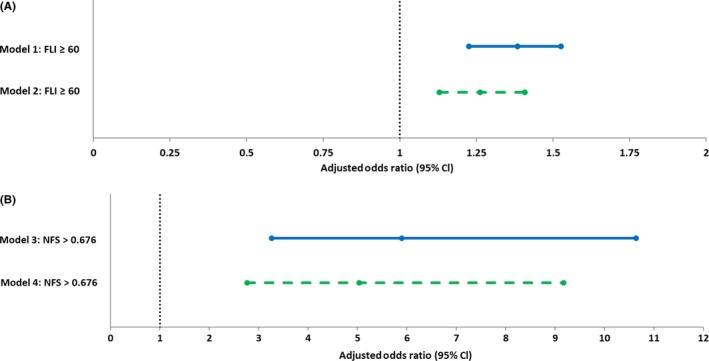
(A) Forest plot of adjusted odds ratios of positive association of the Fatty Liver Index (FLI) with indication for statin therapy based on Table 6; Model 1: adjusted for age, sex, current smoking, metabolic syndrome and impaired renal function; Model 2: adjusted for age, sex, current smoking, individual metabolic syndrome criteria (abdominal obesity, hyperglycaemia, hypertension, elevated triglycerides and low HDL cholesterol) and impaired renal function. (B) Forest plot of adjusted odds ratios of positive association of the NAFLD Fibrosis Score (NFS) with indication for statin therapy based on Table 6; Model 3: adjusted for age, sex, current smoking, metabolic syndrome and impaired renal function; Model 4: adjusted for age, sex, current smoking, individual metabolic syndrome criteria (abdominal obesity, hyperglycaemia, hypertension, elevated triglycerides and low HDL cholesterol) and impaired renal function
The AUCs of the multivariable models (Table 6), which include FLI and NFS as significant predictive variables regarding indication of statin therapy, demonstrate excellent accuracy with the following results: Model 1, AUC = 0.815 (95% CI: 0.808‐0.822, P < 0.001); Model 2, AUC = 0.823 (95% CI: 0.816‐0.830, P < 0.001); Model 3, AUC = 0.856 (95% CI: 0.845‐0.867, P < 0.001) and Model 4, AUC = 0.869 (95% CI: 0.858‐0.879, P < 0.001).
4. DISCUSSION
In this cross‐sectional analysis in a large population‐based cohort study, we demonstrated that statin‐naive subjects with suspected NAFLD, and suspected advanced fibrosis, have a significantly increased cardiovascular risk, and consequently a higher indication for statin therapy. Furthermore, in multivariable regression analyses, indication for statin therapy was independently associated with an elevated FLI as well as NFS. In this study, we have used the FLI and NFS in line with recommendations of international guidelines which advocate to use biomarker‐derived algorithms in order to categorize subjects with probable NAFLD and fibrosis in large‐scale studies.31 Taken together, the present study demonstrates that an elevated FLI and NFS are independently associated with a higher need for statin therapy. We advocate that care for this high‐risk group of subjects should be improved.
Statins have a pivotal role in primary and secondary prevention of cardiovascular diseases by decreasing the synthesis of cholesterol in the liver by inhibition of the mevalonate pathway through a competitive inhibitory effect on 3‐hydroxy‐3‐methylglutaryl CoA reductase, which results in lowering of LDL, VLDL and consequently in lower triglycerides.38 Also, statins have an effect on inhibition of inflammatory pathways, reduction of endothelial dysfunction, antioxidative effects, increased bioavailability of nitric oxide and stabilization of atherosclerotic plaques, all resulting in significant reduction of cardiovascular morbidity and mortality.13, 38
In NAFLD, cardiovascular events are the main cause of mortality, probably driven by increased prevalence of T2D, MetS and plasma lipoprotein abnormalities that predispose to atherosclerotic cardiovascular disease.1 Accumulating evidence demonstrates that statin therapy in NAFLD is safe and not to an important extent associated with hepatotoxicity.16, 17, 18, 19 Severe liver injury caused by statins is rare, and in NAFLD, statin therapy should in fact be encouraged since the risk of cardiovascular mortality is much higher than its potential side effects.19, 31 However, statin therapy may lead to a slightly increased risk of development of T2D, but this seems largely outweighed by the cardiovascular benefit.39
In the last decade, accumulating evidence has become available that statin therapy may even have beneficial effects in NAFLD. In human studies, the administration of statins resulted in an improvement of ultrasonographic amount of hepatic steatosis.40 Studies with biopsy‐proven NAFLD and NASH showed mainly positive results of statin use: reduced formation of steatosis and fibrosis and a protective effect on liver damage in NASH.41, 42, 43 In two large clinical trials, normalization of transaminase levels was found in patients treated with simvastatin or atorvastatin, with concomitant increased cardiovascular benefit in patients with mildly‐to‐moderately elevated baseline transaminases.17, 44 Furthermore, a meta‐analysis demonstrated that statin use is probably associated with lower risk of hepatic decompensation, reduction of portal hypertension and lowering of mortality in patients with chronic liver disease.24
