Abstract
Accumulation of alpha‐synuclein protein aggregates is the hallmark neuropathologic feature of synucleinopathies such as Parkinson’s disease. Rare point mutations and multiplications in SNCA, the gene encoding alpha‐synuclein, as well as other genetic alterations are linked to familial Parkinson’s disease cases with high penetrance and hence constitute major genetic risk factors for Parkinson’s disease. However, the preponderance of cases seems sporadic, most likely based on a complex interplay between genetic predispositions, aging processes and environmental influences. Deciphering the impact of these environmental factors and their interactions with the individual genetic background in humans is challenging and often requires large cohorts, complicated study designs, and longitudinal set‐ups. In contrast, rodent models offer an ideal system to study the influence of individual environmental aspects under controlled genetic background and standardized conditions. In this review, we highlight findings from studies examining effects of environmental enrichment mimicking stimulation of the brain by its physical and social surroundings as well as of environmental stressors on brain health in the context of Parkinson’s disease. We discuss possible internal molecular transducers of such environmental cues in Parkinson’s disease rodent models and emphasize their potential in developing novel avenues to much‐needed therapies for this still incurable disease.
Keywords: alpha‐synuclein, enriched environment, epigenetics, neurodegeneration, Parkinson’s disease, stress
Abbreviations used
- 6‐OHDA
6‐hydroxydopamine
- AD
Alzheimer’s disease
- α-syn
alpha‐synuclein
- BDNF
brain‐derived neurotrophic factor
- EE
environmental enrichment
- EHMT2
euchromatic histone‐lysine N‐methyltransferase 2
- GCs
glucocorticoids
- GR
glucocorticoid receptor
- HD
Huntington’s disease
- IGF‐1
insulin‐like growth factor‐1
- MPTP
1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine
- NGF
nerve growth factor
- PD
Parkinson’s disease
- RE1
restrictive element 1
- REST
restrictive element 1‑silencing transcription factor
- SNAP25
synaptosomal‐associated protein
- VEGF
vascular endothelial growth factor
Alpha‐synuclein (α‐syn) is a key presynaptic protein in Parkinson’s disease (PD) and other synucleinopathies. Misfolding and aggregation of α‐syn is a central event in synucleinopathies, and the presence of eosinophilic intracellular inclusions that contain abundant levels of α‐syn reflects the main neuropathological hallmark of these diseases, known as Lewy pathology (Spillantini et al., 1997; Spillantini et al., 1998; Shults, 2006). Yet, the factors triggering α‐syn pathology and the resulting cellular toxicity are still largely enigmatic.
The physiological function of alpha‐synuclein
α‐syn belongs to the synuclein family of proteins together with its close homologs beta‐ and gamma‐synuclein. Synuclein proteins are prevalently expressed in the nervous system (Jakes et al., 1994; Lavedan et al., 1998a; Lavedan et al., 1998b) and localize preferentially to presynaptic terminals (Jakes et al., 1994; George, 2002; Ninkina et al., 2012). Despite its ubiquitous neuronal expression, α‐syn has also been detected in peripheral tissues and blood cells (Askanas et al., 2000; Shin et al., 2000; Ltic et al., 2004; Nakai et al., 2007). In neuronal cells, the apparent nuclear and synaptic localization of α‐syn suggests a physiological function in both compartments. Despite some controversy about α‐syn’s nuclear localization, current reports support the existence of α‐syn in the nucleus and its potential direct interaction with DNA (Goers et al., 2003; Ma et al., 2014b; Pinho et al., 2019). In addition, nuclear α‐syn has been detected in transgenic models such as mice (Masliah et al., 2000; Goers et al., 2003), Drosophila (Takahashi et al., 2003), and cells (McLean et al., 2000; Specht et al., 2005), as well as in human brain samples (Siddiqui et al., 2012). Apparently, the abnormal accumulation of α‐syn in the nucleus is associated with DNA damage and neurotoxicity (Kontopoulos et al., 2006; Padmaraju et al., 2011; Ma et al., 2014a). Besides the potential binding of α‐syn to DNA and direct regulation of gene expression under specific conditions (Martins et al., 2011; Siddiqui et al., 2012), α‐syn may also interfere with epigenetic processes regulating gene expression (Kontopoulos et al., 2006; Desplats et al., 2011). These processes do not necessarily require the physical presence of α‐syn inside the nucleus as their regulation may also rely on a number of cellular mediators bridging the effects of α‐syn to the nucleus (Jin et al., 2011).
The high concentration of α‐syn in presynaptic terminals, its association with synaptic vesicles (Maroteaux et al., 1988; Kahle et al., 2000), interaction with presynaptic proteins and function as a SNARE chaperon essential for vesicle fusion (Burre et al., 2010; Chen et al., 2013; Zaltieri et al., 2015) suggest a role of α‐syn in regulating synaptic homeostasis and neurotransmitters release including dopamine. While knockout models for α‐syn exhibit normal basal hippocampal synaptic transmission, significant impairments in hippocampal synaptic responses are evident under conditions capable of exhausting docked as well as reserve pool vesicles (Cabin et al., 2002). A slower refilling of the docked pools from the reserve pool, indicates a role for endogenous α‐syn in synaptic vesicles trafficking and maintenance (Abeliovich et al., 2000; Cabin et al., 2002). A reduction in the distal pool of synaptic vesicles upon α‐syn antisense oligonucleotide treatment in primary hippocampal neurons supports the capacity of α‐syn to regulate the vesicle pool size at synaptic terminals (Murphy et al., 2000). Moreover, synuclein knockout mice show decreased SNARE‐complex assembly (Burre et al., 2010) and changes in synaptic structure and size (Greten‐Harrison et al., 2010) together with impairments in learning and memory tasks (Kokhan et al., 2011; Kokhan et al., 2012). These reports indicate that synucleins may not be vital to the basic machinery of synaptic transmission but rather contribute to the long‐term maintenance of synaptic functions (Chandra et al., 2004).
The effect of α‐syn on the synaptic release machinery has been further investigated in α‐syn overexpressing models, where the consequent functional deficits based on α‐syn excess can be related to either toxic loss or gain of function of this protein (Benskey et al., 2016; Collier et al., 2016). α‐syn overexpression inhibits neurotransmitter release possibly due to impairments in synaptic vesicle recycling (Nemani et al., 2010) and alterations in presynaptic proteins linked to exocytosis and endocytosis processes (Scott et al., 2010). Moreover, overexpression of wildtype α‐syn, but not the A30P mutant, results in a decrease of dopamine release that correlates with a decreased density of dopaminergic vesicles (Gaugler et al., 2012). Data from this report further suggest that the affinity of α‐syn protein for membranes plays an important role in the observed presynaptic pathology (Gaugler et al., 2012). A reduction in dopamine reuptake and a dysfunction of the dopamine transporter in dopaminergic neurons have also been observed upon wildtype α‐syn overexpression in rats (Lundblad et al., 2012). Altogether, despite the progress made in revealing the physiological function of α‐syn using knockout and overexpression models, many questions remain unanswered and require further research. A deeper insight into aspects of α‐syn functions and cellular distribution will aid in understanding the contribution of this protein under pathological conditions.
The role of alpha‐synuclein in Parkinson’s disease
Traditionally described as the most common synucleinopathy and neurodegenerative movement disorder, PD is estimated to affect 2–3% of the population aged 65 years and above and its prevalence continues to increase with advancing age (Pringsheim et al., 2014; Poewe et al., 2017). A progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta and depletion of striatal dopamine drive the manifestation of motor symptoms, such as bradykinesia, muscle rigidity, and tremor. Besides cardinal motor symptoms, a plethora of non‐motor and neuropsychiatric features accompany the disease from prodromal to advanced stages, likely involving additional brain circuitries besides the dopaminergic system (Goldman and Postuma, 2014; Poewe et al., 2017; Przedborski, 2017). The characteristic Lewy pathology of both familial and sporadic forms of PD, is thought to precede the occurrence of motor symptoms and starts in the prodromal phase (Spillantini et al., 1997; Spillantini et al., 1998; Shults, 2006).
On a genetic level, point mutations in SNCA (A30P, E46K, H50Q, G51N, A53E, and A53T) are linked to rare familial cases of PD (Polymeropoulos et al., 1997; Kruger et al., 1998; Zarranz et al., 2004; Lesage et al., 2013; Proukakis et al., 2013; Pasanen et al., 2014). In addition, duplication and triplication of this locus cause familial PD in a gene dose‐dependent manner with early onset, varying pathology and clinical features (Singleton et al., 2003; Chartier‐Harlin et al., 2004; Ibanez et al., 2004). Further, increased susceptibility to sporadic PD is associated with several genetic variants in the SNCA gene (Satake et al., 2009; Simon‐Sanchez et al., 2009). Similarly, genetic variants in LRRK2, MAPT, and VPS13C genes, also linked to familial cases of PD, are associated with increased risk for sporadic forms (Satake et al., 2009; Simon‐Sanchez et al., 2009; Nalls et al., 2014). Despite the high disease penetrance of familial cases, clear monogenetic families are very rare, and the relative population risk based on genetic alterations alone seems to be limited to around 10% (Lesage and Brice, 2009). Thus, the great majority of PD patients do not have a straightforward genetic SNCA predisposition. However, all PD patients have α‐syn neuropathology, which in fact is the defining neuropathological hallmark needed for the definite post‐mortem diagnosis of PD.
