Abstract
Antibiotic stewardship programs have traditionally focused on reducing hospital antibiotic use. However, reducing community antibiotic prescribing could have substantial impacts in both hospital and community settings. We developed a deterministic model of transmission of extended-spectrum beta-lactamase–producing Escherichia coli in both the community and hospitals. We fit the model to existing, national-level antibiotic use and resistance prevalence data from Sweden. Across a range of conditions, a given relative change in antibiotic use in the community had a greater impact on resistance prevalence in both the community and hospitals than an equivalent relative change in hospital use. However, on a per prescription basis, changes in antibiotic use in hospitals had the greatest impact. The magnitude of changes in prevalence were modest, even with large changes in antimicrobial use. These data support the expansion of stewardship programs/interventions beyond the walls of hospitals, but also suggest that such efforts would benefit hospitals themselves.
Keywords: antibiotic resistance, antibiotic stewardship, dynamic modeling, transmission
Reducing antibiotic use in the community setting can have a major impact on the prevalence of extended-spectrum beta-lactamase–producing Escherichia coli in both community and hospital settings. Further expansion of community-focused antibiotic stewardship interventions/studies is warranted.
In the wake of globally-increasing rates of antibiotic-resistant infections, mechanisms to monitor and direct the appropriate use of antimicrobials by health-care practitioners have become increasingly important [1]. Antimicrobial stewardship programs (ASPs), typically operated by infectious diseases clinicians and pharmacists, apply a variety of approaches to modify the behavior of prescribing physicians in a given clinical setting. Their aims include optimizing the drug choice for a given clinical scenario, encouraging shorter durations of treatment, and encouraging treatment with the narrowest spectrum antimicrobials possible. ASPs have grown rapidly over the last decade and are considered mandatory in acute-care settings in many high-income countries [1]. The initial outcomes evaluated in ASP studies have focused on process-related outcomes, including behavioral (adoption of stewardship advice) and economic indices (drug/hospitalization costs) [2]. However, attempts to evaluate the effects of changes in antimicrobial use on antibiotic resistant organism (ARO) colonization or infection in hospital and community settings are complicated by the unknown expected effect sizes, small sample sizes, costs of large-scale interventions, and heterogeneity of interventions. As a result, it is not clear where our efforts are most effectively concentrated.
Gram-negative bacteria expressing extended-spectrum beta-lactamases (ESBLs) have emerged as a major cause of morbidity and mortality [3]. ESBLs can hydrolyze a number of different broad-spectrum beta-lactam antimicrobials, are often resistant to standard first-line beta-lactam therapy, and can be transmitted among host populations through either the sharing of resistance mechanisms within and between species of enteric Gram-negative bacteria (Enterobacteriaceae) or through the transmission of resistant strains/lineages [4]. Infections caused by ESBL-producing Gram-negative bacteria (particularly Escherichia coli) have been increasing in the United States and globally, in both community and hospital settings. In some countries, the asymptomatic carriage of ESBL-producing Enterobacteriaceae has been found to occur in up to 70% of individuals [4]. Recent studies have demonstrated the transmission of ESBL-producing Enterobacteriaceae between patients in hospitalized settings, as well as in community environments [5, 6]. These studies support the notion that acquisition is due, in large part, to the person-to-person transmission of resistant strains, from asymptomatic carriage in the gut. In acute-care settings, this may be facilitated by shared health-care personnel and equipment. Colonization with ESBL-producing Gram-negative bacteria is a major risk factor for subsequent, invasive infection and is an important measure of the population burden of disease.
Dynamic modeling, using mechanistic assumptions to construct nonlinear models, offers an approach to simulating the potential outcomes of large-scale interventions, and has been applied to prospectively evaluate the likely effects of interventions focused on reducing the burden of infectious diseases [7, 8]. In particular, such models can capture the effects of interventions on both program participants and nonparticipants (ie, “herd effects”). Previous studies have modeled the transmission of both Gram-positive [9, 10] and Gram-negative bacterial pathogens [5, 11] in both community and hospital settings. These models often employ compartmental models, using deterministic and/or stochastic approaches. When modeling selection effects, multi-strain models are employed to permit competition between strains [12]. Given the lack of empirical data on the expected impacts of implementing antimicrobial stewardship on antimicrobial resistance, in either community or hospital settings, there are clear opportunities for dynamic models [13] to help guide future antimicrobial stewardship approaches [14]. In this paper, we describe the development and validation of an ESBL-producing E. coli compartmental transmission model, fit to high-quality historical data from Sweden. We use this model to evaluate the expected impacts of changes in antimicrobial use, in the community and the hospital, over time.
METHODOLOGY
Model Design
A deterministic, compartmental model was developed (Supplementary Equations 1–16) based on a general, 2-strain competition model that also allowed for the direct replacement of 1 strain with the other upon exposure. This model is based off of well-described model structures, used for evaluating the evolution and spread of antibiotic resistance [12]. The model structure and superstructure are shown in Supplementary Figures 1 and 2, and could be applicable to a variety of pathogens (Gram-positive and Gram-negatives) that transmit within and between community and hospital environments. We then used this model to simulate the transmission of ESBL-producing E. coli within and between these environments. There were 3 groups considered in the base model: (1) the hospital short-stay group, consisting of initially-admitted, acute-care hospital inpatients; (2) the hospital long-stay group, consisting of patients moving from a short to a long stay after admission to a hospital; and (3) the community group, consisting of the remainder of the population. An individual in any group could take 1 of 3 states: either colonized with a resistant (ESBL-producing), potentially-pathogenic E. coli strain (CR: colonized resistant); colonized with a susceptible (non-ESBL producing), potentially-pathogenic E. coli strain (CS); or not colonized with an E. coli strain with pathogenic potential (NC) but potentially colonized with a commensal strain. This strain structure reflects the notion that highly-transmissible and -pathogenic lineages of E. coli can compete with other potentially-pathogenic strains of E. coli to occupy a particular niche [15]. Patients entered the hospital (for a short stay) with rate ɑ and transitioned from a short stay to a long stay with rate ɑls. Patients transitioned back from a long to a short stay, according to an expected long length of stay (and rate δl), and were similarly discharged from a short stay to the community, according to an expected length of admission (and discharge rate δs). Mortality could occur in any individual, with differing rates between the hospital (μh) and community (μc). “Birth” rates (b) were assumed to be equal to the death rate, where births only occurred in the community group in those not receiving antibiotics. The numbers of colonized (CR and CS) and non-colonized (NC) individuals that entered (were “born” into) the community (not receiving antibiotics) group were proportional to the existing distribution within this group, reflecting the maternal-fetal transmission of flora. We also assumed that each compartment could be divided into 2 additional substates: receiving antibiotics or not receiving antibiotics. We assumed that when a patient is receiving antibiotics, they could not be colonized by a susceptible strain. We included 2 terms to incorporate selection due to antibiotic use [12]: (1) a term governing the increased rate of decolonization (when receiving antibiotics) in those individuals colonized with a susceptible organism (CS), called Ga; and (2) a term that reflected enhanced shedding effects (when receiving antibiotics) [12, 16] in those individuals colonized with a resistant organism (CR), called GS, which would lead to enhanced transmission from this group. We also included a fitness cost of resistance, whereby the loss of colonization with the resistant organism is faster than with the susceptible organism by a factor (Ft), regardless of whether the host was receiving antibiotics. Lastly, given the uncertainty over the true transmission model structure in Gram-negative pathogens, we allowed for the direct replacement of 1 C strain with the other, which operated under an attenuated transmission parameter (modified by parameter P) and was subject to shedding effects (Gs). Further model assumptions are outlined in the Supplementary Materials. Parameter estimates are summarized in Table 1, and descriptions of selection are provided in the Supplementary Materials.
Table 1.
Model Parameter Values
| Parameter | Value | Range | Units |
|---|---|---|---|
| Hospital mortality rate (μh) | 0.924 | … | year-1 |
| Community mortality rate (μc) | 0.0077 | … | year-1 |
| Birth rate (b) | a | … | year-1 |
| Hospital admission rate (α)b | 0.15 | … | year-1 |
| Long-stay admission rate (αls)b | 3 | … | year-1 |
| Short length-of-stay discharge rate (δS) | 81 | … | year-1 |
| Long length-of-stay-discharge rate (δl) | 14.6 | … | year-1 |
| Hospital loss-of-colonization rate (Ch) | … | 1.5–3.7 | year-1 |
| Community loss-of-colonization rate (Cc) | 3.7 | … | year-1 |
| Community transmission parameter (Bc) | … | 4–8 | year-1 |
| Hospital transmission parameter (BH) | … | 8–16 | year-1 |
| Rate of starting antibiotics in hospital (AH) | c | … | year-1 |
| Rate of starting antibiotics in community (AC) | c | … | year-1 |
| Rate of discontinuing antibiotics (d) | 52 | … | year-1 |
| Proportion of transmission through direct route (P) | … | 0–0.75 | … |
| Selection effects: acquisition scaling parameter (Ga) | … | 1–4 | … |
| Selection effects: shedding scaling parameter (Gs) | … | 1–4 | … |
| Fitness cost (Ft) | … | 1–1.025 | … |
Ranges reflect the upper and lower bounds of parameter estimates used during model fitting.
aQuasi-steady state assumption: see equations in Supplementary Material.
bCalibrated to achieve ratio of short-to-long length of stay (5–6:1) and outpatient to inpatient (450–550:1).
cVarying by year.
Initial Conditions
The proportion of individuals colonized with ESBL-producing E. coli in the community is highly variable (range 0–70%) [4]. We chose initial prevalence values for both the community and hospital settings, corresponding to those from Sweden for 2001 [4, 17]. We assumed a roughly equal proportion of colonized, non–ESBL-producing E. coli (CS) and NC individuals as the baseline.
Evaluating Relative Effects of Antimicrobial Use Across Parameter Ranges
For the purpose of this paper, the dynamic model was populated with point estimates of specific parameters (Table 1) and initial conditions for ESBL-producing Escherichia coli. Parameters with the greatest uncertainty were allowed to vary within ranges, as shown in Table 1. These included the hospital and community transmission parameters, acquisition and shedding selection parameters, fitness, proportion of direct pathway, and duration of colonization in hospital. All modeling was performed in R (version 1.1.383, Vienna, Austria), and all ordinary differential equations were solved numerically, based on initial conditions and using the lsoda function in the R package deSolve [18]. We generated effect ratios of ESBL-producing E. coli colonization in order to illustrate the relative impacts of antimicrobial use in community and hospital settings. We calculated the effect ratio for a particular setting (community or hospital) as the change in prevalence (at the end of the observed data time period) in that setting for a relative (or absolute) change in antimicrobial use in the community, divided by the change in prevalence in that setting for a relative (or absolute) change in antimicrobial use in the hospital. An effect ratio value greater than 1 indicates that, for the particular setting, a change in antibiotic use of the specified relative amount in the community had a greater effect on prevalence than a change in antibiotic use of the specified relative amount in the hospital setting. An effect ratio value less than 1 indicates that, for the particular setting, a change in antibiotic use of the specified relative amount in the hospital had a greater effect on prevalence than a change in antibiotic use of the specified relative amount in the community setting.
Model Fitting Approach
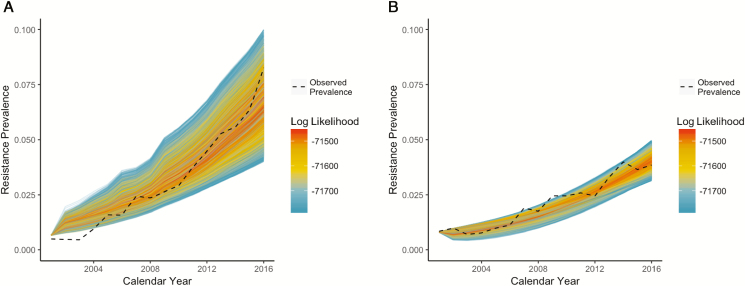
We fit the model to observed antibiotic resistance prevalence data for hospital and community settings within Sweden from 2001–2016, using a Bayesian melding-like approach [19]. Good model fits for both inpatient and outpatient settings were achieved for a variety of parameter sets, across a range of plausible parameter values (Figure 1; Supplemental Figure 5). Full details of the model fitting process are outlined in the Supplementary Materials.
