Abstract
Background
Hepatocellular adenoma (HCA) larger than 5 cm in diameter has an increased risk of haemorrhage and malignant transformation, and is considered an indication for resection. As an alternative to resection, transarterial embolization (TAE) may play a role in prevention of complications of HCA, but its safety and efficacy are largely unknown. The aim of this study was to assess outcomes and postembolization effects of selective TAE in the management of HCA.
Methods
This retrospective, multicentre cohort study included patients aged at least 18 years, diagnosed with HCA and treated with TAE. Patient characteristics, 30‐day complications, tumour size before and after TAE, symptoms before and after TAE, and need for secondary interventions were analysed.
Results
Overall, 59 patients with a median age of 33.5 years were included from six centres; 57 of the 59 patients were women. Median tumour size at time of TAE was 76 mm. Six of 59 patients (10 per cent) had a major complication (cyst formation or sepsis), which could be resolved with minimal therapy, but prolonged hospital stay. Thirty‐four patients (58 per cent) were symptomatic at presentation. There were no significant differences in symptoms before TAE and symptoms evaluated in the short term (within 3 months) after TAE (P = 0·134). First follow‐up imaging was performed a median of 5·5 months after TAE and showed a reduction in size to a median of 48 mm (P < 0·001).
Conclusion
TAE is safe, can lead to adequate size reduction of HCA and, offers an alternative to resection in selected patients.
Abstract
Antecedentes
El adenoma hepatocelular (hepatocellular adenoma, HCA) > 5 cm de diámetro tiene un riesgo mayor de hemorragia y transformación maligna, y se considera una indicación de resección. Como alternativa a la resección, la embolización transarterial (transarterial embolization, TAE) puede desempeñar un papel en la prevención de complicaciones del HCA, pero su seguridad y eficacia son en gran parte desconocidas. El objetivo de este estudio fue evaluar los resultados y los efectos de la embolización de la TAE selectiva en el tratamiento del HCA.
Métodos
Se realizó un estudio de cohortes retrospectivo y multicéntrico que incluyó pacientes de edad ≥ 18 años, diagnosticados de HCA y tratados con TAE. Se analizaron las características de los pacientes, las complicaciones a los 30 días, el tamaño del tumor antes y después de la TAE, los síntomas antes y después de la TAE y la necesidad de intervenciones secundarias.
Resultados
En total, 59 pacientes con una mediana de edad de 33,5 años se incluyeron en seis centros; 57 de 59 pacientes eran mujeres. La mediana del tamaño del tumor en el momento de la TAE fue de 76 mm. Seis de 59 (10%) pacientes tuvieron una complicación mayor (es decir, formación de quistes o sepsis), que pudo resolverse con una terapia mínima, pero ello prolongó la estancia hospitalaria. Treinta y cuatro de 59 (58%) pacientes presentaban síntomas en el momento del diagnóstico. No hubo diferencias significativas en los síntomas antes de la TAE y los síntomas evaluados a corto plazo (< 3 meses) después de la TAE (P > 0,050). La primera prueba de imagen de seguimiento se realizó tras una mediana de 5,5 meses después de la TAE y mostró una reducción en el tamaño del adenoma con una mediana de 48 mm (P < 0,001).
Conclusión
La TAE es un procedimiento seguro y puede llevar a una reducción adecuada del tamaño del HCA. Esta intervención mínimamente invasiva ofrece una alternativa a la resección.
Introduction
Hepatocellular adenoma (HCA) is an uncommon benign tumour of the liver that mostly occurs in middle‐aged women1, 2. Over the past few decades, its rising incidence has been associated with the widespread use of oral contraceptives and the obesity pandemic1, 2, 3, 4, 5, 6. Other less common aetiologies include use of anabolic androgens, a history of maturity‐onset diabetes of the young and glycogen storage disease7, 8, 9, 10, 11.
HCA is a hypervascular lesion with an exclusively arterial blood supply, resulting in increased intratumoral pressure, rendering these tumours susceptible to life‐threatening bleeding3, 5, 12. The reported risk of spontaneous bleeding ranges from 20 to 40 per cent13, 14, 15. The risk of bleeding is increased in larger‐diameter tumours, in tumours showing exophytic growth, and an increased risk has also been reported in the sonic hedgehog (sh‐HCA) and inflammatory (I‐HCA) subtypes16, 17, 18, 19, 20, 21, 22, 23, 24. Malignant transformation into hepatocellular carcinoma (HCC) has been documented in 4·3 per cent of patients with HCA25. An established risk factor for malignant transformation is an HCA diameter larger than 5 cm12, 18, 26. Another important risk factor for malignant transformation is a β‐catenin (exon 3) mutation27, 28, 29. MRI, as a non‐invasive technique, has become a useful diagnostic tool in identifying HCA subtypes30, 31.
Patients with HCA may experience complaints such as nausea, pain and tiredness. Although correlation of these symptoms with the presence of HCA remains difficult, they may severely influence patients' quality of life32. Persistence of these complaints, even when the tumour diameter does not exceed 5 cm, may be an indication for resection in selected patients to achieve symptom relief12, 16, 19, 21, 33. To date, elective resection remains the standard treatment for patients with HCA larger than 5 cm, to prevent potential bleeding and malignant transformation18, 20, 21, 23. However, elective liver resection for non‐ruptured benign tumours is associated with a reported major morbidity rate of up to 7·2 per cent34. Transarterial embolization (TAE) offers an attractive minimally invasive alternative treatment for non‐bleeding HCA35, 36, 37. As the majority of patients with HCA are young, otherwise healthy women, cosmetic outcomes can be considered important. TAE could be used in an elective setting to avoid unnecessary resection, in an open or laparoscopic approach.
