Abstract
BACKGROUND
Pseudocereals are nutrient‐rich grains with high mineral content but also phytate content. Phytate is a mineral absorption inhibitor. The study's aim was to evaluate phytate degradation during spontaneous fermentation and during Lactobacillus plantarum 299v® fermentation of quinoa, canihua, and amaranth grains and flours. It also aimed to evaluate the accessibility of iron, zinc, and calcium and to estimate their bioavailability before and after the fermentation of flours with starter culture. Lactic acid, pH, phytate, and mineral content were analyzed during fermentation.
RESULTS
Higher phytate degradation was found during the fermentation of flours (64–93%) than during that of grains (12–51%). Results suggest that phytate degradation was mainly due to endogenous phytase activity in different pseudocereals rather than the phytase produced by added microorganisms. The addition of Lactobacillus plantarum 299v® resulted in a higher level of lactic acid (76.8–82.4 g kg−1 DM) during fermentation, and a relatively quicker reduction in pH to 4 than in spontaneous fermentation. Mineral accessibility was increased (1.7–4.6‐fold) and phytate : mineral molar ratios were reduced (1.5–4.2‐fold) in agreement with phytate degradation (1.8–4.2‐fold) in fermented flours. The reduced molar ratios were still above the threshold value for the improved estimated mineral bioavailability of mainly iron.
CONCLUSION
Fermentation proved to be effective for degrading phytate in pseudocereal flours, but less so in grains. Fermentation with Lactobacillus plantarum 299v® improved mineral accessibility and estimated bioavailability in flours. © 2019 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: pseudocereals, phytate, lactic acid fermentation, mineral accessibility, estimated bioavailability
Introduction
Pseudocereals are nutrient‐rich grains, which belong to the Amaranthaceae family.1 Quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and canihua (Chenopodium pallidicaule) originated in the Andean region of South America.1, 2 The main quinoa producers are Peru, Bolivia, and Ecuador.3 These grains have three main parts: the seed coat or pericarp, which is formed by cellulose, hemicellulose, some proteins, minerals, and lignin; the embryo, which contains lipids, proteins, and minerals; and the endosperm, which contains starch and proteins.4
All three pseudocereals are good sources of macro‐ and micronutrients, with protein contents comparable to conventional cereals and excellent nutritional properties connected to the high quality of the protein.1 Pseudocereals are also particularly good sources of minerals, with iron, zinc, calcium, magnesium, manganese, and copper content that is higher than conventional cereals.4, 5 Like cereals, however, pseudocereals also contain mineral absorption inhibitors such as phytate (myo‐inositol hexakisphosphate, IP6),5, 6 which is the main phosphorous storage compound in mature grains.7 Phytic acid binds to positively charged divalent cations such as iron, zinc, calcium, and proteins, forming phytate complexes (IP6) that are stable at intestinal pH (6–7), thus inhibiting the absorption of minerals in the small intestine.8, 9 Iron and zinc are essential for optimal physiological function and to sustain life, but their deficiencies are still a worldwide public health concern, and are particularly significant for pregnant women and children under 5 years.10 The inhibitory effect of phytate on minerals is known to follow a dose‐dependent response; thus, the phytate : mineral molar ratio is often used as an indicator of mineral bioavailability.11 The degradation of phytate content is thus very important to decrease its negative effect on mineral bioavailability. Phytate can be hydrolyzed by phytase enzymes; in this process, divalent minerals are released in their free form for absorption, and lower myo‐inositol phosphates with potential antioxidant benefits for human health are also formed.12 Phytase can be endogenous, can present naturally in raw crops, and it can also be produced by microorganisms such as lactic acid bacteria or yeast present in crops or added as starter culture.13 Phytase activity depends on substrate, pH, temperature, water content, particle size, process, and processing time.14 The endogenous phytase activity of raw crops can be increased using processing techniques such as soaking, malting, hydrothermal processing, and lactic acid fermentation.15
Lactic acid fermentation is commonly used in food production to preserve foods, enhance food safety, improve nutritive value, and modify sensory properties.16 During fermentation, the production of organic acids, mainly lactic acid, leads to a decrease in pH, which favors the increase in activity of endogenous phytase.15 Lactic acid fermentations are carried out by microorganisms present in raw crops or by starter culture.16 The starter cultures most commonly used in the fermentation of foods belong to lactic acid bacteria (LAB); more specifically, Lactobacillus plantarum has been shown to be effective in reducing anti‐nutrients in the fermentation of pearl millet,17 and quinoa flour.18 To date, very few studies have focused on the use of fermentation to reduce the mineral absorption inhibitors in pseudocereals, in particular those found in amaranth and canihua. Because of this, together with the fact that the consumption of quinoa and amaranth has increased in recent years due to both their nutritive value and their potential as functional gluten‐free foods,19 it is of utmost importance to study the processing techniques further to decrease the phytate content of these pseudocereals. The aim of the present study was therefore to evaluate the degradation of phytate in grains and flours of pseudocereals (quinoa, canihua, and amaranth) during spontaneous fermentation and with Lactobacillus plantarum 299v® fermentation (Part 1). The study also aimed to evaluate iron, zinc, and calcium accessibility and to estimate bioavailability in pseudocereal flours before and after fermentation with Lactobacillus plantarum 299v® (Part 2).
