ABSTRACT
Village chicken or Ayam Kampung, common to Southeast Asian countries, has always been regarded as superior in comparison to commercial broiler chicken in terms of wholesomeness and health benefits. The current study investigates the prevalence and risk factors of Salmonella among village chicken flocks from the central and southern states of Peninsular Malaysia. A total of 35 village flocks were sampled from Selangor (n = 19), Melaka (n = 10), Johor (n = 4), and Negeri Sembilan (n = 2). In total, 1,042 samples were collected; these included cloacal swabs (n = 675), eggs (n = 62), pooled drinking water (n = 175), pooled feeds (n = 70), and pooled flies (n = 60). Isolation of Salmonella from cloacal swabs, poultry drinking water, and feeds was carried out according to the protocols and recommendations of the World Organization for Animal Health (OIE) terrestrial manual. The prevalence of Salmonella at an individual bird-level was 2.5% (17/675, 95% CI: 1.6 to 4.0). All eggs screened were negative; in the case of environmental samples, however, Salmonella was detected in 5.14% (9/175), 7.14% (5/70), and 5.0% (3/60) for water, feed, and flies, respectively. A total of 34 isolates and 8 Salmonella serotypes were identified. Weltevreden (20.6%) was the most common, followed by Typhimurium and Agona (17.6%), Albany and Enteritidis (8.8%), Molade (5.9%), Corvallis and Schleissheim (2.9%), and others grouped as Salmonella spp. (11.8%). Multivariable logistic regression models revealed that Salmonella positivity among flocks could be strongly predicted by storage of feeds (uncovered feeds; OR = 10.38; 95% CI: 1.25 to 86.39; p = 0.030) and uncovered water tanks (uncovered tank; OR = 6.43; 95% CI: 1.02 to 40.60; p = 0.048). The presence of Salmonella in village chickens in the study area was lower than that of commercial chickens in Malaysia.
Keywords: village chicken, Salmonella, risk factor, prevalence, Peninsular Malaysia
INTRODUCTION
Native “village” chicken or Ayam Kampung is defined as a hybrid, the result of natural cross-breeding between the Malay fowl, jungle fowl, and mixed exotic breeds brought in during periods of European colonization (Azahan and Zahari, 1983; Azahan, 1994). The plumage color of village chickens varies considerably with the most common being a black-red variety (Azahan et al., 1980; Azahan and Zahari, 1983; Azahan, 1994). In Southeast Asia and other developing countries, village chickens are mainly raised in the backyard, variously providing both a side income and source of protein for local households (Aini, 1990; Padhi, 2016). These chickens are free to roam and scavenge for food, and are fed leftovers and other household scraps to supplement their dietary feed requirements. However, since the emergence of highly pathogenic avian influenza, the traditional practice of rearing small flocks of free-range chickens has been largely discouraged (Safman, 2010).
Village chicken is common to many Southeast Asian countries, including Malaysia, and has always been regarded as superior in comparison to commercial broiler chicken in terms of wholesomeness and health benefits (Aini, 1990; Hassan et al., 2005). These chickens are customarily raised with little to no antibiotics or other drugs. In recent years, rising awareness of issues pertaining to animal welfare and the drugs used in commercial poultry production (i.e., intensive production) has resulted in a rapid increase in the demand for free-range native chickens, which consumers considered to be safer and more wholesome (Hassan et al., 2005; Miao et al., 2005; Rahman and Haziqah, 2015). Therefore Ayam Kampung products cover a larger niche market as compared to previous years because of emergent food safety and animal welfare concerns.
Worldwide, Salmonella is the most common pathogen causing foodborne illnesses, with most infections linked to the consumption of poultry or poultry products (Mezal et al., 2014; Saravanan et al., 2015; Wang et al., 2015). The presence of Salmonella in commercial poultry, its meat, and poultry products has been well studied (Rusul et al., 1996; Najwa et al., 2015; Thung et al., 2016). Moreover, several studies from across the globe have studied the risk factors associated with Salmonella contamination in broiler chickens (Rose et al., 1999; Arsenault et al., 2007; Marin et al., 2011). The reported prevalence of Salmonella in live commercial poultry in Malaysia was reported at 14.9% (n = 1911) (Ong et al., 2014). However, little information is available with respect to native chickens. Native chickens generally have considerably greater exposure to environmental elements due to having been free-ranged. In addition, native chickens are not subjected to selective breeding aimed at producing pathogen-free or disease-free flocks. Therefore, native chickens may represent a greater risk for Salmonella infection. The aim of this study is to determine the prevalence of Salmonella among the local native chickens, and to identify those factors that play a role in the perpetuation of the pathogen in these flocks.
MATERIALS AND METHODS
Study Area
Malaysia is located north of the equator, and is bordered to the north by Thailand and in the south by Singapore. Malaysia is a country divided by the South China Sea, with Peninsular Malaysia to the west, and East Malaysia, comprising Sabah and Sarawak, to the east. Peninsular Malaysia is made up of 11 states. This study sampled farms from 4 states, namely Selangor, Johor, Melaka, and Negeri Sembilan in the central and southern regions of the country. These states have a cumulative 220 village chicken farms, comprising approximately 24.86% (n = 885) of the total recorded village chicken farms in Peninsular Malaysia.
Sample Size
The sample size was calculated using Epi info statistical software (Atlanta, GA). The calculation was based on the assumption of an expected prevalence of 14.9% and 3.1%, respectively, for cloacal swabs and eggs (Ong et al., 2014). With a desired absolute precision of 5%, 95% level of confidence, and a large population size, sample sizes of at least n = 235 and n = 56 were required for cloacal swabs and eggs, respectively. The study population comprises village chickens from central and southern states in Peninsular Malaysia. These states were selected based on a sampling frame obtained from the Ayam Kampung Association comprising details of all village chicken farmers. Village chicken farms were randomly selected from each of the 4 study sites using Microsoft Excel 2011. In total, 35 village chicken flocks were selected and visited from Selangor (n = 19), Melaka (n = 10), Johor (n = 4), and Negeri Sembilan (n = 2) during the study period. The majority of farms investigated in this study were from Selangor because Selangor is home to the largest number of village flocks as compared to other states in this study.