Conceptually, tumour regression with a potential reduction in the risk of bleeding supports the use of TAE as an elective treatment for HCAs with a diameter of at least 5 cm. The aim of this study was to assess the safety and efficacy of TAE in the management of HCAs, and to describe its postembolization effects in a multicentre retrospective cohort study.
Methods
Approval was obtained from the medical ethics committee of each participating centre. The medical ethics committee declared this a non‐WMO study, which is the Dutch term for a study that does not directly involve human subjects through intervention and thus was exempt from formal ethics approval.
Study design and inclusion
This international retrospective multicentre cohort study included patients diagnosed with HCA who underwent TAE between 2001 and 2018 in a collaboration of four centres participating in the Dutch Benign Liver Tumour Group (DBLTG). In addition, international data were accrued from Beaujon Hospital in Paris (France) and Southampton General Hospital (UK). All participating centres collected data from patients' (electronic) records and charts. All data were processed and stored anonymously. Patients were excluded if essential data on the TAE procedure or outcomes were lacking. The STROBE guidelines38 were adhered to in this study. Details of data storage and ethical approval are available in the study protocol (Appendix S1, supporting information).
Definitions and outcomes
Data on the following patient characteristics were collected: age, sex, BMI, oral contraceptive or steroid use, co‐morbidities associated with HCA, hepatitis B and C viral status, presence of solitary or multiple tumours, and the liver segments involved. In addition, information on the type of imaging used for diagnosis was documented (contrast‐enhanced (CE) MRI, MRI without contrast, CE‐CT, CT without contrast, and regular ultrasound examination). Data on subtype of HCA based on imaging, subtype based on assessment of biopsy or resection specimen, and molecular subtype based on molecular diagnostics were collected. Subtypes were noted as a baseline characteristic, but not used in subgroup analyses owing to the limited number of patients available for this study. When no material for pathology was present, HCA subtype was determined based on CE‐MRI. Subtypes were classified as steatotic (H‐HCA), inflammatory (I‐HCA), β‐catenin‐activated (β‐HCA), and combined inflammatory and β‐catenin‐activated HCA (β‐IHCA)39, 40; sh‐HCA could not be identified because molecular diagnostics for this subtype had not yet been implemented at time of diagnosis of most patients. Unclassified adenomas were adenomas for which no subtype could be determined. Data on the presence of bleeding (clinical, subclinical, bleeding after biopsy), indication for TAE (elective because of size, elective because of symptoms, elective because of (impending) bleeding, acute because of bleeding or poor surgical candidate) and management (TAE, surgery after TAE, radiofrequency ablation (RFA) and TAE combined, TAE and RFA combined followed by surgery) were collected. The type of embolization agent used was also recorded.
Primary outcomes
The primary outcome measures were safety, defined as procedure‐related morbidity consisting of postembolization syndrome (right upper quadrant pain, fever, nausea/vomiting)41, decrease in renal function42, sepsis (life‐threatening organ dysfunction caused by a dysregulated host response to infection)43, and adverse events (abscess formation, bleeding, malignant transformation). Complications were graded according to the Society of Interventional Radiology Classification System for Complications by Outcome (SIR)44. Minor complications were classified as: A, no therapy, no consequence; and B, nominal therapy, no consequence, including overnight admission for observation only. Major complications were classified as: C, requiring therapy, minor hospital admission (less than 48 h); D, requiring major therapy, unplanned increase in level of care, prolonged hospital stay (48 h or more); E, permanent adverse sequelae; and F, death. Duration of hospital stay was also recorded.
Secondary outcomes
The secondary outcomes related to efficacy: the short‐term effects of TAE on symptoms and size of both bleeding and non‐bleeding HCAs. To this end, data on symptoms before and within 3 months after TAE were obtained. In addition, any decrease in tumour size was registered; tumour size was defined as the diameter of the largest tumour present. Tumour size was measured at diagnostic imaging, last imaging before TAE, first follow‐up after TAE and last follow‐up. Tumour growth or regression was assessed using the Response Evaluation Criteria in Solid Tumours (RECIST)45. Large (intra)tumoral haematomas were not included in total tumour size. If adenomatous tissue was not visible in the HCA because of haematoma, the patient was not included in this study. The overall success rate was based on how often surgery was prevented by TAE. The following data were recorded for patients who subsequently underwent surgery: indication for surgery, symptoms before and after surgery, type of surgery, perioperative and postoperative complications, duration of hospital stay and the occurrence of incisional hernias. All outcomes were analysed in two categories: for bleeding (confirmed on imaging, being the indication for TAE) and non‐bleeding HCAs.
Statistical analysis
Outcomes were reported for the complete cohort, and stratified according to whether the tumour was bleeding. Categorical data are presented as proportions; continuous data are reported as mean(s.d.) if normally distributed, and as median (i.q.r.) if not. Normality was assessed using both Kolmogorov–Smirnov and Shapiro–Wilk tests for normality. Values for asymmetry and kurtosis of between –2 and + 2 were considered acceptable as proof of normal univariable distribution46. The Wilcoxon signed‐rank test was used for analysis of data without a normal distribution (tumour size), and McNemar's test for paired nominal data (symptoms before and after surgery). Two‐tailed P < 0·050 was considered statistically significant. SPSS® for Windows® version 24.0 (IBM, Armonk, New York, USA) was used.