Figure 1.
Model simulations for the 5% of runs with the highest log likelihood, with color gradients proportional to the log likelihood, for (A) hospital and (B) community antibiotic resistance prevalence. The dashed black lines indicate observed data from Sweden.
RESULTS
We performed 200000 realizations of the model (Figure 1; Supplementary Figures 3 and 4) over a 16-year period, with each realization corresponding to a unique set of model parameters. Using the best-fitting parameter sets from these realizations, we explored the potential impact of changes in antibiotic use on the ESBL-producing E. coli prevalence in hospital and community settings. We found that the hospital prevalence of ESBL-producing E. coli was generally greater than the community prevalence (Figure 1). Moreover, across different scenarios of relatively large changes in antibiotic use (up to 25%) for the 16-year period, corresponding magnitudes of absolute change in ESBL-producing E. coli prevalence were modest, ranging from less than 0.2% up to 3.1%.
Changes in ESBL-producing Escherichia coli Prevalence
For the 16-year period evaluated, using a likelihood-weighted average output of the best-fitting parameter sets (likelihood weights >0.001), a 10% and 25% relative reduction in both community and hospital antimicrobial use resulted in absolute reductions in ESBL-producing E. coli prevalences of 0.8%/1.4% (community/hospital) and 1.7%/3.1% (community/hospital), respectively.
For a 10% and 25% relative reduction in hospital antibiotic use, while maintaining baseline community antimicrobial use, we found absolute reductions in ESBL-producing E. coli prevalences of 0.2%/0.7% (community/hospital) and 0.5%/1.6% (community/hospital), respectively.
For a 10% and 25% reduction in relative community antibiotic use, while maintaining baseline hospital antimicrobial use, there were absolute reductions in ESBL-producing E. coli prevalences of 0.5%/0.8% (community/hospital) and 1.2% /1.8% (community/hospital), respectively.
Increases in baseline antimicrobial use in the community had a greater impact on the prevalences of ESBL-producing E. coli (in both the hospital and community) than similar relative increases in hospital antibiotic use (Figure 2). Increases in hospital antibiotic use resulted in attenuated increases in the community ESBL-producing E. coli prevalence, whereas increases in community antibiotic use amplified increases in the hospital ESBL-producing E. coli prevalence (Figure 2).
Figure 2.
The likelihood-weighted model output under different relative changes in antibiotic prescription rate. Only parameter sets with likelihood weights greater than 0.001 contributed to the output. Simulated (A) community and (B) hospital prevalences of ESBL-producing Escherichia coli correspond to relative changes in both community and antibiotic use. Simulated community prevalence of ESBL-producing E. coli, corresponding to relative changes in (C) only hospital and (E) only community antimicrobial use. Simulated hospital prevalence of ESBL-producing E. coli, corresponding to relative changes in (D) only hospital and (F) only community antimicrobial use.
Effect Ratios for Comparison of Setting Specific Changes in Antibiotic Use
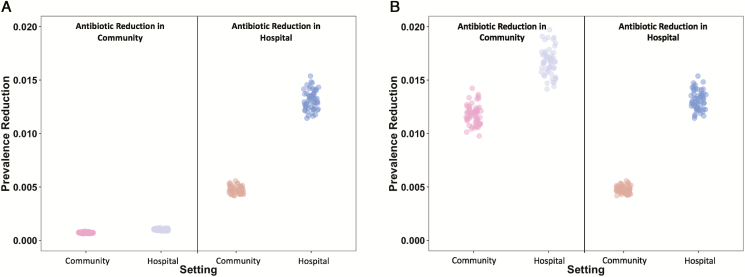
A change in community antibiotic use had greater impacts on hospital and community ESBL-producing E. coli prevalences than similar relative changes in hospital use (effect ratios >1; Figures 3 and 4). However, on a per prescription level, changes in hospital antibiotic use had greater reductions in both hospital and community prevalences (effect ratios <1; Figures 3 and 4).
Figure 3.
Effect ratios for all parameter sets with likelihood weights greater than 0.001. Effect ratios greater than 1 favor antibiotic reductions in the community for a given setting, and those less than 1 favor reductions in hospital antibiotic use for a given setting. (A) Equivalent absolute change in antibiotic use (corresponding to a 20% change in hospital antibiotic use). (B) Equivalent relative 20% change in antibiotic use.
Figure 4.
Prevalence reductions for all parameter sets with likelihood weights greater than 0.001. (A) Equivalent absolute reduction in antibiotic use (corresponding to a 20% change in hospital antibiotic use). (B) Equivalent relative 20% reduction in antibiotic use.
DISCUSSION
For the models described in this paper, community antibiotic use is the dominant factor governing the prevalence of ESBL-producing E. coli colonization of individuals in both community and hospital environments. These findings reflect the flux of patients to and from hospitals, coupled with long durations of colonization, such that many patients acquire resistant, colonizing strains in the community, and then carry them on admission into a hospital [20]. Hospital antibiotic use thereafter modulates the hospital prevalence of resistant colonizers on this background of immigration from the community. These results are clinically relevant, because the prevalence of resistant colonizers is believed to be related to the incidence of resistant, hospital-associated infections [21], where the enteric flora is a source for hospital-associated/-acquired pathogens.
From the epidemic curves (Figures 1 and 2), we see that prevalence increases gradually, over long periods of time, and this fits with the gradual increases in ESBL prevalence seen in many countries [4]. But we also see that prevalence can ultimately reach high levels, depending upon the underlying parameters and the extent of antimicrobial use, and this is also in keeping with the reported high prevalence of resistance in certain regions [4]. This could reflect differences in factors such as transmission parameters, duration of colonization, and antibiotic use between countries. Few investigators have identified significant fitness costs associated with the production of ESBLs [22, 23] and, as a result, even substantial reductions in antimicrobial use may have limited impacts on the longterm prevalence of resistance.
The findings of this model are relevant in a number of ways to current practices of antibiotic use and misuse, as well as current approaches to measuring the impacts of hospital and community stewardship programs. Firstly, we see that community-directed approaches, as have been highlighted in the literature [14, 24], are predicted to have the greatest impact on not only community-associated ESBL-producing E. coli infections, but also hospital-associated infections. Moreover, our findings can explain the inconsistent impacts of hospital-based antimicrobial stewardship interventions on hospital-wide resistance outcomes [25], given the relatively small effect sizes over large spans of time for relatively large reductions in antibiotic use. However, it should be noted that, as a result of the smaller numbers of antibiotic prescriptions in the hospital relative to the community as a whole, on a per prescription basis the greatest impact is found by reducing antibiotic use in the hospital, and this likely relates to the high transmission rates in that setting. This supports the importance of ongoing/existing hospital-based stewardship programs [26], and illustrates that maximum value will likely be obtained with interventions/studies that operate across scales [13], with combined stewardship in the community and the hospital.
Although this study was designed to evaluate ESBL-producing E. coli, these results may be generally applicable to other Gram-negatives and antibiotics in which the predominant mechanism of acquiring a resistant infection is through transmission. When de novo resistance formation is possible, along with patients infected with organisms whose origin is more likely from the hospital environment (eg, Pseudomonas spp. or Acinetobacter spp.), this model would likely underestimate the impact of changes in hospital antibiotic use.
These models have a number of limitations. Firstly, we have only explored the heterogeneity of hospital length of stay by modeling 2 lengths-of-stay categories. It is possible a more complicated length-of-stay distribution pattern may give differing outcomes, likely accentuating the effect of antibiotic selection pressure in those patients with extreme lengths of stay. Furthermore, we did not consider other settings within the continuum of care that may be relevant to resistance, such as long-term care facilities. Our model also does not explicitly consider transmission between/from health-care personnel; however, if part of the patient-to-patient spread in the hospital is mediated by health-care personnel/equipment, our mass action–based approach is a reasonable approximation. While we have not directly incorporated the environment or food as sources of transmission, these routes have not been consistently demonstrated as major contributors to resistant E. coli transmission [27, 28] and, as such, have not been included in our model. Lastly, we did not model the possibility of horizontal gene transfer across organisms; however, this is not the predominant means of antibiotic resistance transmission [29].
In summary, our model findings support the ongoing use of hospital-based ASPs, but highlight the potential, significant impact of community-based stewardship interventions on resistance prevalence in both community and hospital settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Sonja Löfmark, Erika Olsson, and Hanna Billström from the Public Health Agency of Sweden for their support in collecting antimicrobial use and antimicrobial resistance prevalence data.
Financial support. This work was supported by the Cooperative agreement U01 CK000538-01 from the Centers for Disease Control and Prevention and grant number U54GM088558 from the National Institute Of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of General Medical Sciences or the National Institutes of Health.
Potential conflicts of interest. D. R. M. is supported by a Canadian Institutes of Health Research Fellowship Award. M. L. reports grants from the National Institutes of Health/National Institutes of General Medical Sciences during the conduct of the study; personal fees from Affinivax, Merck, and Antigen Discovery; grants and personal fees from Pfizer; and grants from PATH Vaccine Solutions, outside the submitted work. W. P. H. reports personal fees from F. Hoffmann-La Roche Ltd, outside the submitted work. D. N. F. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012; 33:322–7. [DOI] [PubMed] [Google Scholar]
- 2. Karanika S, Paudel S, Grigoras C, Kalbasi A, Mylonakis E. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother 2016; 60:4840–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melzer M, Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Infect 2007; 55:254–9. [DOI] [PubMed] [Google Scholar]
- 4. Woerther PL, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended-spectrum β-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev 2013; 26:744–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haverkate MR, Platteel TN, Fluit AC, et al. Quantifying within-household transmission of extended-spectrum β-lactamase-producing bacteria. Clin Microbiol Infect 2017; 23:46.e1–7. [DOI] [PubMed] [Google Scholar]
- 6. Hilty M, Betsch BY, Bögli-Stuber K, et al. Transmission dynamics of extended-spectrum β-lactamase-producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clin Infect Dis 2012; 55:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Juusola JL, Brandeau ML, Owens DK, Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med 2012; 156:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Echevarria D, Gutfraind A, Boodram B, et al. Mathematical modeling of hepatitis C prevalence reduction with antiviral treatment scale-up in persons who inject drugs in metropolitan Chicago. PLOS One 2015; 10:e0135901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cobey S, Baskerville EB, Colijn C, Hanage W, Fraser C, Lipsitch M. Host population structure and treatment frequency maintain balancing selection on drug resistance. J R Soc Interface 2017; 14:1–9. Available at: http://dx.doi.org/10.1098/rsif.2017.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang Q, Lipsitch M, Hanage WP. Impact of host heterogeneity on the efficacy of interventions to reduce Staphylococcus aureus carriage. Infect Control Hosp Epidemiol 2016; 37:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hughes J, Huo X, Falk L, et al. Benefits and unintended consequences of antimicrobial de-escalation: Implications for stewardship programs. PLOS One 2017; 12:e0171218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spicknall IH, Foxman B, Marrs CF, Eisenberg JN. A modeling framework for the evolution and spread of antibiotic resistance: literature review and model categorization. Am J Epidemiol 2013; 178:508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGregor JC, Furuno JP. Optimizing research methods used for the evaluation of antimicrobial stewardship programs. Clin Infect Dis 2014; 59(Suppl 3):S185–92. [DOI] [PubMed] [Google Scholar]
- 14. Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA 2013; 309:2345–52. [DOI] [PubMed] [Google Scholar]
- 15. Riley LW. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin Microbiol Infect 2014; 20:380–90. [DOI] [PubMed] [Google Scholar]
- 16. Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000; 343:1925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis 2016; 63:310–8. [DOI] [PubMed] [Google Scholar]
- 18. Petzold L, Hindmarsh A LSODA, Ordinary Differential Equation Solver for Stiff or Non-Stiff System. (Sep 2005). Available at: http://www.nea.fr/abs/html/uscd1227.html
- 19. McCormick AW, Abuelezam NN, Rhode ER, et al. Development, calibration and performance of an HIV transmission model incorporating natural history and behavioral patterns: application in South Africa. PLOS One 2014; 9:e98272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doi Y, Park YS, Rivera JI, et al. Community-associated extended-spectrum β-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis 2013; 56:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodríguez-Baño J, López-Cerero L, Navarro MD, Díaz de Alba P, Pascual A. Faecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence, risk factors and molecular epidemiology. J Antimicrob Chemother 2008; 62:1142–9. [DOI] [PubMed] [Google Scholar]
- 22. Schaufler K, Semmler T, Pickard DJ, et al. Carriage of extended-spectrum beta-lactamase-plasmids does not reduce fitness but enhances virulence in some strains of pandemic E. coli lineages. Front Microbiol 2016; 7:1–12. Available at: http://dx.doi.org/10.3389/fmicb.2016.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cottell JL, Webber MA, Piddock LJ. Persistence of transferable extended-spectrum-β-lactamase resistance in the absence of antibiotic pressure. Antimicrob Agents Chemother 2012; 56:4703–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA 2016; 315:562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:990–1001. [DOI] [PubMed] [Google Scholar]
- 26. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vento TJ, Cole DW, Mende K, et al. Multidrug-resistant gram-negative bacteria colonization of healthy US military personnel in the US and Afghanistan. BMC Infect Dis 2013; 13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Börjesson S, Ny S, Egervärn M, et al. Limited dissemination of extended-spectrum β-lactamase- and plasmid-encoded AmpC-producing Escherichia coli from food and farm animals, Sweden. Emerg Infect Dis 2016; 22:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haverkate MR, Dautzenberg MJ, Ossewaarde TJ, et al. Within-host and population transmission of blaOXA-48 in K. pneumoniae and E. coli. PLOS One 2015; 10:e0140960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep 2016; 64:1–119. [PubMed] [Google Scholar]
- 31. Trends in Inpatient Hospital Deaths: National Hospital Discharge Survey, 2000–2010 Available at: http://www.cdc.gov/nchs/products/databriefs/db118.htm. Accessed 9 April 2018. [PubMed]
- 32. Swedres-Svarm reports - SVA Available at: http://www.sva.se/en/antibiotics/svarm-reports. Accessed 8 July 2018.
- 33. Zahar JR, Lanternier F, Mechai F, et al. Duration of colonisation by Enterobacteriaceae producing extended-spectrum beta-lactamase and risk factors for persistent faecal carriage. J Hosp Infect 2010; 75:76–8. [DOI] [PubMed] [Google Scholar]
- 34. Feldman N, Adler A, Molshatzki N, et al. Gastrointestinal colonization by KPC-producing Klebsiella pneumoniae following hospital discharge: duration of carriage and risk factors for persistent carriage. Clin Microbiol Infect 2013; 19:E190–6. [DOI] [PubMed] [Google Scholar]
- 35. Apisarnthanarak A, Bailey TC, Fraser VJ. Duration of stool colonization in patients infected with extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin Infect Dis 2008; 46:1322–3. [DOI] [PubMed] [Google Scholar]
- 36. Hilty M, Betsch BY, Bögli-Stuber K, et al. Transmission dynamics of extended-spectrum β-lactamase-producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clin Infect Dis 2012; 55:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harris AD, Perencevich EN, Johnson JK, et al. Patient-to-patient transmission is important in extended-spectrum beta-lactamase-producing Klebsiella pneumoniae acquisition. Clin Infect Dis 2007; 45:1347–50. [DOI] [PubMed] [Google Scholar]
- 38. Haverkate MR, Platteel TN, Fluit AC, et al. Quantifying within-household transmission of extended-spectrum β-lactamase-producing bacteria. Clin Microbiol Infect 2017; 23:46.e1–e7. [DOI] [PubMed] [Google Scholar]
- 39. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012: Statistical Brief #180. In: Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, Maryland: Agency for Healthcare Research and Quality (US), 2014:1–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.