Material and Methods
Materials
Three types of pseudocereals were used: quinoa (Chenopodium quinoa), canihua (Chenopodium pallidicaule), and amaranth (Amaranthus caudatus). Part 1 was performed in Sweden with quinoa grains of Bolivian origin purchased at the ICA supermarket in Lund, Sweden, in 2015. Canihua and amaranth grains were purchased from a commercial supplier, Productos Alimenticios Andes Trópico, in Cochabamba, Bolivia, and transported to Sweden in the same year. All the samples were separated into batches of 500 g, packed under vacuum, and stored in the darkness at 4 °C. The moisture content of samples was between 84.6 and 102 g kg−1. Quinoa, canihua, and amaranth flours were prepared in a laboratory‐scale mill (Laboratory Mill 120, Perten Instruments AB, Sweden) and sifted through a 500 μm sieve. Part 2 was performed in Cochabamba, Bolivia, and quinoa, canihua, and amaranth flours were purchased from the commercial supplier Productos Alimenticios Andes Trópico in that city in 2016. According to the information provided by this supplier, quinoa grains were collected in Oruro, Bolivia, canihua grains in Cochabamba, Bolivia, and amaranth grains in Chuquisaca, Bolivia.
Lactobacillus plantarum 299v® (ProbiMage, Sweden) was used as a starter culture for the fermentation of pseudocereals. This strain was isolated from healthy human intestinal mucosa and grown in oat substrate.20
Fermentation process
Figure 1 shows all of the fermentations performed with quinoa, canihua, and amaranth. In Part 1 of the study, spontaneous fermentation and fermentation with Lactobacillus plantarum 299v® (Lp299v®) were performed in parallel on quinoa, canihua, and amaranth grains, and flours. Both types of fermentation were stopped 24 h after fermentation with Lp299v® reached pH ≤4 (36–48 h, in total). Fermentation was stopped by drying the suspension at 60 °C. Based on the results of phytate degradation found in Part 1, Part 2 of the study focused on the Lp299v® fermentation of flour to evaluate mineral accessibility and to estimate mineral bioavailability.
Figure 1.
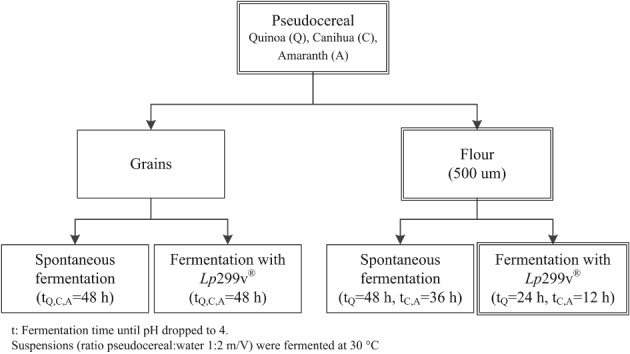
Fermentation process for quinoa, canihua, and amaranth. Part 1 of the study (single‐ and double‐line boxes) includes spontaneous fermentation and Lp299v® fermentation of grains and flours for all three pseudocereals. Part 2 (double line boxes) includes fermentation of quinoa, canihua, and amaranth flour with Lp299v®.