Results
Overall, 60 patients were identified, of whom 59 met the inclusion criteria (Table 1). Twenty‐three patients underwent TAE to treat a bleeding HCA, and 36 had elective TAE for a non‐bleeding HCA. One patient was excluded because portal vein embolization was reported and not TAE. Overall, median age was 33·5 years and 57 of 59 patients (97 per cent) were women. Median tumour size at the time of diagnosis was 84 mm, 85 mm for bleeding HCAs and 83 mm for non‐bleeding HCAs. Median tumour size measured just before TAE was 76 mm overall, 82 mm for bleeding HCAs and 70 mm for non‐bleeding lesions (Fig. 1); these measurements were made 5·7 months after diagnosis, and were not significantly different from those obtained at the time of diagnosis. The most frequently used embolization agent was polyvinyl alcohol in the bleeding group (9 of 23), whereas microspheres were most commonly used in the non‐bleeding group (14 of 36).
Table 1.
Baseline characteristics in patients with bleeding and non‐bleeding hepatocellular adenomas
| Overall (n = 59) | Bleeding (n = 23) | Non‐bleeding (n = 36) | |
|---|---|---|---|
| Age (years) * | 33·5 (26·1–41·2) | 34·4 (25·0–42·7) | 32·7 (26·7–39·9) |
| BMI (kg/m 2 ) * | 29·8 (25·3–37·9) | 30·1 (26·3–32·0) | 28·7 (24·3–39·5) |
| Tumour size at diagnosis (mm) * | 84 (59–100) | 85 (58–100) | 83 (57–106) |
| Sex ratio (F : M) | 57 : 2 | 23 : 0 | 34 : 2 |
| Oral contraceptive | |||
| Stopped at time of diagnosis | 43 (73) | 18 (78) | 25 (69) |
| Stopped before diagnosis | 10 (17) | 4 (17) | 6 (17) |
| Unknown | 4 (7) | 1 (4) | 3 (8) |
| Anabolic steroids | 1 (2) | 0 (0) | 1 (3) |
| Never used | 1 (2) | 0 (0) | 1 (3) |
| Co‐morbidities | |||
| Diabetes mellitus type I | 3 (5) | 2 (9) | 1 (3) |
| Diabetes mellitus type II | 2 (3) | 1 (4) | 1 (3) |
| MODY 3 | 1 (2) | 0 (0) | 1 (3) |
| Glycogen storage disease | 1 (2) | 0 (0) | 1 (3) |
| Insulin resistance and PCOS | 1 (2) | 0 (0) | 1 (3) |
| None | 51 (86) | 20 (87) | 31 (86) |
| Viral status | |||
| Hepatitis B‐positive† | 2 (3) | 1 (4) | 1 (3) |
| Hepatitis C‐positive | 0 (0) | 0 (0) | 0 (0) |
| Tumour pattern | |||
| Solitary | 24 (41) | 8 (35) | 16 (44) |
| Multiple | 35 (59) | 15 (65) | 20 (56) |
| Tumour location | |||
| Both | 30 (51) | 14 (61) | 16 (44) |
| Right | 19 (32) | 6 (26) | 13 (36) |
| Left | 9 (15) | 2 (9) | 7 (19) |
| Unknown | 1 (2) | 1 (4) | 0 (0) |
| Imaging | |||
| CE‐MRI | 50 (85) | 18 (78) | 32 (89) |
| CE‐CT | 4 (7) | 3 (13) | 1 (3) |
| Ultrasound imaging and CE‐CT | 4 (7) | 1 (4) | 3 (8) |
| Ultrasound imaging | 1 (2) | 1 (4) | 0 (0) |
| Molecular subtype ‡ | |||
| HCA – subtype not specified | 33 (56) | 18 (78) | 15 (42) |
| I‐HCA | 15 (25) | 4 (17) | 11 (31) |
| H‐HCA | 5 (8) | 0 (0) | 5 (14) |
| β‐IHCA | 3 (5) | 0 (0) | 3 (8) |
| U‐HCA | 1 (2) | 1 (4) | 0 (0) |
| Uncertain diagnosis | 1 (2) | 0 (0) | 1 (3) |
| I‐HCA + HCC | 1 (2) | 0 (0) | 1 (3) |
| Bleeding | |||
| No bleeding | 28 (47) | 0 (0) | 28 (78) |
| Clinical bleeding | 20 (34) | 20 (87) | 0 (0) |
| Subclinical bleeding | 6 (10) | 1 (4) | 5 (14) |
| Bleeding after biopsy | 5 (8) | 2 (9) | 3 (8) |
| Indication for TAE § | |||
| Size, elective | 31 (53) | 0 (0) | 31 (86) |
| Haemorrhage, acute | 19 (32) | 19 (83) | 0 (0) |
| Haemorrhage, elective | 4 (7) | 4 (17) | 0 (0) |
| Symptoms | 4 (7) | 0 (0) | 4 (11) |
| Poor surgical candidate | 1 (2) | 0 (0) | 1 (3) |
| Management | |||
| TAE | 36 (61) | 14 (61) | 22 (61) |
| TAE then surgery | 19 (32) | 6 (26) | 13 (36) |
| TAE and RFA | 3 (5) | 2 (9) | 1 (3) |
| TAE and RFA then surgery | 1 (2) | 1 (4) | 0 (0) |
| Embolic agent | |||
| PVA | 22 (37) | 9 (39) | 13 (36) |
| Microspheres | 16 (27) | 2 (9) | 14 (39) |
| (Platinum) coils | 6 (10) | 5 (22) | 1 (3) |
| Gelatine sponge/foam | 3 (5) | 2 (9) | 1 (3) |
| Foam + PVA | 3 (5) | 2 (9) | 1 (3) |
| Coils + microspheres | 1 (2) | 1 (4) | 0 (0) |
| Unknown | 8 (14) | 2 (9) | 6 (17) |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.).