Study Design
Selected farms were contacted via telephone and an appointment to visit the farm was set. A cross-sectional study was employed whereby all samples, as well as data on farm management and production systems, were collected during the same visit. Farm management systems were categorized as either free-range, semi-intensive, or intensive. For free-range, birds are let free to roam about and scavenge, fed leftovers and other household scraps, provided with little or no man-made shed, and roost in the evening in a common area. This is the most common system used in resource-poor village households (Lawal et al., 2016); consequently, most native chickens are raised according to this management system. For semi-intensive, birds are housed during the night and released the following morning to scavenge. The house can vary from crude wire-meshed to more sophisticated structures. We found a sizeable number of village chicken farmers to be practicing this system. While little attention is given to chicken vaccination or other treatments in the case of both free-range and semi-intensive system, intensively farmed birds are housed throughout their life cycle and undergo a basic scheduled of preventive measures and vaccinations to guard against poultry and other diseases. Other good farm management practices, such as all-in-all-out, may also be practiced in intensive production systems. The housing type varies from cages to more sophisticated housing as seen in large commercial production systems. In general, this system is the least common for raising village chickens in the region. However, with the increasing interest and demand for village chicken products, this type of production system is fast becoming more prevalent.
Sample Collection from Village Flocks
Sample collection was performed between November 2016 and February 2018. Cloacal swabs were collected using sterile swab sticks (Oxoid, Cheshire, England), and immersed in 10 mL buffered peptone water (BPW, Oxoid). Pooled feed samples were collected from feeders located within the poultry pens using sterile polythene bags. Five 10 mL samples of pooled poultry drinking water were collected using 10 mL sterile syringes from drinkers. Eggs were purchased whenever available at the farms and packed using sterile polythene bags. All samples collected during visitation were immediately transported in an ice-cooled box to the Veterinary Public Health Laboratory at the Faculty of Veterinary Medicine, Universiti Putra Malaysia, for further processing or kept in an isolated refrigerator for sample storage until processed the following day. Flies were collected from the study sites as described elsewhere (Choo et al., 2011).
Isolation of Salmonella
Cloacal Swabs, Flies, Poultry Drinking Water, and Feeds
Isolation of Salmonella from cloacal swabs, poultry drinking water, and feeds was carried out according to the protocols and recommendations of the OIE terrestrial manual (www.oie.int) and ISO 6579:2002. Briefly, for isolation from cloacal swabs, the swab sticks immersed in 10 mL BPW (Oxoid) were incubated at 37°C for 18 to 24 h. For isolation from flies, trapped flies were processed as previously described (Choo et al., 2011), immersed in 10 mL BPW (Oxoid), then incubated at 37°C for 18 to 24 h. For isolation from drinking water, 10 mL of the water collected from the farms were each added to 90 mL of BPW and subsequently incubated at 37°C for 18 to 24 h. Similarly, the feeds collected from the farms were first weighed to 10 g using weighing balance, after which the 10 g of feed was inoculated into 90 mL of BPW (Oxoid) and incubated at 37°C for 18 to 24 h. All samples were further processed for Salmonella identification as described below.
Isolation of Salmonella from the Eggshell Surface and Egg Yolk
All broken or cracked egg samples were discarded. Eggshell surface was swabbed using a sterile cotton swab soaked in sterile normal saline solution. The swabbed stick was immersed into 10 mL Tryptic soy broth (TSB) (Oxoid) and incubated at 37°C for 24 h. For the egg yolk, isolation was done as recommended by the US Food and Drug Administration Bacteriological Analytical Manual, and by a previous study (Zhang et al., 2013). Briefly, the egg sample surface was disinfected by immersing whole eggs in a 3:1 disinfectant solution consisting of 3 parts 70% ethyl alcohol and 1 part iodine/potassium iodide solution for 10 s (Zhang et al., 2013). Surface-disinfected eggs were air-dried at room temperature before eggshell was cracked. Egg yolks were stirred in a stomacher for thorough mixing. 10 mL of the mixed egg yolk content was then inoculated into 90 mL of TSB (Oxoid) and incubated at 37°C for 24 h.
Identification of Salmonella
Following the initial pre-enrichment in BPW (Oxoid), and in TSB (Oxoid) in the case of egg samples, 0.1 mL of the pre-enriched samples were added to 10 mL of Rappaport Vassiliadis broth (RVB) (Oxoid) and incubated at 42°C for 24 h. RVB enriched samples were streaked onto 2 selective media, namely Brilliant Green Agar (BGA) supplemented with Novobiocin antibiotic (Oxoid), and Xylose Lysine Desoxycholate (XLD) (Oxoid). Plates were then incubated at 37°C for 18 to 24 h. Spherical transparent red halo colonies with typical black centers on XLD, and moist spherical red/pink colored colonies with reddening of the media on BGA plates, were selected as presumptive Salmonella colonies and further sub-cultured on nutrient agar plates (NA, Oxoid) and incubated at 37°C for 18 to 24 h. A total of 3 suspected colonies each from XLD and BGA plates (positive) were picked for sub-culturing on NA agar plates. Following incubation of the NA agar plates, triplicate suspected colonies were subjected to serological tests and further biochemical characterization as per the standard methods (Choo et al., 2011).
Serological and Biochemical Confirmation of Suspected Salmonella Colonies
For serological identification, Polyvalent Antiserum “O” was used according to the manufacturer's instructions. Biochemical confirmation of the presumptive Salmonella isolates was done as described elsewhere (Choo et al., 2011). Interpretations followed standard recommended guidelines (Mikoleit, 2014). Confirmation to serogroup level was by poly “O” and poly “H” (Phase 1 and Phase 2) antiserum. All isolates were sent to the Veterinary Research Institute in Ipoh (Peninsular Malaysia) for further confirmation and serotyping. A flock was defined as Salmonella-positive if at least 1 cloacal sample was positive regardless of the positivity of other environmental samples from the same flock.
Data Collection
A questionnaire pertaining to village chicken production was developed and piloted with a sample of village chicken farmers in the state of Selangor. Questionnaire items were refined based on the feedback received from farmers. Informed consent was obtained from each farmer before being administered the questionnaire. The questionnaire was written in both English and Bahasa Melayu languages. Information collected from the questionnaire included data on flock size, income level (Malaysian Ringgit), feed and water sources, storage of water and feed, poultry production system, the presence of wild birds, and other farm management practices, such as the management of sick birds, the disposal of dead birds in the flock, etc.