Part 1, spontaneous fermentation and fermentation with Lp299v® of quinoa, canihua, and amaranth grains and flours
Spontaneous fermentation was performed with pseudocereal grains and flour. A suspension of either pseudocereal grains or flour was prepared with demineralized water (ratio 1:2 w/V) in plastic containers, and it was placed in an oven (Oven Termaks TS 8056, Bergen, Norway) at 30 °C for 48 h. Samples were withdrawn at 0, 4, 8, 12, 24, and 48 h for analysis of pH and total acidity, which was expressed as lactic acid content. The Lp299v® fermentation of grains and flours followed the same method with the difference that the suspensions were inoculated with 7.35 Log10 CFU Lp299v® g−1 DM. After fermentation, the suspension of grains in water had approximately 600 g kg−1 of moisture content and the suspension of flour in water had approximately 700 g kg−1 of moisture content. The samples were then dried in a hot oven (Oven Termaks TS 8056, Bergen, Norway) at 60 °C for 4 h. The dried samples (grains and flours) were ground (500 μm, stainless steel sieve, Laboratory Mill 120, Perten Instruments AB, Sweden) and stored in plastic bags at 4 °C for further analysis of phytate and mineral content.
To determine phytase activity of Lp299v® during fermentation, quinoa grains were toasted at 120 °C for 5 min to inactivate microorganism and enzymes. Toasted grains were milled before they were used as substrate for fermentation. A suspension of toasted quinoa flour was inoculated with 7.35 Log10 CFU Lp299v® g−1 DM and was fermented for 10 h at 30 °C. pH, lactic acid, and phytate were evaluated before and after fermentation.
Part 2, Lp299v® fermentation of quinoa, canihua, and amaranth flours to evaluate mineral accessibility and to estimate mineral bioavailability
Lp299v® fermentation was carried out in flours as describe above. The suspensions were fermented until pH was ≤4, quinoa flour was fermented for 24 h, and canihua and amaranth flours for 12 h at 30 °C. pH, lactic acid, phytate, and mineral contents were evaluated before and after fermentation.
Iron, zinc, and calcium accessibility was determined by in vitro gastrointestinal digestion using dissolution equipment (model PTWS 800 D, Pharma Test, Hainburg, Germany). In vitro gastrointestinal digestion was performed according to the method described by Ulmius et al.21 with slight modifications. Five grams of sample was mixed with 200 mL simulated gastric fluid (0.4 g NaCl, 1.4 mL HCl 1 mol L–1, pH 1.2, in 200 mL deionized water) and 0.64 g pepsin (the pepsin was from gastric mucose, ≥ 250 units mg−1solid) (Sigma‐Aldrich, St. Louis, USA). The mixture was digested for 64 min at 37 °C under constant stirring (100 rpm) in a shaking water bath. Then, 50 mL small intestinal boost (0.66 g NaOH, 1.7 g KH2PO4 in 50 mL deionized water) was added, together with 0.375 g bile salts (porcine bile extract) (Sigma‐Aldrich), and pH was adjusted to 6.8 with 2 mol L–1 NaOH. Pancreatin (from porcine pancreas, 8xUSP specification) (Sigma‐Aldrich) was added and intestinal digestion was carried out for 128 min. At the end of the intestinal digestion, an aliquot of 50 mL was withdrawn and centrifuged at 3270×g (Hettich Zentrifugen, model Rotinal 38, Tuttlingen, Germany) for 2 min. The supernatant was recovered and kept in a boiling water bath for 10 min to inactivate the enzymes and then centrifuged at 3270×g for 7 min to separate remaining particles and denaturated proteins. Supernatant was collected and stored at −20 °C for further mineral content analysis. Iron, zinc, and calcium accessibility was calculated with Eqn (1):
| (1) |
Iron, zinc, and calcium bioavailability were estimated using the molar ratio of phytate : mineral before and after fermentation of flours with Lp299v®. Phy:Fe, Phy:Zn, Phy:Ca, and Phy·Ca:Zn molar ratios were calculated using Eqns (2) to (5):
| (2) |
| (3) |
| (4) |
| (5) |
where: 660 = the molecular weight of phytate; 55.8 = the molecular weight of iron; 65.4 = the molecular weight of zinc; 40.0 = the molecular weight of calcium.
