Abstract
Anxiety Disorders often show a chronic course, even when treated with one of the various effective treatments available. Lack of treatment effect could be due to Treatment Resistance (TR). Consensus on a definition for TR Anxiety Disorders (TR‐AD) is highly needed as currently many different operationalizations are in use. Therefore, generalizability in current TR‐AD research is suboptimal, hampering improvement of clinical care. The objective of this review is to evaluate the currently used definitions of TR‐AD by performing a systematic review of available literature. Out of a total of n = 13 042, 62 studies that operationalized TR‐AD were included. The current review confirms a lack of consensus on TR‐AD criteria. In 62.9% of the definitions, TR was deemed present after the first treatment failure. Most studies (93.0%) required pharmacological treatment failures, whereas few (29.0%) required psychological treatment failures. However, criteria for what constitutes “treatment failure” were not provided in the majority of studies (58.1%). Definitions for minimal treatment duration ranged from at least 4 weeks to at least 6 months. Almost half of the TR‐AD definitions (46.8%) required elevated anxiety severity levels in TR‐AD. After synthesis of the results, the consensus definition considers TR‐AD present after both at least one first‐line pharmacological and one psychological treatment failure, provided for an adequate duration (at least 8 weeks) with anxiety severity remaining above a specified threshold. This definition could contribute to improving course prediction and identifying more targeted treatment options for the highly burdened subgroup of TR‐AD patients.
Keywords: anxiety disorders, assessment/diagnosis, CBT/ cognitive behavioral therapy, pharmacotherapy, treatment resistance
1. INTRODUCTION
Up till now, a widely used definition for treatment resistance in anxiety disorders does not exist (Barton, Karner, Salih, Baldwin, & Edwards, 2014; Chen & Tsai, 2016; Roy‐Byrne, 2015; Starcevic, 2008; Stein, 2004). This is surprising because it is well known that a substantial proportion of adults with Anxiety Disorders experience suboptimal treatment results after evidence‐based treatments (Bruce et al., 2005; Huh, Goebert, Takeshita, Lu, & Kang, 2011; Ramsawh, Raffa, Edelen, Rende, & Keller, 2009). Selective Serotonin Reuptake Inhibitors (SSRIs) and Serotonin Norepinephrine Reuptake Inhibitors (SNRIs) are widely regarded the first‐line pharmacological treatments for Anxiety Disorders (Bandelow et al., 2008; Bandelow et al., 2012; Farach et al., 2012). Cognitive Behavioural Therapy (CBT) is the first‐line psychological treatment option for Anxiety Disorders (Taylor, Abramowitz, & McKay, 2012). First‐line treatments show a moderate effect size in meta‐analytic comparisons with placebo (Carpenter et al., 2018; Jakubovski, Johnson, Nasir, Müller‐Vahl, & Bloch, 2018). After first‐line treatment up till 30–60% of patients have substantial and impairing remaining symptoms (Bandelow et al., 2008; Bruce et al., 2005; Tyrer, Seivewright, & Johnson, 2004).
A wide variety of terms are in use for the phenomenon of suboptimal treatment results in anxiety disorders: “refractory anxiety”, “treatment resistance”, “medication resistance”, “treatment refractory cases”, “remaining symptomatic” and “persistent symptoms” (Baldwin, Stein, & Hermann, 2016; Bystritsky, Stein, & Hermann, 2016; Craske, Bystritsky, Stein, & Hermann, 2016; Craske, Stein, & Hermann, 2016; Ipser et al., 2006; Roy‐Byrne, Stein, & Hermann, 2016). In other psychiatric disorders the term “treatment resistance” (TR) is preferred to describe a subgroup of patients who have a prior history of unfavorable treatment effects (Conway, George, & Sackeim, 2017; Fekadu et al., 2009; Fogelson & Leuchter, 2017; Howes et al., 2016), which also implies having less favorable future treatment effects (Conway et al., 2017; Fekadu et al., 2009; Fogelson & Leuchter, 2017; Howes et al., 2016). The varying terminology reflects the absence of consensus regarding the criteria for TR (Barton et al., 2014; Cirillo, Freire, & Freire, 2016; Perna & Caldirola, 2017; Stein & Seedat, 2004; Yoshinaga et al., 2016). This lack of consensus on criteria for TR‐AD was first recognized in 2004; however, 14 years later still no consensus exists (Barton et al., 2014; Chen & Tsai, 2016; Roy‐Byrne, 2015; Starcevic, 2008; Stein & Seedat, 2004; Van Ameringen & Mancini, 2001).
Most authors define Treatment Resistant Anxiety Disorders (TR‐AD) as the persistence of anxiety symptoms, or as the absence of response, recovery or remission of the disorder after some form of active treatment (Barton et al., 2014; Davies, Dubovsky, Gabbert, & Champman, 2000; Holt & Lydiard, 2007; Pollack, Smoller, Otto, & Rosenbaum, 1996; Stein, 2004; Zoun et al., 2016). These active treatments should represent evidence‐based treatment regimes, provided at an adequate dosage and for an adequate duration (Fava, Rafanelli, & Tomba, 2012; Roy‐Byrne, 2015). However, the absence of anxiety symptoms does not always indicate full disorder remission (Bystritsky, 2006; Chen & Tsai, 2016). A substantial amount of residual disease burden may be present in persisting behavioural changes such as avoidance, or in altered cognitive functioning, for instance in excessive rumination. Additional emphasis on functional recovery is therefore advocated by a number of authors when assessing TR‐AD (Bystritsky, 2006; Chen & Tsai, 2016). No systematic review into the definition for TR‐AD is yet performed.
The aim of this study is to summarize and discuss the different criteria used for TR‐AD. To do this, we will perform a systematic literature review. Second, by summarizing and comparing the different criteria used for TR in anxiety disorders, we aim to propose a consensus definition for TR‐AD.
2. METHODS
The methods for this systematic review were specified in advance in a study protocol which was documented in the PROSPERO database (reference number CRD42017055864). The current paper was drafted in accordance with the PRISMA guidelines for reporting on systematic reviews (Liberati et al., 2009).
2.1. Literature search
A systematic search across MEDLINE, PubMed (non‐MEDLINE), EMBASE, PsycINFO, and Web of Science for available literature until April 2018 was performed. To derive all articles that might include a definition for TR in anxiety disorders we searched for Anxiety Disorders (according to DSM‐5, American Psychiatric Association, 2013) in combination with various free‐text synonyms for “treatment resistance” (see Panel 1) for the full search query).
Panel 1.
Overview of search terms used in this systematic review (formatted for MEDLINE)
| (("Anxiety Disorders"[Mesh:NoExp] OR "Agoraphobia"[Mesh] OR "Anxiety, Separation"[Mesh] OR "Neurocirculatory Asthenia"[Mesh] OR "Neurotic Disorders"[Mesh] OR "Panic Disorder"[Mesh] OR "Phobic Disorders"[Mesh] OR anxiety disorder* [tiab] OR generalized anxiety disorder* [tiab] OR generalised anxiety disorder* [tiab] OR anxiety state* [tiab] OR agoraphobi* [tiab] OR panic* [tiab] OR phobi* [tiab] OR selective mutis* [tiab])) |
| AND |
| (“Retreatment” [Mesh] OR "Drug Resistance" [Mesh:NoExp] OR “Drug tolerance” [Mesh] OR treatment resistan* [tiab] OR refractor* [tiab] OR poor respon* [tiab] OR partial respon* [tiab] OR non‐respon* [tiab] OR nonrespon* [tiab] OR loss of respons* [tiab] OR medication resistan* [tiab] OR drug resistan* [tiab] OR tachyphyl* [tiab] OR resilien* [tiab] OR persistan* [tiab] OR immune [tiab] OR insusceptib* [tiab] OR irresponsive* [tiab] OR unreceptive* [tiab] OR resistive [tiab] OR unsuccessful treatment* [tiab] OR treatment failur* [tiab] OR failed treatment* [tiab] OR "Patient Dropouts"[Mesh] OR patient dropout* [tiab] OR treatment dropout* [tiab] OR "Patient Compliance"[Mesh] OR non‐complian* [tiab] OR noncomplian* [tiab] OR non‐adheren* [tiab] OR nonadheren* [tiab] OR remaining symptom* [tiab] OR pseudo‐resistan* [tiab] OR dropping out [tiab] OR augmentation [tiab] OR inadequate respon* [tiab] OR intractab* [tiab] OR partially respon* [tiab] OR resistant patient* [tiab] OR remain symptom* [tiab] OR remaining symptom* [tiab] OR non‐remitting [tiab] OR nonremitting [tiab] OR partial improvement* [tiab] OR incomplete respon* [tiab] OR residual symptom* [tiab] OR anxiolytic toleran* [tiab]) |
All publication types in English were included with the exception of conference summaries, editorials, columns, book reviews and manifestos as these were unlikely to include a full description of a TR‐AD definition. Studies were selected when they included adults or elderly persons with anxiety disorders (Panic Disorder (with or without Agoraphobia, PD(A)), Social Anxiety Disorder (SAD), Generalized Anxiety Disorder (GAD), Specific Phobia (SP), Selective Mutism, and Separation Anxiety). No restrictions in presence of comorbidity were used. Exclusion criteria included studies with an average study population below 21 years, and studies reporting primarily on posttraumatic stress disorder (PTSD) or Obsessive‐Compulsive Disorder (OCD), because these are no longer classified as Anxiety Disorders.