One hepatitis B‐positive patient had suspicion of hepatocellular carcinoma (HCC).
Based on histopathological analysis, and, if unavailable, on MRI.
Thirty‐five of 36 patients in the non‐bleeding group had tumours larger than 5 cm. MODY, maturity‐onset diabetes of the young; PCOS, polycystic ovary syndrome; CE, contrast‐enhanced; HCA, hepatocellular adenoma; I‐HCA, inflammatory HCA; H‐HCA, steatotic HCA; β‐IHCA, combined inflammatory and β‐catenin‐activated inflammatory HCA; U‐HCA, unclassified HCA; TAE, transarterial embolization; RFA, radiofrequency ablation; PVA, polyvinyl alcohol.
Figure 1.
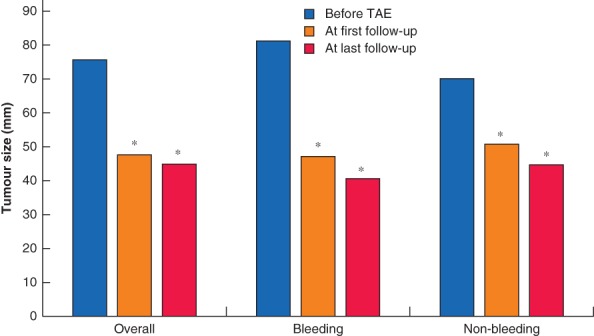
Median tumour size before and after transarterial embolization *P < 0·001 versus before transarterial embolization (TAE) (Wilcoxon signed‐rank test).
Safety of transarterial embolization
Seven of 59 patients (12 per cent) had complications, of whom six (10 per cent) had an SIR D complication (Table S1, supporting information). Of these, two patients developed an intrahepatic cyst, which required drainage because of infection. Two other patients developed sepsis, which was treated successfully with supportive care including antibiotics. One patient died 3 months after TAE; this patient underwent emergency TAE for a bleeding HCA because of haemorrhagic shock. After TAE, the patient was transferred to the ICU of another hospital, but subsequently developed aspiration pneumonia and died. Further details of this patient were not available owing to anonymous data collection and lack of consent to obtain individual data from the other hospital. During follow‐up after TAE, no patient experienced clinically overt bleeding or rebleeding of a treated HCA. Postembolization syndrome (right upper quadrant pain, fever, nausea/vomiting) was observed in one patient, which resolved without intervention. No clinically relevant disturbances of liver function tests were reported. Short‐term pain occurred after TAE in ten patients (17 per cent).
Symptoms before and after transarterial embolization
There were no significant differences in the overall incidence of symptoms before TAE compared with short‐term evaluation within 3 months of TAE (58 versus 37 per cent; P = 0·134). Nor were any significant differences noted before and after TAE in the bleeding and non‐bleeding groups (Table 2). A flow chart summarizing the relief of preprocedural complaints and development of new complaints is shown in Fig. 2.
Table 2.
Symptoms before and after transarterial embolization
| Overall (n = 59) | Bleeding (n = 23) | Non‐bleeding (n = 36) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before TAE | After TAE | P * | Before TAE | After TAE | P * | Before TAE | After TAE | P * | |
| Short‐term symptoms | 34 (58) | 22 (37) | 0·134 | 17 (74) | 8 (35) | 0·109 | 17 (47) | 14 (39) | 0·774 |
| Pain | 27 (46) | 15 (25) | 15 (65) | 3 (13) | 12 (33) | 12 (33) | |||
| None | 16 (27) | 24 (41) | 5 (22) | 9 (39) | 11 (31) | 15 (42) | |||
| Multiple | 5 (9) | 1 (2) | 1 (4) | 0 (0) | 4 (11) | 1 (3) | |||
| Nausea | 1 (2) | 2 (3) | 1 (4) | 2 (9) | 0 (0) | 0 (0) | |||
| Tiredness | 0 (0) | 4 (7) | 0 (0) | 3 (13) | 0 (0) | 1 (3) | |||
| Missing | 10 (17) | 13 (22) | 1 (4) | 6 (26) | 9 (25) | 7 (19) | |||
Values in parentheses are percentages. TAE, transarterial embolization.
McNemar's test for paired nominal data.
Figure 2.
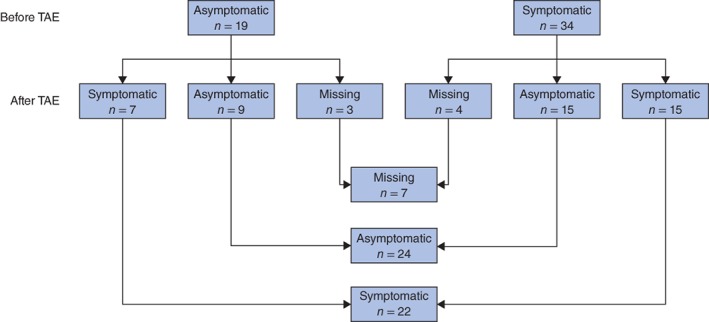
Patients with symptoms before and after transarterial embolization The newly developed complaints among seven patients who were asymptomatic before transarterial embolization (TAE) lasted for only a short time. In 15 patients who were symptomatic before TAE, complaints either persisted or were replaced by short‐term complaints; five of these 15 patients had short‐term complaints only.