Environmental influences in Parkinson’s disease
As the majority of PD cases are sporadic with yet unknown etiology, the risk to develop PD seemingly arises from a complex interplay of genetic predispositions, aging processes, and environmental factors, resulting in a combined effect of both genetic and environmental elements that ultimately determine an individual’s susceptibility to the disease. In line with this idea, epidemiological evidence points towards a strong environmental component in disease etiology (Ascherio and Schwarzschild, 2016; Bellou et al., 2016). Studies involving monozygotic or dizygotic twins, moreover, point at the relevant contribution of genetic and environmental factors. There, too, the evidence implies a strong environmental influence (Wirdefeldt et al., 2004) as similar concordance rates for monozygotic and dizygotic twins have been observed (Tanner et al., 1999). Accumulating evidence points at several environmental agents that potentially modify the risk of developing the disease. Most prominently, an increased risk for PD is associated with exposure to pesticides, herbicides, and insecticides (Brown et al., 2005). Although debatable, living in a rural environment and drinking well water has been associated with a higher risk for PD, possibly due to the exposure to pesticides or other contaminants (Brown et al., 2005; Ascherio and Schwarzschild, 2016). Furthermore, there is growing evidence for the role of heavy metals and exposure to organic solvents as putative risk factors for PD and Parkinsonism (Smargiassi et al., 1998; Racette et al., 2001; Goldman et al., 2012; Racette, 2014). Besides the impact of environmental chemicals, clinical evidence indicates a role for environmental stress in PD onset (Zou et al., 2013) supported by views on an analogy between stress‐induced pathological consequences and the neuronal deterioration observed in PD (Smith et al., 2002). In contrast, seemingly lowering the risk for PD, coffee consumption, smoking, higher serum urate levels, and physical activity are inversely associated with PD (Hernan et al., 2002; Ascherio and Schwarzschild, 2016). It is important to point out that in this context, the causality of these protective effects is highly debated, mainly because of the paucity of well‐designed studies in humans. Nevertheless, compelling evidence supports protective potentials of physical activity owing to highly significant associations with a reduced risk of developing PD (Bellou et al., 2016). Taken together, an individual’s susceptibility to PD is likely influenced by a combination of life style factors and environmental exposures that act simultaneously or in sequence and interact with the individual’s genetic makeup rendering the organism more vulnerable to deleterious processes and neuronal attrition with advancing age (Carvey et al., 2006; Sulzer, 2007).
Epigenetics and alpha‐synuclein (patho‐)physiology
Owing to the dynamic nature of the epigenome and the susceptibility of the epigenetic landscape to the environment (Ost et al., 2014; Allis and Jenuwein, 2016), epigenetic adaptations driven by environmental influences may hold the key to understanding the impact of the latter in health and disease. Changes in the epigenome have been linked to α‐syn and been related to several neurodegenerative disorders (Feng et al., 2015; Landgrave‐Gomez et al., 2015; Hwang et al., 2017; Pavlou et al., 2017). Evaluating genome‐wide DNA methylation profiles, differential methylation has been observed both in blood and brain tissue from PD patients (Masliah et al., 2013). On bulk level, α‐syn has been proposed to alter DNA methylation by sequestering the maintenance DNA methyltransferase (Dnmt1) to the cytoplasm (Desplats et al., 2011). Consistently, global DNA hypomethylation is accompanied by reduction of nuclear DNA methyltransferase 1 in human brain samples of patients with PD or dementia with Lewy bodies (Desplats et al., 2011). Furthermore, α‐syn seemingly interacts with histones, first described in the nuclei of nigral neurons from toxin‐treated mice (Goers et al., 2003). Such interactions between α‐syn and histones have been proposed to be toxic to neurons due to reduced histone acetylation, as rescue was observed when treating with histone deacetylase inhibitors (HDACis) in both cell culture and transgenic flies (Kontopoulos et al., 2006). In addition, several transcription factors coordinating epigenetic regulation of genes essential for neuronal function have been associated with chromatin remodeling. Among such transcriptional regulators is the restrictive element 1‑silencing transcription factor (REST; also known as neuron‐restrictive silencer factor, NRSF), which acts via epigenetic mechanisms to instruct the expression of genes involved in neuronal function and survival (Noh et al., 2012). REST’s role in maintaining normal brain development and function (Ballas and Mandel, 2005; Ooi and Wood, 2007; Baldelli and Meldolesi, 2015) implies that a dysregulation to this system might contribute to brain disorders and neurodegeneration. Indeed, REST seems dysregulated in the striatal tissue of Huntington’s disease (HD) patients and HD mouse models (Zuccato et al., 2003), where nuclear availability of REST in striatal neurons is modulated through its interaction with the huntingtin protein. Recent studies have further highlighted the essential role of REST in healthy aging and implicated that dysregulation of REST is associated with cognitive decline and AD (Lu et al., 2014). In the context of PD, 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) treatment evokes REST expression and changes its subcellular distribution in dopaminergic cells (Yu et al., 2009). In turn, REST depletion exacerbates the detrimental effect of MPTP treatment in mice implying a potential protective role for REST in neurons of the substantia nigra (Yu et al., 2013). REST acts to repress gene expression by binding to restrictive element 1 (RE1) within the promoters of target genes. It recruits co‐repressors and promotes epigenetic remodeling to initiate gene silencing through a number of mechanisms such as recruiting histone deacetylases, histone methyltransferases, and histone demethylases (Huang et al., 1999; Zhang et al., 2002; Lee et al., 2005; Kazantsev and Thompson, 2008). In a transgenic α‐syn Drosophila model and inducible SH‐SY5Y neuroblastoma cells, α‐syn overexpression increases levels of histone marks associated with heterochromatin, together with levels of EHMT2 (euchromatic histone‐lysine N‐methyltransferase 2, also known as G9a) that acts to modify H3K9 (Sugeno et al., 2016). Indeed, a reduction of dimethylated H3K9 has been observed after EHMT2 inhibition in α‐syn expressing cells. Moreover, REST and H3K9 dimethylation are significantly enriched at RE1 sites of the synaptosomal‐associated protein (SNAP25), and their occupancy is further increased after α‐syn induction. This effect is accompanied by reduced SNAP25 expression consistent with the repressive function of H3K9me2 and the REST complex.
In conclusion, disturbances of epigenetic marks in PD likely play a role in disease unfolding and manifestation. Environment‐dependent epigenetic adaptations that impact the underlying gene expression are appealing regulatory layers that might harbor clues to the strong environmental component in PD. The reversibility of such epigenetic events by targeted therapies and environmental interventions offers great potential for new therapeutic strategies for this yet incurable disease.
Using animal models to study environmental impacts
Studying the role of environmental exposures and their interaction with the individual's genetic background in humans is challenging and often requires large cohorts, complicated study designs, and long follow‐ups (years or even decades). In addition, many studies suffer from lack of consistency and specificity, possibly due to our highly complex living conditions that include a vast number of environmental variables and stimuli of different quality and quantity. As an alternative strategy, studying the impact of environmental modulation in disease models offers advantages with respect to I. Control and standardization of range and duration of environmental impacts. II. Focus on a specific stimulus on behavioral, structural, molecular, and functional levels. III. Trace the interaction between specific genetic features and environmental exposures.
In particular, rodent models are suitable to decipher the impact of environmental factors in PD as toxin‐induced as well as genetic models mimic the disease. Given the central role for α‐syn in PD pathogenesis, α‐syn transgenic models have emerged as valuable models in PD research and are well suited to study the role of environmental factors in disease unfolding. In this work, we highlight findings from studies examining environmental interventions in PD rodent models and point out how they relate to observations in PD patients. Specifically, we will focus on environmental enrichment, as a factor with protective impact on PD, and environmental stress, as a factor proposed to increase the risk of PD, and discuss the possible internal molecular transducers of such environmental influences in PD.
Physical activity and environmental enrichment
Environmental enrichment (EE), a term that has been coined for an enhanced motor and cognitive stimulation, is known to have a broad impact on the organism acting both peripherally and centrally. In the brain, the benefits of such an approach extend to several areas and involve structural and functional modulations that affect brain performance and resilience (Nithianantharajah and Hannan, 2006) (Fig. 1). Mimicking EE in laboratory rodents is achieved by placing objects, varying in shape, size, color, and texture, as well as running wheels in housing cages to enable more physical activity (Nithianantharajah and Hannan, 2006). The cages are typically larger in the enriched condition compared to standard housing, offering the possibility to house a higher number of animals in one cage resulting in enhanced social interactions. In addition, an EE also aims at greater cognitive stimulation and requires constant novel inputs for the animals achieved by regularly changing and rearranging objects in the EE cages.
Figure 1.
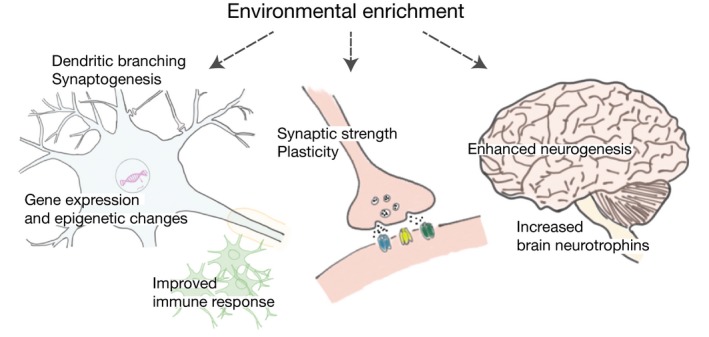
Environmental enrichment induces structural and functional modulations in the brain. The benefits of environmental enrichment (EE) include increased production and maturation of new neurons, enhanced levels of brain growth factors, synaptic strength, and plasticity. In addition, EE improves the immune condition of the brain, modulates gene expression, elicits epigenetic changes, and augments dendritic branching and synaptogenesis.
As one of the best studied brain regions in this context, the hippocampus represents a central brain hub for integrating sensory information from external stimuli (Kempermann et al., 1997). In the hippocampus, EE increases production and maturation of new neurons (Kempermann et al., 1997; Kempermann et al., 2002; Bruel‐Jungerman et al., 2005) possibly mediated by the growth factor VEGF (vascular endothelial growth factor) (During and Cao, 2006), to enhance hippocampal synaptic strength, and to modify long‐term potentiation (LTP) (Duffy et al., 2001; Foster and Dumas, 2001; Artola et al., 2006). Furthermore, enrichment enhances levels of synaptic proteins (Frick and Fernandez, 2003; Nithianantharajah et al., 2004) and brain neurotrophins [such as BDNF (brain‐derived neurotrophic factor) and NGF (nerve growth factor)] (Pham et al., 1999; Ickes et al., 2000) in the hippocampus and other brain regions, and modulates expression of genes linked to neuronal structure and synaptic transmission (Rampon et al., 2000; Wassouf et al., 2018). Moreover, exposure to environmental enrichment augments cortical and hippocampal dendritic branching and synaptogenesis (van Praag et al., 2000). Similarly, enhanced physical activity increases cellular and synaptic plasticity by modulating synaptic structure and strength (Farmer et al., 2004; Eadie et al., 2005), elevating synaptic protein levels (Farmer et al., 2004; Vaynman et al., 2006), and promoting neurogenesis (van Praag et al., 1999; Trejo et al., 2001; Fabel et al., 2003). An increase in dendritic length and complexity has also been linked to enhanced physical activity (Eadie et al., 2005). Consistent with effects of EE, physical activity alone induces growth factors of several classes, including BDNF, IGF‐1 (insulin‐like growth factor‐1), and VEGF, that work in concert and through downstream signaling cascades to mediate the effects of the environment on the brain (Trejo et al., 2001; Berchtold et al., 2005; Kuipers and Bramham, 2006; Ding et al., 2006a; Ding et al., 2006b). Given their broad influence, it is plausible that such growth factors orchestrate the wide‐ranging impact of exercise and EE on brain functions and resilience (Cotman et al., 2007). In this context, the enhancement in growth factor function induced by the environment can be due to either increasing growth factor levels itself or indirectly by improving the immune condition of the brain (Cotman et al., 2007) as proinflammatory cytokines are able to impair growth factor signaling (Venters et al., 2001; Tong et al., 2008). Consequently, such morphological and molecular changes in response to EE and physical activity elicit behavioral improvement in learning and memory (Duffy et al., 2001; Tang et al., 2001; Vaynman et al., 2004; van Praag et al., 2005), neuropsychiatric behavior (Blumenthal et al., 1999; Roy et al., 2001; Benaroya‐Milshtein et al., 2004; Singh et al., 2005), and protect from age‐related and disease‐related neurodegeneration (Colcombe and Kramer, 2003; Weuve et al., 2004; Nithianantharajah and Hannan, 2011; Hentrich et al., 2018). The benefits of such environmental stimuli can be passed to the next generation as shown recently in mice (Benito et al., 2018). As as intergenerational effect, the exposure to EE enhances hippocampal synaptic plasticity and improves cognitive abilities mediated through miR212/132 (Benito et al., 2018).
Environmental enrichment and brain degeneration
After the first evidence that environmental enrichment delays disease onset in a preclinical model of a brain disorder, extensive evidence on the benefits of EE in the context of neurodegenerative disorders has been rapidly accumulating (Nithianantharajah and Hannan, 2006; Wassouf et al., 2018). The global impact of EE on brain health, such as stimulation of neurotrophic factors, immune system improvements, and neurogenesis, induces changes in brain functions and strengthens neuronal and synaptic connectivity, which may provide compensatory capacities and facilitate neuroplasticity that decelerates pathogenetic processes associated with brain neurodegeneration, a theory referred to as ‘brain reserve’ or ‘cognitive reserve’ (Stern, 2002; Valenzuela and Sachdev, 2006; Nithianantharajah and Hannan, 2009). In humans, the association between physical activity and a reduced risk for dementia and Alzheimer’s disease (AD) is evident in several clinical studies (Hamer and Chida, 2009; Brown et al., 2013), and have also demonstrated a role for physical activity protecting from cognitive decline and maintaining a superior cognitive functioning in verbal memory, attention, executive functions, and general cognitive capabilities. Studies in AD mice, moreover, have shown broad behavioral and cellular alterations induced by EE (Cracchiolo et al., 2007; Mirochnic et al., 2009), including enhanced learning and memory (Arendash et al., 2004), marked reduction in cerebral Aß levels and amyloid deposits, and increased neprilysin activity (Lazarov et al., 2005).
As mentioned above, recent meta‐analyses have highlighted physical activity to have one of the most significant associations with a reduced risk of developing PD (Yang et al., 2015; Bellou et al., 2016). Specifically, a medium level of daily physical activity seems inversely associated with PD risk. Likewise, for PD patients, exercise exerts beneficial influences on quality of life and physical competencies, including leg strength, balance, and gait (Goodwin et al., 2008), in line with known benefits of physiotherapy in PD patients (Keus et al., 2007). Equally, treadmill exercise improves gait and postural stability in PD patients with mild to moderate impairments (Herman et al., 2007; Herman et al., 2009).
Environmental enrichment in PD models
The impact of EE in PD was initially shown (Bezard et al., 2003; Tillerson et al., 2003; Faherty et al., 2005; Jadavji et al., 2006) and further followed‐up (Anastasia et al., 2009; Goldberg et al., 2011; Klaissle et al., 2012; Jungling et al., 2017) in toxin‐induced rodent models after neurotoxin insults with MPTP or 6‐hydroxydopamine (6‐OHDA), which cause damage of nigrostriatal dopaminergic neurons and depletion of striatal dopamine. Specifically, EE exposure in these models increases resistance to MPTP‐induced neuronal insult (Bezard et al., 2003; Faherty et al., 2005), and protects from or ameliorates behavioral impairments, including Parkinson’s related motor deficits in gait and balance, as a result of 6‐OHDA or MPTP insult (Tillerson et al., 2003; Jadavji et al., 2006; Pothakos et al., 2009; Goldberg et al., 2011). Preservation of dopaminergic neurons, dopaminergic fibers in the striatum, and dopamine transporters, decreased loss of striatal dopamine along with enhanced neurotrophic factors such as BDNF and glial cell‐derived neurotrophic factor (GDNF) have been further associated with EE exposure in these models (Bezard et al., 2003; Cohen et al., 2003; Tillerson et al., 2003; Faherty et al., 2005; Tajiri et al., 2010). Moreover, physical exercise increases dopamine production in remaining dopaminergic neurons in PD (Sutoo and Akiyama, 2003), and modifies glutamate signaling, either by influencing expression of glutamate receptors or the presynaptic storage and release of glutamate (VanLeeuwen et al., 2010). The beneficial impact of EE might also involve the immune system, counteracting the activation of the immune response observed in the context of PD pathology (Huang and Halliday, 2012; Heneka et al., 2014). Notably, an alternative mechanism for the EE‐induced neuroprotection in the toxin‐models of PD may be through lowering dopamine transporter (DAT) levels, which is required for 6‐OHDA and MPTP uptake and neurotoxicity (O'Dell et al., 2007; Petzinger et al., 2007).
To better understand these principles, it is essential to extend these investigations to genetic animal models of PD. While several genetic PD models, for example for SNCA, are available, reports on EE impact in such models of familial PD forms are still scarce. Recently, a study by Minakaki and colleagues has shown benefits of physical exercise in human alpha‐synuclein expressing mice with motor enhancement, particularly in gait and posture, and augmented striatal tyrosine hydroxylase levels after treadmill exercise (Minakaki et al., 2019). Further, EE exposure improves olfactory function and alleviates oxidative stress and levels of nitrated α‐syn in human A53T α‐syn overexpressing mice (Wi et al., 2018). In a recent study from our lab, we investigated the influences of a long‐term EE in a mouse model overexpressing the human wildtype SNCA gene under its native regulatory elements to trace molecular events that connect EE influences to α‐syn biology (Wassouf et al., 2018). Environmental enrichment led to widespread prevention of SNCA‐induced disturbances in the hippocampal transcriptome including disturbances in microglia and astrocytes. These preventive effects were accompanied by sustained activation of a group of immediate early genes (IEGs), including the transcription factors EGR1 and NURR1/NR4A2 (Wassouf et al., 2018). Such approaches have the potential to highlight intriguing regulatory networks that might harbor attractive therapeutic points of action to mimic beneficial impacts of EE and open novel avenues for preventing, delaying, or treating synucleopathies and related disorders early on.
Environmental stress and brain health
Our brain is constantly changing with experience and the discussed EE effects on brain health and disease are examples for the brain’s plasticity and adaptability. The brain’s adaptation to the surrounding environment further involves perceiving and responding to environmental stressors (McEwen et al., 2015). While healthy brains are capable of coping and adapting to stressors, an individual resilience or vulnerability to stress can be shaped by several factors, such as type, duration, and intensity of the stressor, the individual’s gender, genetic and epigenetic background, as well as early life experiences (McEwen, 2007; Sotiropoulos et al., 2008; McEwen and Morrison, 2013; Bale and Epperson, 2015; Chen and Baram, 2016; Sousa, 2016). Further, stress response can be impaired by aging processes or neurodegenerative diseases and render individuals susceptible to detrimental physical and mental consequences (Hatzinger et al., 1995; Hartmann et al., 1997; Charlett et al., 1998; Peskind et al., 2001; Aguilera, 2011). Regulating stress response in the brain involves hypothalamic and extra‐hypothalamic structures that express glucocorticoid receptor (GR) alone or together with mineralocorticoid receptor. Both receptors respond to glucocorticoids (GC) with varying affinity and mediate GCs’ broad effects in the brain (Veldhuis et al., 1982; McEwen et al., 1986), which may involve neuronal and glial cells (Ahima et al., 1991). Because of higher mineralocorticoid receptor affinity for GC (10 fold higher), these receptors are occupied at basal GC levels, while GR is activated only at a certain GC threshold (Reul and de Kloet, 1985; De Kloet et al., 1998).
Environmental stress and neurodegeneration
Several lines of evidence propose a link between prolonged stress exposure causing elevated stress hormones (GCs) and certain brain disorders including occurrence and progression of neurodegenerative diseases (Mejia et al., 2003; Smith et al., 2008; Simard et al., 2009; de Pablos et al., 2014; Di Meco et al., 2014). The pathological consequences of prolonged stress and elevated stress hormones on the brain comprise altering a wide range of neuronal (and non‐neuronal) functions and structures (McEwen et al., 2015; Vyas et al., 2016) through mechanisms that involve transcriptomic and epigenetic modulations, among others (Meaney and Szyf, 2005; Gray et al., 2014; Hunter et al., 2015). These effects are mediated by glucocorticoid receptors acting as transcription factors together with other interacting cellular mediators. The resulting neuronal attrition and brain pathology due to stress/GC elevation resembles those of neurodegenerative disorders, like distorted neuronal architecture, synaptic damage, mitochondrial dysfunction, altered neurogenesis, and activated neuroinflammatory response (McEwen et al., 2015; Vyas et al., 2016) (Fig. 2). In fact, clinical data and experimental studies exploring the role of stress and GCs in neurodegenerative diseases such as PD, AD, and dementia further support these commonalities (Smith et al., 2002; Mejia et al., 2003; Metz, 2007; Pienaar et al., 2008; Simard et al., 2009; Zou et al., 2013).
Figure 2.
