ABSTRACT
The objective of this article is to evaluate whether the tumoricidal activity of mouse IFN R−/− nature killer (NK) cells is induced by Newcastle disease virus hemagglutinin‐neuraminidase (NDV‐HN) stimulation, and to investigate what is the mechanism of the HN‐stimulated NK cells to kill mouse hepatoma cell line in vitro. The mouse IFN R−/− NK cells were stimulated for 16 hr with 500 ng/mL NDV‐HN in 1640 medium. Quantify the cytotoxic activities of NK cells against mouse hepatoma cells (Hepa1‐6) by flow cytometry. Granzymes B (GrB) and Fas/FasL concentrations in the supernatants of IFN R−/− NK cells medium were determined by specific ELISA assay. The expression of cell surface GrB and Fas was determined by Western blot. NDV‐HN stimulation enhanced tumoricidal activity of IFN R−/− NK cells toward Hepa1‐6 in vitro. Treating with anti‐HN neutralizing mAb induced significant decline in the cytotoxicity of IFN R−/− NK cells toward Hepa1‐6 cell line (P < 0.05). After treating with anti‐HN protein (1 μL/mL), Syk‐specific inhibitor Herbimycin A(250 ng/mL) and NF‐κB inhibitor PDTC (500 ng/mL) downregulated the tumoricidal activity of HN‐stimulated IFN R−/− NK cells (P < 0.05). Moreover, significant suppressions in the production of GrB and Fas/FasL were observed in HN‐stimulated IFN R−/− NK cells (P < 0.05). Thus, we concluded that killer activation receptors pathway is involved in the IFN‐γ‐independent GrB and Fas/FasL expression of NDV‐HN‐stimulated IFN R−/− NK cells, and these are activated by Syk and NF‐κB. Anat Rec, 302:1718–1725, 2019. © 2019 The Authors. The Anatomical Record published by Wiley Periodicals, Inc. on behalf of American Association for Anatomy
Keywords: Newcastle disease virus, HN protein, NK cells, hepatoma cell line, killing effect
Hepatocellular carcinoma (HCC) represents a major worldwide health concern, ranking among the top five most prevalent malignancies (Parkin et al., 2001). Many factors are involved in liver carcinogenesis, including HBV and HCV infection, alcohol abuse, nonalcoholic fatty liver disease, dietary habits, and so on (Mormone et al., 2011). Although surgical resection and liver transplantation are considered to be curative, the majority of HCC cases are detected at an advanced stage, at which time the treatment options are extremely limited (Llovet et al., 2003).
Viral therapy that effectively improves the patient's innate immunity against tumor cells is used as one of the late oncolytic cancer measurements. Newcastle disease virus (NDV) is an avian pathogen that belongs to the rubulavirus genus of the paramyxoviridae family. Cassel and Garrett showed in 1965 that the outbreak of Newcastle disease inhibited metastasis in patients with advanced gastric carcinoma (Cassel and Garrett, 1965). After this report, NDV had attracted more attention in the antitumor effect, and this research is currently in the clinical stage. NDV can selectively replicate in tumor cells when it induces tumor cell death. It has been considered that the selective antitumor activity of NDV is based on cancer‐specific defects in the interfere on pathway (Song et al., 2013). Hemagglutinin‐neuraminidase (HN) is the primary component of the large spike in glycoprotein on the NDV envelopes, has a length of 1,734 bp (encoding 577 amino acids) and a molecular weight of 63 kD, and can control hemagglutinin and neuraminidase activity. Many studies show that the HN protein plays a critical role in the antitumor effects of NDV (Sui et al., 2010). HN hydrolyzes the surface sialic acid of the host cell, exposes biological recognition sites, and induces tumor necrosis factor‐associated apoptosis‐inducing ligand (TRAIL) expression at the surface of mononuclear cells in the peripheral blood (Rajmani et al., 2015). In addition, the localization of HN on the tumor cell membrane also leads to the formation of the same recognition sites, which can improve the cytotoxic effects of the host's immune system against tumor cells.
Nature killer (NK) cells make an important impact on the immune response to infections and malignancies by cytolysis of infected or transformed cells and by secretion of immune mediators (Leung 2014). NK cells release cytotoxic granules containing perforin and granzymes. Perforin leads to the perforation of target cells, and granzymes B (GrB) permeated into target cells and subsequently induced apoptosis (Lieberman 2003; Voskoboinik et al., 2006). In addition to the perforin/granzyme pathway, the engagement of tumor necrosis factor (TNF) receptor superfamily members, such as Fas/CD95, TRAIL receptors, and TNFR1, on tumor cells by the corresponding ligands (FasL, TRAIL and TNF) expressed on or secreted by NK cells contributes to NK cytotoxicity under certain circumstances (Zamai et al., 1998; Voskoboinik et al., 2006). The mouse liver NK cells were shown to upregulate TRAIL after Interferon (IFN)‐γ stimulation in vivo, which contributed to NK cell cytotoxicity in the reduction of liver metastases NK cell cytotoxicity and the reduction of liver metastases. Song et al. (Song et al., 2013) found that NDV stimulation enhanced the killing ability of mouse spleen NK cells toward mouse hepatoma cell line, and triggered TRAIL expression in mouse spleen NK cells could be mediated by IFN‐γ induction. On the other hand, our laboratory found that killing activation receptors pathway is one of the IFN‐γ‐independent TRAIL pathways in NDV‐stimulated NK cells through the activation of spleen tryosine kinases (Syk) and NF‐κB.
In this study, we examined the tumoricidal activity of NDV‐ and HN‐stimulated IFN R−/− NK cells. Furthermore, we evaluated whether IFN‐γ‐independent TRAIL is crucial for the tumoricidal activity of NDV‐ and HN‐stimulated IFN R−/− NK cells, and if so, what is the mechanism of IFN‐γ‐independent TRAIL regulation in the NDV‐ and HN‐stimulated IFN R−/− NK cells.
MATERIALS AND METHODS
Cell Lines
The mouse IFN R−/− NK cell line was provided by Saiqi (Shanghai, China) Biological Engineering. The mouse hepatoma cell line Hepa 1‐6 was obtained from our laboratory stock. The cell line was maintained in suspension under tissue‐culture conditions (humidified incubator with 5% CO2 at 37°C). The cells were passaged every 3–4 days in RPMI 1640 medium (Hyclone) containing 2 mM L‐glutamine, 10% fetal calf serum (Hyclone), and antibiotics (100 IU/mL penicillin and 100 IU/mL streptomycin).
Virus, Protein
The avirulent, nonlytic NDV 7793 strain (NDV7793) was obtained from our laboratory stock. A stock of infectious virus was propagated in embryonated chicken eggs, harvested from the allantoic fluid, and purified by centrifugation (300–400 g, 30 min, 4°C) and then ultracentrifugation (50,000 g, 60 min, 4°C). The sediment was resuspended in phosphate‐buffered saline (PBS) and purified twice over sucrose (35%) via ultracentrifugation (97,000 g, 60 min, 4°C). The virus was resuspended in PBS buffer containing 0.1% ethylenediaminetetraacetic acid. NDV7793 was quantified by a hemagglutination test in which one hemagglutination unit (HU) is defined as the smallest virus concentration leading to visible chicken erythrocyte agglutination.
HN, a recombinant protein, was induced and expressed by IPTG induction from E. coli strain BL21 (DE3) carrying a recombinant HN gene of NDV expression plasmid pET‐HNa.
Reagents
Propidium iodide (PI) and carboxyfluorescein diacetate succinimidyl ester (CFSE) were purchased from Sigma–Aldrich (MO). Anti‐β actin Ab, antimouse GrB Ab, antimouse Fasl Ab, and antimouse Fas Ab were purchased from Novus (CO). Syk kinase inhibitor Herbimycin A (88‐H2030‐35A) and Anti‐HN Ab were purchased from Sigma–Aldrich (CO). NF‐κB inhibitor Pyrrolidinedithiocarbamate (PDTC) (93–1,676‐100) was purchased from Millipore (MA). A cocktail of protease inhibitor and phosphatase inhibitors, enhanced chemiluminescence (ECL) Plus Western blot detection reagents, was purchased from BOSTER (Wuhan, China). GrB, Fas enzyme‐linked immunosorbent assay (ELISA) kit was purchased from CUSABIO (Wuhan, China). 3‐(4,5‐Dimethyl‐2‐thiazolyl)‐2,5‐diphenyl‐2‐H‐tetrazolium bromide (MTT) was purchased from Sigma (CO).
NK Cells Preparation and Activation
IFN R−/− NK cells were stimulated for 16 hr, respectively, with NDV 7793 (25HU/105cells), HN soluble protein (500 ng/mL), or with PBS in 1640 medium. Cells were collected by centrifugation (300–400 g,10 min,4°C), washed twice in PBS, and used for Western blot or flow cytometry assay.
Blocking Experiments
IFN R−/− NK cells were cultured in the presence of Syk‐specific inhibitor Herbimycin A (250 ng/mL), NF‐κB inhibitor PDTC (500 ng/mL), and anti‐HN protein (1 μL/mL), respectively, for 1 hr. Then, IFN R−/− NK cells were stimulated as above.
Cytotoxicity Assay
To quantify the cytotoxic activities of inhibitors against mouse IFN R−/− NK cells, IFN R−/− NK cells (104 cells/well) were incubated with varying concentration of Syk‐specific inhibitor Herbimycin A (1 μg/ml、5 μg/ml、10 μg/ml) and NF‐κB inhibitor PDTC (1 μg/ml、5 μg/ml、10 μg/mL), respectively, for16 hr. MTT (5 mg/mL) of 0.5% was then added to each well followed by incubation at 37°C for 4 hr. Following incubation, the medium containing MTT was removed and 150 μL DMSO was added. The optical density of the homogenous purple solutions was measured using a microplate reader (Thermo, CA) at 450 nm. The optical density of the formazan formed in the control (medium only) cells was taken as 100% viability. All cytotoxicity assays were performed in 96‐well, round‐bottom plates in triplicate. Cell viability = (average absorbance of experimental group/average absorbance of control group) × 100%.
Flow Cytometric Assay of Cytotoxicity
IFN R−/− NK cells were used as the effector, and Hepa 1‐6 cells served as target, effector, and target cells were mixed in complete RPMI 1640 medium at IFN R−/− NK: Hepa 1‐6 = 1:1,5:1,10:1 in triplicates, with 1 × 105 target cells added per well. In short, Hepa 1‐6 cells were collected and rinsed twice with PBS, and then incubated with 5 μM/L CFSE at 37°C and 5% CO2 incubator. After that, cold ice was added to complete RPMI 1640 medium to the cells and remained at 4°C for 5 min followed by centrifugate at 800 g for 5 min. The cells were incubated at 37°C for 4 hr in Falcon tubes (Becton Dickinson Labware, Lincolin Park, NJ) in a final volume of 200 μL per well in a humidified 5% CO2 and then added to a different number of activated IFN R−/− NK cells. At the end of the incubation time, the tubes were then put in ice and incubated with 15 μL of 4 μM PI for 15 min, followed by flow cytometric analysis within 1 hr. CFSE/PI double‐stained target cells were excited by an argon‐ion laser emitting at 488 nm. CFSE were detected in the FL1 channel (530/30‐nm bandpass filter), while PI was detected in the FL3 channel (670‐nm long pass). All samples were analyzed on an FACS Calibur (BD Bioscience), using the software Cell Quest ver.5.2.1 (BD Bioscience) for acquisition and data analysis.
Enzyme‐Linked Immunosorbent Assay
IFN R−/− NK cells were used as the effector, and Hepa 1‐6 cells served as target, effector, and target cells were mixed in complete RPMI 1640 medium at IFN R−/− NK cells: Hepa 1‐6 cells = 5:1 in triplicates, with 1 × 105 target cells added per well. Hepa 1‐6 cells were incubated at 37°C for 4 hr in Falcon tubes in a final volume of 200 μL per well in a humidified 5% CO2 and then added to different number of activated IFN R−/− NK cells. The secretion cytokines (GrB, Fas) were measured by an indirect ELISA. Supernatant was measured using the ELISA protein assay kit according to the manufacturer's instructions. Color development was measured using a microplate reader (Thermo, CA) at 450 nm.
Western Blot Analysis
IFN R−/− NK cells were used as the effector, and Hepa 1‐6 cells served as target, effector, and target cells were mixed in complete RPMI 1640 medium at IFN R−/− NK cells: Hepa 1‐6 cells = 5:1 in triplicates, with 1 × 105 target cells added per well. Hepa 1‐6 cells were incubated at 37°C for 4 hr in Falcon tubes in a final volume of 200 μL per well in a humidified 5% CO2 and then added to different number of activated IFN R−/− NK cells. To assess GrB and Fas/FasL phosphorylation levels, cells were collected through centrifugation (300 g, 10 min, 4°C) and then washed with ice‐cold PBS twice. The cell pellets were resuspended in 100 μL lysis buffer per 1 × 107 cells (30 mM Tris‐Hcl pH 7.5,120 mM NaCl, 10% glycerol and 1% Triton X‐100) with the addition of protease inhibitor according to the manufacturer's instruction. After 30‐min ice‐water bath incubation, the lysates were collected and heated to 100°C for 5 min to denaturalize the proteins.
For Western blot analysis, the resulting lysates were supplemented with fivefold concentrated standard reducing sample buffer. Subsequently, lysates containing 60 μg of protein, as determined by the bicinchoninic acid method (Enhance BCA protein Assay Kit, Bryotime, China), were separated on SDS‐PAGE. After protein transfer onto nitrocellulose membrane by electroblotting, the membrane was blocked with 5% skim milk powder in Tris buffered saline tween (TBST) solution for 1 hr at room temperature. Then, the membrane was incubated in TBST containing 5% skimmed milk and 1 μg/mL primary Abs against mouse GrB and against mouse Fas/FasL (anti‐β‐actin Ab was used for loading control), rocked gently for 12 hr at 4°C, rinsed with TBST three times for 5 min, and then incubated with HRP‐conjugated secondary Abs at dilution 1:2000 in TBST for 1 hr at room temperature. After washing three times for 5 min each time with TBST, the blots on membrane were developed by ECL method using super signal west dura substrate (Thermo Scientific 37071) following the manufacturer's protocol.
Statistical Analysis
Data are expressed as means ± SD of the mean of separate experiments (n ≥ 3). Student's t‐test was applied for comparison of the means of two groups, and ANOVA was used for the means of multiple groups. Values of P < 0.05 were considered statistically significant.
RESULTS
Effect of Syk Inhibitor Herbimycin A and NF‐κB Inhibitor PDTC on the Survival of IFN R−/− NK Cells
To test whether Syk inhibitor Herbimycin A and NF‐κB inhibitor PDTC have the toxicity to IFN R−/− NK cells, IFN R−/− NK cells were stimulated by Herbimycin A and PDTC for 16 hr and then survival rates of IFN R−/− NK cells were evaluated using the MTT assay. The different concentration of Herbimycin A and PDTC had no obvious inhibitory effect on the survival of IFN R−/− NK cells (Table 1).
Table 1.
The effect of Herbimycin A and PDTC, respectively, on the survival rate of IFN R−/− NK cells
| 16 hr | ||||
|---|---|---|---|---|
| Group (μg/mL) | 0 | 1 | 5 | 10 |
| Herbimycin A | 100 | 98.12 ± 0.01* | 98.42 ± 0.01* | 98.36 ± 0.01* |
| PDTC | 100 | 97.67 ± 0.01** | 97.77 ± 0.02** | 96.07 ± 0.02** |
P > 0.05 versus Herbimycin A group (0 ug/mL).
P > 0.05 versus PDTC group (0 ug/mL).
HN‐Stimulated IFN R−/− NK Cells Kill Mouse Hepatoma Cells
To test whether NDV‐ or HN‐stimulated IFN R−/− NK cells have the ability to kill mouse hepatoma cells, IFN R−/− NK cells were stimulated by NDV or HN for 16 hr and then incubated with tumor cells for 4 hr. Mortality rates of tumor cells were evaluated using the CFSE/PI double‐stained. The hepatoma cells could be killed by NDV‐ or HN‐stimulated IFN R−/− NK cells. The PI positive target cells of Hepa 1‐6 hepatoma cells were up to 26.5% and 26.83%, at the effector‐to‐target (E:T) cell ratio of 5:1, respectively (P < 0.01). We observed similar findings in the groups in which the effector‐to‐target (E:T) cell ratio is 10:1, and the PI positive target cells of Hepa 1‐6 hepatoma cells were up to 42.00% and 51.47%, respectively (P < 0.01). And E:T = 10:1, which is much more sensitive to IFN R−/− NK‐mediated cytotoxicity than the E:T = 5:1 (Fig. 1). These data confirmed that HN increased the capacity of IFN R−/− NK cells to cytotoxic targets, and showed no significant difference compared with NDV positive group (P > 0.05).
Figure 1.
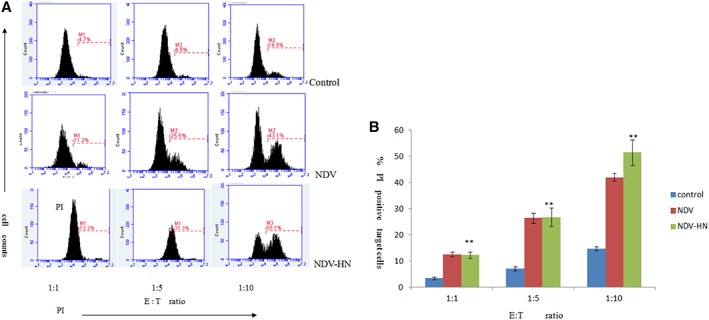
Original images (A) and summarized data (B) showing tumoricidal activity of mouse IFN R−/− NK cells toward Hepa 1‐6 hepatoma cell line. Mouse IFN R−/− NK cells were stimulated with NDV‐HN (500 ng/mL) for 16 hr in 1640 medium. Mouse IFN R−/− NK cells were harvested by centrifugation, and cocultured with Hepa 1‐6 hepatoma cells for 4 hr at the indicated E:T ratios. As a negative control, unstimulated mouse IFN R−/− NK cells were cocultured with target tumor cells for 4 hr. As a positive control, NDV‐stimulated (25 HU/mL) mouse IFN R−/− NK cells were cocultured with target tumor cells for 4 hr. Quantify the cytotoxic activities of mouse IFN R−/− NK cells against mouse hepatoma cells (Hepa 1‐6 cell line) by flow cytometry. Bars represent the mean ± SD of 3 wells, and similar results were obtained in three independent experiments. **P < 0.01 versus Unstimulated.
Tumoricidal Activity of HN‐Stimulated IFN R−/− NK Cell Was Inhibited by Syk Kinase and NF‐κB Inhibitor
The PI positive target cells rate for the killing inhibitive of HN‐stimulated IFN R−/− NK cells was different. IFN R−/− NK cells were preincubated with specific Syk kinase inhibitors Herbimycin A, NF‐κB inhibitor PDTC with HN, and Anti‐HN portein; the PI positive rate of these tumor cells were reduced (Fig. 2) with each E:T cell ratio(P < 0.05). On the other hand, unstimulated IFN R−/− NK cells were less lethal to the tumor cells. These results suggested that capacity of HN‐stimulated IFN R−/− NK cells to cytotoxic their targets were dramatically decreased by inhibition of Syk kinase and NF‐κB activity.
Figure 2.
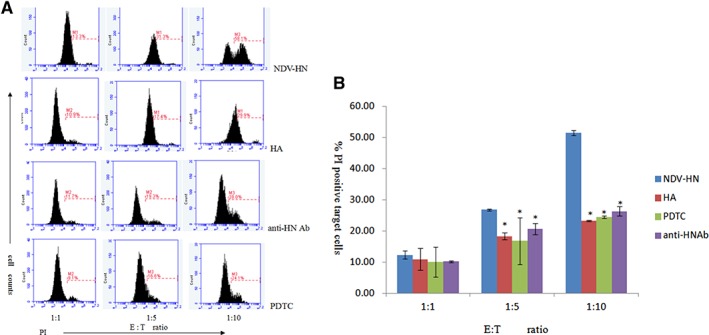
Original images (A) and summarized data (B) showing the correlation between tumoricidal activity upregulation and Syk, NF‐κB pathways in mouse IFN R−/− NK cells toward Hepa 1‐6 hepatoma cell lines. Mouse IFN R−/− NK cells were precultured with the PDTC (500 ng/mL) or herbimycin A (250 ng/mL), prior to incubation with NDV‐HN (500 ng/mL) or a mixture NDV (25 HU/mL) and neutralizing antibodies against HN (1 μL/mL). Precultured or noncultured mouse IFN R−/− NK cells were stimulated with NDV‐HN (500 ng/mL) for 16 hr, then harvested by centrifugation, and cocultured with Hepa 1‐6 hepatoma cells for 4 hr at the indicated E:T ratios. Quantify the cytotoxic activities of mouse IFN R−/− NK cells against mouse hepatoma cells (Hepa 1‐6 cell line) by flow cytometry. Bars represent the mean ± SD of 3 wells, and similar results were obtained in three independent experiments. *P < 0.05 versus NDV‐HN stimulated.
The Effect of GrB and Fas in IFN R−/− NK Cells by NDV or HN Stimulated
We investigated whether NDV or HN pretreatment could stimulate the release of cytokines by IFN R−/− NK cells, and GrB and Fas release in the supernatants of cultured IFN R−/− NK cells stimulated with NDV or HN was measured by ELISA. On the other hand, IFN R−/− NK cells were treated with anti‐HN portein, Herbimycin A, and PDTC for inhibiting Syk kinase and NF‐κB also needed measured GrB and Fas release. NDV or HN stimulation induced a significantly higher soluble GrB and Fas release, compared to the unstimulated IFN R−/− NK cells (P < 0.01) (Fig. 3). In contrast, when IFN R−/− NK cells were cultured in the presence of both Herbimycin A and PDTC, and then to stimulated by HN, as shown in Figure 4 increased of GrB and Fas release into the culture supernatant was obviously inhibited(P < 0.05). All the data suggesting that NDV and HN pretreatment caused increased cytokine secretion in IFN R−/− NK cells, anti‐HN portein, Herbimycin A, and PDTC could restrain this secretion. Thereby, GrB and Fas was involved in the regulation of potentiating IFN R−/− NK cells cytotoxic effects to hepatoma cells after NDV or HN stimulation. Therefore, a clear decrease was observed when Syk kinase and NF‐κB were inhibited, indicating that Syk kinase and NF‐κB signal pathway play a substantial role of IFN R−/− NK cells to cytotoxic their targets by HN stimulated.
Figure 3.
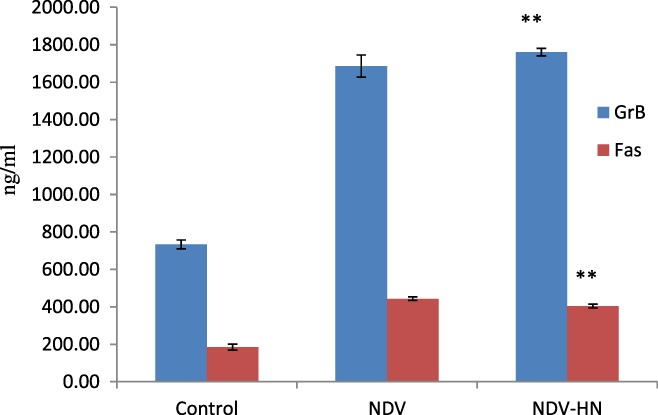
The concentration of soluble GrB and Fas in mouse IFN R−/− NK cells medium. Mouse IFN R−/− NK cells were stimulated with NDV‐HN (500 ng/mL) for 16 hr. Supernatant was harvested, and concentration of soluble GrB and Fas was determined by ELISA assay. As a negative control, unstimulated mouse IFN R−/− NK cells were used. As a positive control, NDV (25 HU/mL)‐stimulated mouse IFN R−/− NK cells were used. Bars represent the mean ± SD of triplicate wells, and similar results were obtained in three independent experiments. **P < 0.01 versus Unstimulated.
Figure 4.
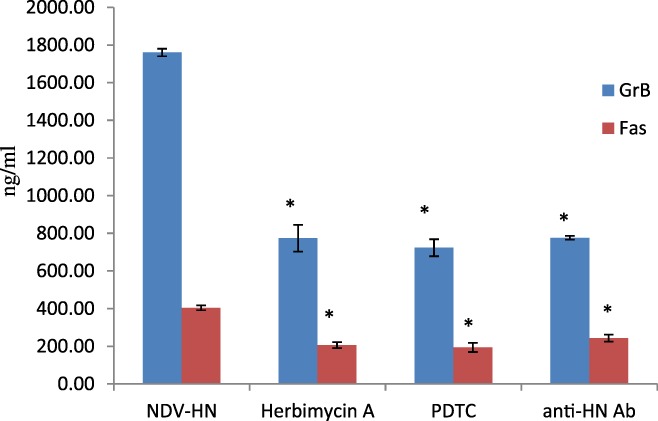
The correlation between GrB or Fas upregulation and Syk, NF‐κB pathways in mouse IFN R−/− NK cells. Mouse IFN R−/− NK cells were precultured with the PDTC (500 ng/mL) or herbimycin A (250 ng/mL), prior to incubation with NDV‐HN (500 ng/mL) or a mixture NDV (25 HU/mL) and neutralizing antibodies against HN (1 μL/mL). Precultured or noncultured mouse IFN R−/− NK cells were stimulated with NDV‐HN (500 ng/mL) for 16 hr. Supernatant was harvested, and concentration of soluble GrB and Fas was determined by ELISA assay. Bars represent the mean ± SD of 3 wells, and similar results were obtained in three independent experiments. *P < 0.05 versus NDV‐HN stimulated.
GrB and Fas/Fasl Expression Is Upregulated in NDV or HN‐Stimulated IFN R−/− NK Cells
Most literature on NK cell cytotoxicity focused on rapid target killing by way of lytic granule transfer and subsequent GrB‐mediated proteolysis. Fas plays an important role in the regulation of immune response and apoptosis. In this case, we sought to explore how GrB and Fas/FasL expression was upregulated in IFN R−/− NK cells with NDV or HN stimulation. We analyzed GrB and Fas protein expression in IFN R−/− NK cells by using Western blot analysis. The results showed clear GrB and Fas/FasL protein expression in IFN R−/− NK cells, 16 hr after stimulation with NDV or HN. Compared with the unstimulated IFN R−/− NK cells, we observed significant increase (Fig. 5) in the protein expression levels in NDV or HN‐stimulated IFN R−/− NK cells. In conclusion, these results clearly demonstrate that NDV‐HN‐stimulated IFN R−/− NK cells induce tumoricidal activity to mouse hepatoma cells, and that GrB and Fas/FasL act as the main effector molecule of this tumoricidal effect.
Figure 5.
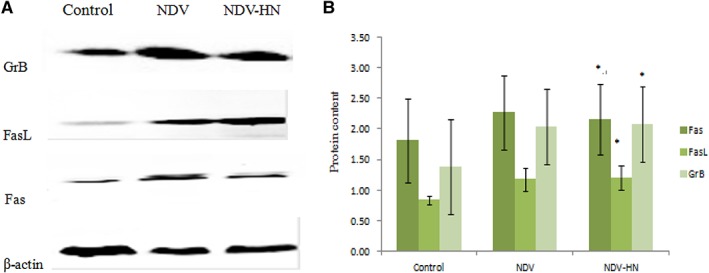
Original images (A) and summarized data (B) showing GrB and Fas/FasL proteins expression in mouse IFN R−/− NK cells. Mouse IFN R−/− NK cells were stimulated with NDV‐HN (500 ng/mL) for 16 hr. Cells were lysed, and GrB‐ and Fas/FasL‐specific Western blot analysis was performed. As a negative control, unstimulated mouse IFN R−/− NK cells were used. As a positive control, NDV (25 HU/mL) stimulated mouse IFN R−/− NK cells were used. β‐actin was used as a loading control. GrB and Fas/FasL expressions in stimulated mouse IFN R−/− NK cells (stimulated by NDV or NDV‐HN) were obviously higher than unstimulated mouse IFN R−/− NK cells. Similar results were obtained in three independent experiments, and bars represent the mean ± SD of 3 wells. *P < 0.05versus Unstimulated.
Syk Kinase and NF‐κB Inhibitor Downregulates GrB and Fas/FasL Expression in HN‐Stimulated IFN R−/− NK Cells
To investigate whether Syk and NF‐κB pathways were intracellular signal events mediating HN stimulation on IFN R−/− NK cells, blocking experiments were examined using various anti‐HN proteins, Syk kinases, and NF‐κB inhibitors. HN stimulation of IFN R−/− NK cells protein expression of GrB and Fas/FasL was analyzed by Western blot analysis. We determined that Both Syk and NF‐κB pathways played a critical role in up‐regulated GrB and Fas/FasL protein expression in HN stimulated IFN R−/− NK cells. A clear decrease was observed in the GrB and Fas/FasL level of HN‐stimulated IFN R−/− NK cells pretreated with various inhibitors, compared with the control (Fig. 6). We provided evidence that the HN‐induced expression of TRAIL on IFN R−/− NK cells was dependent on Syk and NF‐κB signal pathways.
Figure 6.
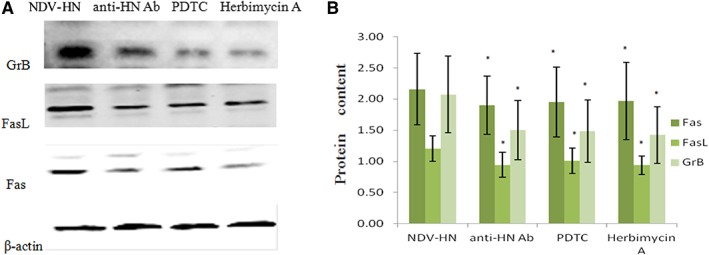
Original images (A) and summarized data (B) showing the correlation between GrB or Fas/FasL upregulation and Syk, NF‐κB pathways in mouse IFN R−/− NK cells. Mouse IFN R−/− NK cells were precultured with PDTC (500 ng/mL) or herbimycin A (250 ng/mL), prior to incubation with NDV‐HN (500 ng/mL) or a mixture NDV (25 HU/mL) and neutralizing antibodies against HN (1 μL/mL). Precultured or noncultured mouse IFN R−/− NK cells were stimulated with NDV‐HN (500 ng/mL) for 16 hr. Cells were lysed, and GrB‐ and Fas/FasL‐specific Western blot analysis was performed. β‐actin was used as a loading control. GrB and Fas/FasL proteins expressions in precultured mouse IFN R−/− NK cells were obviously lower than noncultured mouse IFN R−/− NK cells. Similar results were obtained in three independent experiments, and bars represent the mean ± SD of 3 wells. *P < 0.05 versus NDV‐HN stimulated.
DISCUSSION
NDV has attracted much attention as a promising oncolytic virus. Studies have shown that NK cells were activated when NDV infected tumor cells (Jarahian et al., 2009). Many previously reports showed that NDV was a strong inducer of Types I and II IFNs in both mice and human lymphocytes (Wertz et al., 1994). The HN molecule, the envelope protein of NDV, has received considerable attention so far, primarily due to its mediating viral initial binding to the host cells. To date, it was found that HN acted as a ligand for NKp46 and NKp44 receptors on human NK cells and directly activated NK cells for contribution to the antitumor effects of NDV (Jarahian et al., 2009). Our present work shows that NDV and HN could directly trigger TRAIL upregulation in NK cells of mice through IFNγ‐independent way. Both Syk and NF‐κB pathways played a role in the regulation of TRAIL expression in mice HN‐activated NK cells. Although the NDV and HN triggering the tumoricidal activity of NK cells by TRAIL induction have been demonstrated through the IFNγ pathway and in the IFNγ‐independent way, there are very few studies exploring the mechanisms underlying its actions. CFSE/PI double‐staining was measured to assess the degree of mortality of Hepa 1‐6 cells induced by HN‐stimulated IFN‐R−/− NK cells. The results revealed significant IFN‐R−/− NK cells cytotoxicity on Hepa 1‐6 cells after treated by HN by a dose‐dependent manner. Although our finding shows that under low NK‐to‐target cell ratio (1:1), there are no difference compared to the unstimulated control, which is probably that Hepa 1‐6 cells are less sensitive than other mouse hepatoma cells (e.g., Novikoff cell line).
Furthermore, our past studies have shown that this direct activation happened without IFN‐γ mediation due to the failure of IFN R−/− NK cells to sense any IFN‐γ signals. In addition, HN‐treated IFN R−/− NK cells showed significantly higher activation of Syk and NF‐κB. Here, we found that pharmacologic exposure to herbimycin A, a known Syk family kinase inhibitor, and NF‐κB inhibitor PDTC partially inhibited the stimulation of the IFN R−/− NK cells with HN killing activity toward mouse hepatoma cell lines. These data suggested that the activation of both and NF‐κB factor were requisite signaling events in regulation of TRAIL in HN‐induced NK cells directly without IFN participation.
The main mechanism of NK cells used to kill tumor cells and virus‐infected cells was through Fas and FasL interaction. In addition, perforin/GrB‐induced apoptosis is the main pathway used by cytotoxic lymphocytes to eliminate virus‐infected. Upon release, perforin forms pores in the plasma membrane of the target cell, creating an aqueous channel through which granzymes and associated molecules can enter and induce either apoptosis or osmotic cell lysis. FasL is a transmembrane protein that is expressed by lymphocytes, mainly by CD4+ and CD8+ T cells and B cells, after engagement of the Ag‐specific T or B cell receptor and macrophages and also by NK cells (Abrams, 2005). Fas plays an important role in the regulation of immune response and apoptosis via inflammatory cytokines. A study revealed an intricate control of tumor growth by NK cell uniquely through the FasL mechanism (Dupaul‐Chicoine et al., 2015), suggesting that the FasL mechanism may indeed be exploited to provide new targets and strategies for engineering primary NK cells for adoptive cell transfer therapy.
Our findings provide evidence that HN regulates the IFN R−/− NK cells' immune response activity by regulating the cytokine release and receptor expression through different mechanisms. In this study, we found that GrB expression was upregulated in IFN R−/− NK cells pretreated with HN, while Fas/ FasL levels were increased in IFN R−/− NK cells stimulated with HN, providing evidence that HN enhances mouse hepatoma cells apoptosis induced by IFN R−/− NK cells via activation of Fas/FasL signaling and increase of GrB expression. This phenomenon reminds us that HN enhanced cytotoxicity of IFN R−/− NK cells against Hepa 1‐6 cells by upregulating the expression of Fas/FasL on the IFN R−/− NK cells surface and also upregulating the GrB expression on IFN R−/− NK cells. In addition, in the present study, we observed that treatment with Herbimycin A and PDTC, the expression of GrB and Fas/FasL of IFN R−/− NK cells, was decreased significantly. Therefore, Syk and NF‐κB pathways are one possible mechanism involving in the antitumor effects of HN stimulation IFN R−/− NK cells.
Our results demonstrated that the antitumor properties of NDV and HN strain are to trigger the tumoricidal activity of NK cells induction through the IFNγ‐independent pathway. We found that GrB and Fas/FasL could be regulated by HN stimulation in IFN R−/− NK cells. These findings certificated that the upregulation of GrB and Fas/FasL expression of IFN R−/− NK cells could enhance cytotoxicity of NK cells on tumor cells. The Syk and NF‐κB pathways play a critical role in the NDV‐stimulated NK cells, which leads to an increase in the mortality rate of tumor cells. These findings may contribute to the immune‐stimulation of NDV for oncolytic activity.
ACKNOWLEDGMENTS
We thank Qiguang Huang and Zhenping Lai for excellent technical assistance.
LITERATURE CITED
- Abrams SI. 2005. Positive and negative consequences of Fas/Fas ligand interactions in the antitumor response. Front Biosci 10:809–821. [DOI] [PubMed] [Google Scholar]
- Cassel WA, Garrett RE. 1965. Newcastle disease virus as an antineoplastic agent. Cancer 18:863–868. [DOI] [PubMed] [Google Scholar]
- Dupaul‐Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, Rodrigue‐Gervais IG, Breton V, Colpitts SL, Beauchemin N, et al. 2015. The nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity 43:751–763. [DOI] [PubMed] [Google Scholar]
- Jarahian M, Watzl C, Fournier P, Arnold A, Djandji D, Zahedi S, Cerwenka A, Paschen A, Schirrmacher V, Momburg F. 2009. Activation of natural killer cells by newcastle disease virus hemagglutinin‐neuraminidase. J Virol 83:8108–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W. 2014. Infusions of allogeneic natural killer cells as cancer therapy. Clin Cancer Res 20:3390–3400. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. 2003. Hepatocellular carcinoma. Lancet 362:1907–1917. [DOI] [PubMed] [Google Scholar]
- Lieberman J. 2003. The ABCs of granule‐mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol 3:361–370. [DOI] [PubMed] [Google Scholar]
- Mormone E, George J, Nieto N. 2011. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact 193(3):225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. 2001. Estimating the world cancer burden: Globocan 2000. Int J Cancer 94:153–156. [DOI] [PubMed] [Google Scholar]
- Rajmani RS, Singh PK, Ravi KG, Saxena S, Singh LV, Kumar R, Sahoo AP, Gupta SK, Chaturvedi U, Tiwari AK. 2015. In‐vitro characterization and evaluation of apoptotic potential of bicistronic plasmid encoding HN gene of Newcastle disease virus and human TNF‐α. Anim Biotechnol 26(2):112–119. [DOI] [PubMed] [Google Scholar]
- Song DZ, Liang Y, Xiao Q, Yin J, Gong JL, Lai ZP, Zhang ZF, Gao LX, Fan XH. 2013. TRAIL is Involved in the Tumoricidal activity of mouse natural killer cells stimulated by Newcastle disease virus in vitro. Anat Rec 296:1552–1560. [DOI] [PubMed] [Google Scholar]
- Sui H, Bai Y, Wang K, Li X, Song C, Fu F, Zhang Y, Li L. 2010. The anti‐tumor effect of Newcastle disease virus HN protein is influenced by differential subcellular targeting. Cancer Immunol Immunother 59:989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoboinik I, Smyth MJ, Trapani JA. 2006. Perforin‐mediated target‐cell death and immune homeostasis. Nat Rev Immunol 6:940–952. [DOI] [PubMed] [Google Scholar]
- Wertz K, Büttner M, Mayr A, Kaaden OR. 1994. More than one component of the Newcastle disease virus particle is capable of interferon induction. Vet Microbiol 39:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. 1998. Natural killer (NK) cell‐mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med 188:2375–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]