Outcomes of transarterial embolization and surgery
Data on TAE and surgical intervention are presented in Table 3. Most patients received only a single session of TAE (36 of 59, 61 per cent). Median overall duration of hospital stay after TAE was 3 days; patients with a bleeding HCA had a significantly longer hospital stay than those with a non‐bleeding tumour (median 12 versus 2 days; P = 0·013).
Table 3.
Outcomes of transarterial embolization and surgery
| Overall (n = 59) | Bleeding (n = 23) | Non‐bleeding (n = 36) | |
|---|---|---|---|
| TAE | |||
| No. of sessions required | |||
| 1 | 36 (61) | 13 (57) | 23 (64) |
| 2 | 6 (10) | 2 (9) | 4 (11) |
| 3 | 5 (8) | 2 (9) | 3 (8) |
| 4 | 1 (2) | 0 (0) | 1 (3) |
| Not reported | 11 (19) | 6 (26) | 5 (14) |
| Duration of hospital stay (days)* | 3·0 (1·0–10·5) | 12·0 (4·8–18·3) | 2·0 (1·0–5·0) |
| Surgery | 20 (34) | 7 (30) | 13 (36) |
| Indication for surgery | |||
| Persisting size | 9 (45) | 1 (14) | 8 (62) |
| Bleeding | 4 (20) | 4 (57) | 0 (0) |
| HCC suspected | 3 (15) | 1 (14) | 2 (15) |
| Growth | 2 (10) | 0 (0) | 2 (15) |
| Persisting symptoms | 1 (5) | 0 (0) | 1 (8) |
| Pregnancy wish | 1 (5) | 1 (14) | 0 (0) |
| Type of surgery | |||
| Open segment resection | 9 (45) | 4 (57) | 5 (38) |
| Open hemihepatectomy | 6 (30) | 2 (29) | 4 (31) |
| Laparoscopic hemihepatectomy | 5 (25) | 1 (14) | 4 (31) |
| Perioperative complications | |||
| None | 18 (90) | 6 (86) | 12 (92) |
| Bleeding | 2 (10) | 1 (14) | 1 (8) |
| Postoperative complications | |||
| None | 14 (70) | 3 (43) | 11 (85) |
| Bleeding (Clavien–Dindo I) | 2 (10) | 2 (29) | 0 (0) |
| Abscess (Clavien–Dindo III) | 2 (10) | 2 (29) | 0 (0) |
| Thrombosis (Clavien–Dindo II) | 1 (5) | 0 (0) | 1 (8) |
| Thrombosis and sepsis (Clavien–Dindo II) | 1 (5) | 0 (0) | 1 (8) |
| Duration of hospital stay (days)* | 10·0 (7·5–11·0) | 10·0 (10·0–41·0) | 8·0 (4·0–10·0) |
| Incisional hernia† | 2 (10) | 1 (14) | 1 (8) |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.).
All symptomatic. TAE, transarterial embolization.
One‐third of patients (20 of 59) required resection following initial TAE. Sixteen of these patients had received one session of TAE, whereas three patients had undergone multiple sessions; the number of TAE sessions was not reported for one patient.
Efficacy of transarterial embolization
Changes in tumour size during follow‐up are reported in Fig. 1, and Fig. S1 and Table S2 (supporting information). Overall median tumour size at the time of TAE was 76 mm. First follow‐up imaging a median of 5·5 months after TAE (Table S3, supporting information) showed a reduction in tumour size of 37 per cent, to a median of 48 mm (P < 0·001). At last follow‐up imaging, a median of 27·4 months after TAE, tumour size had reduced by 41 per cent to a median of 45 mm (P < 0·001). Diagnosis and subtypes are summarized in Table S4 (supporting information).
In the subgroup analyses, bleeding HCAs showed similar regression, from 82 mm at the time of TAE, to 47 mm (43 per cent reduction) at first follow‐up (18 patients) (P < 0·001), and 41 mm (50 per cent reduction) at the end of follow‐up (12 patients) (P < 0·001). A similar pattern was observed in the subgroup of non‐bleeding HCAs; median size was 70 mm at the time of TAE, decreasing to 51 mm (27 per cent reduction) at first follow‐up (35 patients) (P < 0·001), and 45 mm (36 per cent reduction) at last follow‐up (21 patients) (P < 0·001).
Of 36 patients (61 per cent) who had no procedure other than TAE during the study interval, 28 had a tumour of 5 cm or larger before embolization. The tumour had decreased to smaller than 5 cm in 17 of the 36 patients after 6 months, and in 27 patients by last follow‐up. No significant differences were found regarding size reduction and regrowth after TAE for the different embolization agents (Table S5, supporting information).
Malignant transformation
Two patients had an uncertain diagnosis on histopathological assessment. The histopathology report stated that a lesion resected before TAE was an I‐HCA/highly differentiated HCC in one patient. This patient had multiple tumours in both liver lobes, and underwent embolization of the remaining lesions, which could not be removed during surgery. No material was available for pathological analysis of these tumours. Although the previous pathology report suggested the presence of I‐HCA/HCC, the clinical course of this patient was uneventful after TAE. The remaining lesions regressed after TAE, no metastasis developed, there were no paraneoplastic signs or raised tumour marker levels, and the patient was still alive after a follow‐up of 4 years with no signs of disease. In the other patient, an uncertain diagnosis of HCA or HCC was based on indistinctive pathological features after hemihepatectomy. This patient was a male body builder and anabolic steroid user, who received TAE for treatment of an HCA in the other liver lobe. Two other patients with biopsy‐proven β‐HCA underwent TAE and showed no signs of malignant transformation or regrowth during follow‐up, after 40 and 39 months respectively.