2.2. Eligibility assessment
Eligibility assessment on title and abstract was performed independently by two reviewers (WB, GW, JG) by using the Cochrane‐supported review program Covidence (www.covidence.org). Disagreements were resolved by consensus after discussion. A flow chart for inclusion of eligible studies according to PRISMA guidelines is provided in Figure 1. Full‐text screening was performed independently by two reviewers (WB and JG). During the full‐text screening phase, articles were excluded if a full‐text version could not be retrieved or if any of the exclusion criteria were present. Studies were included if their definition for TR‐AD could be implicitly deduced from inclusion criteria used in a study. Reviews, meta analyses and book chapters were included if they provided their own definition for TR‐AD but were excluded if they repeated other studies’ definitions without providing rationale for choosing this definition over others. As the vast majority of studies used TR and “refractory” interchangeably we chose to regard them as synonyms and will refer to these phenomena as TR‐AD.
Figure 1.
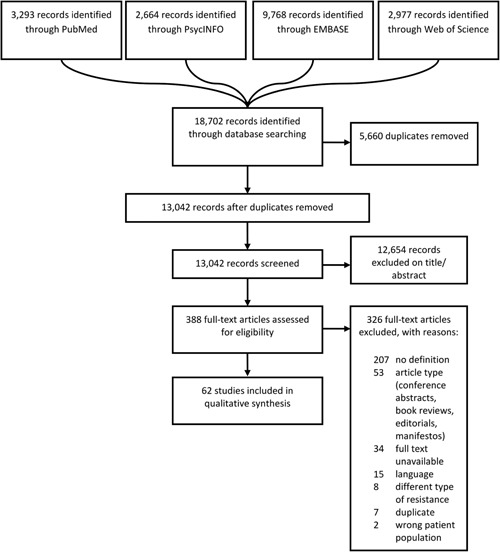
PRISMA flow chart for study inclusion
2.3. Data extraction
From trials we extracted data on study characteristics: number of subjects, population of interest, intervention, comparator condition, follow‐up period, primary outcomes and results; from reviews we extracted data on study design and population of interest. With regard to the definitions for TR‐AD, we extracted data on nine predefined putative criteria for the definition, based on criteria used in the Maudsley Staging Method for treatment resistant depressive disorders (Fekadu et al., 2009). In addition, we extracted one TR‐AD criterion (treatment response), that was not predefined in our study protocol. The ten criteria were: minimal number of failed treatments, failed psychotherapy trials, failed pharmacological trials, failed other biological treatments, minimal length of treatment, treatment response criterion (i.e., which posttreatment change constitutes response/failure), minimal duration of anxiety disorder, severity of symptoms, presence of functional impairment, and presence of comorbidity. We evaluated which of these ten criteria were present in TR‐AD definitions across included studies (yes/no). Specific values for each criterion were extracted as well.
2.4. Quality of definitions
We assessed the definition quality in each included study. As there are no formal risk of bias tools available for the purpose of our study, and as we are not interested in potential sources of study outcome bias we assessed definition quality in two ways; first, by counting the total number of TR‐AD criteria included in each study's definition, second, by determining the degrees of precision with which the definition for TR‐AD is presented in each paper. The total number of TR‐AD criteria was a count variable counting presence of all ten dichotomized TR‐AD criteria. Degrees of precision was categorized into “high”, “medium” and “low”. Precision was considered “high” if a study provided an explicit definition for TR‐AD, for example in this study by De Salas‐Cansado et al. (2013):
Refractory was defined as subjects with persistent symptoms/suboptimal response, a Hamilton‐anxiety (HAM‐A) scale score ≥ 16 and a Clinic Global Impression (CGI) score ≥ 3 at baseline, after a standard dose regimen of any anti‐anxiety drug, alone or in combination, for at least 6 months, given before the baseline study visit. (p987).
The degree of precision was deemed “medium” if the criteria were only implicitly attributable to the concept of TR‐AD, or if multiple terms were used interchangeably, for instance in a study by Lohoff, Etemad, Mandos, Gallop, and Rickels (2010) in patients with “refractory GAD”:
Subjects also had to have treatment failure of at least 1 adequate trial of an SSRI, an SNRI, a BZ, or a combination of these agents. Patients who were on an SSRI, an SNRI, a BZ, or a combination of these agents before enrollment had to be on a stable dose for 4 weeks. Inclusion further required a total score of 16 or higher on the Hamilton Anxiety Scale (HAM‐A) and a score of 4 or greater on the Clinical Global Impression Severity of Illness Scale (CGI‐S) (p186).
Finally, if the study only provided a description of the concept of TR‐AD, without operationalizing it in specific criteria, the degree of precision was deemed “low”, for instance:
“failure of an adequate clinical trial of medication” (Stein, 2004).
2.5. Data synthesis
To synthesize the results of the systematic review into a new operationalization for TR‐AD, frequencies for presence of each individual TR‐AD criterion were assessed. The most frequently used values for each individual criterion were considered the most appropriate operationalization for that criterion and were chosen for the consensus definition. However, if an unspecified category for a certain criterion (e.g., “unspecified type of pharmacological treatment”) was the most frequently used value, we did not consider this category for the new definition if a more specified value was available. In addition, criteria that were included only in a small minority (<10%) of the studies were not used for the new definition, as they were then judged to be lacking a convincing empirical basis.
2.6. Statistical analyses
To test associations between total number of criteria provided in definitions, degrees of precision and publication year, we performed Kruskall‐Wallis tests for differences in mean rank. We hypothesized that higher definition quality studies (i.e., more total criteria or a higher degree of precision) would be the most recent studies. Fisher's Exact tests were performed to investigate whether the two definition quality variables were associated with different frequencies for values of each TR‐AD criterion. For instance: did high definition quality studies more often require a higher number of failed treatments or more often mention a SSRI/SNRI failure as requisite for TR‐AD compared with lower definition quality studies?
3. RESULTS
3.1. Study selection
The electronic database search yielded 18,702 results. After deduplication 13,042 entries remained. During title and abstract screening, 12,654 studies were excluded. We assessed 388 full‐text studies, of which 207 did not contain a definition, 53 were a wrong article type (conference abstracts and editorials), 34 could not be retrieved, 15 did not meet language requirements, 8 reported on a different type of “resistance” (e.g., “resistance” in the psychodynamic paradigm), 7 were previously unrecognized duplicates and 2 reported on a different patient population. This resulted in the final inclusion of 62 studies (for a flow chart see Figure 1).
3.2. Characteristics of included studies
Included studies were published between 1986 and 2018. They consisted of eight narrative reviews (Bakker, Van Balkom, & Stein, 2005; Bandelow et al., 2008; Bystritsky, 2006; Chen & Tsai, 2016; Holt & Lydiard, 2007; Lorenz, Jackson, & Saitz, 2010; Pollack, 2009; Starcevic, 2008), five systematic reviews (Barton et al., 2014; Cosci & Fava, 2013; Ipser et al., 2006; Patterson & Van Ameringen, 2016; Samuel, Zimovetz, Gabriel, & Beard, 2011), of which three also performed meta‐analyses (Barton et al., 2014; Ipser et al., 2006; Patterson & Van Ameringen, 2016), seven treatment guidelines/algorithms (Bandelow, 2008; Bandelow, Zohar, Hollander, Kasper, & Moller, 2002; National Institute for Health & Clinical Excellence, 2011; Stein et al., 2001; Stein et al., 2010; Stein, 2003; Stein, 2004), three book chapters (Baldwin, Polkinghorn, Lerer, & Stahl, 2005; Deligiannidis & Rothschild, 2010; Van Ameringen, Mancini, Patterson, & Stein, 2009), 21 open‐label trials (Gabriel & Violato, 2011; Gabriel, 2010; George et al., 2008; Glue et al., 2017, 2018; Heldt et al., 2003; Heldt et al., 2006; Hoge et al., 2008; Hollifield, Thompson, Ruiz, & Uhlenhuth, 2005; Katzman et al., 2008; Kinrys, Vasconcelos E Sa, & Nery, 2007; Menza, Dobkin, & Marin, 2007; Ociskova, Prasko, Latalova, Kamaradova, & Grambal, 2016; Pallanti & Quercioli, 2006; Pollack, Otto, Kaspi, Hammerness, & Rosenbaum, 1994; Simon et al., 2006; Snyderman et al., 2005; Solbakken & Abbass, 2015, 2016; Worthington III, Kinrys, Wygant, & Pollack, 2005; Yoshinaga et al., 2016), eight RCTs (Brawman‐Mintzer, Knapp, & Nietert, 2005; Castle, Gray, Neehoff, & Glue, 2017; Gloster et al., 2015; Hirschmann et al., 2000; Lohoff et al., 2010; Pollack et al., 2006; Rickels et al., 2012; Simon et al., 2009), four retrospective cohorts (Bakish et al., 1995; Cowley, Ha, & Roy‐Byrne, 1997; Durham, Higgins, Chambers, Swan, & Dow, 2012; Kinrys et al., 2007), one prospective cohort (Milrod et al., 2016), three case series (Aarre, 2003; Otto, Pollack, Penava, & Zucker, 1999; Tesar & Rosenbaum, 1986), one cost‐effectiveness analysis (De Salas‐Cansado et al., 2013), and one trial protocol (Zoun et al., 2016). Thirty‐three studies pertained to PD, 34 to GAD, 21 to SAD, two to SP, and five to Anxiety Disorders in general. For a summary of study characteristics see Table 1. For full details, see eTable 1 (trials, cohort studies and meta‐analyses) and eTable 2 (reviews, treatment guidelines and book chapters).