Previous studies that have investigated the indication for statin therapy in subjects with NAFLD are scarce. To date, our study is the only study assessing the indication for statin therapy in statin‐naive subjects with suspected NAFLD from the general population. Del Ben et al assessed cardiovascular risk and statin therapy indication in 605 subjects referred to the outpatient clinic for screening of suspected metabolic diseases. They described that 44% of NAFLD patients with indication for statin use were on therapy.27 However, this study was performed in a selected high metabolic risk group. Blais et al conducted a medical record review of 255 dyslipidaemic NAFLD patients from a Veteran database, where only 59.6% received appropriate statin therapy. Interestingly, from this group, 38.1% received statin dose reduction or even discontinuation at time of NAFLD detection and the most important determinant for inappropriate statin use was recognition of NAFLD,25 both probably because of fear for hepatotoxicity. In a correspondence letter, Taii et al described in 9960 patients with NAFLD and high LDL cholesterol that 71% of patients received statin therapy, but additional cardiovascular or metabolic information from these subjects was unfortunately not presented.26
Our study has several strengths. Considering a sample size of over 34 000 individuals, this is the largest study to date reporting on the indication for statin therapy in subjects with NAFLD and fibrosis. Furthermore, this enabled careful calculations on effect sizes, sufficiently powered subgroup analysis and multivariable models presenting excellent predictive performance. Additionally, the Lifelines Cohort Study population has been well characterized, with extensive validated questionnaires and standardized measurements and all laboratory measurements were performed in a single reference laboratory.28, 29 From that, a complete cardiovascular risk profile of all subjects could be realized.
Several other methodological aspects and limitations also need to be addressed. First, its cross‐sectional design does not allow cause‐effect relationships to be established with certainty. Second, immigrants were excluded in order to select a Western European population. While this likely limits extrapolation of our findings to other ethnicities, this was done in view of the limited percentage of immigrants in our region and our choice to use the FLI and NFS for NAFLD and advanced fibrosis assessment. Third, since ancestry, alcohol intake, medication use and medical history were based on self‐administered questionnaires, misreporting by individuals cannot be excluded. However, considering the large number of subjects, this limitation does not significantly affect the interpretation of the presented results. Fourth, the proportion of subjects with suspected fibrosis in our study is quite low amounting to only 1%. This low percentage of individuals with suspected fibrosis could probably be explained by exclusion of all subjects using statins, where subjects using statins have a predefined high cardiovascular risk and increased risk of progressive NAFLD. Fifth, in the ESC/EAS Guideline for the Management of Dyslipidaemias, the estimated 10‐year cardiovascular risk depends on systolic blood pressure, irrespective of antihypertensive drugs. Therefore, use of short‐term antihypertensive drugs could have underestimated the risk of cardiovascular disease and classification into a lower cardiovascular risk category could have occurred. Finally, the FLI and NFS are not an absolute measure of hepatic fat accumulation and level of fibrosis and thus some over‐ and underestimation of NAFLD and advanced fibrosis could have occurred. While histological examination of a liver biopsy is still the gold standard for diagnosing NAFLD and fibrosis, a liver biopsy has well‐known limitations with respect to invasiveness and sampling variability. As an alternative, imaging techniques are time consuming, expensive and also not feasible in large observational studies. Given these considerations, serum biomarkers are the preferred diagnostic tool for large‐scale screening studies and the FLI and NFS seem to perform best in European subjects, which is probably related to the ethnical difference in fat distribution.31, 32, 34
In conclusion, because of increased cardiovascular risk in NAFLD, a substantial proportion of subjects with suspected NAFLD and fibrosis could benefit from lipid‐lowering treatment and statin therapy should thus be encouraged.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
EB, AB, RD, DZ, VM and HB were involved in the concept and design of study. EB and DZ collected and analysed the data. EB, AB, RD, HM, DZ, VM and HB were involved in data interpretation and drafted the manuscript. All authors have revised and approved the submitted manuscript.