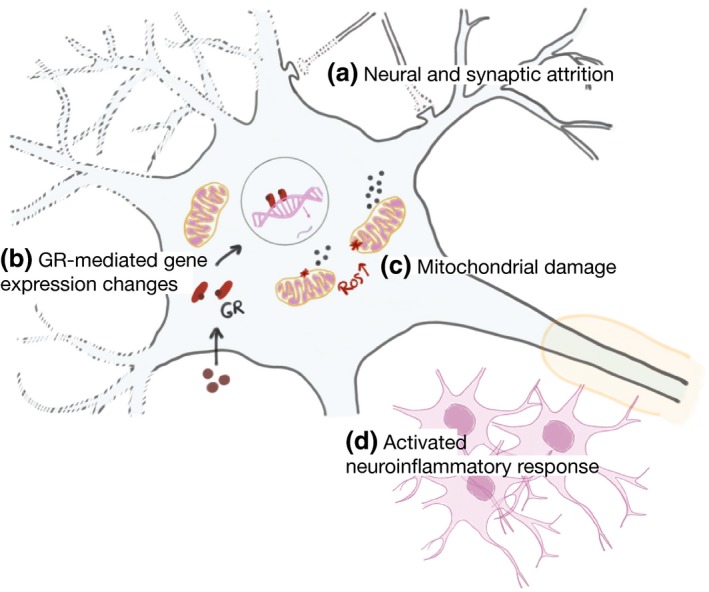
Pathological consequences of environmental stress and elevated stress hormones on the brain. (a) Neural and synaptic attrition. (b) Glucocorticoids (GCs) are released as a result of stress and their actions are mediated by binding to glucocorticoid receptors (GRs), GRs act as transcription factors causing changes in gene expression. (c) Mitochondrial damage and increase of ROS production. (d) Activated neuroinflammatory response. ROS: Reactive oxygen species.
Environmental stress in PD models
Combining stress exposure with MPTP or 6‐OHDA insults in toxin‐induced animal models of PD exacerbates neuronal damage, aggravates motor deficits, and triggers neuroinflammatory response in rodents (Pienaar et al., 2008; Smith et al., 2008; Lauretti et al., 2016). These findings and several others (Mizoguchi et al., 2000; Rasheed et al., 2010; de Pablos et al., 2014) have unveiled an overt susceptibility of the dopaminergic circuitries to stress‐induced cytotoxicity. Such vulnerability of the dopaminergic system can be related to prolonged release of catecholamines such as dopamine, GCs, and glutamate as a result of chronic stress (Smith et al., 2002). Particularly, catecholamines, including dopamine, are prone to auto‐oxidation, which can ultimately lead to neurodegeneration (Goldstein, 2011). In fact, mild chronic stress has already been reported to trigger oxidative stress (Lucca et al., 2009). Chronic stress, moreover, induces a proinflammatory response and release of cytokines and chemokines that result in activation of the hypothalamic‐pituitary‐adrenal (HPA) axis (Haddad et al., 2002). On a related note, changes in GR have been described in PD patients and MPTP‐intoxicated models, and sustained proinflammatory response resulting in dopaminergic neurons degeneration has been observed after specific modulation of the microglial GR in MPTP‐treated mice (Ros‐Bernal et al., 2011). Altogether, stress and sustained elevation of GCs potentially impact motor as well as non‐motor features of PD such as anxiety and depression already surfacing during PD’s prodromal phase (Goldman and Postuma, 2014; Bellou et al., 2016). Some PD characteristic traits associated with anxiety seemingly result from damage to the mesolimbic dopaminergic projections involved in reward and motivation, such as lower novelty seeking and higher harm avoidance (Menza et al., 1993; Kaasinen et al., 2001; Costa and Caltagirone, 2015).
Similar to studies on environmental enrichment, the majority of reports examining the impact of environmental stress in PD models focus on the effects on dopaminergic neurons and dopamine depletion triggered by neurotoxins in toxin‐induced models. Whether environmental stressors have the same impact in genetic models of PD is still poorly investigated. In a study by Wu and colleagues, chronic stress has been shown to trigger motor impairment and degeneration of dopaminergic neurons accompanied by an increase of abnormal α‐syn inclusions and activation of a proinflammatory response in A53T mice (Wu et al., 2016). In the same genetic model, chronic restrain stress accelerates motor deficits and aggravates pathological signs linked to PD, possibly via the activation of RTP801, also known as the stress‐responsive gene DNA damage‐inducible transcript 4 (DDIT4) (Zhang et al., 2018). Inhibition of RTP801 alleviates neurodegeneration and PD‐like symptoms in stress‐treated A53T mice (Zhang et al., 2018). Further, higher anxiety‐related behavior and abnormal response to immobilization stress have been described in A53T mice, which were accompanied by aberrant regulation of dopamine β‐hydroxylase (Kim et al., 2014). Another study from our lab has investigated the effects of chronic unpredictable stress exposure on a genetic α‐syn model (Wassouf et al., 2019). In this work, we provide evidence on an altered stress response in SNCA‐overexpressing mice complemented by aberrant anxiety‐related behavior. These phenotypic manifestations precedes the presence of motor impairments and concurres with modulations of the striatal transcriptome affecting genes linked to neuroinflammation, synaptic signaling, and neurotransmission in SNCA‐overexpressing mice. Importantly, further research that delves into exploring the molecular transducers of the environmental influences will aid shedding light on the intriguing interplay of environment and SNCA related processes.
Conclusion and outlook
While evidence is converging on the impact of a variety of environmental influences on cause and course of neurodegenerative disorders, there seems to be no single universal environmental factor triggering these disorders. Instead, a combination of environmental elements, genetic predispositions, and aging processes seemingly prime the demise of a specific subset of neurons in these diseases. Identifying the molecular events that govern such demise and that mediate the impact of each of these interacting factors may shed light on potential molecular targets to reverse a physiological state to ultimately prevent, attenuate, or cure these diseases. Because of the dynamic nature of the transcriptome and the epigenome in response to environmental stimuli, these regulatory layers may harbor attractive targets for molecular modulation that eventually exerts its effects on functional and phenotypic endpoints. Hence, several research projects have been and further should be geared towards identifying such molecular targets that either mimic or oppose the environmental or disease attributes. Importantly, translating these findings to clinical trials entails conducting animal studies with high relevance to the human conditions as discussed before (Nithianantharajah and Hannan, 2006). In this context, a valid question is raised, namely whether standard housing for rodents with typically low levels of environmental stimulation is an appropriate control housing or rather a deprived condition compared to environmental enrichment which may better mimic our daily complex and stimulating lives. The observed beneficial effects of EE, in this case, may only be due to worsening of disease‐related phenotypes in animals housed in deprived standard‐housing conditions. Yet, the observed effects of EE remain relevant corroborated by reports employing higher levels of enrichment referred to ‘super enrichment’ (Mazarakis et al., 2014). Accounting for such considerations when interpreting findings from animal studies is vital to facilitate translation to clinical practice and define the type of environmental intervention necessary to achieve the functional benefits we aim for in patients.
Acknowledgments and conflict of interest disclosure
We thank O. Riess and T. Hentrich for helpful advice and discussions. This work was supported by the decipherPD transnational consortium on Epigenomics of Complex Diseases (BMBF grant number 01KU1503). The authors declare that they have no competing interests.
References
- Abeliovich A., Schmitz Y., Farinas I., et al. (2000) Mice lacking alpha‐synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252. [DOI] [PubMed] [Google Scholar]
- Aguilera G. (2011) HPA axis responsiveness to stress: implications for healthy aging. Exp. Gerontol. 46, 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima R., Krozowski Z. and Harlan R. (1991) Type I corticosteroid receptor‐like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J. Comp. Neurol. 313, 522–538. [DOI] [PubMed] [Google Scholar]
- Allis C. D. and Jenuwein T. (2016) The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17, 487–500. [DOI] [PubMed] [Google Scholar]
- Anastasia A., Torre L., de Erausquin G. A. and Masco D. H. (2009) Enriched environment protects the nigrostriatal dopaminergic system and induces astroglial reaction in the 6‐OHDA rat model of Parkinson's disease. J. Neurochem. 109, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash G. W., Garcia M. F., Costa D. A., Cracchiolo J. R., Wefes I. M. and Potter H. (2004) Environmental enrichment improves cognition in aged Alzheimer's transgenic mice despite stable beta‐amyloid deposition. NeuroReport 15, 1751–1754. [DOI] [PubMed] [Google Scholar]
- Artola A., von Frijtag J. C., Fermont P. C., Gispen W. H., Schrama L. H., Kamal A. and Spruijt B. M. (2006) Long‐lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur. J. Neurosci. 23, 261–272. [DOI] [PubMed] [Google Scholar]
- Ascherio A. and Schwarzschild M. A. (2016) The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Alvarez R. B., McFerrin J. and Broccolini A. (2000) Novel immunolocalization of alpha‐synuclein in human muscle of inclusion‐body myositis, regenerating and necrotic muscle fibers, and at neuromuscular junctions. J. Neuropathol. Exp. Neurol. 59, 592–598. [DOI] [PubMed] [Google Scholar]
- Baldelli P. and Meldolesi J. (2015) The transcription repressor REST in adult neurons: physiology, pathology, and diseases. eNeuro 2, ENEURO.0010-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T. L. and Epperson C. N. (2015) Sex differences and stress across the lifespan. Nat. Neurosci. 18, 1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N. and Mandel G. (2005) The many faces of REST oversee epigenetic programming of neuronal genes. Curr. Opin. Neurobiol. 15, 500–506. [DOI] [PubMed] [Google Scholar]
- Bellou V., Belbasis L., Tzoulaki I., Evangelou E. and Ioannidis J. P. (2016) Environmental risk factors and Parkinson's disease: an umbrella review of meta‐analyses. Parkinsonism Relat Disord. 23, 1–9. [DOI] [PubMed] [Google Scholar]
- Benaroya‐Milshtein N., Hollander N., Apter A., Kukulansky T., Raz N., Wilf A., Yaniv I. and Pick C. G. (2004) Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur. J. Neurosci. 20, 1341–1347. [DOI] [PubMed] [Google Scholar]
- Benito E., Kerimoglu C., Ramachandran B., et al. (2018) RNA‐dependent intergenerational inheritance of enhanced synaptic plasticity after environmental enrichment. Cell Rep. 23, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey M. J., Perez R. G. and Manfredsson F. P. (2016) The contribution of alpha synuclein to neuronal survival and function ‐ Implications for Parkinson's disease. J. Neurochem. 137, 331–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold N. C., Chinn G., Chou M., Kesslak J. P. and Cotman C. W. (2005) Exercise primes a molecular memory for brain‐derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 133, 853–861. [DOI] [PubMed] [Google Scholar]
- Bezard E., Dovero S., Belin D., Duconger S., Jackson‐Lewis V., Przedborski S., Piazza P. V., Gross C. E. and Jaber M. (2003) Enriched environment confers resistance to 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci 23, 10999–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal J. A., Babyak M. A., Moore K. A., et al. (1999) Effects of exercise training on older patients with major depression. Arch. Intern. Med. 159, 2349–2356. [DOI] [PubMed] [Google Scholar]
- Brown R. C., Lockwood A. H. and Sonawane B. R. (2005) Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect 113, 1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. M., Peiffer J. J. and Martins R. N. (2013) Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer's disease? Mol. Psychiatry 18, 864–874. [DOI] [PubMed] [Google Scholar]
- Bruel‐Jungerman E., Laroche S. and Rampon C. (2005) New neurons in the dentate gyrus are involved in the expression of enhanced long‐term memory following environmental enrichment. Eur. J. Neurosci. 21, 513–521. [DOI] [PubMed] [Google Scholar]
- Burre J., Sharma M., Tsetsenis T., Buchman V., Etherton M. R. and Sudhof T. C. (2010) Alpha‐synuclein promotes SNARE‐complex assembly in vivo and in vitro. Science 329, 1663–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin D. E., Shimazu K., Murphy D., et al. (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha‐synuclein. J. Neurosci. 22, 8797–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvey P. M., Punati A. and Newman M. B. (2006) Progressive dopamine neuron loss in Parkinson's disease: the multiple hit hypothesis. Cell Transplant. 15, 239–250. [DOI] [PubMed] [Google Scholar]
- Chandra S., Fornai F., Kwon H. B., et al. (2004) Double‐knockout mice for alpha‐ and beta‐synucleins: effect on synaptic functions. Proc. Natl Acad. Sci. USA 101, 14966–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlett A., Dobbs R. J., Purkiss A. G., Wright D. J., Peterson D. W., Weller C. and Dobbs S. M. (1998) Cortisol is higher in parkinsonism and associated with gait deficit. Acta Neurol. Scand. 97, 77–85. [DOI] [PubMed] [Google Scholar]
- Chartier‐Harlin M. C., Kachergus J., Roumier C., et al. (2004) Alpha‐synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169. [DOI] [PubMed] [Google Scholar]
- Chen Y. and Baram T. Z. (2016) Toward understanding how early‐life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology 41, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Wislet‐Gendebien S., Samuel F., Visanji N. P., Zhang G., Marsilio D., Langman T., Fraser P. E. and Tandon A. (2013) alpha‐Synuclein membrane association is regulated by the Rab3a recycling machinery and presynaptic activity. J. Biol. Chem. 288, 7438–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. D., Tillerson J. L., Smith A. D., Schallert T. and Zigmond M. J. (2003) Neuroprotective effects of prior limb use in 6‐hydroxydopamine‐treated rats: possible role of GDNF. J. Neurochem. 85, 299–305. [DOI] [PubMed] [Google Scholar]
- Colcombe S. and Kramer A. F. (2003) Fitness effects on the cognitive function of older adults: a meta‐analytic study. Psychol. Sci. 14, 125–130. [DOI] [PubMed] [Google Scholar]
- Collier T. J., Redmond D. E., Jr , Steece‐Collier K., Lipton J. W. and Manfredsson F. P. (2016) Is alpha‐synuclein loss‐of‐function a contributor to Parkinsonian pathology? Evidence from non‐human primates. Front Neurosci. 10, 12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A. and Caltagirone C. (2015) Individual differences in approach‐avoidance aptitude: some clues from research on Parkinson's disease. Front. Syst. Neurosci. 9, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Berchtold N. C. and Christie L. A. (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472. [DOI] [PubMed] [Google Scholar]
- Cracchiolo J. R., Mori T., Nazian S. J., Tan J., Potter H. and Arendash G. W. (2007) Enhanced cognitive activity–over and above social or physical activity–is required to protect Alzheimer's mice against cognitive impairment, reduce Abeta deposition, and increase synaptic immunoreactivity. Neurobiol. Learn Mem. 88, 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P., Spencer B., Coffee E., Patel P., Michael S., Patrick C., Adame A., Rockenstein E. and Masliah E. (2011) Alpha‐synuclein sequesters Dnmt1 from the nucleus: a novel mechanism for epigenetic alterations in Lewy body diseases. J. Biol. Chem. 286, 9031–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q., Vaynman S., Akhavan M., Ying Z. and Gomez‐Pinilla F. (2006a) Insulin‐like growth factor I interfaces with brain‐derived neurotrophic factor‐mediated synaptic plasticity to modulate aspects of exercise‐induced cognitive function. Neuroscience 140, 823–833. [DOI] [PubMed] [Google Scholar]
- Ding Y. H., Li J., Zhou Y., Rafols J. A., Clark J. C. and Ding Y. (2006b) Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr. Neurovasc Res. 3, 15–23. [DOI] [PubMed] [Google Scholar]
- Duffy S. N., Craddock K. J., Abel T. and Nguyen P. V. (2001) Environmental enrichment modifies the PKA‐dependence of hippocampal LTP and improves hippocampus‐dependent memory. Learn Mem. 8, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During M. J. and Cao L. (2006) VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr. Alzheimer Res. 3, 29–33. [DOI] [PubMed] [Google Scholar]
- Eadie B. D., Redila V. A. and Christie B. R. (2005) Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol. 486, 39–47. [DOI] [PubMed] [Google Scholar]
- Fabel K., Fabel K., Tam B., Kaufer D., Baiker A., Simmons N., Kuo C. J. and Palmer T. D. (2003) VEGF is necessary for exercise‐induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18, 2803–2812. [DOI] [PubMed] [Google Scholar]
- Faherty C. J., Raviie Shepherd K., Herasimtschuk A. and Smeyne R. J. (2005) Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Brain Res. Mol. Brain Res. 134, 170–179. [DOI] [PubMed] [Google Scholar]
- Farmer J., Zhao X., van Praag H., Wodtke K., Gage F. H. and Christie B. R. (2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague‐Dawley rats in vivo. Neuroscience 124, 71–79. [DOI] [PubMed] [Google Scholar]
- Feng Y., Jankovic J. and Wu Y. C. (2015) Epigenetic mechanisms in Parkinson's disease. J. Neurol. Sci. 349, 3–9. [DOI] [PubMed] [Google Scholar]
- Foster T. C. and Dumas T. C. (2001) Mechanism for increased hippocampal synaptic strength following differential experience. J. Neurophysiol. 85, 1377–1383. [DOI] [PubMed] [Google Scholar]
- Frick K. M. and Fernandez S. M. (2003) Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol. Aging 24, 615–626. [DOI] [PubMed] [Google Scholar]
- Gaugler M. N., Genc O., Bobela W., et al. (2012) Nigrostriatal overabundance of alpha‐synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol. 123, 653–669. [DOI] [PubMed] [Google Scholar]
- George J. M. (2002) The synucleins. Genome Biol 3, Reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goers J., Manning‐Bog A. B., McCormack A. L., Millett I. S., Doniach S., Di Monte D. A., Uversky V. N. and Fink A. L. (2003) Nuclear localization of alpha‐synuclein and its interaction with histones. Biochemistry 42, 8465–8471. [DOI] [PubMed] [Google Scholar]
- Goldberg N. R., Haack A. K. and Meshul C. K. (2011) Enriched environment promotes similar neuronal and behavioral recovery in a young and aged mouse model of Parkinson's disease. Neuroscience 172, 443–452. [DOI] [PubMed] [Google Scholar]
- Goldman J. G. and Postuma R. (2014) Premotor and nonmotor features of Parkinson's disease. Curr. Opin. Neurol. 27, 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. M., Quinlan P. J., Ross G. W., et al. (2012) Solvent exposures and Parkinson disease risk in twins. Ann. Neurol. 71, 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. S. (2011) Stress, allostatic load, catecholamines, and other neurotransmitters in neurodegenerative diseases. Endocr. Regul. 45, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin V. A., Richards S. H., Taylor R. S., Taylor A. H. and Campbell J. L. (2008) The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta‐analysis. Mov. Disord. 23, 631–640. [DOI] [PubMed] [Google Scholar]
- Gray J. D., Rubin T. G., Hunter R. G. and McEwen B. S. (2014) Hippocampal gene expression changes underlying stress sensitization and recovery. Mol. Psychiatry. 19, 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten‐Harrison B., Polydoro M., Morimoto‐Tomita M., et al. (2010) alphabetagamma‐Synuclein triple knockout mice reveal age‐dependent neuronal dysfunction. Proc. Natl Acad. Sci. USA 107, 19573–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J. J., Saade N. E. and Safieh‐Garabedian B. (2002) Cytokines and neuro‐immune‐endocrine interactions: a role for the hypothalamic‐pituitary‐adrenal revolving axis. J. Neuroimmunol. 133, 1–19. [DOI] [PubMed] [Google Scholar]
- Hamer M. and Chida Y. (2009) Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol. Med. 39, 3–11. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Veldhuis J. D., Deuschle M., Standhardt H. and Heuser I. (1997) Twenty‐four hour cortisol release profiles in patients with Alzheimer's and Parkinson's disease compared to normal controls: ultradian secretory pulsatility and diurnal variation. Neurobiol. Aging 18, 285–289. [DOI] [PubMed] [Google Scholar]
- Hatzinger M., Z'Brun A., Hemmeter U., Seifritz E., Baumann F., Holsboer‐Trachsler E. and Heuser I. J. (1995) Hypothalamic‐pituitary‐adrenal system function in patients with Alzheimer's disease. Neurobiol. Aging 16, 205–209. [DOI] [PubMed] [Google Scholar]
- Heneka M. T., Kummer M. P. and Latz E. (2014) Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477. [DOI] [PubMed] [Google Scholar]
- Hentrich T., Wassouf Z., Riess O. and Schulze‐Hentrich J. M. (2018) SNCA overexpression disturbs hippocampal gene expression trajectories in midlife. Aging 10, 4024–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman T., Giladi N., Gruendlinger L. and Hausdorff J. M. (2007) Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson's disease: a pilot study. Arch. Phys. Med. Rehabil. 88, 1154–1158. [DOI] [PubMed] [Google Scholar]