Table 1.
Study characteristics for included studies
| Study characteristics | n | % |
|---|---|---|
| Publication type | ||
| Book chapter | 3 | 4.8 |
| Case series | 3 | 4.8 |
| Cost‐effectiveness analysis | 1 | 1.6 |
| Narrative review | 8 | 12.9 |
| Open‐label trial | 21 | 33.9 |
| Prospective cohort study | 1 | 1.6 |
| Randomized controlled trial | 8 | 12.9 |
| Retrospective cohort study | 4 | 6.5 |
| Systematic review | 2 | 3.2 |
| Systematic review + meta‐analysis | 3 | 4.8 |
| Trial protocol | 1 | 1.6 |
| Treatment guidelines/algorithms | 7 | 11.3 |
| Population of interest a | ||
| Anxiety disorders (in general) | 5 | 8.1 |
| Generalized anxiety disorder | 34 | 54.8 |
| Panic disorder | 33 | 53.2 |
| Social anxiety disorder | 21 | 33.9 |
| Specific phobia | 5 | 8.1 |
| Type of intervention used (if any) | ||
| Adjunctive psychotherapy | 8 | 12.9 |
| Any therapy | 1 | 1.6 |
| Any adjunctive therapy | 1 | 1.6 |
| Combination treatment: pharmacological and psychological | 4 | 6.5 |
| Either pharmacologic monotherapy or pharmacologic augmentation therapy | 1 | 1.6 |
| Pharmacologic augmentation or combination treatment | 17 | 27.4 |
| Pharmacologic monotherapy | 7 | 11.3 |
| Nerve vagus stimulation | 1 | 1.6 |
| Self‐management | 1 | 1.6 |
| Degree of precision of included definitions | ||
| High | 13 | 21.0 |
| Medium | 44 | 71.0 |
| Low | 5 | 8.1 |
Some studies described more than one population of interest.
3.3. Definition quality
The total number of criteria per study ranged from one to six (mean = 3.58; SD = 1.31). With respect to the assessment of the degree of precision for TR‐AD definitions it appeared that 13 studies (21.0%) provided a high degree of precision, 44 (71.0%) a medium degree, and 5 (8.1%) a low degree of precision.
There was a significant association between total number of criteria and year of publication (χ 2(df = 5) = 13.01; p = 0.02): the studies with the highest number of criteria were, on average, the most recent. For degrees of precision no association with publication date existed (χ 2(df = 2) = 2.13; p = 0.34). Neither studies with a higher total number of criteria, nor studies with a higher degree of precision provided a different perspective on the ten TR‐AD criteria. Since definition quality did not change operationalizations for TR‐AD, all studies were used in the synthesis of results.
3.4. Main results
By applying a systematic review approach it became apparent that a large majority of studies on the topic of TR‐AD (n = 207) do not provide a definition for the phenomenon of TR‐AD. Furthermore, the included studies (n = 62) yielded many different definitions for the concept of TR‐AD (see eTable 3 for all definitions). Trials often used the presence of one failed pharmacological treatment as an adequate definition for TR‐AD. Other studies provided additional criteria. When the frequencies for each of the ten extracted TR‐AD criteria were compared across included studies, some distinctive patterns arose (see Table 1).
Table 2.
Criteria included in definitions for treatment resistance anxiety disorders
| Treatment resistance definition criteria | n | % |
|---|---|---|
| Minimal number of failed treatments | ||
| Not part of definition | 9 | 14.5 |
| Included in definition | 53 | 85.5 |
| Unspecified or varying number | 7 | 11.3 |
| 1 failed treatment | 39 | 62.9 |
| 2 failed treatments | 3 | 4.8 |
| 3 or more failed treatments | 4 | 6.5 |
| Failed psychotherapy trials | ||
| Not part of definition | 44 | 71.0 |
| Included in definition | 18 | 29.0 |
| Any | 9 | 14.5 |
| At least one failed CBT trial | 7 | 11.3 |
| Varying number (stepped‐care or staging approach) | 2 | 3.2 |
| Failed pharmacological trials | ||
| Not part of definition | 4 | 6.5 |
| Included in definition | 58 | 93.5 |
| Unspecified number or type of failed pharmacological treatment | 27 | 43.5 |
| At least one failed SSRI/SNRI trial | 15 | 24.2 |
| At least one failed other pharmacotherapeutic trial | 14 | 22.6 |
| Varying number or types (stepped‐care or staging approach) | 2 | 3.2 |
| Other biological treatments | ||
| Not part of definition | 62 | 100 |
| Included in definition | 0 | 0 |
| Minimal length of treatment | ||
| Not part of definition | 28 | 45.2 |
| Included in definition | 34 | 54.8 |
| >4 weeks | 8 | 12.9 |
| >6 weeks | 3 | 4.8 |
| >8 weeks a | 15 | 24.2 |
| >11 weeks or 20 sessions of CBT | 1 | 1.6 |
| >12 weeks | 3 | 4.8 |
| >4 months | 2 | 3.2 |
| >6 months | 2 | 3.2 |
| Treatment response criterion | ||
| Not part of definition | 36 | 58.1 |
| Included in definition | 26 | 41.9 |
| Cut‐off values for effective/ failed treatment provided b | 26 | 41.9 |
| Minimal duration of anxiety disorder | ||
| Not part of definition | 60 | 96.8 |
| Included in definition | 2 | 3.2 |
| >1 year | 1 | 1.6 |
| >2 years | 1 | 1.6 |
| Severity of symptoms | ||
| Not part of definition | 33 | 53.2 |
| Included in definition | 29 | 46.8 |
| Aspecific criterion (e.g., “severe”) | 1 | 1.6 |
| Specific criterion (cut‐off values) provided c | 28 | 45.2 |
| Functional impairment | ||
| Not part of definition | 57 | 91.9 |
| Included in definition | 5 | 8.1 |
| Aspecific criterion (e.g., “marked impairments”) | 4 | 6.5 |
| Specific criteria (cut‐off values) provided d | 1 | 1.6 |
| Presence of comorbidity | ||
| Not part of definition | 61 | 98.4 |
| Included in definition | 1 | 1.6 |
| Comorbidity as exclusion criterion for TR‐AD | 1 | 1.6 |
Note: CBT: cognitive behavioral therapy; CGI‐I:Clinical Global Impression Improvement Scale; CGI‐S: Clinical Global Impression Severity Scale; HAM‐A: Hamilton Anxiety Rating Scale; LSAS: Leibowitz Social Anxiety Scale; PD(A): panic disorder (with or without agoraphobia); PDSS: Panic Disorder Severity Scale; SAD:social anxiety disorder; SDS: Sheehan Disability Scale; SSRI: selective serotonin reuptake inhibitor; SNRI: selective norepinephrine reuptake inhibitor.
including studies with minimal treatment duration of “2 months.”
the most often used criteria were: ∆HAM‐A < 50% or CGI‐I < 2.
the most often used criteria for severe symptomatology were HAM‐A < 16 or CGI‐S ≥ 4 (for all Anxiety Disorders), PDSS > 3 or any PDSS item > 1 (for PD(A)), LSAS > 60 (for SAD).
one study used SDS > 1 on each item as criterion for functional impairments.
The minimal number of required failed treatments, regardless of treatment type, was reasonably consistent across studies: 39 studies (62.9%) required one treatment failure for TR‐AD, with other studies varying between two (n = 3) and five (n = 1) failed previous treatments. Failed psychotherapy trials were only included in 18 studies (29.0%). These studies all regarded CBT an appropriate treatment, with seven studies (11.3%) restricting TR‐AD to CBT failure alone, whilst others (n = 9; 14.5%) also regarded other psychological treatments appropriate.