Supporting information
ACKNOWLEDGEMENTS
The authors wish to acknowledge the services of the Lifelines Cohort Study and Biobank, the contributing research centres delivering data, the participating general practitioners and pharmacists and all the study participants.
van den Berg EH, Wolters AAB, Dullaart RPF, et al. Prescription of statins in suspected non‐alcoholic fatty liver disease and high cardiovascular risk, a population‐based study. Liver Int. 2019;39:1343–1354. 10.1111/liv.14116
Handling Editor: Luca Valenti
Funding information
The Lifelines Cohort Study was supported by the Netherlands Organization for Scientific Research [grant 175.010.2007.006]; the Economic Structure Enhancing Fund of the Dutch government; the Ministry of Economic Affairs, Education, Culture and Science and Health, Welfare and Sports; the Northern Netherlands Collaboration of Provinces; the Province of Groningen; University Medical Center Groningen; the University of Groningen; the Dutch Kidney Foundation; the Dutch Diabetes Research Foundation and the National Consortium for Healthy Ageing and the BioSHaRE‐EU consortium (KP7, project reference 261433). The funders had no role in study design, data collection and analyses, decision to publish or preparation of the manuscript.
REFERENCES
- 1. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686‐690. 10.1038/nrgastro.2013.171 [DOI] [PubMed] [Google Scholar]
- 2. Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta‐analysis. J Gastroenterol Hepatol. 2016;31(5):936‐944. 10.1111/jgh.13264 [DOI] [PubMed] [Google Scholar]
- 3. Nass KJ, van den Berg EH, Faber KN, Schreuder TCMA, Blokzijl H, Dullaart RPF. High prevalence of apolipoprotein B dyslipoproteinemias in non‐alcoholic fatty liver disease: the lifelines cohort study. Metab Clin Exp. 2017;72:37‐46. 10.1016/j.metabol.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 4. van den Berg EH, Amini M, Schreuder TCMA, et al. Prevalence and determinants of non‐alcoholic fatty liver disease in lifelines: a large Dutch population cohort. PLoS ONE. 2017;12(2):e0171502 10.1371/journal.pone.0171502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bril F, Sninsky JJ, Baca AM, et al. Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD. J Clin Endocrinol Metab. 2016;101(2):644‐652. 10.1210/jc.2015-3111 [DOI] [PubMed] [Google Scholar]
- 6. Adiels M, Taskinen M‐R, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755‐765. 10.1007/s00125-005-0125-z [DOI] [PubMed] [Google Scholar]
- 7. Cali AMG, Zern TL, Taksali SE, et al. Intrahepatic fat accumulation and alterations in lipoprotein composition in obese adolescents: a perfect proatherogenic state. Diabetes Care. 2007;30(12):3093‐3098. 10.2337/dc07-1088 [DOI] [PubMed] [Google Scholar]
- 8. Hamaguchi M, Kojima T, Takeda N, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13(10):1579‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non‐alcoholic fatty liver disease: causal effect or epiphenomenon?. Diabetologia. 2008;51:1947‐1953. [DOI] [PubMed] [Google Scholar]
- 10. Fracanzani AL, Burdick L, Raselli S, et al. Carotid artery intima‐media thickness in nonalcoholic fatty liver disease. The American Journal of Medicine. 2008;121(1):72‐78. 10.1016/j.amjmed.2007.08.041 [DOI] [PubMed] [Google Scholar]
- 11. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology. 2017;65(5):1557‐1565. 10.1002/hep.29085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology. 2015;61(5):1547‐1554. 10.1002/hep.27368 [DOI] [PubMed] [Google Scholar]
- 13. Authors/Task Force Members ,Catapano AL, Graham I, et al. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;2016(253):281‐344. 10.1016/j.atherosclerosis.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 14. Calderon RM, Cubeddu LX, Goldberg RB, Schiff ER. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: a therapeutic dilemma. Mayo Clin Proc. 2010;85(4):349‐356. 10.4065/mcp.2009.0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rzouq FS, Volk ML, Hatoum HH, Talluri SK, Mummadi RR, Sood GK. Hepatotoxicity fears contribute to underutilization of statin medications by primary care physicians. Am J Med Sci. 2010;340(2):89‐93. 10.1097/MAJ.0b013e3181e15da8 [DOI] [PubMed] [Google Scholar]
- 16. Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology. 2006;44(2):466‐471. 10.1002/hep.21248 [DOI] [PubMed] [Google Scholar]
- 17. Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long‐term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post‐hoc analysis. Lancet. 2010;376(9756):1916‐1922. 10.1016/S0140-6736(10)61272-X [DOI] [PubMed] [Google Scholar]
- 18. Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126(5):1287‐1292. [DOI] [PubMed] [Google Scholar]
- 19. Eslami L, Merat S, Malekzadeh R, Nasseri‐Moghaddam S, Aramin H. Statins for non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;(12);CD008623 10.1002/14651858.CD008623.pub2 [DOI] [PubMed] [Google Scholar]