The calculated molar ratios were then compared with the recommended ratios for the adequate bioavailability of these minerals. The following molar ratios have been suggested: phytate : iron (Phy:Fe) < 1; phytate:zinc (Phy : Zn) < 15; phytate·calcium:zinc (Phy·Ca:Zn) < 200; and phytate : calcium (Phy:Ca) < 0.17.11, 22
Analytical procedures
Moisture determination
The moisture content was determined by measuring water loss after drying. Five g (±0.0001 g) of each sample was weighed and dried at 105 °C (heating oven model ED23 Binder, Tuttlingen, Germany) until a constant weight was obtained.23
pH and total acidity determination
The determination of pH and total acidity was carried out in duplicate following the method described by Nuobariene et al.24 Briefly, 10 g of sample was suspended with 90 mL of deionized water and homogenized under a stirrer for 120 s. The pH value was recorded with a pH meter (Denver Instrument, VB‐10 Ultra Basic, New York, USA). The total acidity was determined by titration with 0.1 N sodium hydroxide using phenolphthalein as the indicator, until a faint pink color persisted for 30 s. The total acidity was expressed as g kg−1 DM of lactic acid, where 1.0 mL of 0.1 N NaOH was equivalent to 9.0 × 10−3 g lactic acid.23
Mineral content determination
The content of minerals in pseudocereals was determined by the procedure described by Lazarte et al.5 Duplicate samples of 0.5 g (±0.0001 g) were placed in Teflon vessels and mixed with 5 mL HNO3 (65% V/V, Merck, Darmstadt, Germany) and 2 mL H2O2 (30% V/V, Merck, Darmstadt, Germany). After 1 h of acid digestion in a microwave reaction system (Model Multiwave PRO, Anton Paar Co., Ashland, VA, USA), each sample was diluted to 25 mL with deionized water. Iron, zinc, and calcium were analyzed by flame atomic absorption spectrophotometry with air‐acetylene flame (Model AAnalyst 200, Perkin Elmer Corp., Norwalk, CT, USA) at 213.9 nm, 248.3 nm, and 422.7 nm wavelength, respectively. Calcium was analyzed after addition of La2O3 (10 g L−1) (Sigma‐Aldrich) to each sample to prevent phosphorous interference. All materials and glassware were washed with a solution of nitric acid (30 g L−1) and double‐rinsed with distilled water and deionized water to avoid any mineral contamination.
Phytate content determination
Phytate content, as myo‐inositol hexakisphosphate, was analyzed using high‐performance ion chromatography (HPIC) following the method described by Carlsson et al.25 and modified by Lazarte et al.5 Duplicate samples of 0.5 g were extracted with 20 mL of 0.5 mol L–1 HCl for 2 h at room temperature under constant stirring. Supernatant was recovered after centrifugation (Model Allegra® X‐15r, Beckman Coulter, Brea, CA, USA) at 2851 g for 10 min at 20 °C. Supernatant was frozen overnight, thawed, and centrifuged (Model Optima™ LE‐80 k, Beckman Coulter Brea, CA, USA) at 12348 g for 10 min at 20 °C. Supernatant (2 mL) was filtered through a 0.2 μm syringe filter disk and 50 μL of supernatant were injected and analyzed by HPIC with an OmniPac PA‐100 (4 × 250 mm) analytical column and a PA‐100 (4 × 50 mm) guard column (Dionex Corp., Sunnyvale, CA, USA). Detection and quantification of phytate were made after a post‐column reaction with 0.1 g L−1 Fe(NO3)3.9H2O (99.99% trace metal basis) (Sigma‐Aldrich) in 20 g L−1 HClO4; the absorbance was monitored at 290 nm in a UV detector (Waters 486, tunable absorbance detector, Waters Corporation, Milford, MA, USA). Phytate dodecasodium salt hydrate (Sigma‐Aldrich, Staad, Switzerland) was used as standard. The limit of detection of the method was 0.13 g kg−1.