Data Analysis
Data was entered and managed in Microsoft Office Excel 2011. Descriptive statistics were utilized to determine the prevalence and proportions of Salmonella positivity according to the independent variables. Chi-square, Fisher's exact test, or simple logistic regression was used for the univariable exploratory analysis to identify risk factors associated with the outcome variable. Risk factors were analyzed by multivariable logistic regression models using forward Wald stepwise regression on variables with p < 0.05 from the univariable analysis. Risk of Salmonella infection amongst birds was expressed as an adjusted odds ratio (OR) with 95% confidence interval. All analyses were performed using SPSS version 22.0 (IBM, Armonk, NY) at the significance level α = 0.05.
RESULTS
Farm Demography and Distribution in the Central and Southern Peninsular Malaysia
According to the sampling frame provided, there were 222 farms located in central and southern Peninsular Malaysia (Table 1). Selangor had the highest number of village chicken farms (n = 122), followed by Melaka (n = 85), Johor (n = 11), and Negeri Sembilan (n = 2). Although Johor has fewer village chicken farms, it has the highest density of birds as compared to the other states combined. The majority of village chicken flocks in Selangor are backyard operations, with flock sizes ranging from as low as 10 birds, to as high as several hundred. Majority of the farms we sampled followed intensive production system (n = 15, 43%), followed by semi-intensive (n = 12; 34%), and free-range (n = 8; 23%).
Table 1.
Description of village chicken farms in the central and southern regions of Peninsular Malaysia.
| Farm locations | Number of farms | Relative % | Flock size1 | Range1 |
|---|---|---|---|---|
| Selangor | 122 | 55.5 | 94,178 | 4–15,000 |
| Negeri Sembilan | 2 | 0.9 | 21,200 | 1,200–20,000 |
| Melaka | 85 | 38.6 | 25,911 | 20–4,000 |
| Johor | 11 | 5 | 563,000 | 8,000–100,000 |
| Total | 220 | 100 | 704,289 | 4–100,000 |
Number of village chickens raised.
Salmonella Prevalence and Serotypes
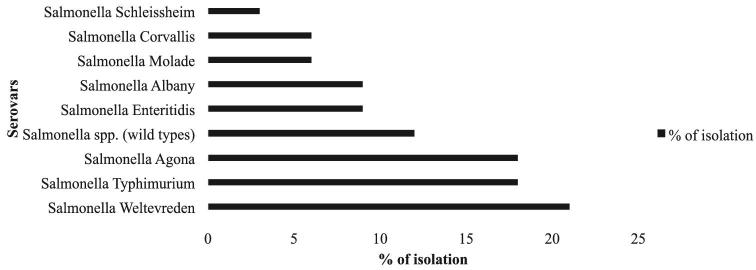
Of the 35 farms screened, 15 farms were positive, giving a farm-level prevalence of 42.9% (95% CI: 28.0, 59.2). Of the 675 cloacal swabs sampled from all farms, 17 (2.5%; 95% CI 1.6, 4.0) were positive for Salmonella (Table 2). All 62 eggs screened were negative. The Salmonella detection rates from pooled poultry drinking water, feeds, and flies were 5.14%, 7.14%, and 5.0%, respectively (Table 2). According to the management practices, the percentage of Salmonella positive was 75%, 25%, and 40% for free-range (n = 8), semi-intensive (n = 12), and intensive (n = 15) management system, respectively (Table 3). In total, 34 Salmonella isolates were recovered: 18 isolates from cloacal swabs, 9 from pooled poultry drinking water, 5 from pooled feed and 3 fly samples (Table 2). Of the 34 isolates, we identified S. Weltevreden (n = 7), S. Agona (n = 6), S. Typhimurium (n = 6), S. Albany (n = 3), S. Enteritidis (n = 3), S. Molade (n = 2), S. Corvallis (n = 2), and S. Schleissheim (n = 1). Other unidentified serotypes were grouped as Salmonella spp (n = 4) (Figure 1).
Table 2.
Salmonella from “Ayam kampung” farms in the central and southern regions of Peninsular Malaysia (n = 35).
| Sources | Total | Positive (%) |
|---|---|---|
| Cloacal swab | 675 | 17 (2.75) |
| Eggs | 62 | 00 |
| Poultry drinking water1 | 175 | 9 (5.14) |
| Feeds | 70 | 5 (7.14) |
| Flies2 | 60 | 3 (5.00) |
| Overall | 1,042 | 34 (3.26) |
5 samples of water 10 mLs each was obtained from each farm.
2 pooled samples of 5 flies each was sampled from each farm.
Table 3.
Univariable analysis for risk factors associated with the occurrence of Salmonella amongst village chickens from the central and southern Peninsular Malaysia.
| Variables | Frequency | Positive (%) | Chi-square (χ²) | P-value |
|---|---|---|---|---|
| Flock size (number of birds in the farm) | ||||
| <500 | 18 | 9 (50.0) | 0.787 | 0.675 |
| 500–1,000 | 6 | 2 (33.3) | ||
| >1,000 | 11 | 4 (36.4) | ||
| Income level (Ringgit) | ||||
| <500 | 16 | 7 (43.8) | 0.048 | 0.976 |
| 500–1,200 | 9 | 4 (44.4) | ||
| >1,200 | 10 | 4 (40.0) | ||
| Level of education of the farmer | ||||
| Primary school | 2 | 0 | 3.241 | 0.356 |
| Secondary | 9 | 5 (55.6) | ||
| Certificate | 9 | 5 (55.6) | ||
| University degree | 15 | 5 (33.3) | ||
| Source of feeds in the farm1 | ||||
| Homemade | 20 | 12 (60.0) | 5.872 | 0.053 |
| Commercially prepared | 12 | 2 (16.7) | ||
| Table scraps | 3 | 1 (33.3) | ||
| Storage of feeds in the farm | ||||
| Covered | 21 | 13 (61.9) | 9.343 | 0.002 |
| Uncovered | 14 | 2 (14.3) | ||
| Use of additives/growth promoters | ||||
| Yes | 24 | 8 (33.3) | 2.828 | 0.093 |
| No | 11 | 7 (63.6) | ||
| Source of water in the farm | ||||
| Rain water | 2 | 1 (50.0) | 2.106 | 0.349 |
| Well water | 9 | 2 (22.2) | ||
| Tap/Borehole | 24 | 12 (50.0) | ||
| Storage of water in the farm | ||||
| Covered tank storage | 16 | 10 (62.5) | 4.644 | 0.031 |
| Uncovered tank storage | 19 | 5 (26.3) | ||
| Poultry production system | ||||
| Free-range | 8 | 6 (75.0) | 4.988 | 0.083 |
| Semi-intensive | 12 | 3 (25.0) | ||
| Intensive | 15 | 6 (40.0) | ||
| Frequency of poultry manure disposal | ||||
| Disposed weekly | 10 | 6 (60.0) | 2.622 | 0.270 |
| Disposed monthly | 17 | 5 (29.4) | ||
| Never | 8 | 4 (50.0) | ||
| Frequency of cleaning house litters | ||||
| Weekly | 9 | 1 (11.1) | 5.213 | 0.074 |
| Monthly | 23 | 12 (52.2) | ||
| Never | 3 | 2 (66.7) | ||
| Wild birds in the farm | ||||
| Yes | 21 | 11 (52.4) | 1.944 | 0.163 |
| No | 14 | 4 (28.6) | ||
| Accessibility of the farm to the public | ||||
| Presence of fence | 27 | 12 (44.4) | 0.122 | 0.727 |
| No fence | 8 | 3 (37.5) | ||
| Presence of other animals (Dogs, Cats, Goats) in the farm | ||||
| Yes | 21 | 9 (42.9) | 0.000 | 1.000 |
| No | 14 | 6 (42.9) | ||
| Management of sick birds in the farm | ||||
| Isolated from healthy birds | 20 | 5 (25.0) | 6.076 | 0.014 |
| No isolation | 15 | 10 (66.7) | ||
| Disposal of dead birds in the farm | ||||
| Disposed inside farm premises | 27 | 14 (51.9) | 3.902 | 0.048 |
| Disposed outside farm premises | 8 | 1 (12.5) | ||
| Control of vermins in the farm | ||||
| Yes | 20 | 9 (45.0) | 0.087 | 0.767 |
| No | 15 | 6 (40.0) | ||
| Use of disinfectants and footbaths | ||||
| Yes | 6 | 2 (33.3) | 0.268 | 0.605 |
| No | 29 | 13 (44.8) | ||
| Use of antibiotics in the farm | ||||
| Yes | 18 | 10 (55.5) | 2.440 | 0.118 |
| No | 17 | 5 (29.4) | ||
| Vaccination of birds in the farm | ||||
| Yes | 18 | 8 (44.4) | 0.038 | 0.845 |
| No | 17 | 7 (41.2) | ||
| Veterinary consultancy services | ||||
| Yes | 19 | 7 (36.8) | 1.703 | 0.427 |
| No | 16 | 8 (50.0) | ||
| Salmonella isolated from environmental samples2 | ||||
| Yes | 10 (28.6) | 1.680 | 0.266 | |
| No | 7 (20.0) | |||
Homemade poultry feeds are made by village farmers at home with organic ingredients and other raw materials from local feed stores.
Environmental samples comprised poultry drinking water, poultry feeds, and flies in and around the pens.
Figure 1.
Distribution of the serotypes of Salmonella isolated from village chickens in Peninsular Malaysia.
Univariable Analysis of Risk Factors for Salmonella
Univariable analysis revealed risk factors associated with salmonellosis in village chickens. These risk factors included sources of feeds (χ² = 5.872; p = 0.053), farm feed storage (χ² = 9.343; p = 0.002), farm water storage (χ² = 4.644; p = 0.031), sick bird management practices (χ² = 6.076; p = 0.014), and the disposal of dead birds from the farm (χ² = 3.902; p = 0.048) (Table 3).
Multiple Logistic Regression of Risk Factors for Salmonella
Multivariable regression revealed that uncovered feed storage (OR = 10.4; 95% CI: 1.25 to 86.39; p = 0.030) and uncovered water storage (OR = 6.4; 95% CI: 1.02 to 40.6; p = 0.048) were significantly associated with an increased odds of Salmonella positivity (Table 4). Village chickens in farms with uncovered feeds storage were approximately 10 times more likely to test positive for Salmonella as compared to those from farms with covered storage. Likewise, village chickens from farms with uncovered water storage were approximately 6 times more likely to test positive for Salmonella as compared to those from farms with covered water storage (Table 4). However, while sick bird management practices (i.e., not isolating sick birds from healthy birds; OR = 2.56; 95% CI: 0.40 to 16.22; p = 0.318) fitted the final regression model, the result was not significant (Table 4).
Table 4.
Multiple logistic regression of risk factors associated with the occurrence of salmonellosis in village chickens from the central and southern Peninsular Malaysia.
| Variables | B | S.E | Wald | df | P-value | Adjusted OR1 | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| Storage of feeds (Uncovered) | 2.341 | 1.081 | 4.690 | 1 | 0.030 | 10.387 | 1.25−86.39 |
| Storage of water (Uncovered tank storage) | 1.860 | 0.941 | 3.911 | 1 | 0.048 | 6.425 | 1.02−40.60 |
| Management of sick birds | 0.940 | 0.942 | 0.995 | 1 | 0.318 | 2.560 | 0.40−16.22 |
| (Number isolation of sick birds) |
OR, Odds ratio.
DISCUSSION
Village chickens and their products have been gaining in popularity in Malaysia because of their presumed wholesomeness and safety. This study found that although the flock-level prevalence for Salmonella was quite high, chicken-level prevalence was much lower at 2.5% (n = 675). This prevalence is much lower than that previously published by Ong et al. for local commercial chickens—14.9% (n = 1911) in poultry farms (Ong et al., 2014). This finding was unexpected because village chickens are raised with few structured disease controls or Salmonella control programs. Moreover, the farms have minimum or non-existent biosecurity measures, thus exposing chickens to various environmental elements before they attain marketable weight. However, we believe that overcrowding and stress in commercial production may explain these observed differences. It has been reported that overcrowding and high-density population increases Salmonella transmission among commercially-raised poultry despite preventative measures (Bailey, 1988; Huneau-Salaün et al., 2009; Foley et al., 2011; Im et al., 2015; Ansari et al., 2017). Moreover, high-density production leads to stress, thus lowering individual ability to resist infection (Gomes et al., 2014; Gast et al., 2017).
Compared to other studies of Salmonella in backyard chicken, we found higher Salmonella levels than a South Australian that reported an estimated animal-level prevalence of 0.02% after screening (n = 115) pooled cloacal swab samples from 30 backyard flocks (Manning et al., 2015). However, our estimate is lower than findings reported from West Bengal in India (15%; n = 40, Samanta et al., 2014), and from Iran (5.8%; n = 85) following testing of 422 cloacal swab samples from 35 backyard flocks (Jafari et al., 2007). Our findings are in fact similar to those in Paraguay (3.5%; n = 400) of 50 backyard flocks (Leotta et al., 2010), and the central region of Saudi Arabia (2.2%) obtained from broiler parent flocks (Saad et al., 2007). Notwithstanding, the aforementioned comparisons should be regarded with caution given various differences in the techniques used for isolation, limited sampling areas (as seen in the study from Paraguay, which used a relatively small sample size, and the study from Iran), and differences in the population dynamics of dominant Salmonella serovars in each country. Other authors (e.g., Carrique-Mas and Davies, 2008) have explained that the prevalence of Salmonella measured in birds using cloacal swabs is often low because of, among other factors, the intermittent shedding of the organism and relatively low number of organisms excreted by infected birds. In our study, more than half of the farmers surveyed during the study stated that they used antibiotics when their birds are sick, which could have probably reduce the recovery rate of Salmonella in our study. In addition, diverse age range of sampled chickens could also be a contributing factor to the differences observed. Moreover, lack of further enrichment step with either selenite/tetrathionate broth after the pre-enrichment with buffered peptone water could be another reason for the lower isolation rate observed in this study.
We recovered 8 serotypes of Salmonella enterica, with a predominance of Weltevreden, Agona, and Typhimurium. S. Typhimurium—serotypes with well-established histories of causing various human infections, and frequently isolated among clinical cases in Malaysia (Ministry of Health, Malaysia, 2015; Karim et al., 2017). One recent study reported that S. Typhimurium was among the most commonly associated non-typhoidal Salmonella poultry-related salmonellosis in the United States (Shah et al., 2016). Previous studies have not reported the other 2 aforementioned serotypes to be as predominant in ready-to-eat foods, vegetables, beef, raw meat, carcasses, or live birds in Malaysia (Rusul et al., 1996; Ong et al., 2014; Najwa et al., 2015; Nidaullah et al., 2017). Weltevreden and Agona serotypes are pathogenic and have caused multiple foodborne disease outbreaks in Malaysia (Ministry of Health, Malaysia, 2015; Karim et al., 2017), and elsewhere (Centers for Disease Control and Prevention, 2006; Bruun et al., 2009; Whelan et al., 2010; Russo et al., 2013; Mba-Jonas et al., 2018). For instance, an earlier study based on clinical records sourced from the Institute of Medical Research between 1983 and 1992 reported that serotype Weltevreden was among the most predominant Salmonella isolated from clinical cases in humans (Yasin et al., 1997). Recently, Malaysia's Ministry of Health reported on a foodborne outbreak associated with a local dish called—laksa—that resulted in 2 deaths. The laboratory results from the clinical samples confirmed Salmonella enterica serovar Weltevreden as the causal agent of the foodborne infection. On the other hand, S. Agona is among the most common non-typhoidal serovars of Salmonella isolated from humans in Europe, and was responsible for the 2008 pan-European outbreak (McCusker et al., 2014).
In this study, we recorded 5.14%, 7.14%, and 5.0% Salmonella in samples of drinking water, feeds, and flies, respectively (Table 2). The isolation rates of 5.14% from drinking water and 7.14% from poultry feeds were lower than the 36% reported in commercial poultry drinking water in Thailand (Sasipreeyajan et al., 1996), and 22.2% reported from 36 bulk commercial poultry feeds in Nigeria (Okoli et al., 2006). However, the isolation rates reported in our study were also slightly higher than 3.3% (poultry water) and 2.5% (poultry feeds) as reported from layer farms in Northern India (Singh et al., 2013). Contaminated poultry feed and drinking water are critical sources of Salmonella infection and have been associated with transmission and the perpetuation of Salmonella in poultry farms (Frederick and Huda, 2011). Although we did not find a significant association of Salmonella being present in the environmental samples, to the farm-level Salmonella prevalence in this study, several previous studies highlight the importance of environmental contamination (Sasipreeyajan et al., 1996; Okoli et al., 2006; Singh et al., 2013; Ong et al., 2014; Samanta et al., 2014).
Other studies have reported Salmonella in eggs (Assefa et al., 2011; Murakami et al., 2013; Singh et al., 2013; Ayachi et al., 2015); however, our study did not. This finding is consistent with the low levels of Salmonella infection found among village flocks. Nevertheless, we screened only 62 eggs from the flocks in this study, which may be too small to detect Salmonella in the eggs.
Uncovered storage of both poultry drinking water and feeds allows complete access to rodents, flies, and the droppings of wild bird. Rodents and wild birds are known Salmonella carriers (Andrés-Barranco et al., 2014; Mustaffa et al., 2014; Matias et al., 2016; Ribas et al., 2016). A number of studies have highlighted the role of flies, rodents, and wild birds in the transmission and perpetuation of Salmonella infection among poultry flocks and other farm animals. Rodents can carry Salmonella in their intestinal tracts asymptomatically (Zamora-Sanabria and Alvarado, 2017), and are the reported vectors and amplifiers of Salmonella in farm animals (Meerburg and Kijlstra, 2007). Rodent droppings can remain a source of Salmonella contamination for up to 3 mo following infection with the feaces of sick animals (Davies and Wray, 1995; Zamora-Sanabria and Alvarado, 2017). On the other hand, flies also act as mechanical vectors, aiding transmission of the bacteria from one farm to another. Heavy fly populations have been identified as a risk factor for Salmonella in poultry, dairy cattle, swine, and feedlot cattle (Vanselow et al., 2007). Wild birds and other wildlife can introduce and disseminate the bacteria via the contamination of feed, water, or direct environmental contamination (Zamora-Sanabria and Alvarado, 2017).