- Herman T., Giladi N. and Hausdorff J. M. (2009). Treadmill training for the treatment of gait disturbances in people with Parkinson’s disease: a mini‐review. J. Neural. Trans. (Vienna, Austria : 1996) 116, 307–318. [DOI] [PubMed] [Google Scholar]
- Hernan M. A., Takkouche B., Caamano‐Isorna F. and Gestal‐Otero J. J. (2002) A meta‐analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann. Neurol. 52, 276–284. [DOI] [PubMed] [Google Scholar]
- Huang Y. and Halliday G. M. (2012) Aspects of innate immunity and Parkinson's disease. Front Pharmacol. 3, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Myers S. J. and Dingledine R. (1999) Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat. Neurosci. 2, 867–872. [DOI] [PubMed] [Google Scholar]
- Hunter R. G., Gagnidze K., McEwen B. S. and Pfaff D. W. (2015) Stress and the dynamic genome: Steroids, epigenetics, and the transposome. Proc. Natl Acad. Sci. USA 112, 6828–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J. Y., Aromolaran K. A. and Zukin R. S. (2017) The emerging field of epigenetics in neurodegeneration and neuroprotection. Nat. Rev. Neurosci. 18, 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez P., Bonnet A. M., Debarges B., Lohmann E., Tison F., Pollak P., Agid Y., Durr A. and Brice A. (2004) Causal relation between alpha‐synuclein gene duplication and familial Parkinson's disease. Lancet 364, 1169–1171. [DOI] [PubMed] [Google Scholar]
- Ickes B. R., Pham T. M., Sanders L. A., Albeck D. S., Mohammed A. H. and Granholm A. C. (2000) Long‐term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp. Neurol. 164, 45–52. [DOI] [PubMed] [Google Scholar]
- Jadavji N. M., Kolb B. and Metz G. A. (2006) Enriched environment improves motor function in intact and unilateral dopamine‐depleted rats. Neuroscience 140, 1127–1138. [DOI] [PubMed] [Google Scholar]
- Jakes R., Spillantini M. G. and Goedert M. (1994) Identification of two distinct synucleins from human brain. FEBS Lett 345, 27–32. [DOI] [PubMed] [Google Scholar]
- Jin H., Kanthasamy A., Ghosh A., Yang Y., Anantharam V. and Kanthasamy A. G. (2011) alpha‐Synuclein negatively regulates protein kinase Cdelta expression to suppress apoptosis in dopaminergic neurons by reducing p300 histone acetyltransferase activity. J. Neurosci. 31, 2035–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungling A., Reglodi D., Karadi Z. N., Horvath G., Farkas J., Gaszner B. and Tamas A. (2017) Effects of postnatal enriched environment in a model of Parkinson's disease in adult rats. Int. J. Mol. Sci. 18, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V., Nurmi E., Bergman J., Eskola O., Solin O., Sonninen P. and Rinne J. O. (2001) Personality traits and brain dopaminergic function in Parkinson's disease. Proc. Natl Acad. Sci. USA 98, 13272–13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle P. J., Neumann M., Ozmen L., et al. (2000) Subcellular localization of wild‐type and Parkinson's disease‐associated mutant alpha ‐synuclein in human and transgenic mouse brain. J. Neurosci. 20, 6365–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev A. G. and Thompson L. M. (2008) Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat. Rev. Drug. Discov. 7, 854–868. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H. G. and Gage F. H. (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gast D. and Gage F. H. (2002) Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long‐term environmental enrichment. Ann. Neurol. 52, 135–143. [DOI] [PubMed] [Google Scholar]
- Keus S. H., Bloem B. R., Hendriks E. J., Bredero‐Cohen A. B. and Munneke M. (2007) Evidence‐based analysis of physical therapy in Parkinson's disease with recommendations for practice and research. Mov. Disord. 22, 451–460, quiz 600. [DOI] [PubMed] [Google Scholar]
- Kim S., Park J. M., Moon J. and Choi H. J. (2014) Alpha‐synuclein interferes with cAMP/PKA‐dependent upregulation of dopamine beta‐hydroxylase and is associated with abnormal adaptive responses to immobilization stress. Exp. Neurol. 252, 63–74. [DOI] [PubMed] [Google Scholar]
- Klaissle P., Lesemann A., Huehnchen P., Hermann A., Storch A. and Steiner B. (2012) Physical activity and environmental enrichment regulate the generation of neural precursors in the adult mouse substantia nigra in a dopamine‐dependent manner. BMC Neurosci. 13, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E. R., Vreugdenhil E., Oitzl M. S. and Joels M. (1998) Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301. [DOI] [PubMed] [Google Scholar]
- Kokhan V. S., Van'kin G. I., Ninkina N. N., Shelkovnikova T. A. and Bachurin S. O. (2011) Impaired spatial and working memory in ageing mice with targeted inactivation of alpha‐synuclein gene. Dokl Biol. Sci. 441, 354–356. [DOI] [PubMed] [Google Scholar]
- Kokhan V. S., Afanasyeva M. A. and Van'kin G. I. (2012) alpha‐Synuclein knockout mice have cognitive impairments. Behav. Brain Res. 231, 226–230. [DOI] [PubMed] [Google Scholar]
- Kontopoulos E., Parvin J. D. and Feany M. B. (2006) Alpha‐synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 15, 3012–3023. [DOI] [PubMed] [Google Scholar]
- Kruger R., Kuhn W., Muller T., Woitalla D., Graeber M., Kosel S., Przuntek H., Epplen J. T., Schols L. and Riess O. (1998) Ala30Pro mutation in the gene encoding alpha‐synuclein in Parkinson's disease. Nat. Genet. 18, 106–108. [DOI] [PubMed] [Google Scholar]
- Kuipers S. D. and Bramham C. R. (2006) Brain‐derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr. Opin. Drug Discov. Devel. 9, 580–586. [PubMed] [Google Scholar]
- Landgrave‐Gomez J., Mercado‐Gomez O. and Guevara‐Guzman R. (2015) Epigenetic mechanisms in neurological and neurodegenerative diseases. Front. Cell Neurosci. 9, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauretti E., Di Meco A., Merali S. and Pratico D. (2016) Chronic behavioral stress exaggerates motor deficit and neuroinflammation in the MPTP mouse model of Parkinson's disease. Transl. Psychiat. 6, e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavedan C., Leroy E., Dehejia A., Buchholtz S., Dutra A., Nussbaum R. L. and Polymeropoulos M. H. (1998a) Identification, localization and characterization of the human gamma‐synuclein gene. Hum. Genet. 103, 106–112. [DOI] [PubMed] [Google Scholar]
- Lavedan C., Leroy E., Torres R., Dehejia A., Dutra A., Buchholtz S., Nussbaum R. L. and Polymeropoulos M. H. (1998b) Genomic organization and expression of the human beta‐synuclein gene (SNCB). Genomics 54, 173–175. [DOI] [PubMed] [Google Scholar]
- Lazarov O., Robinson J., Tang Y. P., Hairston I. S., Korade‐Mirnics Z., Lee V. M., Hersh L. B., Sapolsky R. M., Mirnics K. and Sisodia S. S. (2005) Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 120, 701–713. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Wynder C., Cooch N. and Shiekhattar R. (2005) An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature 437, 432–435. [DOI] [PubMed] [Google Scholar]
- Lesage S. and Brice A. (2009) Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 18, R48–R59. [DOI] [PubMed] [Google Scholar]
- Lesage S., Anheim M., Letournel F., et al. (2013) G51D alpha‐synuclein mutation causes a novel parkinsonian‐pyramidal syndrome. Ann. Neurol. 73, 459–471. [DOI] [PubMed] [Google Scholar]
- Ltic S., Perovic M., Mladenovic A., Raicevic N., Ruzdijic S., Rakic L. and Kanazir S. (2004) Alpha‐synuclein is expressed in different tissues during human fetal development. J. Mol. Neurosci. 22, 199–204. [DOI] [PubMed] [Google Scholar]
- Lu T., Aron L., Zullo J., et al. (2014) REST and stress resistance in ageing and Alzheimer's disease. Nature 507, 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucca G., Comim C. M., Valvassori S. S., Reus G. Z., Vuolo F., Petronilho F., Dal‐Pizzol F., Gavioli E. C. and Quevedo J. (2009) Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem. Int. 54, 358–362. [DOI] [PubMed] [Google Scholar]
- Lundblad M., Decressac M., Mattsson B. and Bjorklund A. (2012) Impaired neurotransmission caused by overexpression of alpha‐synuclein in nigral dopamine neurons. Proc. Natl Acad. Sci. USA 109, 3213–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K. L., Song L. K., Yuan Y. H., Zhang Y., Han N., Gao K. and Chen N. H. (2014a) The nuclear accumulation of alpha‐synuclein is mediated by importin alpha and promotes neurotoxicity by accelerating the cell cycle. Neuropharmacology 82, 132–142. [DOI] [PubMed] [Google Scholar]
- Ma K. L., Song L. K., Yuan Y. H., Zhang Y., Yang J. L., Zhu P. and Chen N. H. (2014b) Alpha‐Synuclein is prone to interaction with the GC‐box‐like sequence in vitro. Cell Mol. Neurobiol. 34, 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L., Campanelli J. T. and Scheller R. H. (1988) Synuclein: a neuron‐specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 8, 2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M., Rosa A., Guedes L. C., et al. (2011) Convergence of miRNA expression profiling, alpha‐synuclein interacton and GWAS in Parkinson's disease. PLoS ONE 6, e25443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A. and Mucke L. (2000) Dopaminergic loss and inclusion body formation in alpha‐synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269. [DOI] [PubMed] [Google Scholar]
- Masliah E., Dumaop W., Galasko D. and Desplats P. (2013) Distinctive patterns of DNA methylation associated with Parkinson disease: identification of concordant epigenetic changes in brain and peripheral blood leukocytes. Epigenetics 8, 1030–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarakis N. K., Mo C., Renoir T., van Dellen A., Deacon R., Blakemore C. and Hannan A. J. (2014) 'Super‐Enrichment' reveals dose‐dependent therapeutic effects of environmental stimulation in a transgenic mouse model of Huntington's disease. J. Huntington's Dis. 3, 299–309. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. and Morrison J. H. (2013) The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., De Kloet E. R. and Rostene W. (1986) Adrenal steroid receptors and actions in the nervous system. Physiol. Rev. 66, 1121–1188. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Bowles N. P., Gray J. D., Hill M. N., Hunter R. G., Karatsoreos I. N. and Nasca C. (2015) Mechanisms of stress in the brain. Nat. Neurosci. 18, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P. J., Ribich S. and Hyman B. T. (2000) Subcellular localization of alpha‐synuclein in primary neuronal cultures: effect of missense mutations. J. Neural. Transm. Suppl. 58, 53–63. [DOI] [PubMed] [Google Scholar]
- Meaney M. J. and Szyf M. (2005) Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 7, 103–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meco A., Joshi Y. B. and Pratico D. (2014) Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer's disease with plaques and tangles. Neurobiol. Aging 35, 1813–1820. [DOI] [PubMed] [Google Scholar]
- Mejia S., Giraldo M., Pineda D., Ardila A. and Lopera F. (2003) Nongenetic factors as modifiers of the age of onset of familial Alzheimer's disease. Int. Psychogeriatr. 15, 337–349. [DOI] [PubMed] [Google Scholar]
- Menza M. A., Golbe L. I., Cody R. A. and Forman N. E. (1993) Dopamine‐related personality traits in Parkinson's disease. Neurology 43, 505–508. [DOI] [PubMed] [Google Scholar]
- Metz G. A. (2007) Stress as a modulator of motor system function and pathology. Rev. Neurosci. 18, 209–222. [DOI] [PubMed] [Google Scholar]
- Minakaki G., Canneva F., Chevessier F., et al. (2019) Treadmill exercise intervention improves gait and postural control in alpha‐synuclein mouse models without inducing cerebral autophagy. Behav. Brain Res. 363, 199–215. [DOI] [PubMed] [Google Scholar]
- Mirochnic S., Wolf S., Staufenbiel M. and Kempermann G. (2009) Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus 19, 1008–1018. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K., Yuzurihara M., Ishige A., Sasaki H., Chui D. H. and Tabira T. (2000) Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J. Neurosci. 20, 1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. D., Rueter S. M., Trojanowski J. Q. and Lee V. M. (2000) Synucleins are developmentally expressed, and alpha‐synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 20, 3214–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M., Fujita M., Waragai M., Sugama S., Wei J., Akatsu H., Ohtaka‐Maruyama C., Okado H. and Hashimoto M. (2007) Expression of alpha‐synuclein, a presynaptic protein implicated in Parkinson's disease, in erythropoietic lineage. Biochem. Biophys Res. Commun. 358, 104–110. [DOI] [PubMed] [Google Scholar]
- Nalls M. A., Pankratz N., Lill C. M., et al. (2014) Large‐scale meta‐analysis of genome‐wide association data identifies six new risk loci for Parkinson's disease. Nat. Genet. 46, 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani V. M., Lu W., Berge V., Nakamura K., Onoa B., Lee M. K., Chaudhry F. A., Nicoll R. A. and Edwards R. H. (2010) Increased expression of alpha‐synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65, 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkina N., Peters O. M., Connor‐Robson N., Lytkina O., Sharfeddin E. and Buchman V. L. (2012) Contrasting effects of alpha‐synuclein and gamma‐synuclein on the phenotype of cysteine string protein alpha (CSPalpha) null mutant mice suggest distinct function of these proteins in neuronal synapses. J. Biol. Chem. 287, 44471–44477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J. and Hannan A. J. (2006) Enriched environments, experience‐dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J. and Hannan A. J. (2009) The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog. Neurogibol. 89, 369–382. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J. and Hannan A. J. (2011) Mechanisms mediating brain and cognitive reserve: experience‐dependent neuroprotection and functional compensation in animal models of neurodegenerative diseases. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 331–339. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J., Levis H. and Murphy M. (2004) Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD‐95 proteins. Neurobiol. Learn Mem. 81, 200–210. [DOI] [PubMed] [Google Scholar]
- Noh K. M., Hwang J. Y., Follenzi A., Athanasiadou R., Miyawaki T., Greally J. M., Bennett M. V. and Zukin R. S. (2012) Repressor element‐1 silencing transcription factor (REST)‐dependent epigenetic remodeling is critical to ischemia‐induced neuronal death. Proc. Natl Acad. Sci. USA 109, E962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell S. J., Gross N. B., Fricks A. N., Casiano B. D., Nguyen T. B. and Marshall J. F. (2007) Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience 144, 1141–1151. [DOI] [PubMed] [Google Scholar]
- Ooi L. and Wood I. C. (2007) Chromatin crosstalk in development and disease: lessons from REST. Nat. Rev. Genet. 8, 544–554. [DOI] [PubMed] [Google Scholar]
- Ost A., Lempradl A., Casas E., et al. (2014) Paternal diet defines offspring chromatin state and intergenerational obesity. Cell 159, 1352–1364. [DOI] [PubMed] [Google Scholar]
- de Pablos R. M., Herrera A. J., Espinosa‐Oliva A. M., Sarmiento M., Munoz M. F., Machado A. and Venero J. L. (2014) Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J. Neuroinflammation 11, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmaraju V., Bhaskar J. J., Prasada Rao U. J., Salimath P. V. and Rao K. S. (2011) Role of advanced glycation on aggregation and DNA binding properties of alpha‐synuclein. J. Alzheimers Dis. 24(Suppl 2), 211–221. [DOI] [PubMed] [Google Scholar]
- Pasanen P., Myllykangas L., Siitonen M., Raunio A., Kaakkola S., Lyytinen J., Tienari P. J., Poyhonen M. and Paetau A. (2014) Novel alpha‐synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson's disease‐type pathology. Neurobiol. Aging 35, 2180.e2181–2185. [DOI] [PubMed] [Google Scholar]
- Pavlou M. A. S., Pinho R., Paiva I. and Outeiro T. F. (2017) The yin and yang of α‐synuclein‐associated epigenetics in Parkinson’s disease. Brain 140, 878–886. [DOI] [PubMed] [Google Scholar]
- Peskind E. R., Wilkinson C. W., Petrie E. C., Schellenberg G. D. and Raskind M. A. (2001) Increased CSF cortisol in AD is a function of APOE genotype. Neurology 56, 1094–1098. [DOI] [PubMed] [Google Scholar]
- Petzinger G. M., Walsh J. P., Akopian G., et al. (2007) Effects of treadmill exercise on dopaminergic transmission in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐lesioned mouse model of basal ganglia injury. J. Neurosci. 27, 5291–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T. M., Ickes B., Albeck D., Soderstrom S., Granholm A. C. and Mohammed A. H. (1999) Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience 94, 279–286. [DOI] [PubMed] [Google Scholar]
- Pienaar I. S., Kellaway L. A., Russell V. A., Smith A. D., Stein D. J., Zigmond M. J. and Daniels W. M. (2008) Maternal separation exaggerates the toxic effects of 6‐hydroxydopamine in rats: implications for neurodegenerative disorders. Stress 11, 448–456. [DOI] [PubMed] [Google Scholar]
- Pinho R., Paiva I., Jercic K. G., et al. (2019) Nuclear localization and phosphorylation modulate pathological effects of alpha‐synuclein. Hum. Mol. Genet. 28, 31–50. [DOI] [PubMed] [Google Scholar]
- Poewe W., Seppi K., Tanner C. M., Halliday G. M., Brundin P., Volkmann J., Schrag A.‐E. and Lang A. E. (2017) Parkinson disease. Nat. Rev. Dis. Prim. 3, 17013. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Lavedan C., Leroy E., et al. (1997) Mutation in the alpha‐synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Pothakos K., Kurz M. J. and Lau Y. S. (2009) Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson's disease with severe neurodegeneration. BMC Neurosci. 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Christie B. R., Sejnowski T. J. and Gage F. H. (1999) Running enhances neurogenesis, learning, and long‐term potentiation in mice. Proc. Natl Acad. Sci. USA 96, 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G. and Gage F. H. (2000) Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198. [DOI] [PubMed] [Google Scholar]
- van Praag H., Shubert T., Zhao C. and Gage F. H. (2005) Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 25, 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T., Jette N., Frolkis A. and Steeves T. D. (2014) The prevalence of Parkinson's disease: a systematic review and meta‐analysis. Mov. Disord. 29, 1583–1590. [DOI] [PubMed] [Google Scholar]
- Proukakis C., Dudzik C. G., Brier T., MacKay D. S., Cooper J. M., Millhauser G. L., Houlden H. and Schapira A. H. (2013) A novel alpha‐synuclein missense mutation in Parkinson disease. Neurology 80, 1062–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S. (2017) The two‐century journey of Parkinson disease research. Nat. Rev. Neurosci. 18, 251. [DOI] [PubMed] [Google Scholar]
- Racette B. A. (2014) Manganism in the 21st century: the Hanninen lecture. Neurotoxicology 45, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette B. A., McGee‐Minnich L., Moerlein S. M., Mink J. W., Videen T. O. and Perlmutter J. S. (2001) Welding‐related parkinsonism: clinical features, treatment, and pathophysiology. Neurology 56, 8–13. [DOI] [PubMed] [Google Scholar]
- Rampon C., Jiang C. H., Dong H., Tang Y. P., Lockhart D. J., Schultz P. G., Tsien J. Z. and Hu Y. (2000) Effects of environmental enrichment on gene expression in the brain. Proc. Natl Acad. Sci. USA 97, 12880–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed N., Ahmad A., Pandey C. P., Chaturvedi R. K., Lohani M. and Palit G. (2010) Differential response of central dopaminergic system in acute and chronic unpredictable stress models in rats. Neurochem. Res. 35, 22–32. [DOI] [PubMed] [Google Scholar]
- Reul J. M. and de Kloet E. R. (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117, 2505–2511. [DOI] [PubMed] [Google Scholar]
- Ros‐Bernal F., Hunot S., Herrero M. T., et al. (2011) Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc. Natl Acad. Sci. USA 108, 6632–6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy V., Belzung C., Delarue C. and Chapillon P. (2001) Environmental enrichment in BALB/c mice: effects in classical tests of anxiety and exposure to a predatory odor. Physiol. Behav. 74, 313–320. [DOI] [PubMed] [Google Scholar]
- Satake W., Nakabayashi Y., Mizuta I., et al. (2009) Genome‐wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat. Genet. 41, 1303–1307. [DOI] [PubMed] [Google Scholar]
- Scott D. A., Tabarean I., Tang Y., Cartier A., Masliah E. and Roy S. (2010) A pathologic cascade leading to synaptic dysfunction in alpha‐synuclein‐induced neurodegeneration. J. Neurosci. 30, 8083–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin E. C., Cho S. E., Lee D. K., Hur M. W., Paik S. R., Park J. H. and Kim J. (2000) Expression patterns of alpha‐synuclein in human hematopoietic cells and in Drosophila at different developmental stages. Mol. Cells 10, 65–70. [DOI] [PubMed] [Google Scholar]
- Shults C. W. (2006) Lewy bodies. Proc. Natl Acad. Sci. USA 103, 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A., Chinta S. J., Mallajosyula J. K., Rajagopolan S., Hanson I., Rane A., Melov S. and Andersen J. K. (2012) Selective binding of nuclear alpha‐synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson's disease. Free Radic. Biol. Med. 53, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M., Hudon C. and van Reekum R. (2009) Psychological distress and risk for dementia. Current Psychiatry Rep. 11, 41–47. [DOI] [PubMed] [Google Scholar]
- Simon‐Sanchez J., Schulte C., Bras J. M., et al. (2009) Genome‐wide association study reveals genetic risk underlying Parkinson's disease. Nat. Genet. 41, 1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. A., Stavrinos T. M., Scarbek Y., Galambos G., Liber C. and Fiatarone Singh M. A. (2005) A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 60, 768–776. [DOI] [PubMed] [Google Scholar]
- Singleton A. B., Farrer M., Johnson J., et al. (2003) α‐Synuclein Locus Triplication Causes Parkinson's Disease. Science 302, 841. [DOI] [PubMed] [Google Scholar]
- Smargiassi A., Mutti A., De Rosa A., De Palma G., Negrotti A. and Calzetti S. (1998) A case‐control study of occupational and environmental risk factors for Parkinson's disease in the Emilia‐Romagna region of Italy. Neurotoxicology 19, 709–712. [PubMed] [Google Scholar]
- Smith A. D., Castro S. L. and Zigmond M. J. (2002) Stress‐induced Parkinson's disease: a working hypothesis. Physiol. Behav. 77, 527–531. [DOI] [PubMed] [Google Scholar]
- Smith L. K., Jadavji N. M., Colwell K. L., Katrina Perehudoff S. and Metz G. A. (2008) Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson's disease. Eur. J. Neurosci. 27, 2133–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos I., Cerqueira J. J., Catania C., Takashima A., Sousa N. and Almeida O. F. (2008) Stress and glucocorticoid footprints in the brain‐the path from depression to Alzheimer's disease. Neurosci. Biobehav. Rev. 32, 1161–1173. [DOI] [PubMed] [Google Scholar]
- Sousa N. (2016) The dynamics of the stress neuromatrix. Mol. Psychiatry 21, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht C. G., Tigaret C. M., Rast G. F., Thalhammer A., Rudhard Y. and Schoepfer R. (2005) Subcellular localisation of recombinant alpha‐ and gamma‐synuclein. Mol. Cell Neurosci. 28, 326–334. [DOI] [PubMed] [Google Scholar]
- Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R. and Goedert M. (1997) Alpha‐synuclein in Lewy bodies. Nature 388, 839–840. [DOI] [PubMed] [Google Scholar]
- Spillantini M. G., Crowther R. A., Jakes R., Hasegawa M. and Goedert M. (1998) alpha‐Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc. Natl Acad. Sci. USA 95, 6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2002) What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. [PubMed] [Google Scholar]
- Sugeno N., Jackel S., Voigt A., Wassouf Z., Schulze‐Hentrich J. and Kahle P. J. (2016) alpha‐Synuclein enhances histone H3 lysine‐9 dimethylation and H3K9me2‐dependent transcriptional responses. Sci. Rep. 6, 36328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 30, 244–250. [DOI] [PubMed] [Google Scholar]
- Sutoo D. and Akiyama K. (2003) Regulation of brain function by exercise. Neurobiol. Dis. 13, 1–14. [DOI] [PubMed] [Google Scholar]
- Tajiri N., Yasuhara T., Shingo T., et al. (2010) Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res. 1310, 200–207. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Kanuka H., Fujiwara H., Koyama A., Hasegawa M., Miura M. and Iwatsubo T. (2003) Phosphorylation of alpha‐synuclein characteristic of synucleinopathy lesions is recapitulated in alpha‐synuclein transgenic Drosophila. Neurosci. Lett. 336, 155–158. [DOI] [PubMed] [Google Scholar]
- Tang Y. P., Wang H., Feng R., Kyin M. and Tsien J. Z. (2001) Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology 41, 779–790. [DOI] [PubMed] [Google Scholar]
- Tanner C. M., Ottman R., Goldman S. M., Ellenberg J., Chan P., Mayeux R. and Langston J. W. (1999) Parkinson disease in twins: an etiologic study. JAMA 281, 341–346. [DOI] [PubMed] [Google Scholar]
- Tillerson J. L., Caudle W. M., Reveron M. E. and Miller G. W. (2003) Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience 119, 899–911. [DOI] [PubMed] [Google Scholar]
- Tong L., Balazs R., Soiampornkul R., Thangnipon W. and Cotman C. W. (2008) Interleukin‐1 beta impairs brain derived neurotrophic factor‐induced signal transduction. Neurobiol. Aging 29, 1380–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo J. L., Carro E. and Torres‐Aleman I. (2001) Circulating insulin‐like growth factor I mediates exercise‐induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21, 1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M. J. and Sachdev P. (2006) Brain reserve and dementia: a systematic review. Psychol. Med. 36, 441–454. [DOI] [PubMed] [Google Scholar]
- VanLeeuwen J. E., Petzinger G. M., Walsh J. P., Akopian G. K., Vuckovic M. and Jakowec M. W. (2010) Altered AMPA receptor expression with treadmill exercise in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine‐lesioned mouse model of basal ganglia injury. J. Neurosci. Res. 88, 650–668. [DOI] [PubMed] [Google Scholar]
- Vaynman S., Ying Z. and Gomez‐Pinilla F. (2004) Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 20, 2580–2590. [DOI] [PubMed] [Google Scholar]
- Vaynman S. S., Ying Z., Yin D. and Gomez‐Pinilla F. (2006) Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 1070, 124–130. [DOI] [PubMed] [Google Scholar]
- Veldhuis H. D., Van Koppen C., Van Ittersum M. and De Kloet E. R. (1982) Specificity of the adrenal steroid receptor system in rat hippocampus. Endocrinology 110, 2044–2051. [DOI] [PubMed] [Google Scholar]
- Venters H. D., Broussard S. R., Zhou J. H., Bluthe R. M., Freund G. G., Johnson R. W., Dantzer R. and Kelley K. W. (2001) Tumor necrosis factor(alpha) and insulin‐like growth factor‐I in the brain: is the whole greater than the sum of its parts? J. Neuroimmunol. 119, 151–165. [DOI] [PubMed] [Google Scholar]
- Vyas S., Rodrigues A. J., Silva J. M., Tronche F., Almeida O. F., Sousa N. and Sotiropoulos I. (2016) Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural. Plast. 2016, 6391686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassouf Z., Hentrich T., Samer S., Rotermund C., Kahle P. J., Ehrlich I., Riess O., Casadei N. and Schulze‐Hentrich J. M. (2018) Environmental enrichment prevents transcriptional disturbances induced by alpha‐synuclein overexpression. Front. Cell Neurosci. 12, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassouf Z., Hentrich T., Casadei N., Jaumann M., Knipper M., Riess O. and Schulze‐Hentrich J. M. (2019) Distinct stress response and altered striatal transcriptome in alpha‐synuclein overexpressing mice. Front Neurosci. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J., Kang J. H., Manson J. E., Breteler M. M., Ware J. H. and Grodstein F. (2004) Physical activity, including walking, and cognitive function in older women. JAMA 292, 1454–1461. [DOI] [PubMed] [Google Scholar]
- Wi S., Lee J. W., Kim M., Park C. H. and Cho S. R. (2018) An enriched environment ameliorates oxidative stress and olfactory dysfunction in Parkinson's disease with alpha‐synucleinopathy. Cell Transplant. 27, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirdefeldt K., Gatz M., Schalling M. and Pedersen N. L. (2004) No evidence for heritability of Parkinson disease in Swedish twins. Neurology 63, 305. [DOI] [PubMed] [Google Scholar]
- Wu Q., Yang X., Zhang Y., Zhang L. and Feng L. (2016) Chronic mild stress accelerates the progression of Parkinson's disease in A53T alpha‐synuclein transgenic mice. Exp. Neurol. 285, 61–71. [DOI] [PubMed] [Google Scholar]
- Yang F., Trolle Lagerros Y., Bellocco R., Adami H. O., Fang F., Pedersen N. L. and Wirdefeldt K. (2015) Physical activity and risk of Parkinson's disease in the Swedish National March Cohort. Brain 138, 269–275. [DOI] [PubMed] [Google Scholar]
- Yu M., Cai L., Liang M., Huang Y., Gao H., Lu S., Fei J. and Huang F. (2009) Alteration of NRSF expression exacerbating 1‐methyl‐4‐phenyl‐pyridinium ion‐induced cell death of SH‐SY5Y cells. Neurosci. Res. 65, 236–244. [DOI] [PubMed] [Google Scholar]
- Yu M., Suo H., Liu M., et al. (2013) NRSF/REST neuronal deficient mice are more vulnerable to the neurotoxin MPTP. Neurobiol. Aging 34, 916–927. [DOI] [PubMed] [Google Scholar]
- Zaltieri M., Grigoletto J., Longhena F., et al. (2015) alpha‐synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J. Cell Sci. 128, 2231–2243. [DOI] [PubMed] [Google Scholar]
- Zarranz J. J., Alegre J., Gomez‐Esteban J. C., et al. (2004) The new mutation, E46K, of alpha‐synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Piston D. W. and Goodman R. H. (2002) Regulation of corepressor function by nuclear NADH. Science 295, 1895–1897. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Chu S. F., Wang S. S., Jiang Y. N., Gao Y., Yang P. F., Ai Q. D. and Chen N. H. (2018) RTP801 is a critical factor in the neurodegeneration process of A53T alpha‐synuclein in a mouse model of Parkinson's disease under chronic restraint stress. Br J. Pharmacol. 175, 590–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K., Guo W., Tang G., Zheng B. and Zheng Z. (2013) A Case of early onset Parkinson's disease after major stress. Neuropsychiatr Dis. Treat 9, 1067–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C., Tartari M., Crotti A., et al. (2003) Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE‐controlled neuronal genes. Nat. Genet. 35, 76–83. [DOI] [PubMed] [Google Scholar]


