Abstract
Aim
To investigate treatment satisfaction with semaglutide, a once‐weekly glucagon‐like peptide‐1 receptor agonist, versus placebo/active comparators in the SUSTAIN clinical trial programme.
Methods
In SUSTAIN 2–5 and 7, the Diabetes Treatment Satisfaction Questionnaire was used to evaluate patient‐perceived treatment satisfaction, hyperglycaemia and hypoglycaemia. Post hoc subgroup analyses were conducted to explore the effects of gastrointestinal adverse events (GI AEs), weight loss (≥5%) or achieving glycaemic (HbA1c < 7%) targets on treatment satisfaction.
Results
Overall treatment satisfaction increased from baseline to end of treatment with all treatments across trials. Improvements were significantly greater with semaglutide versus comparators/placebo in SUSTAIN 2–5 (all P < 0.05), and generally greater in patients who achieved versus did not achieve weight loss and glycaemic targets, often with greater improvements with semaglutide 1.0 mg versus comparator/placebo in both weight loss groups. In SUSTAIN 7, improvements in overall treatment satisfaction were generally similar between semaglutide and dulaglutide, irrespective of weight loss or glycaemic control. In SUSTAIN 7, changes in overall treatment satisfaction score were generally lower in patients with versus without GI AEs at week 16 (except dulaglutide 0.75 mg), but similar by week 40. Perceived hyperglycaemia was significantly reduced from baseline to end of treatment with semaglutide versus all comparators/placebo (all P < 0.05). No differences between treatments were observed for perceived hypoglycaemia.
Conclusions
Semaglutide was associated with significantly greater (SUSTAIN 2–5) or similar (SUSTAIN 7) improvements in overall treatment satisfaction versus comparators/placebo. Improvements in overall treatment satisfaction were generally greater in patients achieving versus not achieving treatment targets.
Clinicaltrials.gov: NCT01930188 (SUSTAIN 2), NCT01885208 (SUSTAIN 3), NCT02128932 (SUSTAIN 4), NCT02305381 (SUSTAIN 5) and NCT02648204 (SUSTAIN 7).
EudraCT: 2012–004827‐19 (SUSTAIN 2), 2012–004826‐92 (SUSTAIN 3), 2013–004392‐12 (SUSTAIN 4), 2013–004502‐26 (SUSTAIN 5) and 2014–005375‐91 (SUSTAIN 7).
Keywords: glucagon‐like peptide‐1 analogue, hypoglycaemia, incretin therapy, type 2 diabetes, weight control
1. INTRODUCTION
Despite the availability of effective glucose‐lowering agents, many patients with type 2 diabetes (T2D) do not achieve glycaemic targets.1, 2, 3 Adherence to and persistence with treatment regimens are important factors for treatment effectiveness.4 Higher treatment satisfaction has been associated with better treatment adherence and improved persistence.5
Ongoing day‐to‐day management of T2D lies with the patient and their caregivers, and treatment guidelines are moving towards an individualized approach to improve treatment satisfaction and adherence.6, 7, 8 Surveys have shown that key determinants of treatment preference among patients with T2D include route of administration (e.g. oral vs. injectable), convenience and low frequency of administration, efficacy (e.g. achievement of glycaemic control) and safety [e.g. adverse events (AEs) including weight gain, gastrointestinal (GI) effects and hypoglycaemia].9, 10, 11, 12 In addition to biomarkers and clinical data, patient‐reported outcomes (PROs) can provide insights into risks and benefits of T2D treatments.9 Consequently, PROs are frequently assessed in T2D trials, including measures such as treatment satisfaction, quality of life, well‐being, health status and impact of weight changes.9
Standardized instruments have been developed for the reliable assessment of PROs in patients with T2D.9 The Diabetes Treatment Satisfaction Questionnaire (DTSQ) is designed to assess patient satisfaction during treatment.13, 14 It is recommended by the World Health Organization and the International Diabetes Federation to evaluate the impact of new treatments on psychological well‐being and patient satisfaction.15 The DTSQ is one of the most widely used PRO tools in phase 3 trials of glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) and novel insulins;9 being disease‐specific, it allows comparisons between different groups of patients with diabetes.13, 15 DTSQ‐measured treatment satisfaction has been shown to be independently associated with factors related to adherence.16
Semaglutide is a GLP‐1RA approved for once‐weekly treatment of T2D.17 The phase 3 clinical trial programme, Semaglutide Unabated Sustainability in Treatment of type 2 diabetes (SUSTAIN), investigated subcutaneous semaglutide once weekly versus placebo or active comparators [sitagliptin, exenatide extended release (ER), insulin glargine (IGlar) and dulaglutide] across the continuum of care in >9000 patients with T2D. Across SUSTAIN, semaglutide consistently provided superior HbA1c and body weight reductions versus comparators.18, 19, 20, 21, 22, 23, 24 The DTSQ (status version) was used to assess PROs in all trials except SUSTAIN 1, in which semaglutide monotherapy was compared with placebo, and SUSTAIN 6, a cardiovascular and long‐term outcomes trial (also vs. placebo).18, 23
The aim of this analysis was to further investigate patient‐perceived treatment satisfaction with semaglutide versus comparators or placebo in the SUSTAIN trials utilizing the DTSQ (SUSTAIN 2–5 and 7) and to explore the effect of specific treatment outcomes, such as GI AEs, weight loss (WL ≥ 5%) or glycaemic (HbA1c < 7%) targets, on treatment satisfaction.