Contrastingly, a large majority (n = 58; 93.5%) required at least one failed pharmacotherapy trial for their definition for TR‐AD. Of these, some studies (n = 15; 24.2%) considered at least one failed SSRI/SNRI trial sufficient to be classified as TR‐AD. A substantial number of studies did not specify type of pharmacotherapeutic treatment failure required for TR‐AD (n = 27; 43.5%), for instance by referring to “first‐line” or “standard” antianxiety treatments. A few used a varying number of treatment types in a stepped‐care or staging algorithm (n = 2; 3.2%) or considered other pharmacotherapeutic treatment failures adequate (n = 14, 22.6%). See eTable 3 for detailed descriptions of types of pharmacotherapy. Whether failed trials were caused by a lack of effect, or a lack of tolerability was usually not reported. Other biological treatments were not included in TR‐AD definitions.
Most studies (n = 34; 54.8%) used a minimal treatment length criterion ranging from 4 weeks to 6 months, while the most often used adequate minimal treatment duration was 8 weeks (n = 15; 24.2%).
A substantial number of studies (n = 26; 41.9%) gave a response criterion. The most commonly used cut‐off values were a <50% posttreatment improvement on the Hamilton Anxiety Rating Scale (HAM‐A) and a posttreatment Clinical Global Impression Improvement scale (CGI‐I) score greater than two (i.e., “minimal improvement”, at best). Severity of anxiety symptoms was often included in definitions (n = 29; 46.8%), with cut‐off scores commonly provided: a HAM‐A score of above 15 (for any Anxiety Disorder), a Clinical Global Impression Severity Scale (CGI‐S) score of four or higher (for any Anxiety Disorder), a total score above 3, or any item above 1 on the Panic Disorder Severity Scale (PDSS) for PD and a score at or above 60 on the Leibowitz Social Anxiety Scale (LSAS) for SAD. For GAD, no disorder‐specific measurement instrument was reported in TR‐AD definitions. Finally, minimal disease duration (n = 2; 3.2%), presence of functional impairments (n = 5; 8.1%) and presence of comorbidity (n = 1; 1.6%) were sparsely included in definitions for TR‐AD. See Table 2 for a summary per TR‐AD criterion, and eTable 4 for a full overview of included TR‐AD criteria per study.
3.5. Synthesis of results
To propose a consensus definition for TR‐AD that reflects the current literature, we included the most prevalent values for all criteria that were provided consistently across studies into the new TR‐AD definition. Failed SSRI/SNRI trials were most often considered as criterion for TR‐AD. Studies typically referred to SSRI/SNRI trials as “first‐line” treatment. Therefore, failure of at least one first‐line treatment (SSRI/SNRI) was included in the new definition. Although psychotherapeutic treatment failure was less often incorporated in TR‐AD definitions, CBT was usually referred to as “first‐line” psychological intervention. Therefore, first‐line psychological interventions (CBT) failure was included in the new definition. Although the most often provided criterion for minimal number of treatments was one, by including both pharmacological and psychological treatment failures into the definition, the minimal number of failed treatments in the new definition rose to at least two. A minimal adequate treatment duration of 8 weeks was included in the consensus definition. In studies that permitted psychotherapy failures as criterion for TR‐AD (n = 21), only five provided a minimal treatment duration criterion, ranging from 4 weeks to 20 sessions CBT (see eTable 4). Therefore, a minimal duration of 8 weeks was maintained for psychotherapy trials.
Absence of treatment response was included in the consensus definition, using the two most commonly provided cut‐off values from studies included in this review. Other biological treatments, minimal duration of Anxiety Disorder, presence of functional impairments and comorbidity were only sporadically included in TR‐AD definitions and therefore were not considered for the consensus TR‐AD definition. See Panel 2 for the full description of this consensus TR‐AD definition with most commonly used cut‐off values for each criterion.
Panel 2.
Proposed operationalization for Treatment Resistant Anxiety Disorders (TR‐AD)
| TR‐AD checklist | ||||
| Failed pharmacotherapeutic treatment | ||||
| □ | At least one first‐line treatment (SSRI, SNRI)1 | |||
| □ | pre‐to posttreatment difference in HAM‐A <50% or posttreatment CGI‐I >2 | |||
| □ | treatment period of at least 8 weeks | |||
| Failed psychotherapeutic treatment | ||||
| □ | At least one first‐line psychotherapeutic treatment (CBT)2 | |||
| □ | pre‐to posttreatment difference in HAM‐A <50% or posttreatment CGI‐I >2 | |||
| □ | provided according to local protocols and for an adequate duration (at least>8 weeks) | |||
| Current severity of anxiety symptoms | ||||
| □ | GAD | HAM‐A >15 | or CGI‐S > 3 | |
| □ | PD | HAM‐A >15 or PDSS >3, or any item >1 | or CGI‐S > 3 | |
| □ | SAD | HAM‐A >15 or LSAS ≥ 60 | or CGI‐S > 3 | |
TR‐AD is present if all six treatment boxes can be checked in addition to at least one symptom severity box
1SSRIs and SNRIs are considered first‐line pharmacotherapeutic treatment options as per 2018 (Bystritsky, Stein, & Hermann, 2016; National Institute for Health and Clinical Excellence, 2011; Roy‐Byrne, Stein, & Hermann, 2016)
2CBT interventions are considered first‐line psychotherapeutic treatment options as per 2018 (Craske, Stein, & Hermann, 2016; National Institute for Health and Clinical Excellence, 2011)
Abbreviations: SAD= Social Anxiety Disorder, PD= Panic Disorder, GAD= Generalized Anxiety Disorder, HAM‐A= Hamilton Anxiety Rating Scale, PDSS= Panic Disorder Severity Scale, LSAS= Leibowitz Social Anxiety Scale, CGI‐S= Clinical Global Impression Severity Scale, CGI‐I= Clinical Global Impression Improvement Scale, SSRI= Selective Serotonin Reuptake Inhibitor, SNRI= Selective Serotonin and Norepinephrine Reuptake Inhibitor, CBT= Cognitive Behavioral Therapy.
4. DISCUSSION
This paper aimed to systematically review different definitions and criteria for treatment resistant Anxiety Disorders (TR‐AD) and showed that the majority of studies do not provide a definition for TR‐AD and that consistency and consensus across TR‐AD definitions in included studies is lacking. The most frequently used definition for TR‐AD simply consists of one failed first‐line pharmacotherapy treatment. Both the lack of consensus in current TR‐AD definitions and the unclear description of TR‐AD in the most used definition make the current attempt of aligning definitions a necessity.
Out of ten putative criteria, we identified six criteria that are regularly integrated into the various different definitions for TR‐AD: minimal number of treatment failures, presence (and type) of psychological treatment failure, presence (and type) of pharmacological treatment failure, minimal treatment duration (>8 weeks), specification of a response criterion (i.e., what constitutes a “failed treatment”), and minimal symptom severity. These criteria were integrated into a consensus definition. Four putative criteria were dismissed: “minimal duration of disorder”, “other biological treatment failures”, “presence of comorbidity”, and “presence of functional impairment” due to the low frequency with which these were mentioned. In selecting the specific cut‐off values for included criteria, we opted to use the most commonly mentioned cut‐off values (the mode). Based on the most recent treatment guidelines, for the purpose of this definition we considered SSRIs and SNRIs as current first‐line pharmacotherapy options, and CBT current first‐line psychotherapy option (Bystritsky et al., 2016; Craske, Stein et al., 2016; National Institute for Health & Clinical Excellence, 2011; Roy‐Byrne et al., 2016). Furthermore, the consensus definition for TR‐AD requires both a failed pharmacotherapeutic and psychotherapeutic trial, as these were both regularly used as criterion for TR‐AD.
This is the first study to systematically assess different criteria for TR‐AD. A systematic approach was complicated by the absence of a risk of bias assessment tool for the purpose of the current study. Tools such as the Cochrane risk of bias tool for randomized studies (Higgins & Green, 2011) or the RoBANS for nonrandomized studies (Kim et al., 2013) determine the level of confidence with which the results of a certain study can be interpreted. However, the data we extracted from studies referred to the definition for treatment resistance they used, not the outcome of the study. Therefore, we chose to assess definition quality by determining the total number of criteria provided and the degree of precision with which the definition was provided. After analyzing these data, it seemed that the quality of included definitions did not impact operationalization of TR‐AD.
A limitation in this study was that although integration of the definitions was done systematically the final consensus definition could still reflect some subjective choices by the authors of the current study. Furthermore, it was apparent that some criteria might have been underreported in the included studies, for instance on minimal treatment duration in CBT. Another limitation in our methodology was the lack of studies that incorporated evaluation of pseudo‐resistance into their TR‐AD definitions. Pseudo‐resistance refers to any nonresponse in treatments that are not used to their full potential. Before treatment resistance can be deemed present, pseudo‐resistance should always be ruled out. In pharmacotherapy trials this could be due to a wrong indication, an inadequate dosage or inadequate duration (Fava et al., 2012; Roy‐Byrne, 2015). In psychotherapy trials this could be due to clinicians not following the treatment protocol or patients not being compliant with homework assignments (Roy‐Byrne, 2015; Taylor et al., 2012). In addition to this, in clinical care it should always be assessed whether the Anxiety Disorder diagnosis is incorrect, whether another comorbid disorder is the primary problem or whether there are exogenous factors like caffeine overuse, alcohol or substance use or medical diagnoses that contribute to treatment resistance (Fava et al., 2012; Roy‐Byrne, 2015). Also, in some studies it was not possible to assess whether previous treatment failures that were counted towards presence of TR‐AD consisted of evidence‐based antianxiety treatments. Finally, although psychological treatments like CBT were repeatedly proven effective in Anxiety Disorders (Bandelow et al., 2015; Carpenter et al., 2018), in many parts of the world they are not readily available (Saxena, Thornicroft, Knapp, & Whiteford, 2007). Therefore, generalizability of our findings may be limited in these regions.
Furthermore, for the purpose of this study we regarded TR‐AD, “refractory anxiety” and other related terms as synonyms. Even though this approach is in line with the majority of the studies, a minority consider TR‐AD and “refractory anxiety” to be different entities. For instance, in a Cochrane review, Ipser et al. (2006) propose the term TR for Anxiety Disorder patients who failed one pharmacologic treatment, whereas “refractory anxiety” refers to Anxiety Disorder patients with more than one failed treatment. Their approach can be viewed as a staging approach, distinguishing patients with end‐stage TR‐AD disorders from those with early stage TR‐AD. This approach is also advocated by Cosci and Fava (2013), who propose a staging model for TR Panic Disorders. In their model, the level of TR increases when more treatment regimens within pharmacologic, psychological and combination treatment have failed. In a number of treatment algorithms, a stepped care approach hints to the author's underlying assumption of a staging model for levels of TR (National Institute for Health & Clinical Excellence, 2011). In staging models, treatment decision making is based on the stage of disease progression in which the patient currently is classified. This could lead to evidence‐based stepped‐care treatment algorithms. We did not incorporate this staging paradigm for TR‐AD into the current paper, as no consensus exists for definitions of TR‐AD, nor for staging approaches in TR‐AD.
Future studies could empirically investigate the consensus definition for TR‐AD. A first step could be to apply the proposed TR‐AD definition to an Anxiety Disorder cohort and evaluate the longitudinal course of patients with TR‐AD compared to patients without TR‐AD. Possibly, this could also yield risk factors for development of TR‐AD. Further research could also focus on the validity of a staging approach in TR‐AD, as suggested by Cosci and Fava (2013) and Ipser et al. (2006).
In depression, a staging paradigm for TR is in use with the Maudsley Staging Method (Fekadu et al., 2009; Peeters et al., 2016; van Belkum et al., 2018). A similar approach could be beneficial for Anxiety Disorders. The criteria comprising TR‐AD that were described in the current paper could be studied on their merits as individual components in a staging method for TR‐AD, to reflect the various degrees of TR‐AD.
5. CONCLUSIONS
The majority of studies on treatment resistant Anxiety Disorders (TR‐AD) do not demarcate this phenomenon. Across studies that do provide a definition for TR‐AD there are many inconsistencies, which are likely to halt progress in Anxiety Disorder research. The current systematic review integrated the current literature into a consensus definition for TR‐AD (see Panel 2). This consensus definition should be regarded as a first step to advance the field further. The definition provided in this paper could contribute in harmonization of the process of evaluating presence of TR‐AD, which is a necessary first step towards improvement of the prognosis for TR‐AD patients.
Supporting information
Supporting information
Bokma WA, Wetzer GAAM, Gehrels JB, Penninx BWJH, Batelaan NM, van Balkom ALJM. Aligning the many definitions of treatment resistance in anxiety disorders: A systematic review. Depress Anxiety. 2019;36:801–812. 10.1002/da.22895
References
REFERENCES
- Aarre, T. F. (2003). Phenelzine efficacy in refractory social anxiety disorder: A case series. Nordic Journal of Psychiatry, 57(4), 313–315. 10.1080/08039480310002110 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (fifth). Washington, DC: American Psychiatric Association. [Google Scholar]
- Bakish, D. , Hooper, C. L. , West, D. L. , Miller, C. , Blanchard, A. , & Bashir, F. (1995). Moclobemide and specific serotonin re‐uptake inhibitor combination treatment of resistant anxiety and depressive disorders. Human Psychopharmacology: Clinical and Experimental, 10(2), 105–109. 10.1002/hup.470100205 [DOI] [Google Scholar]
- Bakker, A. , Van Balkom, A. J. L. M. , & Stein, D. J. (2005). Evidence‐based pharmacotherapy of panic disorder. International Journal of Neuropsychopharmacology, 8(3), 473–482. 10.1017/S1461145705005201 [DOI] [PubMed] [Google Scholar]
- Baldwin, D. , Stein, M. B. , & Hermann, R. (2016). Generalized anxiety disorder in adults: Epidemiology, pathogenesis, clinical manifestations, course, assessment, and diagnosis. November 22, 2016, Retrieved from. https://www.uptodate.com/contents/generalized‐anxiety‐disorder‐in‐adults‐epidemiology‐pathogenesis‐clinical‐manifestations‐course‐assessment‐and‐diagnosis
- Baldwin, D.S. , & Polkinghorn, C. (2005). Evidence‐based psychopharmacology In Stein (Ed.), Evidence‐based pharmacotherapy of generalized anxiety disorder. New York, NY: Cambridge University Press; Retrieved from. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2005‐14567‐004&site=ehost‐live. [DOI] [PubMed] [Google Scholar]
- Bandelow, B. (2008). The medical treatment of obsessive‐compulsive disorder and anxiety. CNS Spectrums, 13(9, 14), 37–47. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L352617762 [DOI] [PubMed] [Google Scholar]
- Bandelow, B. , Zohar, J. , Hollander, E. , Kasper, S. , & Moller, H.‐J. (2002). World Federation of Societies of Biological Psychiatry (WfSBP) guidelines for the pharmacological treatment of anxiety, obsessive‐compulsive and psttraumatic stress disorders. The World Journal of Biological Psychiatry, 3(904758812), 171–199. 10.1080/15622970802465807 [DOI] [PubMed] [Google Scholar]
- Bandelow, B. , Zohar, J. , Hollander, E. , Kasper, S. , Möller, H.‐J. , & WFSBP Task force on treatment guidelines for anxiety obsessive‐compulsive post‐traumatic stress disorders (2008). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive‐compulsive and post‐traumatic stress disorders ‐ First revision. World Journal of Biological Psychiatry, 9(4), 248–312. Retrieved from. 10.1080/15622970802465807 [DOI] [PubMed] [Google Scholar]
- Bandelow, B. , Reitt, M. , Röver, C. , Michaelis, S. , Görlich, Y. , & Wedekind, D. (2015). Efficacy of treatments for anxiety disorders: A meta‐analysis. International Clinical Psychopharmacology, 30(4), 183–192. 10.1097/YIC.0000000000000078 [DOI] [PubMed] [Google Scholar]