- 20. Schierwagen R, Maybüchen L, Hittatiya K, et al. Statins improve NASH via inhibition of RhoA and Ras. Am J Physiol Gastrointest Liver Physiol. 2016;311(4):G724‐G733. 10.1152/ajpgi.00063.2016 [DOI] [PubMed] [Google Scholar]
- 21. Trebicka J, Hennenberg M, Odenthal M, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53(4):702‐712. 10.1016/j.jhep.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 22. Trebicka J, Hennenberg M, Laleman W, et al. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho‐kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46(1):242‐253. 10.1002/hep.21673 [DOI] [PubMed] [Google Scholar]
- 23. Tripathi DM, Vilaseca M, Lafoz E, et al. Simvastatin prevents progression of acute on chronic liver failure in rats with cirrhosis and portal hypertension. Gastroenterology. 2018;155(5):1564‐1577. 10.1053/j.gastro.2018.07.022 [DOI] [PubMed] [Google Scholar]
- 24. Kim RG, Loomba R, Prokop LJ, Singh S. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2017;15(10):1521‐1530.e1528. 10.1016/j.cgh.2017.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blais P, Lin M, Kramer JR, El‐Serag HB, Kanwal F. Statins are underutilized in patients with nonalcoholic fatty liver disease and dyslipidemia. Dig Dis Sci. 2016;61(6):1714‐1720. 10.1007/s10620-015-4000-6 [DOI] [PubMed] [Google Scholar]
- 26. Taii Al H, Yaqoob Z, Al‐Kindi SG. Underutilization of statin therapy among patients with NAFLD in the USA: validation with big data. Dig Dis Sci. 2016;61(9):2760 10.1007/s10620-016-4257-4 [DOI] [PubMed] [Google Scholar]
- 27. Del Ben M, Baratta F, Polimeni L, et al. Under‐prescription of statins in patients with non‐alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2017;27(2):161‐167. 10.1016/j.numecd.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 28. Stolk RP, Rosmalen JGM, Postma DS, et al. Universal risk factors for multifactorial diseases: LifeLines: a three‐generation population‐based study. Eur J Epidemiol. 2008;23(1):67‐74. 10.1007/s10654-007-9204-4 [DOI] [PubMed] [Google Scholar]
- 29. Scholtens S, Smidt N, Swertz MA, et al. Cohort profile: LifeLines, a three‐generation cohort study and biobank. Int J Epidemiol. 2015;44(4):1172‐1180. 10.1093/ije/dyu229 [DOI] [PubMed] [Google Scholar]
- 30. Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33 10.1186/1471-230X-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. European Association for the Study of the Liver (EASL). Electronic address: easloffice@easloffice.eu, European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016. 10.1016/j.jhep.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846‐854. 10.1002/hep.21496 [DOI] [PubMed] [Google Scholar]
- 33. Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10(11):666‐675. 10.1038/nrgastro.2013.175 [DOI] [PubMed] [Google Scholar]
- 34. European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL‐ALEH Clinical Practice Guidelines . Non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237‐264. 10.1016/j.jhep.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 35. Vallet‐Pichard A, Mallet V, Nalpas B, et al. FIB‐ 4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32‐36. 10.1002/hep.21669 [DOI] [PubMed] [Google Scholar]
- 36. Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104‐1112. 10.1016/j.cgh.2009.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735‐2752. 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 38. Endo A. The discovery and development of HMG‐CoA reductase inhibitors. J Lipid Res. 1992;33(11):1569‐1582. [PubMed] [Google Scholar]
- 39. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. The Lancet. 2010;375(9716):735‐742. 10.1016/S0140-6736(09)61965-6 [DOI] [PubMed] [Google Scholar]
- 40. Athyros VG, Giouleme O, Ganotakis ES, et al. Safety and impact on cardiovascular events of long‐term multifactorial treatment in patients with metabolic syndrome and abnormal liver function tests: a post hoc analysis of the randomised ATTEMPT study. Arch Med Sci. 2011;7(5):796‐805. 10.5114/aoms.2011.25554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nascimbeni F, Aron‐Wisnewsky J, Pais R, et al. Statins, antidiabetic medications and liver histology in patients with diabetes with non‐alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3(1):e000075 10.1136/bmjgast-2015-000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non‐alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow‐up study. J Hepatol. 2007;47(1):135‐141. 10.1016/j.jhep.2007.02.013 [DOI] [PubMed] [Google Scholar]
- 43. Dongiovanni P, Petta S, Mannisto V, et al. Statin use and non‐alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63(3):705‐712. 10.1016/j.jhep.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 44. Tikkanen MJ, Fayyad R, Faergeman O, et al. Effect of intensive lipid lowering with atorvastatin on cardiovascular outcomes in coronary heart disease patients with mild‐to‐moderate baseline elevations in alanine aminotransferase levels. Int J Cardiol. 2013;168(4):3846‐3852. 10.1016/j.ijcard.2013.06.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