2. MATERIALS AND METHODS
2.1. Trial design
The trial designs of SUSTAIN 2–5 and 7 have been reported previously (Table S1).19, 20, 21, 22, 24 Patients with T2D were randomized to receive semaglutide 0.5 mg, semaglutide 1.0 mg (except SUSTAIN 3, semaglutide 1.0 mg only) or comparator/placebo [sitagliptin 100 mg for 56 week (SUSTAIN 2); exenatide ER 2.0 mg for 56 week (SUSTAIN 3); IGlar for 30 week (SUSTAIN 4); placebo for 30 week (SUSTAIN 5); dulaglutide 0.75 mg or 1.5 mg for 40 week (SUSTAIN 7)].19, 20, 21, 22, 24
In all SUSTAIN trials, semaglutide‐treated patients followed a fixed dose‐escalation to minimize GI AEs. A maintenance dose of semaglutide 0.5 mg was reached after 4 week of semaglutide 0.25 mg. A maintenance dose of semaglutide 1.0 mg was reached after 4 weeks of semaglutide 0.25 mg, followed by 4 week of semaglutide 0.5 mg.19, 20, 21, 22, 24
Trial medication was added on to oral glucose‐lowering therapy (SUSTAIN 2–4 and 7) and basal insulin (SUSTAIN 5; Table S1). Background therapy remained unchanged, unless the criteria for rescue treatment were met.19, 20, 21, 22, 24
2.2. Patient population
Key patient inclusion criteria were similar across SUSTAIN 2–5 and 7; age ≥ 18 years and inadequately controlled T2D (HbA1c 7.0–10.0%, SUSTAIN 4 and 5 and 7.0–10.5%, SUSTAIN 2, 3 and 7).19, 20, 21, 22, 24 Key exclusion criteria included: estimated glomerular filtration rate <60 mL/min/1.73 m2 (SUSTAIN 2, 3 and 7) or <30 mL/min/1.73 m2 (SUSTAIN 4 and 5); acute coronary or cerebrovascular event <90 days before randomization (not specified in SUSTAIN 7); or severe chronic heart failure (New York Heart Association class IV).19, 20, 21, 22, 24
Trials were conducted in compliance with the International Conference on Harmonization Good Clinical Practice guidelines25, 26 and the Declaration of Helsinki.27 The protocols were approved by local ethics committees and institutional review boards.
2.3. Study endpoints and DTSQ assessments
Prespecified study endpoints were similar across the trials.19, 20, 21, 22, 24 The primary endpoint was change in HbA1c from baseline to end of treatment. Secondary endpoints included change in body weight from baseline to end of treatment and other efficacy, safety and tolerability variables.
The DTSQ status version (DTSQs) comprises six questions to assess patient‐perceived treatment satisfaction; each question is scored on a scale of 0 to 6, with higher scores indicating better treatment satisfaction. Scores are added to provide an overall treatment satisfaction score ranging from 0 to 36. Two additional questions to assess perceived hyperglycaemia and hypoglycaemia are treated separately, with lower scores indicating a perception of blood glucose levels that are closer to ideal (Table S2).13, 15
In all trials, the DTSQs was completed by patients at randomization and at end of treatment (either planned or at premature discontinuation), preferably before any other trial‐related activities.
In SUSTAIN 7, DTSQs assessments were also completed at week 16 (corresponding to weeks 12 and 8 after dose‐escalation in the 0.5 mg and 1.0 mg semaglutide treatment groups, respectively; there was no dose escalation with dulaglutide).
2.4. Statistical analyses
Responses to DTSQs questions were analyzed based on the full analysis set from the observation period, including all patients on treatment without the addition of rescue medication who had completed the DTSQ assessment at the end of treatment. The observation period was prespecified and implemented to avoid potential interference because of the initiation of rescue medication.
In SUSTAIN 2–5, postbaseline DTSQ responses were analyzed using an analysis of covariance model, with treatment and country as fixed factors and baseline value as covariate. In addition, SUSTAIN 4 had stratum [pretrial oral glucose‐lowering drug at screening (metformin, or metformin and sulphonylurea)] as a fixed factor, and SUSTAIN 5 had stratification variables [HbA1c level at screening (≤8% or >8%) and use of metformin (yes or no)] as fixed factors. Mean estimates were adjusted according to observed baseline distributions.
In SUSTAIN 7, postbaseline DTSQ responses were analyzed using a mixed model for repeated measurements, with treatment and country as fixed factors and baseline value as covariate, all nested within visit. As prespecified in SUSTAIN 7, semaglutide 0.5 mg was compared with dulaglutide 0.75 mg, and semaglutide 1.0 mg was compared with dulaglutide 1.5 mg.
From these models, the adjusted estimated treatment differences between semaglutide and comparators/placebo were calculated, and presented together with an associated two‐sided 95% confidence interval and unadjusted two‐sided P‐value.
To determine whether changes in treatment satisfaction could be considered to constitute meaningful improvements, a distribution‐based approach with minimally important changes (MICs) was used. MICs have not been defined for the DTSQ; however, they have been previously evaluated for DTSQ using a distribution‐based approach.28 MICs were calculated as 0.5 × standard deviation of the DTSQ score at baseline,28, 29 for both overall treatment satisfaction and individual questions per trial. MICs are applicable to the evaluation of absolute changes from baseline in DTSQ scores, but not for differences between treatments. Baseline standard deviation was calculated from all non‐missing items for the full analysis set. Changes from baseline in DTSQ (individual questions and overall treatment satisfaction) were compared with the MICs, and any changes from baseline greater or equal to the MICs were considered meaningful.