Statistical analysis
The results are presented as means and standard deviations. The effects of fermentation on pH, lactic acid, mineral, and phytate content were tested for significance using the one‐way ANOVA from the SPSS Statistics 24 package (SPSS Inc., IBM Corporation Armok, USA).
Results
Part 1, spontaneous fermentation and fermentation with Lp299v® of quinoa, canihua, and amaranth grains and flours
Figures 2(A), 3(A), and 4(A) show the effects on phytate content of spontaneous fermentation and fermentation with Lp299v® of quinoa, canihua, and amaranth grains and flours. Figures 2(B), 3(B), and 4(B) show the changes in pH and total acidity, expressed as lactic acid content, in the samples during fermentation. In all three pseudocereals, phytate degradation after fermentation (Figs 2(A), 3(A), and 4(A)) was higher when the flours were fermented (64% to 93%) than in grain fermentation (12% to 51%). Regarding the type of fermentation, there were no significant differences in phytate degradation after spontaneous fermentation and fermentation with Lp299v®. However, there were differences in phytate content after fermentation of different pseudocereals. The highest phytate degradation was reached in canihua flour (88–93%) and quinoa flour (83–85%), and the lowest degradation was found in amaranth flour (64% to 80%). The initial pH for all pseudocereal flours (Figs 2(B), 3(B), and 4(B)) ranged between 6.43 and 6.82. The pH of flours fermented with Lp299v® was between 3.78 and 3.81, and it was significantly (P < 0.05) lower than the pH of those that were spontaneously fermented (3.98–4.22). Flours fermented with Lp299v® had significantly higher lactic acid content (76.8–82.4 g kg−1 DM) than those that were spontaneously fermented (52.3–70.5 g kg−1 DM). As expected, there were no significant changes in mineral content before and after fermentation of all three pseudocereals (data not shown).
Figure 2.
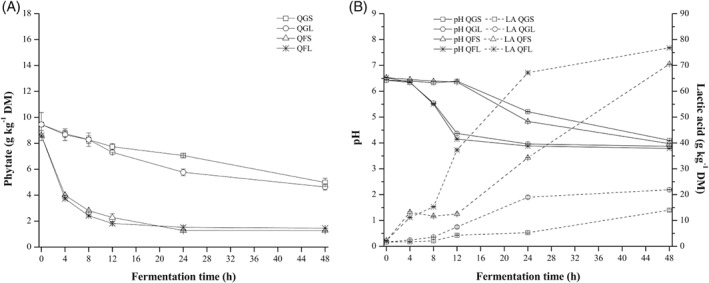
Fermentation of quinoa (grains and flour) – spontaneous fermentation and with starter culture Lp299v®. (A) Effect on phytate content. (B) Effect on pH and lactic acid content. QGS, Quinoa grains spontaneous; QGL, Quinoa grains with starter; QFS, Quinoa flour spontaneous; QFL, Quinoa flour with starter.
Figure 3.
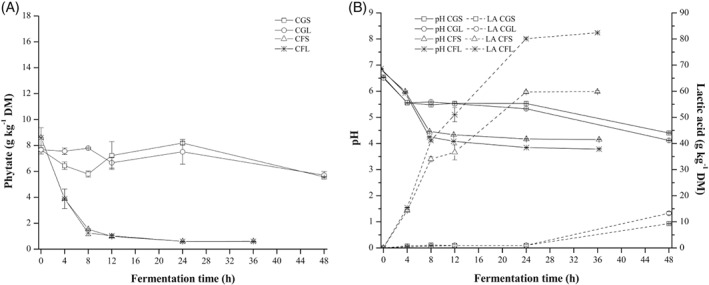
Fermentation of canihua (grains and flour) – spontaneous fermentation and with starter culture Lp299v®. (A) Effect on phytate content. (B) Effect on pH and lactic acid content. CGS, