Abstract
Objective
To investigate whether early improvement, measured after two electroconvulsive therapy (ECT) sessions, is a good predictor of eventual remission in severely depressed in‐patients receiving ECT.
Method
A prospective cohort study was performed that included 89 major depressive disorder in‐patients treated with bilateral ECT. Sensitivity, specificity, and predictive values were computed for various definitions of early improvement (15%, 20%, 25%, and 30% reduction on the Montgomery Asberg depression rating scale (MADRS) score) after 1 week (i.e. two sessions) of ECT regarding prediction of remission (final MADRS score ≤ 9).
Results
A 15% reduction in MADRS score appeared to be the best definition of early improvement, with modest sensitivity (51%) and relatively good specificity (79%). Kaplan–Meier analysis showed a more than 2‐week shorter time to remission in patients with early improvement compared with patients lacking early improvement.
Conclusion
Early improvement during an ECT course may be assessed after two ECT sessions. Such improvement, defined as a 15% reduction in the MADRS score, is a moderately sensitive predictor for eventual remission in an in‐patient population with severe major depression.
Keywords: electroconvulsive therapy, major depression, early improvement, remission
Significant outcomes.
Early improvement after two ECT sessions is a predictor for final remission
Attainment of remission occurs more than 2 weeks earlier in patients with early remission
Lack of early improvement may contribute to treatment decision‐making in patients receiving ECT.
Limitations.
The sample size of the study is relatively small.
The homogeneity of the sample may limit generalizability of the results to patients with less severe major depression.
The exclusion of patients refusing further ECT sessions may have overestimated the efficacy of ECT.
Introduction
Electroconvulsive therapy (ECT) is an effective acute treatment for patients with severe major depressive disorder in need of rapid response, either with or without medication resistance. In severe major depression, ECT has the highest rates of response and remission of any form of antidepressant treatment, with 70–90% of those treated showing response 1. Some even report clinical improvement after the first ECT session 2.
Following extensive research on the prediction of outcome of ECT in major depression, several clinical predictors for higher efficacy of ECT have been determined. These include the absence of medication resistance 3, 4, psychotic symptoms 4, 5, advanced age 5, and shorter duration of the index episode 4. With regard to polarity of mood disorder, this variable appears to have no influence on the efficacy of ECT. Whether the presence of melancholic features is a predictor for a higher efficacy of ECT remains uncertain 5.
Early improvement as a predictor of the final outcome in the treatment of depression has been investigated. Some studies showed that the largest improvement on ECT may take place during the first weeks of the ECT course 6, 7. Most studies investigating the predictive value of early improvement employed the Hamilton Depression Rating Scale (HAM‐D) 8. Several studies found that the best predictor of eventual response or remission was the reduction in HAM‐D score after 3‐6 ECT sessions 9, 10. More recently, Lin et al. (2019), investigating 104 patients, showed that final response and remission could be predicted by an early improvement of 30% after six ECT sessions, but that early improvement after three sessions had less discriminative value. Another recent study found that a 30% improvement after six ECT sessions appeared to be a good predictor of remission in depressed in‐patients 11. The assessment of early improvement may be clinically relevant, since this may guide decision‐making during the ECT course with regard to electrode placement, dosing, and treatment schedule. However, six ECT sessions might be midway, rather than early, in the ECT course. Therefore, it may not be the optimal time point to measure early improvement.
Aim of the study
The primary aim of the present study was to assess whether early improvement, measured after two electroconvulsive therapy (ECT) sessions, is a good predictor of eventual remission in severely depressed in‐patients receiving ECT. The cutoff will be chosen based on the area under the curve, which represents a balance between sensitivity and specificity. The secondary aim was to analyze differences in time to remission between patients with and without early improvement.
Methods
The PROspective Study on Patients receiving ECT (PROSPECT) is a prospective study of depressed patients treated with ECT at the Department of Psychiatry of the Erasmus Medical Center from January 2006 to date 12. The end date for the present sample was December 2015. Patients are included in the PROSPECT cohort if they meet the DSM‐IV criteria 13 for major depressive disorder (MDD) with or without psychotic features and have a score of ≥17 on the 17‐item Hamilton Rating Scale for Depression (HAM‐D) 8. Diagnoses are based on clinical observations during a routine drug‐free observation period. A diagnosis of mood‐congruent psychotic depression is made only when the patient shows definite mood‐congruent delusions and/or hallucinations. A subset of adult PROSPECT patients (aged ≥ 18 years) was selected for the current analyses. Excluded were all patients with alcohol or drug dependence, a diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, psychotic disorder not otherwise specified, obsessive‐compulsive disorder, dementia, and other neurological disorders. To avoid electrode placement acting as a confounding factor, only patients treated with bitemporal ECT were selected. Although both a recent randomized trial 14 and a recent meta‐analysis found no difference in efficacy between high‐dose unilateral and bitemporal ECT in MDD 15, there might be differences in terms of speed of response. To avoid bias, of those patients receiving more than one ECT course during this period, only the first treatment course was included in the analysis.
The study was conducted in accordance with the latest version of the Declaration of Helsinki, and informed consent of the participants was obtained after the nature of the procedures had been fully explained. Since all data were obtained as part of standard psychiatric care, medical ethical review was not deemed necessary.
ECT protocol
Patients were withdrawn from all psychotropic medication at least 5 days prior to the first ECT treatment, and the majority of patients were maintained medication‐free during the course of ECT. In case of severe agitation, incidental use of haloperidol was allowed, whereas the use of benzodiazepines during ECT was not allowed. All patients were treated with bilateral ECT, administered twice weekly with a brief‐pulse constant current apparatus (Thymatron IV, Somatics, IL, USA). We used a pulse width of 0.5 ms and a pulse amplitude of 900 mA. Seizure threshold, defined as the stimulus dosage that elicited a seizure of at least 25 s according to the cuff method, was determined during the first session with empirical stimulus titration. If the starting stimulus dose failed to elicit a seizure of at least 25 s, stimulus charge was increased according to the titration schedule and the patient was restimulated after 30 s. For the second treatment, the stimulus dosage was set at 1.5 times the seizure threshold. During the course of ECT, stimulus dosage settings were adjusted upward to maintain a seizure duration of at least 25 s as measured with the cuff method. Anesthesia was achieved after premedication with 0.2 mg glycopyrrolate, with intravenous administration of etomidate (0.2 mg/kg), alfentanil (7–10 μg/kg), and succinylcholine (0.5–1.0 mg/kg). During the procedure, patients were ventilated by mask until the resumption of spontaneous respiration. Physiological monitoring included pulse oximetry, non‐invasive blood pressure measurement, electrocardiogram, and electroencephalogram. The number of ECT treatments was determined by clinical observation; a minimum of 10 bilateral treatments was required before evaluation as a non‐responder. ECT was continued until patients were either asymptomatic (i.e. a MADRS score ≤ 9) or had not shown any further improvement as measured by the MADRS over the course of three consecutive treatments (i.e. a MADRS score ≥ MADRS score 2 weeks previously).
Outcome measurement
The Montgomery Asberg Depression Rating Scale (MADRS) was used to evaluate the severity of MDD and was routinely performed 1–3 days prior to the ECT and 1–3 days after treatment termination to evaluate the efficacy of ECT. Since the MADRS 16 is more sensitive to detect small changes over time 17, it was performed weekly, during ECT treatment to evaluate the time to remission. The MADRS was performed by two independent residents, who were not involved in decisions about the duration of the ECT course. To ensure comparable ratings, inter‐rater training sessions took place once a month, aiming at a difference of ≤2 points in total MADRS score. The primary outcome criterion for efficacy is the proportion of patients in remission (dichotomous) as measured by the MADRS. The secondary outcome criterion is time to remission as measured by the MADRS. Remission was defined as a MADRS score of ≤9 at the end of the ECT course. Also, a baseline MADRS score was taken before the start of the treatment. The MADRS scores were used for the present study; percentage improvement after 1 week of treatment was calculated with the MADRS score in week one (i.e. after receiving two ECT sessions) relative to the MADRS score at baseline, resulting in two study groups (early improvement vs. no early improvement).
Statistical analysis
Statistical analyses were performed with spss, version 24.0. Alpha was set at 0.05. Differences with regard to sociodemographic, clinical, and outcome variables between subgroups of patients were tested with t‐tests or an anova test for continuous variables, and Pearson's chi‐square and Fisher's exact test (FET) for categorical variables. To determine the best definition of early improvement after 1 week of treatment, four definitions of early improvement were used (15%, 20%, 25%, and 30% reduction in baseline MADRS score) and analyzed in relation to the following measures: sensitivity (proportion of remitters, who achieved early improvement), specificity (proportion of non‐remitters who did not achieve early improvement), positive predictive value (PPV, proportion of patients with early improvement, who remitted), and negative predictive value (NPV, proportion of patients without early improvement who did not remit). Furthermore, a receiver operating (ROC) analysis was performed. The area under the ROC curve (AUC) was used to assess the accuracy of the analysis to distinguish remitters from non‐remitters. Differences in the rate of remission between the two subgroups were determined using the chi‐square test. Finally, differences in the time to remission between the subgroups were analyzed using the Kaplan–Meier method 18, 19.
Data imputation was done for patients with missing values: for nine patients, the mean MADRS score was calculated for single or multiple missing values, provided that the baseline MADRS was known. In one patient, last observation carried backwards was done because the baseline MADRS score was the only missing value and no other missing data during the subsequent treatment course; we decided to adopt a last observation carried backward imputation method instead of excluding this case.
Results
Patient characteristics
Between 2006 and 2015, 190 patients received ECT; of these, 85 were excluded because they did not meet the inclusion criteria or they fulfilled one or more exclusion criteria. Six patients refused further ECT sessions or were transferred to another hospital, and 10 patients were excluded due to a missing baseline MADRS score or because no weekly MADRS score was available (e.g. in patients with very severe depression with catatonic features and/or mutism).
Finally, 89 patients were included in the present study (Fig. 1); 65% were female, and mean age was 63.9 (SD 12.4, range 33–96) years. Of these patients, 54 (61%) had depression with psychotic features, 29% had an episode duration >1 year, and the mean baseline MADRS score was 38.3 (SD 8.2). Table 1 presents the clinical characteristics of the total sample, and for the two subgroups with and without remission. No significant differences between the two subgroups were found for age, sex, previous pharmacotherapy failure, episode duration, the presence of psychotic features, and the baseline MADRS score. Response was attained by 77 (87%) patients, while 65 (73%) attained full remission.
Figure 1.
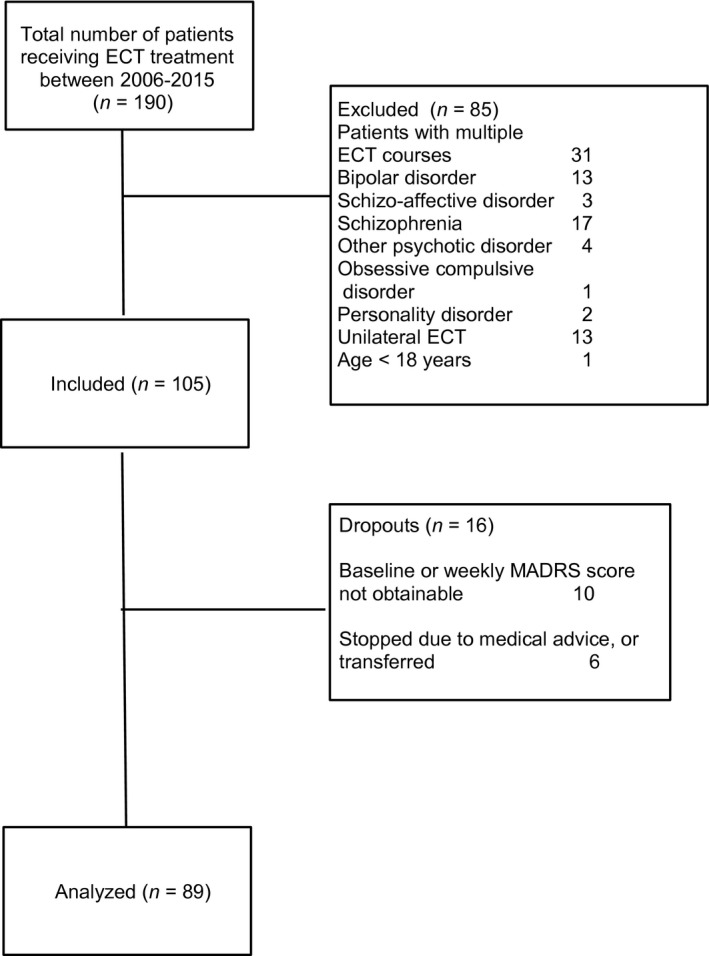
Flowchart of inclusion of study sample.
Table 1.
Clinical characteristics of the study sample (n = 89)
| No remission (n = 24) | Remission (n = 65) | Total (n = 89) | Test | |
|---|---|---|---|---|
| Female, n (%) | 12 (50) | 46 (71) | 58 (65) | Χ 2 = 3.33, P = 0.07 |
| Age in years, mean ± SD | 66.2 ± 15.5 | 63 ± 11.1 | 63.9 ± 12.4 | t = 0.92, P = 0.37 |
| Pharmacotherapy failure, n (%) | 20 (83) | 47 (72) | 67 (75) | Χ 2 = 1.15, P = 0.29 |
| Episode duration > 1 year, n (%) | 8 (33) | 18 (28) | 26 (29) | Χ 2 = 0.27, P = 0.61 |
| Psychotic features, n (%) | 12 (50) | 42 (65) | 54 (61) | Χ 2 = 1.57, P = 0.21 |
| Previous ECT treatment | 6 (25) | 13 (20) | 19 (21) | Χ 2 = 0.26, P = 0.77 |
| Baseline MADRS score, mean ± SD | 38.7 ± 5.5 | 38.1 ± 9.0 | 38.3 ± 8.2 | T = 0.84, P = 0.40 |
| Baseline HAM‐D score, mean ± SD | 28.2 ± 6.2 | 29.7 ± 6.9 | 28.8 ± 6.5 | t = 1.07, P = 0.29 |
| Number of ECT sessions, mean ± SD | 18.0 ± 4.6 | 13.5 ± 4.4 | 14.7 ± 4.8 | t = −4.24, P < 0.001 |
Analysis of four definitions of early improvement
The predictive value of early improvement was analyzed as a predictor of eventual remission. As mentioned, remission was defined as a final MADRS score ≤9. To investigate the predictability of early improvement to predict eventual remission, we calculated sensitivity, specificity, PPV, NPV, and AUC. For early improvement, several definitions were used, that is, a ≥15%, 20%, 25%, or 30% reduction on the MADRS score at week 1, after two ECT sessions (Table 2). The results show that early improvement is a modestly sensitive predictor for eventual remission. Using a higher cutoff level tends to decrease the sensitivity; for example the ≥15% cutoff level for early improvement shows a higher sensitivity compared with the higher cutoff levels of early improvement. To identify a predictor with both acceptable specificity and sensitivity, ROC curves were constructed that compared a ≥15%, 20%, 25%, and 30% reduction on the MADRS score at week 1. The AUC for the predictor 15% improvement at week 1 to predict eventual remission was 0.65, indicating modest predictability. The AUCs for the other definitions of early improvement were only slightly inferior.
Table 2.
Estimates of early improvement (after 1 week of ECT) for prediction of remission
| % Early improvement | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|
| 15 | 0.51 | 0.79 | 0.87 | 0.37 | 0.65 |
| 20 | 0.42 | 0.79 | 0.84 | 0.33 | 0.60 |
| 25 | 0.37 | 0.91 | 0.92 | 0.35 | 0.64 |
| 30 | 0.32 | 0.96 | 0.95 | 0.34 | 0.64 |
PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
Figure 2 shows the relatively poor sensitivity of the predictor: of the 51 patients not achieving early improvement after 1 week of ECT, 32 (63%) were remitters at the end of the ECT course. The fairly good specificity of the predictor is also shown: of the 38 patients achieving early improvement after 1 week of ECT, only 5 (13%) were non‐remitters at the end of the ECT course.
Figure 2.
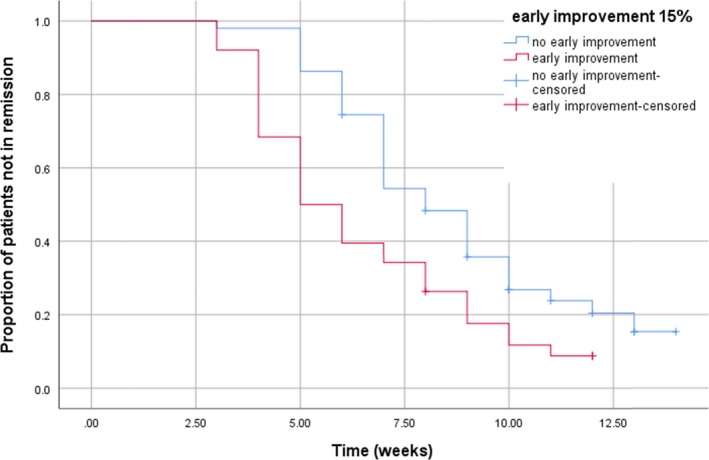
Kaplan–Meier curve: time to remission in both subgroups. Mean time to remission in early improvers: 6.49 weeks, 95% CI 5.61–7.36; In patients without early improvement: 8.82 weeks, 95% CI 7.94–9.69. Breslow test: Χ 2 = 12.67, P = 0.01.
Relation between early improvement and time to remission
A 15% reduction in MADRS score was used as definition of early improvement, in order to assess its influence on the time of the ECT course required to attain full remission. The Kaplan–Meier curve (Fig. 2) shows that remission was achieved more than 2 weeks earlier in patients who attained early improvement (i.e. 15% reduction in the MADRS score) compared with those who did not; this difference was significant (Breslow test Χ 2 = 12.67, P = 0.01).
Moreover, a sensitivity analysis was performed, calculating time to remission with remission defined as an MADRS score ≤ 6. Patients meeting the criterion for early improvement attained remission after 7.72 (95% CI 6.52–8.91) weeks vs. 10.81 (9.84–11.77) weeks in patients without early improvement (Breslow test χ2 = 16.64, P < 0.001).
Discussion
This study shows that early improvement within 1 week (i.e. two sessions) of bilateral ECT is a moderately sensitive predictor for time to remission in an in‐patient population with severe major depression. However, 87% of the patients that achieved early improvement became remitters, whereas as much as 63% of the patients without early improvement attained eventual remission. Therefore, lack of early improvement is not useful to predict who will not benefit from ECT. Regarding the secondary aim of the present study, remission was attained more than two weeks earlier in patients with early improvement. A faster resolution of depressive symptoms is associated with a better prognosis 20. Therefore, lack of early improvement appears to have clinical relevance. As suggested previously 11, changes in treatment schedule, dosing, or augmentation with an antidepressant may be considered for patients without early improvement. Regarding the best definition of early improvement, it should be noted that the AUCs of the four definitions were similar. The 15% improvement on the MADRS score appears to be the most appropriate definition, since its modest sensitivity (51%) only decreases when using larger percentages of reduction on the MADRS score as a definition of early improvement. Remarkably, patients without early improvement still showed a favorable treatment effect. At the end of the ECT course, 32/51 patients (63%) met the criteria for eventual remission. These results show that continuing the ECT course is a sensible strategy, even for patients failing to achieve early improvement.
Comparison with previous studies
Two recent studies investigated the same topic; in these studies, early improvement was measured after 2 weeks of ECT with a three session/week schedule 10, 11, that is, after six ECT sessions. The authors found a 30% reduction in the HAM‐D score to be the best definition of early improvement. Both studies included severely depressed in‐patients with high baseline HAM‐D scores, similar to the patient sample in the present study. In particular, in the study by Martinez‐Amoros et al., the patient sample appears to be comparable to that of our study, with ≥50% of patients suffering from psychotic depression, and an average age of 66 years. However, because of the difference in the number of ECT sessions after which early improvement was measured, it is difficult to make a meaningful comparison between that study and the present study. Nevertheless, predicting remission after six ECT sessions may be relatively easy, since for many patients this is midway, rather than early, in their ECT course. The possibility of predicting eventual remission particularly early in the ECT course is more surprising, although it has been described previously 2, 7. The previous studies that investigated early improvement as a predictor of response to antidepressant drugs 21, 22, 23 usually defined early improvement as a reduction of ≥20% in the HAM‐D score after 2 weeks of treatment with antidepressants. However, since ECT is used for patients who tend to be more severely depressed, and often in need of a rapid antidepressant effect, measuring early improvement after 2 weeks of ECT may not be the optimal choice. A greater efficacy and faster onset of action of ECT 1, 9 should emerge earlier than after 2 weeks of treatment. When early improvement as predictor is compared with a prediction model for the efficacy of ECT, the Maudsley Staging Method (MSM) 24, three differences between both methods are apparent: the MSM is assessed prior to the ECT course, while early improvement is measured during the ECT course. Moreover, the MSM is a score on a scale, whereas early improvement is a dichotomous predictor. Finally, van Diermen et al. 24 used the MSM to assess the probability of remission with ECT, while early improvement also was used to assess time to remission. In clinical practice, the MSM may be used to support the decision to perform ECT, whereas early improvement may be helpful to assess whether adaptations in the ECT method are necessary.
Strengths and limitations
Strengths of this study include (i) making the diagnosis of major depression during a drug‐free observation period resulting in a homogeneous sample of patients with severe depression and (ii) its prospective design. Furthermore, refraining from the use of benzodiazepines during the ECT course is regarded as a strength, because these agents have a negative effect on the efficacy of right unilateral (RUL) ECT 25; this may also apply to bilateral (BL) ECT. A limitation of the study is the homogeneity of our patient sample, implying that the results may not be generalizable to patients with less severe major depression. Unfortunately, we have no follow‐up data on our sample, which is a limitation. Moreover, the relatively small sample size (n = 89) is another limitation. Since only severely depressed in‐patients receive ECT at our center, the high response (87%) and remission (73%) rate may not be feasible for patients with less severe major depression. Finally, since BL ECT was exclusively used, our findings may not be applicable to patients receiving RUL or bifrontal ECT.
To conclude, early improvement during an ECT course may be assessed after two ECT sessions. Such an improvement is a moderately sensitive predictor for eventual remission in an in‐patient population with severe major depression. Final remission was attained over 2 weeks earlier in patients with early improvement, which may be considered a clinically relevant difference. However, although it takes them more time, eventually the majority of patients without early improvement also became remitters. Therefore, the lack of early improvement should not necessarily be a reason to stop ECT prematurely. This could lead to considering changes in treatment schedule, dosing, or augmentation with an antidepressant.
Declaration of interest
The authors report no financial or any other relationship relevant to the subject of this article.
Birkenhager TK, Roos J, Kamperman AM. Improvement after two sessions of electroconvulsive therapy predicts final remission in in‐patients with major depression.
Data availability
The data concerning the present study are available upon request.
References
- 1. UK, ECT Review Group . Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta‐analysis. Lancet 2003;361:799–808. [DOI] [PubMed] [Google Scholar]
- 2. Rich CL. Recovery from depression after one ECT. Am J Psychiatry 1984;141:1010–1011. [DOI] [PubMed] [Google Scholar]
- 3. Heijnen WT, Birkenhager TK, Wierdsma AI, van den Broek WW. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta‐analysis. J Clin Psychopharmacol 2010;30:616–619. [DOI] [PubMed] [Google Scholar]
- 4. Haq AU, Sitzmann AF, Goldman ML, Maixner DF, Mickey BJ. Response of depression to electroconvulsive therapy: a meta‐analysis of clinical predictors. J Clin Psychiatr 2015;76:1374–1384. [DOI] [PubMed] [Google Scholar]
- 5. van Diermen L, van den Ameele S, Kamperman AM, et al. Prediction of electroconvulsive therapy response and remission in major depression: meta‐analysis. Br J Psychiatry 2018;212:71–80. [DOI] [PubMed] [Google Scholar]
- 6. Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry 2004;65:485–491. [DOI] [PubMed] [Google Scholar]
- 7. Rodger CR, Scott AI, Whalley LJ. Is there a delay in the onset of the antidepressant effect of electroconvulsive therapy? Br J Psychiatry 1994;164:106–109. [DOI] [PubMed] [Google Scholar]
- 8. Bech P, Kastrup M, Rafaelsen OJ. Mini‐compendium of rating scales for states of anxiety depression mania schizophrenia with corresponding DSM‐III syndromes. Acta Psychiatr Scand Suppl 1986;326:1–37. [PubMed] [Google Scholar]
- 9. Segman RH, Shapira B, Gorfine M, Lerer B. Onset and time course of antidepressant action: psychopharmacological implications of a controlled trial of electroconvulsive therapy. Psychopharmacology (Berlin) 1995;119:440–448. [DOI] [PubMed] [Google Scholar]
- 10. Lin H‐S, Lin C‐H. Early improvement in HAMD‐17 and HAMD‐6 scores predicts ultimate response and remission for depressed patients treated with fluoxetine or ECT. J Affect Disord 2019;245:910–997. [DOI] [PubMed] [Google Scholar]
- 11. Martinez‐Amoros E, Goldberg X, Galvez V, et al. Early improvement as a predictor of final remission in major depressive disorder: new insights in electroconvulsive therapy. J Affect Disord 2018;235:169–175. [DOI] [PubMed] [Google Scholar]
- 12. Heijnen WT, Kamperman AM, Tjokrodipo L, Hoogendijk WJ, van den Broek WW, Birkenhager TK. Influence of age on ECT efficacy in depression and the mediating role of psychomotor retardation and psychotic features. J Psychiatr Res 2019;109:41–47. [DOI] [PubMed] [Google Scholar]
- 13. American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edn Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 14. Semkovska M, Landau S, Dunne R, et al. Bitemporal versus high dose unilateral twice‐weekly electroconvulsive therapy for depression (EFFECT‐Dep): a pragmatic, randomized, non‐inferiority trial. Am J Psychiatry 2016;2016:408–417. [DOI] [PubMed] [Google Scholar]
- 15. Kolshus E, Jelovac A, McLoughlin DM. Bitemporal v high‐dose unilateral electroconvulsive therapy for depression: a systematic review and meta‐analysis of randomized controlled trials. Psychol Med 2016;47:1–13. [DOI] [PubMed] [Google Scholar]
- 16. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 17. Carmody TJ, Rush AJ, Bernstein I, et al. The Montgomery Asberg and the Hamilton ratings of depression: a comparison of measures. Eur Neuropsychopharmacol 2006;16:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 19. Kleinbaum DG, Klein M. Survival analysis. A self‐learning text, 3rd edn New York: Springer, 2012. [Google Scholar]
- 20. Gorwood P, Corruble E, Falissard B, Goodwin GM. Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressive outpatients. Am J Psychiatry 2008;165:731–739. [DOI] [PubMed] [Google Scholar]
- 21. Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, Thase ME. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta‐analysis including 6562 patients. J Clin Psychiatry 2009;70:344–353. [DOI] [PubMed] [Google Scholar]
- 22. Henkel V, Seemuller F, Obermeier M, et al. 2009 Does early improvement triggered by antidepressants predict response/remission? Analysis of data from a naturalistic study on a large sample of inpatients with major depression. J Affect Disord 2009;115:439–449. [DOI] [PubMed] [Google Scholar]
- 23. Vermeiden M, Kamperman AM, Vulink ME, van den Broek WW, Birkenhager TK. Early improvement as a predictor of eventual antidepressant treatment response in severely depressed inpatients. Psychopharmacology (Berlin) 2015;232:1347–1356. [DOI] [PubMed] [Google Scholar]
- 24. Van Diermen L, Hebbrecht K, Schrijvers D, et al. The Maudsley staging method as predictor of electroconvulsive therapy effectiveness in depression. Acta Psychiatr Scand 2018;138:605–614. [DOI] [PubMed] [Google Scholar]
- 25. Pettinati HM, Stephens SM, Willis KM, Robin SE. Evidence for less improvement in depression in patients taking benzodiazepines during unilateral ECT. Am J Psychiatry 1990;147:1029–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data concerning the present study are available upon request.