In post hoc analyses, patients in each trial were divided into subgroups based on the presence or absence of GI AEs (nausea, diarrhoea, vomiting, dyspepsia and gastroesophageal reflux), achieving or not achieving WL ≥ 5%, and achieving or not achieving HbA1c < 7%. For subgroup analyses, postbaseline responses were analyzed using the same approach as overall treatment satisfaction, with the addition of the analyzed subgroup event [GI AE (yes/no), achieved WL ≥ 5% (yes/no), achieved HbA1c < 7% (yes/no)] interacting with treatment as fixed factors. SUSTAIN 7 included GI AE (yes/no) prior to week 16 as a fixed factor. Mean estimates were adjusted according to observed baseline distribution.
3. RESULTS
3.1. Patient disposition and baseline characteristics
Overall, 4731 patients with T2D were randomized to once‐weekly semaglutide 0.5 mg or 1.0 mg, or comparator/placebo treatment. Of these, 4711 (99.6%) were exposed to investigational product (1204, semaglutide 0.5 mg; 1604, semaglutide 1.0 mg; and 1903, comparator/placebo), and 4435 (93.7%) completed their respective trial.
Approximately 80% of randomized patients on treatment without rescue medication recorded data for DTSQ at baseline and end of treatment (Table S1). Across trials, ~14% of patients discontinued treatment prematurely, for reasons which included pregnancy, protocol violation and AEs, and ~6% withdrew from the trial, were lost to follow‐up, or had missing follow‐up information (Table S1). Among patients randomized to semaglutide, there were no significant differences between DTSQ completers and non‐completers in key baseline characteristics, except with respect to ethnicity: there was a greater proportion of Hispanic/Latino patients in the DTSQ non‐completer group (Table S3).
Overall, baseline characteristics were similar between treatment groups within each trial, while between‐trial differences reflected differences in eligibility criteria: mean baseline age (55.1–58.8 years), HbA1c (8.1–8.4%) and body mass index (32.2–33.8 kg/m2). Diabetes duration ranged from 6.6 to 13.3 years (Table S4).
3.2. Overall treatment satisfaction (combined DTSQ score)
Overall treatment satisfaction increased from baseline to the end of treatment in all treatment arms (Figure 1A). The magnitude of the increase was significantly greater for semaglutide versus comparators/placebo in SUSTAIN 2–5, and similar for semaglutide and dulaglutide in SUSTAIN 7. The change in overall treatment satisfaction was greater with semaglutide 1.0 mg versus semaglutide 0.5 mg, except in SUSTAIN 7, where improvements were the same with both doses.
Figure 1.

(A) Change in overall treatment satisfaction score from baseline to end of treatment and (B) proportion of patients achieving improvements in overall treatment satisfaction (≥MIC) from baseline to end of treatment. *P < 0.05, **P < 0.01, ***P < 0.001 for semaglutide versus comparator. Overall treatment satisfaction as measured by DTSQ scores ranging from 0 to 36, where higher scores indicate better satisfaction. Bars may appear larger or smaller than the number indicated because of rounding to one decimal point. Mean observed values are based on the FAS and LOCF imputed data. For SUSTAIN 2–5, postbaseline data were analyzed using an ANCOVA model with treatment, country and stratum as fixed factors and baseline value as covariate. Mean estimates were adjusted according to observed baseline distribution. For SUSTAIN 7, postbaseline data were analyzed using an MMRM with treatment and country as fixed factors and baseline value as covariate, all nested within visit. MIC is calculated as 0.5×SD of the baseline score28,29 for each individual trial. Baseline SD is calculated from all non‐missing items for the FAS. Values for sitagliptin (SUSTAIN 2) and placebo (SUSTAIN 5) are pooled (mean) values. Abbreviations: ANCOVA, analysis of covariance; DTSQ, Diabetes Treatment Satisfaction Questionnaire; exenatide ER, exenatide extended release; FAS, full analysis set; IGlar, insulin glargine; LOCF, last observation carried forward; MIC, minimally important change; MMRM, mixed model for repeated measurements; SD, standard deviation
The mean change from baseline in overall treatment satisfaction reached or exceeded the calculated MICs in all treatment arms across trials, except for semaglutide 0.5 mg and placebo in SUSTAIN 5 (Figure 1A). Across all trials, a meaningful improvement in overall treatment satisfaction score (i.e. change ≥MIC) was achieved by 37.7–55.8% of patients treated with semaglutide 0.5 mg, 40.5–62.2% with semaglutide 1.0 mg, 34.2% with placebo, and 46.4–52.8% with active comparators (Figure 1B). The proportion of patients with a meaningful increase in treatment satisfaction score was generally numerically higher with semaglutide versus comparators, except in SUSTAIN 7, in which similar proportions of patients on semaglutide 1.0 mg and dulaglutide 1.5 mg had meaningful improvements (49.5% and 50.7%, respectively).
3.3. Individual DTSQ items
Changes in response to the six individual DTSQ questions contributing to overall treatment satisfaction (Table S2) were generally more favourable for semaglutide versus comparators/placebo for SUSTAIN 2–5, and similar for semaglutide and dulaglutide in SUSTAIN 7 (Figure S1).
3.4. Perceived frequency of hyperglycaemia and hypoglycaemia
Scores for the question on perceived hyperglycaemia decreased (indicating improvement) between baseline and the end of treatment in all treatment arms (Figure 2A). These reductions were significantly greater with semaglutide versus active comparators (including dulaglutide) or placebo. By contrast, perceptions of hypoglycaemia did not improve in all treatment arms, and there were no significant differences between semaglutide and any of the active comparators or placebo in the magnitude of change from baseline (Figure 2B).
Figure 2.