- Bandelow, B. , Sher, L. , Bunevicius, R. , Hollander, E. , Kasper, S. , Zohar, J. , & Vega, J. (2012). Guidelines for the pharmacological treatment of anxiety disorders, obsessive‐compulsive disorder and posttraumatic stress disorder in primary care. International Journal of Psychiatry in Clinical Practice, 16(2), 77–84. 10.3109/13651501.2012.667114 [DOI] [PubMed] [Google Scholar]
- Barton, S. , Karner, C. , Salih, F. , Baldwin, D. S. , & Edwards, S. J. (2014). Clinical effectiveness of interventions for treatment‐resistant anxiety in older people: A systematic review. Health Technology Assessment, 18(50), 1–62. 10.3310/hta18500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawman‐Mintzer, O. , Knapp, R. G. , & Nietert, P. J. (2005). Adjunctive risperidone in generalized anxiety disorder: A double‐blind, placebo‐controlled study. Journal of Clinical Psychiatry, 66(10), 1321–1325. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L41546513 [DOI] [PubMed] [Google Scholar]
- Bruce, S. E. , Yonkers, K. A. , Otto, M. W. , Eisen, J. L. , Weisberg, R. B. , Pagano, M. , & Keller, M. B. (2005). Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: A 12‐year prospective study. American Journal of Psychiatry, 162(6), 1179–1187. 10.1176/appi.ajp.162.6.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky, A. (2006). Treatment‐resistant anxiety disorders. Molecular Psychiatry, 11(9), 805–814. 10.1038/sj.mp.4001852 [DOI] [PubMed] [Google Scholar]
- Bystritsky, A. , Stein, M. B. , & Hermann, R. (2016). Pharmacotherapy for generalized anxiety disorder in adults. November 22, 2016, Retrieved from. https://www‐uptodate‐com.vu‐nl.idm.oclc.org/contents/psychotherapy‐for‐generalized‐anxiety‐disorder‐in‐adults
- Carpenter, J. K. , Andrews, L. A. , Witcraft, S. M. , Powers, M. B. , Smits, J. A. J. , & Hofmann, S. G. (2018). Cognitive behavioral therapy for anxiety and related disorders: A meta‐analysis of randomized placebo‐controlled trials. Depression and Anxiety, 35(6), 502–514. 10.1002/da.22728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle, C. , Gray, A. , Neehoff, S. , & Glue, P. (2017). Effect of ketamine dose on self‐rated dissociation in patients with treatment refractory anxiety disorders. Journal of Psychopharmacology, 31(10), 1306–1311. 10.1177/0269881117725685 [DOI] [PubMed] [Google Scholar]
- Chen, M.‐H. , & Tsai, S.‐J. (2016). Treatment‐resistant panic disorder: Clinical significance, concept and management. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 70, 219–226. 10.1016/j.pnpbp.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Cirillo, P. , & Freire, R. C. R. (2016). Pharmacological treatment of panic disorder with non‐selective drugs In Nardi A. E., Freire R. C. R., Nardi A. E., & Freire R. C. R. (Eds.), Panic disorder: Neurobiological and treatment aspects (pp. 289–301). Switzerland: Springer International Publishing; 10.1007/978-3-319-12538-1_19 [DOI] [Google Scholar]
- Conway, C. R. , George, M. S. , & Sackeim, H. A. (2017). Toward an evidence‐based, operational definition of treatment‐resistant depression. JAMA Psychiatry, 74(1), 9 10.1001/jamapsychiatry.2016.2586 [DOI] [PubMed] [Google Scholar]
- Cosci, F. , & Fava, G. A. (2013). Staging of mental disorders: Systematic review. Psychotherapy and Psychosomatics, 82(1), 20–34. 10.1159/000342243 [DOI] [PubMed] [Google Scholar]
- Cowley, D. S. , Ha, E. H. , & Roy‐Byrne, P. P. (1997). Determinants of pharmacologic treatment failure in panic disorder. Journal of Clinical Psychiatry, 58(12), 555–561. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L28100280 [DOI] [PubMed] [Google Scholar]
- Craske, M. , Stein, M. B. , & Hermann, R. (2016). Psychotherapy for generalized anxiety disorder in adults. November 22, 2016, Retrieved from https://www‐uptodate‐com.vu‐nl.idm.oclc.org/contents/psychotherapy‐for‐generalized‐anxiety‐disorder‐in‐adults
- Craske, M. , Bystritsky, A. , Stein, M. B. , & Hermann, R. (2016). Approach to treating generalized anxiety disorder in adults. November 22, 2016, Retrieved from. https://www.uptodate.com/contents/approach‐to‐treating‐generalized‐anxiety‐disorder‐in‐adults
- Davies, R. , Dubovsky, S. , Gabbert, S. , & Champman, M. (2000). Treatment resistence in anxiety disorders. Bulletin of the Menninger Clinic, 64(3), A22–A36. [PubMed] [Google Scholar]
- De Salas‐Cansado, M. , Álvarez, E. , Olivares, J. M. , Carrasco, J. L. , Ferro, M. B. , & Rejas, J. (2013). Modelling the cost‐effectiveness of pregabalin versus usual care in daily practice in the treatment of refractory generalised anxiety disorder in Spain. Social Psychiatry and Psychiatric Epidemiology, 48(6), 985–996. 10.1007/s00127-012-0606-6 [DOI] [PubMed] [Google Scholar]
- Deligiannidis, K. M. , & Rothschild, A. J. (2010). Mood and anxiety disorders In Rothschild A. J. (Ed.), The evidence‐based guide to antipsychotic medications (1st ed., pp. 45–100). Arlington, VA: American Psychiatric Publishing, Inc; Retrieved from. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2010‐03392‐003&site=ehost‐live [Google Scholar]
- Durham, R. C. , Higgins, C. , Chambers, J. A. , Swan, J. S. , & Dow, M. G. T. (2012). Long‐term outcome of eight clinical trials of CBT for anxiety disorders: Symptom profile of sustained recovery and treatment‐resistant groups. Journal of Affective Disorders, 136(3), 875–881. 10.1016/j.jad.2011.09.017 [DOI] [PubMed] [Google Scholar]
- Farach, F. J. , Pruitt, L. D. , Jun, J. J. , Jerud, A. B. , Zoellner, L. A. , & Roy‐Byrne, P. P. (2012). Pharmacological treatment of anxiety disorders: Current treatments and future directions. Journal of Anxiety Disorders, 26(8), 833–843. 10.1016/j.janxdis.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava, G. A. , Rafanelli, C. , & Tomba, E. (2012). The clinical process in psychiatry: A clinimetric approach. Journal of Clinical Psychiatry, 73(2), 177–184. 10.4088/JCP.10r06444 [DOI] [PubMed] [Google Scholar]
- Fekadu, A. , Wooderson, S. , Donaldson, C. , Markopoulou, K. , Masterson, B. , Poon, L. , & Cleare, A. J. (2009). A multidimensional tool to quantify treatment resistance in depression: The Maudsley staging method. Journal of Clinical Psychiatry, 70(2), 177–184. 10.4088/JCP.08m04309 [DOI] [PubMed] [Google Scholar]
- Fogelson, D. L. , & Leuchter, A. (2017). Defining treatment‐resistant depression. JAMA Psychiatry, 74(7), 758 10.1001/jamapsychiatry.2017.0837 [DOI] [PubMed] [Google Scholar]
- Gabriel, A. (2010). The mixed amphetamine salt extended release (Adderall XR, Max‐XR) as an adjunctive to SSRIs or SNRIs in the treatment of adult ADHD patients with comorbid partially responsive generalized anxiety: An open‐label study. ADHD Attention Deficit and Hyperactivity Disorders, 2(2), 87–92. 10.1007/s12402-010-0025-z [DOI] [PubMed] [Google Scholar]
- Gabriel, A. , & Violato, C. (2011). Adjunctive atomoxetine to SSRIs or SNRIs in the treatment of adult ADHD patients with comorbid partially responsive generalized anxiety (GA): An open‐label study. ADHD Attention Deficit and Hyperactivity Disorders, 3(4), 319–326. 10.1007/s12402-011-0063-1 [DOI] [PubMed] [Google Scholar]
- George, M. S. , Ward, H. E. , Ninan, P. T. , Pollack, M. , Nahas, Z. , Anderson, B. , & Ballenger, J. C. (2008). A pilot study of vagus nerve stimulation (VNS) for treatment‐resistant anxiety disorders. Brain Stimulation, 1(2), 112–121. 10.1016/j.brs.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Gloster, A. T. , Sonntag, R. , Hoyer, J. , Meyer, A. H. , Heinze, S. , Ströhle, A. , & Wittchen, H.‐U. (2015). Treating treatment‐resistant patients with panic disorder and agoraphobia using psychotherapy: A randomized controlled switching trial. Psychotherapy and Psychosomatics, 84(2), 100–109. 10.1159/000370162 [DOI] [PubMed] [Google Scholar]
- Glue, P. , Neehoff, S. M. , Medlicott, N. J. , Gray, A. , Kibby, G. , & McNaughton, N. (2018). Safety and efficacy of maintenance ketamine treatment in patients with treatment‐refractory generalised anxiety and social anxiety disorders. Journal of Psychopharmacology, 32(6), 663–667. 10.1177/0269881118762073 [DOI] [PubMed] [Google Scholar]
- Glue, P. , Medlicott, N. J. , Harland, S. , Neehoff, S. , Anderson‐Fahey, B. , Le Nedelec, M. , & McNaughton, N. (2017). Ketamine's dose‐related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. Journal of Psychopharmacology, 31(10), 1302–1305. 10.1177/0269881117705089 [DOI] [PubMed] [Google Scholar]
- Heldt, E. , Manfro, G. G. , Kipper, L. , Blaya, C. , Isolan, L. , & Otto, M. W. (2006). One‐year follow‐up of pharmacotherapy‐resistant patients with panic disorder treated with cognitive‐behavior therapy: Outcome and predictors of remission. Behaviour Research and Therapy, 44(5), 657–665. 10.1016/j.brat.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Heldt, E. , Manfro, G. G. , Kipper, L. , Blaya, C. , Maltz, S. , Isolan, L. , & Otto, M. W. (2003). Treating medication‐resistant panic disorder: Predictors and outcome of cognitive‐behavior therapy in a Brazilian public hospital. Psychotherapy and Psychosomatics, 72(1), 43–48. 10.1159/000067188 [DOI] [PubMed] [Google Scholar]
- Higgins, J. , & Green, S. (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. Retrieved from. www.handbook.cochrane.org
- Hirschmann, S. , Dannon, P. N. , Iancu, I. , Dolberg, O. T. , Zohar, J. , & Grunhaus, L. (2000). Pindolol augmentation in patients with treatment‐resistant panic disorder: A double‐blind, placebo‐controlled trial. Journal of Clinical Psychopharmacology, 20(5), 556–559. 10.1097/00004714-200010000-00011 [DOI] [PubMed] [Google Scholar]
- Hoge, E. A. , Worthington, J. J., III , Kaufman, R. E. , Delong, H. R. , Pollack, M. H. , Simon, N. M. , & Simon, N. M. (2008). Aripiprazole as augmentation treatment for refractory generalized anxiety disorder and panic disorder. CNS Spectrums, 13(6), 522–527. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L351960777 [DOI] [PubMed] [Google Scholar]
- Hollifield, M. , Thompson, P. M. , Ruiz, J. E. , & Uhlenhuth, E. H. (2005). Potential effectiveness and safety of olanzapine in refractory panic disorder. Depression and Anxiety, 21(1), 33–40. 10.1002/da.20050 [DOI] [PubMed] [Google Scholar]
- Holt, R. L. , & Lydiard, R. B. (2007). Management of treatment‐resistant panic disorder. Psychiatry, 4(10), 48–59. Retrieved from. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2007‐16431‐006&site=ehost‐live [PMC free article] [PubMed] [Google Scholar]
- Howes, O. D. , McCutcheon, R. , Agid, O. , de Bartolomeis, A. , van Beveren, N. J. , Birnbaum, M. L. , & Correll, C. U. (2016). Treatment‐resistant schizophrenia: Treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. American Journal of Psychiatry, 1(9 appi.ajp.2016.1. 10.1176/appi.ajp.2016.16050503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh, J. , Goebert, D. , Takeshita, J. , Lu, B. Y. , & Kang, M. (2011). Treatment of generalized anxiety disorder. The Primary Care Companion for CNS Disorders, 13(2) 10.4088/PCC.08r00709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser, J. C. , Carey, P. , Dhansay, Y. , Fakier, N. , Seedat, S. , & Stein, D. J. (2006). Pharmacotherapy augmentation strategies in treatment‐resistant anxiety disorders (Review). Cochrane Database of Systematic Reviews, (4), 005473 10.1002/14651858.CD005473.pub2 [DOI] [PubMed] [Google Scholar]
- Jakubovski, E. , Johnson, J. A. , Nasir, M. , Müller‐Vahl, K. , & Bloch, M. H. (2018). Systematic review and meta‐analysis: Dose‐response curve of SSRIs and SNRIs in anxiety disorders. Depression and Anxiety, 36(September), 1–15. 10.1002/da.22854 [DOI] [PubMed] [Google Scholar]
- Katzman, M. A. , Vermani, M. , Jacobs, L. , Marcus, M. , Kong, B. , Lessard, S. , & Gendron, A. (2008). Quetiapine as an adjunctive pharmacotherapy for the treatment of non‐remitting generalized anxiety disorder: A flexible‐dose, open‐label pilot trial. Journal of Anxiety Disorders, 22(8), 1480–1486. 10.1016/j.janxdis.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Kim, S. Y. , Park, J. E. , Lee, Y. J. , Seo, H. J. , Sheen, S. S. , Hahn, S. , & Son, H. J. (2013). Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. Journal of Clinical Epidemiology, 66(4), 408–414. 10.1016/j.jclinepi.2012.09.016 [DOI] [PubMed] [Google Scholar]
- Kinrys, G. , Vasconcelos E Sa, D. , & Nery, F. (2007). Adjunctive zonisamide for treatment refractory anxiety. International Journal of Clinical Practice, 61(6), 1050–1053. 10.1111/j.1742-1241.2007.01365.x [DOI] [PubMed] [Google Scholar]
- Kinrys, G. , Worthington, J. J. , Wygant, L. , Nery, F. , Reese, H. , & Pollack, M. H. (2007). Levetiracetam as adjunctive therapy for refractory anxiety disorders. Journal of Clinical Psychiatry, 68(7), 1010–1013. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L47340319 [DOI] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gøtzsche, P. C. , Ioannidis, J. P. A. , & Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine, 6(7), e1000100 10.1016/j.jclinepi.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohoff, F. W. , Etemad, B. , Mandos, L. A. , Gallop, R. , & Rickels, K. (2010). Ziprasidone treatment of refractory generalized anxiety disorder. Journal of Clinical Psychopharmacology, 30(2), 185–189. 10.1097/JCP.0b013e3181d21951 [DOI] [PubMed] [Google Scholar]
- Lorenz, R. A. , Jackson, C. W. , & Saitz, M. (2010). Adjunctive use of atypical antipsychotics for treatment‐resistant generalized anxiety disorder. Pharmacotherapy, 30(9), 942–951. 10.1592/phco.30.9.942 [DOI] [PubMed] [Google Scholar]
- Menza, M. A. , Dobkin, R. D. , & Marin, H. (2007). An open‐label trial of aripiprazole augmentation for treatment‐resistant generalized anxiety disorder [3]. Journal of Clinical Psychopharmacology, 22(2), 207–210. 10.1097/01.jcp.0000248620.34541.bc [DOI] [PubMed] [Google Scholar]
- Milrod, B. , Altemus, M. , Gross, C. , Busch, F. , Silver, G. , Christos, P. , & Schneier, F. (2016). Adult separation anxiety in treatment nonresponders with anxiety disorders: Delineation of the syndrome and exploration of attachment‐based psychotherapy and biomarkers. Comprehensive Psychiatry, 66, 139–145. 10.1016/j.comppsych.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence. (2011). Generalised anxiety disorder and panic disorder in adults: management. CG113. Manchester, United Kingdom. [PubMed]
- Ociskova, M. , Prasko, J. , Latalova, K. , Kamaradova, D. , & Grambal, A. (2016). Psychological factors and treatment effectiveness in resistant anxiety disorders in highly comorbid inpatients. Neuropsychiatric Disease and Treatment, 12, 1539–1551. 10.2147/NDT.S104301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto, M. W. , Pollack, M. H. , Penava, S. J. , & Zucker, B. G. (1999). Group cognitive‐behavior therapy for patients failing to respond to pharmacology for panic disorder: A clinical case series. Behaviour Research and Therapy, 37(8), 763–770. 10.1016/S0005-7967(98)00176-4 [DOI] [PubMed] [Google Scholar]
- Pallanti, S. , & Quercioli, L. (2006). Resistant social anxiety disorder response to Escitalopram. Clinical Practice and Epidemiology in Mental Health, 2(35), 35 10.1186/1745-0179-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, B. , & Van Ameringen, M. (2016). Augmentation strategies for treatment‐resistant anxiety disorders: A systematic review and meta‐analysis. Depression and Anxiety, 33(8), 728–736. 10.1002/da.22525 [DOI] [PubMed] [Google Scholar]
- Peeters, F. P. M. L. , Ruhe, H. G. , Wichers, M. , Abidi, L. , Kaub, K. , van der Lande, H. J. , & Schene, A. H. (2016). The Dutch measure for quantification of treatment resistance in depression (DM‐TRD): An extension of the maudsley staging method. Journal of Affective Disorders, 205, 365–371. 10.1016/j.jad.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Perna, G. , & Caldirola, D. (2017). Management of treatment‐resistant panic disorder. Curr Treat Options Psychiatry, 4(4), 371–386. 10.1007/s40501-017-0128-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack, M. H. (2009). Refractory generalized anxiety disorder. The Journal of Clinical Psychiatry, 70(suppl 2), 32–38. 10.4088/JCP.s.7002.06 [DOI] [PubMed] [Google Scholar]
- Pollack, M. H. , & Smoller, J. W. (1996). Pharmacologic approaches to treatment‐resistant panic disorder In Pollack M. H., Otto M. W., Rosenbaum J. F., Pollack M. H., Otto M. W., & Rosenbaum J. F. (Eds.), Challenges in clinical practice: Pharmacologic and psychosocial strategies (pp. 89–112). New York, NY, US: Guilford Press; Retrieved form. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=1996‐97898‐004&site=ehost‐live [Google Scholar]