CONCLUSION
We observed a lower animal level of prevalence of Salmonella infection among village chickens compared to reported prevalence among commercial broiler chickens. Risk factors for infection include uncovered water and feed storage. These practices allow access of poultry feeds and drinking water to flies, rodents, and wild bird droppings. To improve the safety of village chicken, farmers should be advised to undertake simple good management practices, such as storing feed and water in covered containers, and improving biosecurity to reduce the accessibility of wild birds and animals.
ACKNOWLEDGMENTS
We thank the local Ayam Kampung Association of Peninsular Malaysia and farmers for their kind support and collaboration.
FUNDING
This research project was part of a doctoral study undertaken in the Faculty of Veterinary Medicine, Universiti Putra Malaysia, and was funded by the UPM Research Grant number 9522900. The funding source did not play any role in the conduct of the research, nor in the writing or submission of the manuscript for publication.
Competing Interests
None declared.
REFERENCES
- Aini I. 1990. Indigenous chicken production in south-east Asia. Worlds Poult. Sci. J. 46:51–57. [Google Scholar]
- Andrés-Barranco S., Vico J. P., Garrido V., Samper S., Herrera-León S., de Frutos C., Mainar-Jaime R. C.. 2014. Role of wild bird and rodents in the epidemiology of subclinical Salmonellosis in finishing pigs. Foodborne Pathog. Dis. 11:689–697. [DOI] [PubMed] [Google Scholar]
- Ansari F., Pourjafar H., Bokaie S., Peighambari S. M., Mahmoudi M., Fallah M. H., Tehrani F., Rajab A., Ghafouri S. A., Shabani M.. 2017. Association between poultry density and Salmonella infection in commercial laying flocks in Iran using a kernel density. Pak. Vet. J. 37:299–304. [Google Scholar]
- Arsenault J., Letellier A., Quessy S., Normand V., Boulianne M.. 2007. Prevalence and risk factors for Salmonella spp. and Campylobacter spp. caecal colonization in broiler chicken and turkey flocks slaughtered in Quebec, Canada. Prev. Vet. Med. 81:250–264. [DOI] [PubMed] [Google Scholar]
- Assefa M., Teklu A., Negussie H.. 2011. The prevalence and public health importance of Salmonella from chicken table eggs, Ethiopia. Am. Eurasian J. Agric. Environ. Sci. 11:512–518. [Google Scholar]
- Ayachi A., Bennoune O., Heleili N., Alloui N.. 2015. Minor Salmonella: potential pathogens in eggs in Algeria. J. Infect. Dev. Ctries. 9:1156. [DOI] [PubMed] [Google Scholar]
- Azahan E. A. E. 1994. The red and black-red native chickens of Malaysia. Malaysian Agric. Res. Dev. Inst. Res. J 22:73–78. [Google Scholar]
- Azahan E. A. E., Seet C. P., Zainab O.. 1980. A comparative study of physiological and productive performance of local (Kampung) and commercial chickens. Malaysian Agric. Res. Dev. Inst. Res. J. 52:61–70. [Google Scholar]
- Azahan E. A. E., Zahari W. M.. 1983. Observation on some characteristics of carcass and meat of Malaysian kampong chickens. Mardi Res. Bull. 11:225–232. [Google Scholar]
- Bailey J. S. 1988. Integrated colonization control of Salmonella in poultry. Poult. Sci. 67:928–932. [DOI] [PubMed] [Google Scholar]
- Bruun T., Sørensen G., Forshell L. P., Jensen T., Nygard K., Kapperud G., Lindstedt B. A., Berglund T., Wingstrand A., Petersen R. F., Müller L., Kjelsø C., Ivarsson S., Hjertqvist M., Löfdahl S., Ethelberg S.. 2009. An outbreak of Salmonella Typhimurium infections in Denmark, Norway and Sweden, 2008. Euro Surveill 14 PMID: 19317986. https://www.eurosurveillance.org/content/10.2807/ese.14.10.19147-en#html_fulltext. [PubMed] [Google Scholar]
- Carrique-Mas J. J., Davies R. H.. 2008. Sampling and bacteriological detection of Salmonella in poultry and poultry premises: a review. Rev. Sci. Tech. OIE 27:665–677. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2006. Multistate outbreak of Salmonella typhimurium infections associated with eating ground beef−United States, 2004. MMWR Morb. Mortal Wkly. Re. 55:180–218. [PubMed] [Google Scholar]
- Choo L. C., Saleha A. A., Wai S. S., Fauziah N.. 2011. Isolation of Campylobacter and Salmonella from houseflies (Musca domestica) in a university campus and a poultry farm in Selangor, Malaysia. Trop. Biomed. 28:16–20. [PubMed] [Google Scholar]
- Davies R., Wray C.. 1995. Mice as carriers of Salmonella enteritidis on persistently infected poultry units. Vet. Rec. 137:337–341. [DOI] [PubMed] [Google Scholar]
- Foley S. L., Nayak R., Hanning I. B., Johnson T. J., Han J., Ricke S. C.. 2011. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 77:4273–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick A., Huda N.. 2011. Salmonellas, poultry house environments and feeds: a review. J. Anim. Vet. Adv. 10:679–685. [Google Scholar]
- Gast R. K., Guraya R., Jones D. R., Anderson K. E., Karcher D. M.. 2017. Frequency and duration of fecal shedding of Salmonella enteritidis by experimentally infected laying hens housed in enriched colony cages at different stocking densities. Front. Vet. Sci. 4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A. V. S., Quinteiro-Filho W. M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M. L., Baskeville E., Akamine A. T., Astolfi-Ferreira C. S., Ferreira A. J. P., Palermo-Neto J.. 2014. Overcrowding stress decreases macrophage activity and increases Salmonella Enteritidis invasion in broiler chickens. Avian Pathol. 43:82–90. [DOI] [PubMed] [Google Scholar]
- Hassan L., Suhaimi S. Z., Saleha A. A.. 2005. The detection and comparison of antimicrobial resistance pattern of vancomycin-resistant enterococci and Salmonella isolated from eggs of commercial layers and free-range chickens. J. Vet. Malaysia 17:7–11. [Google Scholar]
- Huneau-Salaün A., Marianne C., Sophie L. B., Françoise L., Isabelle P., Sandra R., Virginie M., Philippe F., Nicolas R.. 2009. Risk factors for Salmonella enterica subsp. enterica contamination in 519 French laying hen flocks at the end of the laying period. Prev. Vet. Med. 89:51–58. [DOI] [PubMed] [Google Scholar]
- Im M. C., Jeong S. J., Kwon Y.-K., Jeong O.-M., Kang M.-S., Lee Y. J.. 2015. Prevalence and characteristics of Salmonella spp. isolated from commercial layer farms in Korea. Poult. Sci. 94:1691–1698. [DOI] [PubMed] [Google Scholar]
- Jafari R. A., Ghorbanpour M., Jaideri A.. 2007. An investigation into Salmonella infection status in backyard chickens in Iran. Int. J. Poult. Sci. 6:227–229. [Google Scholar]
- Karim B. A., Latip A. L., Shukor A. S. A., Rashid N. A., Mohd W. M. W., Kamaludin F.. 2017. A large common source outbreak of Salmonella typhimurium linked to Kuala Terengganu night markets, Malaysia, 2014. OSIR J. 10:1–7. [Google Scholar]
- Lawal J. R., Jajere S. M., Ibrahim U. I., Geidam Y. A., Gulani I. A., Musa G., Ibekwe B. U.. 2016. Prevalence of coccidiosis among village and exotic breed of chickens in Maiduguri, Nigeria, Vet. World 9:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotta G., Suzuki K., Alvarez F. L., Nuñez L., Silva M. G., Castro L., Faccioli M. L., Zarate N., Weiler N., Alvarez M., Copes J.. 2010. Prevalence of Salmonella Spp. in backyard chickens in Paraguay. Int. J. Poult. Sci. 9:533–536. [Google Scholar]
- Manning J., Gole V., Chousalkar K.. 2015. Screening for Salmonella in backyard chickens. Prev. Vet. Med. 120:241–245. [DOI] [PubMed] [Google Scholar]
- Marin C., Balasch S., Vega S., Lainez M.. 2011. Sources of Salmonella contamination during broiler production in Eastern Spain. Prev. Vet. Med. 98:39–45. [DOI] [PubMed] [Google Scholar]
- Matias C. A. R., Pereira I. A., dos Reis E. M. F., Rodrigues D. dos P., Siciliano S.. 2016. Frequency of zoonotic bacteria among illegally traded wild birds in Rio de Janeiro. Braz. J. Microbiol. 47:882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mba-Jonas A., Culpepper W., Hill T., Cantu V., Loera J., Borders J., Saathoff-Huber L., Nsubuga J., Zambrana I., Dalton S., Williams I., Neil K. P.. 2018. A multistate outbreak of human Salmonella agona infections associated with consumption of fresh, whole papayas imported from Mexico—United States, 2011. Clin. Infect. Dis. 66:1756–1761. [DOI] [PubMed] [Google Scholar]
- McCusker M. P., Hokamp K., Buckley J. F., Wall P. G., Martins M., Fanning S.. 2014. Complete genome sequence of Salmonella enterica serovar agona pulsed-field type SAGOXB.0066, cause of a 2008 pan-European outbreak. Genome Announc. 2:e01219–13, 2/1/e01219-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerburg B. G., Kijlstra A.. 2007. Role of rodents in transmission ofSalmonella andCampylobacter. J. Sci. Food Agric. 87:2774–2781. [Google Scholar]
- Mezal E. H., Sabol A., Khan M. A., Ali N., Stefanova R., Khan A. A.. 2014. Isolation and molecular characterization of Salmonella enterica serovar Enteritidis from poultry house and clinical samples during 2010. Food Microbiol. 38:67–74. [DOI] [PubMed] [Google Scholar]
- Miao Z. H., Glatz P. C., Ru Y. J.. 2005. Free-range poultry production − a review. Asian Australas. J. Anim. Sci 18:113–132. [Google Scholar]
- Mikoleit M. L. 2014. Biochemical identification of Salmonella and Shigella using an abbreviated panel of tests. World Health Organization, Global Foodborne Infections Network, Geneva, Switzerland. [Google Scholar]
- Ministry of Health, Malaysia 2015. Health Facts 2015. Health Informatics Centre, Planning and Development Division, Ministry of Health, Malaysia, Kuala Lumpur, Malaysia. [Google Scholar]
- Murakami K., Noda T., Onozuka D., Sera N.. 2013. Salmonella in liquid eggs and other foods in Fukuoka Prefecture, Japan. Int. J. Microbiol. 2013:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustaffa S. S., Saleha A. A., Jalila A.. 2014. Occurrence of antibiotic resistant Salmonella and Campylobacter in wild birds. J. Vet. Malaysia 26:17–19. [Google Scholar]
- Najwa M. S., Rukayadi Y., Ubong A., Loo Y. Y., Chang W. S., Lye Y. L., Thung T. Y., Aimi S. A., Malcolm T. T. H., Goh S. G., Kuan C. H., Yoshitsugu N., Nishibuchi M., Son R.. 2015. Quantification and antibiotic susceptibility of Salmonella spp., Salmonella enteritidis and Salmonella typhimurium in raw vegetables (ulam). Int. Food Res. J. 22:1761–1769. [Google Scholar]
- Nidaullah H., Abirami N., Shamila-Syuhada A. K., Chuah L.-O., Nurul H., Tan T. P., Zainal Abidin F. W., Rusul G.. 2017. Prevalence of Salmonella in poultry processing environments in wet markets in Penang and Perlis, Malaysia. Vet. World 10:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoli C. I., Ndujihe G. E., Ogbuewu I. P.. 2006. Frequency of isolation of salmonella from commercial poultry feeds and their anti-microbial resistance profiles, Imo State, Nigeria. Online J. Health Allied Sci. 5 http://www.ojhas.org/issue18/2006-2-3.htm. [Google Scholar]
- Ong L. P., Muniandy K., How S. P., Yip L. S., Lim B. K.. 2014. Salmonella isolation from poultry farms in Malaysia from 2011 to 2013. Malaysian J. Vet. Res. 5:1–7. [Google Scholar]
- Padhi M. K. 2016. Importance of indigenous breeds of chicken for rural economy and their improvements for higher production performance. Scientifica 2016:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman W. A., Haziqah F.. 2015. Ectoparasitic fauna of scavenging chickens (Gallus domesticus) from Penang Island, Peninsular Malaysia. Malaysian J. Vet. Res. 6:33–42. [Google Scholar]
- Ribas A., Saijuntha W., Agatsuma T., Prantlová V., Poonlaphdecha S.. 2016. Rodents as a source of Salmonella contamination in wet markets in Thailand. Vector Borne Zoonotic Dis. 16:537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N., Beaudeau F., Drouin P., Toux J. Y., Rose V., Colin P.. 1999. Risk factors for Salmonella enterica subsp. enterica contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 39:265–277. [DOI] [PubMed] [Google Scholar]
- Russo E. T., Biggerstaff G., Hoekstra R. M., Meyer S., Patel N., Miller B., Quick R., and The Salmonella Agona Outbreak Investigation Team. 2013. A recurrent, multistate outbreak of Salmonella serotype agona infections associated with dry, unsweetened cereal consumption, United States, 2008. J. Food Prot. 76:227–230. [DOI] [PubMed] [Google Scholar]
- Rusul G., Khair J., Radu S., Cheah C. T., Yassin R. M.. 1996. Prevalence of Salmonella in broilers at retail outlets, processing plants and farms in Malaysia. Int. J. Food Microbiol. 33:183–194. [DOI] [PubMed] [Google Scholar]
- Saad A. M., Almujali D. M., Babiker S. H., Suhaib M. A. M., Adelgadir K. A., Alfadul Y. A.. 2007. Prevalence of Salmonellae in broiler chicken carcasses and poultry farms in the central region of KSA. J. Anim. Vet. Adv. 6:164–167. [Google Scholar]
- Safman R. M. 2010. Avian influenza control in Thailand: balancing the interests of different poultry producers. Pages 169–206 in Avian Influenza: Science, Policy and Politics. Scoones I., ed. Earthscan, London, UK. [Google Scholar]
- Samanta I., Joardar S. N., Das P. K., Sar T. K., Bandyopadhyay S., Dutta T. K., Sarkar U.. 2014. Prevalence and antibiotic resistance profiles of Salmonella serotypes isolated from backyard poultry flocks in West Bengal, India, India. J. Appl. Poult. Res. 23:536–545. [Google Scholar]
- Saravanan S., Purushothaman V., Murthy T. R. G., Sukumar K., Srinivasan P., Gowthaman V., Balusamy M., Atterbury R., Kuchipudi S. V.. 2015. Molecular epidemiology of nontyphoidal Salmonella in poultry and poultry products in India: implications for human health. Indian J. Microbiol. 55:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasipreeyajan J., Jerngklinchan J., Koowatananukul C., Saitanu K.. 1996. Prevalence of Salmonellae in broiler and breeder flocks in Thailand. Trop. Anim. Health Prod. 28:174–180. [PubMed] [Google Scholar]
- Shah D. H., Paul N. C., Sischo W. C., Crespo R., Guard J.. 2016. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult. Sci. 96:687–702. [DOI] [PubMed] [Google Scholar]
- Singh R., Yadav A. S., Tripathi V., Singh R. P.. 2013. Antimicrobial resistance profile of Salmonella present in poultry and poultry environment in north India. Food Control 33:545–548. [Google Scholar]
- Thung T. Y., Mahyudin N. A., Basri D. F., Wan Mohamed Radzi C. W. J., Nakaguchi Y., Nishibuchi M., Radu S.. 2016. Prevalence and antibiotic resistance of Salmonella enteritidis and Salmonella typhimurium in raw chicken meat at retail markets in Malaysia. Poult. Sci. 95:1888–1893. [DOI] [PubMed] [Google Scholar]
- Vanselow B., Hornitzky M., Walker K., Eamens G., Bailey G., Gill P., Coates K., Corney B., Cronin J., Renilson S.. 2007. Salmonella and on-farm risk factors in healthy slaughter-age cattle and sheep in eastern Australia. Aust. Vet. J. 85:498–502. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang B., Wu Y., Zhang Z., Meng X., Xi M., Wang X., Xia X., Shi X., Wang D., Meng J.. 2015. Molecular characterization of Salmonella enterica serovar Enteritidis on retail raw poultry in six provinces and two National cities in China. Food Microbiol. 46:74–80. [DOI] [PubMed] [Google Scholar]
- Whelan J., Noel H., Friesema I., Hofhuis A., de Jager C. M., Heck M., Heuvelink A., van Pelt W.. 2010. National outbreak of Salmonella Typhimurium (Dutch) phage-type 132 in the Netherlands, October to December 2009. Euro Surveill 15https://www.eurosurveillance.org/content/10.2807/ese.15.44.19705-en. [DOI] [PubMed] [Google Scholar]
- Yasin R. M., Jegathesan M., Tiew C. C.. 1997. Salmonella serotypes isolated in Malaysia over the ten-year period 1983-1992. Asia Pac. J. Public Health 9:1–5. [DOI] [PubMed] [Google Scholar]
- Zamora-Sanabria R., Alvarado A. M.. 2017. Preharvest Salmonella risk contamination and the control strategies. Pages 193–213 in Current Topics in Salmonella and Salmonellosis. Mares M., ed. InTechOpen, Rijeka, Croatia. [Google Scholar]
- Zhang G., Thau E., Brown E. W., Hammack T. S.. 2013. Comparison of a novel strategy for the detection and isolation of Salmonella in shell eggs with the Food and Drug Administration Bacteriological Analytical Manual method1. Poult. Sci. 92:3266–3274. [DOI] [PubMed] [Google Scholar]