Change in (A) perceived hyperglycaemia and (B) hypoglycaemia from baseline to end of treatment. *P < 0.05, **P < 0.01, ***P < 0.001 for semaglutide versus comparator. Patients responded on a scale of 0–6 to the question ‘How often have you felt that your blood sugars have been unacceptably high/low recently?’ where 0 was the fewest times and 6 was the most times. Observed ‘on‐treatment without rescue medication data’. Bars may appear larger or smaller than the number indicated because of rounding to one decimal point. For SUSTAIN 2–5, postbaseline data were analyzed using an ANCOVA model with treatment, country and stratum as fixed factors and baseline value as covariate. Mean estimates were adjusted according to observed baseline distribution. For SUSTAIN 7, postbaseline data were analyzed using an MMRM with treatment and country as fixed factors and baseline value as covariate, all nested within visit. Abbreviations: ANCOVA, analysis of covariance; DTSQ, Diabetes Treatment Satisfaction Questionnaire; exenatide ER, exenatide extended release; IGlar, insulin glargine; MMRM, mixed model for repeated measurements
3.5. Subgroup analysis: the effect of outcomes on overall treatment satisfaction
In post hoc analyses of the impact of GI AEs or treatment efficacy (WL ≥ 5% or attainment of HbA1c < 7%) on overall treatment satisfaction, mean scores increased from baseline to the end of treatment in all subgroups, although the increases were not all statistically significant (Figures 3, 4, 5).
Figure 3.
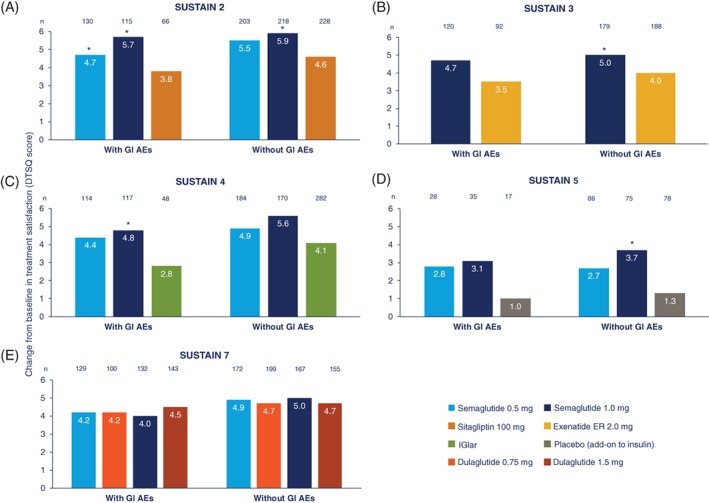
Change in overall treatment satisfaction score from baseline to end of treatment by the presence or absence of GI AEs in (A) SUSTAIN 2, (B) SUSTAIN 3, (C) SUSTAIN 4, (D) SUSTAIN 5 and (E) SUSTAIN 7. *P < 0.05 for semaglutide versus comparator within subgroup; no significant differences were observed in change from baseline with GI AEs and without GI AEs for each treatment arm. Treatment satisfaction as measured by DTSQ scores ranging from 0 to 6, where higher scores indicate better satisfaction. Bars may appear larger or smaller than the number indicated because of rounding to one decimal point. Only trial completers are included in the analysis. Number of patients in each trial are those exposed to at least one dose of trial drug who did not receive rescue medication. For SUSTAIN 2–5, postbaseline data were analyzed using an ANCOVA model with treatment, country and stratum as fixed factors and baseline value as covariate. Mean estimates were adjusted according to observed baseline distribution. For SUSTAIN 7, postbaseline data were analyzed using an MMRM with treatment and country as fixed factors and baseline value as covariate, all nested within visit. Abbreviations: AE, adverse event; ANCOVA, analysis of covariance; DTSQ, Diabetes Treatment Satisfaction Questionnaire; exenatide ER, exenatide extended release; GI, gastrointestinal; IGlar, insulin glargine; MMRM, mixed model for repeated measurements
Figure 4.

Change in overall treatment satisfaction score from baseline to end of treatment in patients who did or did not achieve WL ≥ 5% in (A) SUSTAIN 2, (B) SUSTAIN 3, (C) SUSTAIN 4, (D) SUSTAIN 5 and (E) SUSTAIN 7. *P < 0.05 for semaglutide versus comparator within subgroup; †P < 0.05 for difference in change from baseline between patients who achieved ≥5% WL versus those with WL < 5% for each treatment arm. Treatment satisfaction as measured by DTSQ scores ranging from 0 to 6, where higher scores indicate better satisfaction. Bars may appear larger or smaller than the number indicated because of to rounding to one decimal point. Only trial completers are included in the analysis. Number of patients in each trial are those exposed to at least one dose of the trial drug who did not receive rescue medication. For SUSTAIN 2–5, postbaseline data were analyzed using an ANCOVA model with treatment, country and stratum as fixed factors and baseline value as covariate. Mean estimates were adjusted according to observed baseline distribution. For SUSTAIN 7, postbaseline data were analyzed using an MMRM with treatment and country as fixed factors and baseline value as covariate, all nested within visit. Abbreviations: ANCOVA, analysis of covariance; DTSQ, Diabetes Treatment Satisfaction Questionnaire; exenatide ER, exenatide extended release; IGlar, insulin glargine; MMRM, mixed model for repeated measurements; WL, weight loss
Figure 5.

Change in overall treatment satisfaction from baseline to end of treatment in patients who did or did not achieve HbA1c < 7% in (A) SUSTAIN 2, (B) SUSTAIN 3, (C) SUSTAIN 4, (D) SUSTAIN 5 and (E) SUSTAIN 7. *P < 0.05 for semaglutide versus comparator within subgroup. †P < 0.05 for difference in change from baseline between patients with HbA1c < 7% and HbA1c ≥ 7% for each treatment arm. Treatment satisfaction as measured by DTSQ scores ranging from 0 to 6, where higher scores indicate better satisfaction. Bars may appear larger or smaller than the number indicated because of rounding to one decimal point. Only trial completers are included in the analysis. Number of patients in each trial are those exposed to at least one dose of trial drug who did not receive rescue medication. For SUSTAIN 2–5, postbaseline data were analyzed using an ANCOVA model with treatment, country and stratum as fixed factors and baseline value as covariate. Mean estimates were adjusted according to observed baseline distribution. For SUSTAIN 7, postbaseline data were analyzed using an MMRM with treatment and country as fixed factors and baseline value as covariate, all nested within visit. Abbreviations: ANCOVA, analysis of covariance; DTSQ, Diabetes Treatment Satisfaction Questionnaire; exenatide ER, exenatide extended release; IGlar, insulin glargine; MMRM, mixed model for repeated measurements
3.5.1. GI AEs
In SUSTAIN 2–5, increase in overall treatment satisfaction (DTSQ score) from baseline to end of treatment was similar in both patients with or without GI AEs (Figure 3A–D).
In SUSTAIN 7, the changes in overall treatment satisfaction from baseline to week 16 were significantly lower in patients with versus those without GI AEs for semaglutide (0.5 mg: 2.95 vs. 4.27, P = 0.0322; 1.0 mg: 2.80 vs. 4.03, P = 0.0462) and dulaglutide 1.5 mg (3.56 vs. 4.74, P = 0.0491), but not for dulaglutide 0.75 mg (3.54 vs. 4.62, P = 0.1056). However, by week 40, change from baseline in overall treatment satisfaction was similar for patients with or without GI AEs, in all treatment groups (Figure 3E).
Semaglutide consistently showed greater numerical change from baseline in overall treatment satisfaction score versus comparators/placebo, regardless of the presence or absence of GI AEs (Figure 3A–D). Differences between semaglutide 1.0 mg and comparators/placebo were significant for patients without GI AEs in SUSTAIN 2–5, and for patients with GI AEs in SUSTAIN 2 and 4 (P < 0.05; Figure 3A–D). The majority of other differences between semaglutide and comparator/placebo score were non‐significant. In SUSTAIN 7, changes in score from baseline to end of treatment were similar for semaglutide and dulaglutide, for both the low‐ and high‐dose comparisons (Figure 3E).
3.5.2. Weight loss
In SUSTAIN 2–5, the magnitude of change in overall treatment satisfaction score from baseline to end of treatment was generally greater in patients with WL ≥ 5% versus those with WL < 5% for all treatment arms (Figure 4A–D), but the differences were not statistically significant. In SUSTAIN 7, the change from baseline to end of treatment in overall treatment satisfaction was generally similar in both WL subgroups, except for dulaglutide 1.5 mg, in which the change was significantly greater in patients with WL ≥ 5% versus those with WL < 5% (P < 0.05; Figure 4E).
In SUSTAIN 2–5, changes from baseline in overall treatment satisfaction score were generally greater for semaglutide versus comparator/placebo, in both WL categories. Differences between semaglutide 1.0 mg and comparator were mostly significant (P < 0.05; Figure 4A–D).
In SUSTAIN 7, changes in overall treatment satisfaction score were similar for semaglutide and dulaglutide in both WL categories (Figure 4E).
3.5.3. Glycaemic control
Changes in overall treatment satisfaction score were generally greater in patients with HbA1c < 7% versus those with HbA1c ≥ 7% (Figure 5A–E). However, differences between these groups were significant only for semaglutide 1.0 mg in SUSTAIN 2 and 7, placebo in SUSTAIN 5 and dulaglutide 1.5 mg in SUSTAIN 7 (P < 0.05; Figure 5A,D,E).
There were few significant differences between semaglutide and comparator/placebo in this analysis. In patients with HbA1c < 7%, changes from baseline in overall treatment satisfaction were significantly greater with semaglutide 1.0 mg versus sitagliptin and placebo in SUSTAIN 2 and 5, respectively (P < 0.05; Figure 5A,D), but there were no other significant differences between treatments.
4. DISCUSSION
Efficacy and safety data from clinical trials are key factors for clinicians when considering treatment options for patients with T2D. However, in this era of individualized care, patients' preferences, needs and values should also guide decision‐making.7, 8
Patients will probably adhere more to treatment they are satisfied with,30 so taking their views into consideration may have an important impact in the real‐world setting. Adherence and treatment persistence will probably be lower in a real‐world versus clinical‐trial setting, because patients typically have less contact with their physician and may have more concerns about their treatment, including hypoglycaemia and weight gain.31
The DTSQ was used as it is a validated tool for measuring treatment satisfaction in patients with diabetes.13 Furthermore, the SUSTAIN trials are large, randomized controlled trials with ~80% of the patients participating in PRO assessments. It is possible that the exclusion of patients who did not complete the PRO assessments (20%) may have introduced bias. For example, patients who did not complete the DTSQ because of AEs can be assumed to have had a lower level of treatment satisfaction. Also, there was a greater proportion of Hispanic/Latino patients in the DTSQ non‐completer group than in the completer group, despite the DTSQ being available in both English and Spanish at the US trial sites. Despite a protocol recommendation to complete the DTSQ prior to any trial‐related activity, this may not have been possible for all patients as clinical assessments may sometimes have been prioritized over completion of the DTSQ. In such cases, data from the non‐completers are missing at random and are, therefore, unlikely to introduce bias. The current analysis, which included patients enrolled in SUSTAIN 2–5 and 7, showed that overall treatment satisfaction increased in all treatment arms between baseline and the end of treatment, despite the majority of patients adding an injectable therapy to their oral treatment regimen.19, 20, 21, 22, 24 Furthermore, semaglutide was associated with significantly greater overall treatment satisfaction compared with sitagliptin, exenatide ER, IGlar and placebo added on to basal insulin (SUSTAIN 2–5).
In SUSTAIN 7, an open‐label trial24 in which patients administered treatment with a multi‐use pen device without a hidden needle for semaglutide or a single‐use pen with a hidden needle for dulaglutide, changes in overall treatment satisfaction were similar for both treatments. Previous studies have shown a preference for the dulaglutide device when compared with devices similar to that used with semaglutide.32, 33 However, the current analysis indicated no device preference based on overall treatment satisfaction, as treatments had similar patient‐perceived outcomes. Speculatively, any difference in treatment satisfaction as a result of injection devices may have been offset by the significantly greater glycaemic efficacy and WL reported for semaglutide versus dulaglutide at both low and high doses.24
In a survey comparing exenatide twice daily with liraglutide, the most important drivers of patient preference were efficacy, less frequent dosing, and lower risk of nausea and hypoglycaemia.34 Our post hoc subgroup analyses suggest that glycaemic efficacy and WL may be associated with greater treatment satisfaction. The magnitude of change from baseline in overall treatment satisfaction was greater, although not significantly, in patients who achieved WL ≥ 5% or HbA1c < 7% versus those who did not.
We did not find compelling evidence to support a sustained relationship between the occurrence of GI AEs and treatment satisfaction. In SUSTAIN 7, overall treatment satisfaction was significantly lower at week 16, for both semaglutide and dulaglutide, in patients who had GI AEs compared with those who did not. However, these differences disappeared by week 40, reflecting a transient nature of GI AEs, often of short duration and primarily reported during dose escalation in the weeks following treatment initiation.35 In addition, significantly higher overall treatment satisfaction was reported in patients with HbA1c < 7% versus HbA1c ≥ 7% for semaglutide 1.0 mg and dulaglutide 1.5 mg in SUSTAIN 7. This is in line with the results of previous studies that showed treatment efficacy to be an important driver of overall treatment satisfaction in patients with T2D.10, 11 Differences in treatment satisfaction across subgroups tended to reflect those of the overall population, but did not always reach statistical significance. This may, in part, be attributable to low patient numbers in some subgroups. Notably, there was a consistent and significant difference between semaglutide 1.0 mg and comparator in SUSTAIN 2–5, even in patients who did not achieve WL ≥ 5%. These findings suggest that several factors, including glycaemic control and body weight, are involved in driving treatment satisfaction with semaglutide. Although the achievement of glycaemic targets and of weight‐loss responses appear to be positively associated with treatment satisfaction in the overall SUSTAIN 2–5 and 7 patient population, such findings may vary across different patients. For example, in older patients (aged ≥65 years)—the proportion of whom ranged from 18% in SUSTAIN 2 to 29% in SUSTAIN 5—their vulnerability to hypoglycaemia and age‐related comorbidities may mean that the association between achieving glycaemic targets or weight‐loss responses and treatment satisfaction is different.36, 37 Such subgroup analyses are an area for further investigation.
Changes from baseline in perceived hypoglycaemia were generally low across all treatment arms, with no significant differences between the treatment arms. However, this may reflect the low perception of hypoglycaemia at baseline. It could be speculated that frequent contact with healthcare professionals in the SUSTAIN programme may also have reduced patients' worries around hypoglycaemia. Furthermore, the risk of hypoglycaemia in the general study population was low, and it can be hypothesized that this, in part, resulted from the exclusion of patients with recurrent severe hypoglycaemia and/or hypoglycaemia unawareness from some of the trials (SUSTAIN 4 and 5).
In all of the trials included in this analysis, reductions in HbA1c were significantly greater with semaglutide versus comparators.19, 20, 21, 22, 24 These reductions are reflected in our results by the significant reduction in patient‐perceived hyperglycaemia with semaglutide versus comparators/placebo. These results, and the increase in overall treatment satisfaction observed with semaglutide versus comparators, are consistent with the results of previous studies in which reductions in perceived hyperglycaemia, as measured by DTSQ, were associated with increased treatment satisfaction.38, 39
One limitation of the DTSQ is that the individual questions do not indicate whether changes in overall treatment satisfaction are driven by drug effect, route of administration (oral/injection), or other characteristics specific to the drug class. However, in some SUSTAIN trials (SUSTAIN 2 and 5), a double‐blind, double‐dummy design was used.19 In this setting, patients would not be aware of differences in administration routes between treatment arms. This means that there may be a potential bias in patient‐reported treatment satisfaction in the three SUSTAIN trials (SUSTAIN 3, 4 and 7) in which an open‐label design was necessary, because of the different injection devices used for the trial product in each treatment arm.19 Furthermore, as the DTSQ is patient‐reported, the data should be interpreted with caution. In particular, responses to questions on hypoglycaemia and hyperglycaemia will be influenced by patients' prior experience of glycaemic abnormalities. The greater frequency of clinic visits and contacts with healthcare professionals that patients experience in clinical trials (vs. the intermittent ones that patients have in clinical practice) may, in itself, contribute to improvement in treatment satisfaction. However, the intrinsic design of a randomized parallel‐group clinical trial should ensure that any trial‐related improvements are equally distributed across treatment arms, including the placebo group.40 Cut‐offs for HbA1c and weight loss response were chosen based on clinical relevance and alignment with the primary SUSTAIN trials. While correlation between these outcomes and changes in DTSQ could have also been analyzed, assessing differences between “responders” and “non‐responders” was more relevant in the context of drivers of DTSQ score change from baseline. The rationale is that such subgroup analyses enable us to assess whether those individuals who achieved HbA1c targets (as laid out in guidelines) and weight loss deemed clinically meaningful had greater treatment satisfaction than those who did not.
The clinical significance of changes measured with the DTSQ has been debated. Although there is no predetermined MIC for many PRO tools, including the DTSQ, the use of MICs provides some guidance on DTSQ score changes that an individual would consider meaningful.30 A meaningful increase from baseline in overall treatment satisfaction (≥MIC) was achieved by approximately half of all patients treated with GLP‐1RAs, oral glucose‐lowering drugs or insulin. Although the absolute change from baseline in overall treatment satisfaction was significantly greater with semaglutide versus comparators, the treatment differences were numerically small. To our knowledge, there are currently no MICs or other measures to evaluate clinically meaningful differences in patient‐reported treatment satisfaction or quality of life between treatments. Further research is required to fill the knowledge gap on how treatment differences in DTSQ and other PRO tools translate into clinically meaningful improvements.
In conclusion, the clinical benefits of once‐weekly semaglutide versus comparators/placebo in patients with T2D, as evidenced by the SUSTAIN trial programme, are accompanied by significantly greater (SUSTAIN 2–5) or similar (SUSTAIN 7) improvements in patient‐perceived overall treatment satisfaction measured by DTSQ. Although GI AEs are frequently reported with GLP‐1RA therapy, their presence (or absence) did not significantly diminish long‐term patient‐perceived treatment satisfaction. Treatment satisfaction, in addition to efficacy and safety findings, is an additional factor that should be considered when selecting glucose‐lowering therapy for people with T2D.
CONFLICT OF INTEREST
J.J. reports consultant fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Medtronic and Sanofi; serving on speaker bureau for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Merck; all outside the submitted work. A.L.B. reports personal fees from Novo Nordisk during the conduct of the study. W.H.P. reports serving as a consultant for Novo Nordisk outside the submitted work. R.S. reports personal fees from Novo Nordisk, Eli Lilly and AstraZeneca outside the submitted work. K.U. reports other financial activities with Novo Nordisk and Astra Zeneca outside the submitted work. T.H. has nothing to disclose. J.H.‐B. reports being previously employed by Novo Nordisk. S.T. has nothing to disclose. M.J.D. reports personal fees from Novo Nordisk, Sanofi‐Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca, Janssen, Servier, Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc., and grants from Novo Nordisk, Sanofi‐Aventis, Lilly, Boehringer Ingelheim and Janssen, all outside the submitted work.
AUTHOR CONTRIBUTIONS
All authors contributed to the analysis and interpretation of data, and the writing and critical revision of manuscript at all stages of manuscript development. All authors approved the final version.
Supporting information
Figure S1. Estimated treatment differences for change from baseline to end of treatment in the six individual DTSQ questions contributing to overall treatment satisfaction.
Table S1. Clinical trial design overview and patient disposition in the SUSTAIN 2–5 and 7 trials19, 20, 21, 22, 24.
Table S3. Baseline characteristics by DTSQ completion in the SUSTAIN 2–5 and 7 trials.
Table S4. Baseline characteristics in the SUSTAIN 2–5 and 7 trials19–22,24.
ACKNOWLEDGMENTS
We thank Daniella Pfeifer, PhD (AXON Communications), for medical writing and editorial assistance, who received compensation from Novo Nordisk.
Jendle J, Birkenfeld AL, Polonsky WH, et al. Improved treatment satisfaction in patients with type 2 diabetes treated with once‐weekly semaglutide in the SUSTAIN trials. Diabetes Obes Metab. 2019;21:2315–2326. 10.1111/dom.13816
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13816.
Funding information Novo Nordisk
REFERENCES
- 1. Khunti K, Ceriello A, Cos X, De Block C. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: a meta‐analysis. Diabetes Res Clin Pract. 2018;137:137‐148. [DOI] [PubMed] [Google Scholar]
- 2. Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab. 2017;19:1155‐1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017;8:863‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doggrell SA, Warot S. The association between the measurement of adherence to anti‐diabetes medicine and the HbA1c. Int J Clin Pharmacol. 2014;36:488‐497. [DOI] [PubMed] [Google Scholar]
- 5. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140‐149. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . 1. Improving care and promoting health in populations: standards of medical care in diabetes – 2019. Diabetes Care. 2019;42:S7‐S12. [DOI] [PubMed] [Google Scholar]
- 8. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reaney M, Elash CA, Litcher‐Kelly L. Patient reported outcomes (PROs) used in recent phase 3 trials for type 2 diabetes: a review of concepts assessed by these PROs and factors to consider when choosing a PRO for future trials. Diabetes Res Clin Pract. 2016;116:54‐67. [DOI] [PubMed] [Google Scholar]
- 10. Purnell TS, Joy S, Little E, Bridges JF, Maruthur N. Patient preferences for noninsulin diabetes medications: a systematic review. Diabetes Care. 2014;37:2055‐2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flood EM, Bell KF, de la Cruz MC, Ginchereau‐Sowell FM. Patient preferences for diabetes treatment attributes and drug classes. Curr Med Res Opin. 2017;33:261‐268. [DOI] [PubMed] [Google Scholar]
- 12. Jendle J, Torffvit O, Ridderstrale M, Lammert M, Ericsson A, Bogelund M. Willingness to pay for health improvements associated with anti‐diabetes treatments for people with type 2 diabetes. Curr Med Res Opin. 2010;26:917‐923. [DOI] [PubMed] [Google Scholar]