- Pollack, M. H. , Otto, M. W. , Kaspi, S. P. , Hammerness, P. G. , & Rosenbaum, J. F. (1994). Cognitive behavior therapy for treatment‐refractory panic disorder. Journal of Clinical Psychiatry, 55(5), 200–205. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L24184829 [PubMed] [Google Scholar]
- Pollack, M. H. , Simon, N. M. , Zalta, A. K. , Worthington, J. J. , Hoge, E. A. , Mick, E. , & Oppenheimer, J. (2006). Olanzapine augmentation of fluoxetine for refractory generalized anxiety disorder: A placebo controlled study. Biological Psychiatry, 59(3), 211–215. 10.1016/j.biopsych.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Ramsawh, H. J. , Raffa, S. D. , Edelen, M. O. , Rende, R. , & Keller, M. B. (2009). Anxiety in middle adulthood: Effects of age and time on the 14‐year course of panic disorder, social phobia and generalized anxiety disorder. Psychological Medicine, 39(4), 615–624. 10.1017/S0033291708003954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickels, K. , Shiovitz, T. M. , Ramey, T. S. , Weaver, J. J. , Knapp, L. E. , & Miceli, J. J. (2012). Adjunctive therapy with pregabalin in generalized anxiety disorder patients with partial response to SSRI or SNRI treatment. International Clinical Psychopharmacology, 27(3), 142–150. 10.1097/YIC.0b013e328350b133 [DOI] [PubMed] [Google Scholar]
- Roy‐Byrne, P. (2015). Treatment‐refractory anxiety; definition, risk factors, and treatment challenges. Dialogues in Clinical Neuroscience, 17(2), 191–206. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L605900234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy‐Byrne, P. P. , Stein, M. B. , & Hermann, R. (2016). Pharmacotherapy for panic disorder in adults. November 22, 2016, Retrieved from. https://www‐uptodate‐com.vu‐nl.idm.oclc.org/contents/pharmacotherapy‐for‐panic‐disorder‐in‐adults
- Samuel, M. , Zimovetz, E. A. , Gabriel, Z. , & Beard, S. M. (2011). Efficacy and safety of treatments for refractory generalized anxiety disorder: A systematic review. International Clinical Psychopharmacology, 26(2), 63–68. 10.1097/YIC.0b013e328341bb4a [DOI] [PubMed] [Google Scholar]
- Saxena, S. , Thornicroft, G. , Knapp, M. , & Whiteford, H. (2007). Resources for mental health: Scarcity, inequity, and inefficiency. Lancet, 370(9590), 878–889. 10.1016/S0140-6736(07)61239-2 [DOI] [PubMed] [Google Scholar]
- Simon, N. M. , Hoge, E. A. , Fischmann, D. , Worthington, J. J., III , Christian, K. M. , Kinrys, G. , & Pollack, M. H. (2006). An open‐label trial of risperidone augmentation for refractory anxiety disorders. Journal of Clinical Psychiatry, 67(3), 381–385. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L43556561 [DOI] [PubMed] [Google Scholar]
- Simon, N. M. , Otto, M. W. , Worthington, J. J. , Hoge, E. A. , Thompson, E. H. , LeBeau, R. T. , & Pollack, M. H. (2009). Next‐step strategies for panic disorder refractory to initial pharmacotherapy. The Journal of Clinical Psychiatry, 70(11), 1563–1570. 10.4088/JCP.08m04485blu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman, S. H. , Rynn, M. A. , Rickels, K. , Snydermae, S. H. , Rynn, M. A. , Rickels, K. , & Rickels, K. (2005). Open‐label pilot study of ziprasidone for refractory generalized anxiety disorder. Journal of Clinical Psychopharmacology, 25(5), 497–499. 10.1097/01.jcp.0000177853.15910.de [DOI] [PubMed] [Google Scholar]
- Solbakken, O. A. , & Abbass, A. (2015). Intensive short‐term dynamic residential treatment program for patients with treatment‐resistant disorders. Journal of Affective Disorders, 181, 67–77. 10.1016/j.jad.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Solbakken, O. A. , & Abbass, A. (2016). Symptom‐ and personality disorder changes in intensive short‐term dynamic residential treatment for treatment‐resistant anxiety and depressive disorders. Acta Neuropsychiatrica, 28(05), 257–271. 10.1017/neu.2016.5 [DOI] [PubMed] [Google Scholar]
- Starcevic, V. (2008). Treatment of panic disorder: Recent developments and current status. Expert Review of Neurotherapeutics, 8(8), 1219–1232. 10.1586/14737175.8.8.1219 [DOI] [PubMed] [Google Scholar]
- Stein, D. J. (2003). Algorithm for the pharmacotherapy of anxiety disorders. Current Psychiatry Reports, 5(4), 282–288. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L38899220 [DOI] [PubMed] [Google Scholar]
- Stein, D. J. (2004). Algorithms for primary care: An evidence‐based approach to the pharmacotherapy of depression and anxiety disorders. Primary psychiatry, 11(6), 55–78. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L38879037 [Google Scholar]
- Stein, D. J. , & Seedat, S. (2004). Unresolved questions about treatment‐resistant anxiety disorders. CNS Spectrums, 9(10), 715 Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/15470558 [DOI] [PubMed] [Google Scholar]
- Stein, D. J. , Kasper, S. , Matsunaga, H. , Osser, D. N. , Stein, M. B. , Van Ameringen, M. , & Zhang, M. (2001). Pharmacotherapy of social anxiety disorder: An algorithm for primary care ‐ 2001. Primary Care Psychiatry, 7(3), 107–110. 10.1185/135525701317094368 [DOI] [Google Scholar]
- Stein, D. J. , Baldwin, D. S. , Bandelow, B. , Blanco, C. , Fontenelle, L. F. , Lee, S. , & Van Ameringen, M. (2010). A 2010 evidence‐based algorithm for the pharmacotherapy of social anxiety disorder. Current Psychiatry Reports, 12(5), 471–477. 10.1007/s11920-010-0140-8 [DOI] [PubMed] [Google Scholar]
- Taylor, S. , Abramowitz, J. S. , & McKay, D. (2012). Non‐adherence and non‐response in the treatment of anxiety disorders. Journal of Anxiety Disorders, 26(5), 583–589. 10.1016/j.janxdis.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Tesar, G. E. , & Rosenbaum, J. F. (1986). Successful use of clonazepam in patients with treatment‐resistant panic disorder. Journal of Nervous and Mental Disease, 174(8), 477–482. Retrieved from. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L16040140 [DOI] [PubMed] [Google Scholar]
- Tyrer, P. , Seivewright, H. , & Johnson, T. (2004). The Nottingham study of neurotic disorder: Predictors of 12‐year outcome of dysthymic, panic and generalized anxiety disorder. Psychological Medicine, 34(8), 1385–1394. 10.1017/S0033291704002569 [DOI] [PubMed] [Google Scholar]
- Van Ameringen, M. , & Mancini, C. (2001). Pharmacotherapy of social anxiety disorder at the turn of the millennium. Psychiatric Clinics of North America, 24(4), 783–803. 10.1016/S0193-953X(05)70263-X [DOI] [PubMed] [Google Scholar]
- Van Ameringen, M. , Mancini, C. , & Patterson, B. (2009). Oxford handbook of anxiety and related disorders In Antony M.M., & Stein M.B. (Eds.), Pharmacotherapy for social anxiety disorder and specific phobia. New York, NY: Oxford University Press; Retrieved from. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2008‐13193‐024&site=ehost‐live. [Google Scholar]
- van Belkum, S. M. , Geugies, H. , Lysen, T. S. , Cleare, A. J. , Peeters, F. P. , Penninx, B. W. , & Ruhe, H. G. (2018). Validity of the Maudsley Staging Method in predicting treatment‐resistant depression outcome using the netherlands study of depression and anxiety. Journal of Clinical Psychiatry, 79(1 17m11475. 10.1103/PhysRevLett.113.113903 [DOI] [PubMed] [Google Scholar]
- Worthington III, J. J. , Kinrys, G. , Wygant, L. E. , & Pollack, M. H. (2005). Aripiprazole as an augmentor of selective serotonin reuptake inhibitors in depression and anxiety disorder patients. International Clinical Psychopharmacology, 20(1), 9–11. 10.1097/00004850-200501000-00002 [DOI] [PubMed] [Google Scholar]
- Yoshinaga, N. , Matsuki, S. , Niitsu, T. , Sato, Y. , Tanaka, M. , Ibuki, H. , & Shimizu, E. (2016). Cognitive behavioral therapy for patients with social anxiety disorder who remain symptomatic following antidepressant treatment: A randomized, assessor‐blinded, controlled trial. Psychotherapy and Psychosomatics, 85(4), 208–217. 10.1159/000444221 [DOI] [PubMed] [Google Scholar]
- Zoun, M. H. , Koekkoek, B. , Sinnema, H. , Muntingh, A. D. , van Balkom, A. J. , Schene, A. H. , & Spijker, J. (2016). Effectiveness and cost‐effectiveness of a self‐management training for patients with chronic and treatment resistant anxiety or depressive disorders: Design of a multicenter randomized controlled trial. BMC Psychiatry, 16(1), 216 10.1186/s12888-016-0927-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