Discussion
This study has shown that TAE for large HCA is relatively safe and potentially effective in decreasing tumour size, as tumours decreased to approximately half their size after treatment. This decrease was also clinically relevant, as the tumour was smaller than 5 cm in diameter after embolization in the majority of patients, so did not require resection. The results warrant a prospective study to compare outcomes of TAE with those of (minimally invasive) surgery in the management of HCA.
A systematic review42 comprising 151 patients (95 with non‐bleeding tumours) who underwent TAE for the treatment of HCA identified no deaths. Complete regression on imaging of the tumour was observed in 10 per cent of patients, and a decrease of more than 30 per cent of the tumour diameter in 75 per cent. The review, however, could not distinguish size reduction between bleeding and non‐bleeding tumours42. The authors of a small study47 proposed that bleeding of an HCA leads to size reduction, possibly because of tumour disruption and subsequent destruction of the adenomatous tissue. This may imply that the effect of TAE on regression is merely based on the bleeding event. Therefore, to assess the overall effect of TAE on size reduction, bleeding and non‐bleeding HCAs were compared in the present study. A similar size reduction was seen in both groups.
Whether the decrease in size also reduces the risk of malignant transformation is subject to speculation. Suspicion of malignancy was the reason for resection in some patients in this study, but malignancy could not be excluded in only one male patient. No malignant transformation was observed in two patients with β‐HCA after TAE who were followed up for 40 and 39 months respectively. However, as these patients represent the only cases of β‐HCA treated with TAE to be reported in the literature so far, the implications of positive β‐catenin status after biopsy in regard with treatment with TAE are unknown.
The question remains whether biopsy should be performed routinely preceding TAE to identify β‐HCA, especially as molecular diagnostics are now available. However, there are also disadvantages to routine biopsy, such as bleeding, needle‐track tumour seeding (both of which carry a small risk) and sampling error.
Whether TAE helps to relieve complaints in symptomatic patients remains uncertain. No differences in symptoms before TAE and within 3 months after embolization were found in this study. This is surprising, especially considering that patients with an acute bleeding HCA usually present with severe pain. Although it might be assumed that stopping the bleeding would relieve complaints, a counterargument could be that the haematoma remains in patients with intracapsular bleeding. However, there was a trend suggesting relief of symptoms in the bleeding subgroup in the present study, although differences were not statistically significant.
A limitation of this study is that no validated questionnaire was used and only symptoms developing within 3 months of TAE were evaluated. Newly developed complaints could therefore not be distinguished from pre‐existing ones as some patients crossed over from the asymptomatic to the symptomatic group after final treatment (Fig. 2). Moreover, it is unclear whether any new complaints were transient or not. Pain developing after TAE was most likely caused by intratumoral ischaemia induced by TAE. This should subside after necrosis of the tumour is complete. Follow‐up of at least 1 year is necessary to assess the long‐term impact of TAE on relief of symptoms, using a validated quality‐of‐life questionnaire.
An alternative to both surgery and TAE could be local ablation of the HCA. Percutaneous image‐guided ablation is less invasive than open or laparoscopic surgery. Benefits and harms of percutaneous ablation in patients with HCA have never been assessed in a prospective cohort study, and the largest retrospective study48 included only 18 patients. The complications that occurred in the latter study were mainly formation of cysts because of necrosis. These complications were resolved readily using routine therapeutic interventions. Moreover, percutaneous ablation is typically performed in tumours smaller than 4 cm because of the risk of incomplete ablation49, 50. The majority of HCAs with an indication for intervention exceed 5 cm, but it is uncertain whether incomplete ablation of HCA is an issue. An advantage of TAE is that application is not limited by tumour size or location.
Another limitation of the present study is the lack of a control group. Ideally, patients should be randomized between TAE and sham TAE to assess the true effect of TAE on relief of complaints. To assess the effect on tumour size, however, randomization is not strictly necessary, as a placebo effect will not influence tumour size after TAE. The control group in the study by Klompenhouwer and colleagues51 could be used as a surrogate group when considering tumour size; these authors reported regression of HCA during conservative management. However, in the present series, HCA treated with TAE showed a larger decrease in size than the conservatively managed HCA reported by Klompenhouwer et al.51.
A strength of the present study is the national and international collaboration between centres participating in the DBLTG. This collaboration resulted in an increase in volume and in the number of specialized centres involved in the field of benign liver tumours, increasing the quality of the study. A further strength of this study is its clinical relevance; TAE as primary treatment of HCA has the potential to be considered in future guidelines.
Supporting information
Appendix S1. Research protocol
Table S1. Outcomes of patients with complications
Table S2. Change in tumour size during follow‐up.
Table S3. Follow‐up
Table S4. Diagnosis and subtypes
Table S5. Tumour regression per different embolization agent.
Fig. S1. Tumour size before and after TAE, delta per patient.
Four patients had tumours smaller than five cm; of these, two patients had tumours larger than four cm, and two patients smaller four cm. The patients with tumours below 4 cm were treated because of bleeding. The other 2 patients with tumor size above 4 cm and below 5 cm were treated (i) because of tumour growth, and (ii) because of severe complaints.
Acknowledgements
B.V.v.R. and A.J.K. are joint first authors, and J.V. and T.M.v.G. are joint senior authors, of this article. All data materials and analytical methods are available from the corresponding author on request.
Disclosure: The authors declare no conflict of interest.
References
- 1. Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics 2001; 21: 877–892. [DOI] [PubMed] [Google Scholar]
- 2. Reddy KR, Schiff ER. Approach to a liver mass. Semin Liver Dis 1993; 13: 423–435. [DOI] [PubMed] [Google Scholar]
- 3. Contostavlos DL. Letter: benign hepatomas and oral contraceptives. Lancet 1973; 2: 1200. [DOI] [PubMed] [Google Scholar]
- 4. Klatskin G. Hepatic tumors: possible relationship to use of oral contraceptives. Gastroenterology 1977; 73: 386–394. [PubMed] [Google Scholar]
- 5. Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP et al Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA 1979; 242: 644–648. [PubMed] [Google Scholar]
- 6. Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Tyler CW Jr. The association between oral contraception and hepatocellular adenoma – a preliminary report. Int J Gynaecol Obstet 1977; 15: 143–144. [DOI] [PubMed] [Google Scholar]
- 7. Alshak NS, Cocjin J, Podesta L, van de Velde R, Makowka L, Rosenthal P et al Hepatocellular adenoma in glycogen storage disease type IV. Arch Pathol Lab Med 1994; 118: 88–91. [PubMed] [Google Scholar]
- 8. Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr 1997; 24: 276–279. [DOI] [PubMed] [Google Scholar]
- 9. Reddy BS, Rao CV, Rivenson A, Kelloff G. Inhibitory effect of aspirin on azoxymethane‐induced colon carcinogenesis in F344 rats. Carcinogenesis 1993; 14: 1493–1497. [DOI] [PubMed] [Google Scholar]
- 10. Socas L, Zumbado M, Pérez‐Luzardo O, Ramos A, Pérez C, Hernández JR et al Hepatocellular adenomas associated with anabolic androgenic steroid abuse in bodybuilders: a report of two cases and a review of the literature. Br J Sports Med 2005; 39: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Søe KL, Søe M, Gluud C. Liver pathology associated with the use of anabolic–androgenic steroids. Liver 1992; 12: 73–79. [DOI] [PubMed] [Google Scholar]
- 12. Leese T, Farges O, Bismuth H. Liver cell adenomas. A 12‐year surgical experience from a specialist hepato‐biliary unit. Ann Surg 1988; 208: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herman P, Pugliese V, Machado MA, Montagnini AL, Salem MZ, Bacchella T et al Hepatic adenoma and focal nodular hyperplasia: differential diagnosis and treatment. World J Surg 2000; 24: 372–376. [DOI] [PubMed] [Google Scholar]
- 14. Kerlin P, Davis GL, McGill DB, Weiland LH, Adson MA, Sheedy PF II. Hepatic adenoma and focal nodular hyperplasia: clinical, pathologic, and radiologic features. Gastroenterology 1983; 84: 994–1002. [PubMed] [Google Scholar]
- 15. Nagorney DM. Benign hepatic tumors: focal nodular hyperplasia and hepatocellular adenoma. World J Surg 1995; 19: 13–18. [DOI] [PubMed] [Google Scholar]
- 16. Barthelmes L, Tait IS. Liver cell adenoma and liver cell adenomatosis. HPB (Oxford) 2005; 7: 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charny CK, Jarnagin WR, Schwartz LH, Frommeyer HS, DeMatteo RP, Fong Y et al Management of 155 patients with benign liver tumours. Br J Surg 2001; 88: 808–813. [DOI] [PubMed] [Google Scholar]
- 18. Ault GT, Wren SM, Ralls PW, Reynolds TB, Stain SC. Selective management of hepatic adenomas. Am Surg 1996; 62: 825–829. [PubMed] [Google Scholar]
- 19. Chiche L, Dao T, Salamé E, Galais MP, Bouvard N, Schmutz G et al Liver adenomatosis: reappraisal, diagnosis, and surgical management: eight new cases and review of the literature. Ann Surg 2000; 231: 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Zondervan PE, Tilanus HW et al Indications and long‐term outcome of treatment for benign hepatic tumors: a critical appraisal. Arch Surg 2001; 136: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 21. van der Windt DJ, Kok NF, Hussain SM, Zondervan PE, Alwayn IP, de Man RA et al Case‐orientated approach to the management of hepatocellular adenoma. Br J Surg 2006; 93: 1495–1502. [DOI] [PubMed] [Google Scholar]
- 22. Veteläinen R, Erdogan D, de Graaf W, ten Kate F, Jansen PL, Gouma DJ et al Liver adenomatosis: re‐evaluation of aetiology and management. Liver Int 2008; 28: 499–508. [DOI] [PubMed] [Google Scholar]
- 23. Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR et al Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol 2009; 16: 640–648. [DOI] [PubMed] [Google Scholar]
- 24. Bieze M, Phoa SS, Verheij J, van Lienden KP, van Gulik TM. Risk factors for bleeding in hepatocellular adenoma. Br J Surg 2014; 101: 847–855. [DOI] [PubMed] [Google Scholar]
- 25. Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 2010; 12: 509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weimann A, Ringe B, Klempnauer J, Lamesch P, Gratz KF, Prokop M et al Benign liver tumors: differential diagnosis and indications for surgery. World J Surg 1997; 21: 983–990. [DOI] [PubMed] [Google Scholar]
- 27. Bioulac‐Sage P, Balabaud C, Bedossa P, Scoazec JY, Chiche L, Dhillon AP et al; Laennec and Elves groups. Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol 2007; 46: 521–527. [DOI] [PubMed] [Google Scholar]
- 28. Thompson MD, Monga SP. WNT/beta‐catenin signaling in liver health and disease. Hepatology 2007; 45: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 29. Zucman‐Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S et al Genotype–phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 2006; 43: 515–524. [DOI] [PubMed] [Google Scholar]
- 30. Laumonier H, Bioulac‐Sage P, Laurent C, Zucman‐Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology 2008; 48: 808–818. [DOI] [PubMed] [Google Scholar]
- 31. van Aalten SM, Thomeer MG, Terkivatan T, Dwarkasing RS, Verheij J, de Man RA et al Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology 2011; 261: 172–181. [DOI] [PubMed] [Google Scholar]
- 32. Terkivatan T, de Wilt JH, de Man RA, van Rijn RR, Zondervan PE, Tilanus HW et al Indications and long‐term outcome of treatment for benign hepatic tumors: a critical appraisal. Arch Surg 2001; 136: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 33. van Rosmalen BV, Bieze M, Besselink MG, Tanis P, Verheij J, Phoa SS et al Long‐term outcomes of resection in patients with symptomatic benign liver tumours. HPB (Oxford) 2016; 18: 908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Rosmalen BV, de Graeff JJ, van der Poel MJ, de Man IE, Besselink M, Abu Hilal M et al; Dutch Benign Liver Tumour Group. Impact of open and minimally invasive resection of symptomatic solid benign liver tumours on symptoms and quality of life: a systematic review. HPB (Oxford) 2019; doi: 10.1016/j.hpb.2019.02.022 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35. Stoot JH, van der Linden E, Terpstra OT, Schaapherder AF. Life‐saving therapy for haemorrhaging liver adenomas using selective arterial embolization. Br J Surg 2007; 94: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 36. Erdogan D, van Delden OM, Busch OR, Gouma DJ, van Gulik TM. Selective transcatheter arterial embolization for treatment of bleeding complications or reduction of tumor mass of hepatocellular adenomas. Cardiovasc Intervent Radiol 2007; 30: 1252–1258. [DOI] [PubMed] [Google Scholar]
- 37. Deodhar A, Brody LA, Covey AM, Brown KT, Getrajdman GI. Bland embolization in the treatment of hepatic adenomas: preliminary experience. J Vasc Interv Radiol 2011; 22: 795–799. [DOI] [PubMed] [Google Scholar]
- 38. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nault JC, Bioulac‐Sage P, Zucman‐Rossi J. Hepatocellular benign tumors – from molecular classification to personalized clinical care. Gastroenterology 2013; 144: 888–902. [DOI] [PubMed] [Google Scholar]
- 40. Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF et al Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology 2017; 152: 880–894.e6. [DOI] [PubMed] [Google Scholar]
- 41. Blackburn H, West S. Management of postembolization syndrome following hepatic transarterial chemoembolization for primary or metastatic liver cancer. Cancer Nurs 2016; 39: E1–E18. [DOI] [PubMed] [Google Scholar]
- 42. van Rosmalen BV, Coelen RJS, Bieze M, van Delden OM, Verheij J, Dejong CHC et al Systematic review of transarterial embolization for hepatocellular adenomas. Br J Surg 2017; 104: 823–835. [DOI] [PubMed] [Google Scholar]
- 43. Singer M, Deutschman CS, Seymour CW, Shankar‐Hari M, Annane D, Bauer M et al The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khalilzadeh O, Baerlocher MO, Shyn PB, Connolly BL, Devane AM, Morris CS et al Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol 2017; 28: 1432–1437.e3. [DOI] [PubMed] [Google Scholar]
- 45. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 46. George D, Mallery P. SPSS for Windows Step By Step: A Simple Study Guide and Reference, 17.0 Update, 10th edn. Pearson: Boston, 2010.
- 47. Klompenhouwer AJ, de Man RA, Thomeer MG, Ijzermans JN. Management and outcome of hepatocellular adenoma with massive bleeding at presentation. World J Gastroenterol 2017; 23: 4579–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Vledder MG, van Aalten SM, Terkivatan T, de Man RA, Leertouwer T, Ijzermans JN. Safety and efficacy of radiofrequency ablation for hepatocellular adenoma. J Vasc Interv Radiol 2011; 22: 787–793. [DOI] [PubMed] [Google Scholar]
- 49. Solbiati L, Ierace T, Tonolini M, Osti V, Cova L. Radiofrequency thermal ablation of hepatic metastases. Eur J Ultrasound 2001; 13: 149–158. [DOI] [PubMed] [Google Scholar]
- 50. Künzli BM, Abitabile P, Maurer CA. Radiofrequency ablation of liver tumors: actual limitations and potential solutions in the future. World J Hepatol 2011; 3: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klompenhouwer AJ, Alblas M, van Rosmalen BV, Haring MPD, Venema E, Doukas M et al Development and validation of a model to predict regression of large size hepatocellular adenoma. Am J Gastroenterol 2019; doi: 10.14309/ajg.0000000000000182 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Research protocol
Table S1. Outcomes of patients with complications
Table S2. Change in tumour size during follow‐up.
Table S3. Follow‐up
Table S4. Diagnosis and subtypes
Table S5. Tumour regression per different embolization agent.
Fig. S1. Tumour size before and after TAE, delta per patient.
Four patients had tumours smaller than five cm; of these, two patients had tumours larger than four cm, and two patients smaller four cm. The patients with tumours below 4 cm were treated because of bleeding. The other 2 patients with tumor size above 4 cm and below 5 cm were treated (i) because of tumour growth, and (ii) because of severe complaints.