- 13. Saisho Y. Use of diabetes treatment satisfaction questionnaire in diabetes care: importance of patient‐reported outcomes. Int J Environ Res Public Health. 2018;15:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bradley C. Diabetes treatment satisfaction questionnaire (DTSQ) In: Bradley C, ed. Handbook of Psychology and Diabetes: a Guide to Psychological Measurement in Diabetes Research and Practice. Chur: Harwood Academic Publishers; 1994:111‐132. [Google Scholar]
- 15. Bradley C, Gamsu DS. Guidelines for encouraging psychological well‐being: report of a Working Group of the World Health Organization Regional Office for Europe and International Diabetes Federation European Region St Vincent Declaration Action Programme for Diabetes. Diabet Med. 1994;11:510‐516. [DOI] [PubMed] [Google Scholar]
- 16. Biderman A, Noff E, Harris SB, Friedman N, Levy A. Treatment satisfaction of diabetic patients: what are the contributing factors? Fam Pract. 2009;26:102‐108. [DOI] [PubMed] [Google Scholar]
- 17. Lau J, Bloch P, Schäffer L, et al. Discovery of the once‐weekly glucagon‐like peptide‐1 (GLP‐1) analogue semaglutide. J Med Chem. 2015;58:7370‐7380. [DOI] [PubMed] [Google Scholar]
- 18. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251‐260. [DOI] [PubMed] [Google Scholar]
- 19. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341‐354. [DOI] [PubMed] [Google Scholar]
- 20. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label randomized clinical trial. Diabetes Care. 2018;41:258‐266. [DOI] [PubMed] [Google Scholar]
- 21. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:355‐366. [DOI] [PubMed] [Google Scholar]
- 22. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 24. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275‐286. [DOI] [PubMed] [Google Scholar]
- 25. International Conference on Harmonisation Working Group . ICH harmonised tripartite guideline: guideline for good clinical practice E6 (R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; June 10, 1996; Washington, DC. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed July 1, 2019.
- 26. European Medicines Agency . International Conference on Harmonisation Guideline for Good Clinical Practice E6(R2), 2009. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002874.pdf. Accessed July 1, 2019.
- 27. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 28. Reaney M, Yu M, Lakshmanan M, Pechtner V, van Brunt K. Treatment satisfaction in people with type 2 diabetes mellitus treated with once‐weekly dulaglutide: data from the AWARD‐1 and AWARD‐3 clinical trials. Diabetes Obes Metab. 2015;17:896‐903. [DOI] [PubMed] [Google Scholar]
- 29. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health‐related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582‐592. [DOI] [PubMed] [Google Scholar]
- 30. Davies M, Speight J. Patient‐reported outcomes in trials of incretin‐based therapies in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:882‐892. [DOI] [PubMed] [Google Scholar]
- 31. Marrett E, Stargardt T, Mavros P, Alexander CM. Patient‐reported outcomes in a survey of patients treated with oral antihyperglycaemic medications: associations with hypoglycaemia and weight gain. Diabetes Obes Metab. 2009;11:1138‐1144. [DOI] [PubMed] [Google Scholar]
- 32. Asakura T, Suzuki S, Aranishi T, Cai Z. Comparative usability study of the dulaglutide single‐use pen versus the insulin degludec FlexTouch® among self‐injection‐naive patients with type 2 diabetes mellitus in Japan. Curr Med Res Opin. 2018;34:1117‐1124. [DOI] [PubMed] [Google Scholar]
- 33. Matza LS, Boye KS, Currie BM, et al. Patient perceptions of injection devices used with dulaglutide and liraglutide for treatment of Type 2 diabetes. Curr Med Res Opin. 2018;34:1457‐1464. [DOI] [PubMed] [Google Scholar]
- 34. Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP‐1 products—liraglutide and exenatide—for the treatment of type 2 diabetes. J Med Econ. 2010;13:655‐661. [DOI] [PubMed] [Google Scholar]
- 35. Horowitz M, Aroda VR, Han J, Hardy E, Rayner CK. Upper and/or lower gastrointestinal adverse events with glucagon‐like peptide‐1 receptor agonists: incidences and consequences. Diabetes Obes Metab. 2017;19:672‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8:120‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sinclair AJ, Paolisso G, Castro M, Bourdel‐Marchasson I, Gadsby R, Rodriguez Mañas L. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab. 2011;37:S27‐S38. [DOI] [PubMed] [Google Scholar]
- 38. Nicolucci A, Cucinotta D, Squatrito S, et al. Clinical and socio‐economic correlates of quality of life and treatment satisfaction in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2009;19:45‐53. [DOI] [PubMed] [Google Scholar]
- 39. Boels AM, Vos RC, Hermans TGT, Zuithoff NPA, Müller N, Khunti K, et al; GUIDANCE Study Group. What determines treatment satisfaction of patients with type 2 diabetes on insulin therapy? An observational study in eight European countries. BMJ Open. 2017;7:e016180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spieth PM, Kubasch AS, Penzlin AI, Illigens BM, Barlinn K, Siepmann T. Randomized controlled trials ‐ a matter of design. Neuropsychiatr Dis Treat. 2016;12:1341‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Estimated treatment differences for change from baseline to end of treatment in the six individual DTSQ questions contributing to overall treatment satisfaction.
Table S1. Clinical trial design overview and patient disposition in the SUSTAIN 2–5 and 7 trials19, 20, 21, 22, 24.
Table S3. Baseline characteristics by DTSQ completion in the SUSTAIN 2–5 and 7 trials.
Table S4. Baseline characteristics in the SUSTAIN 2–5 and 7 trials19–22,24.


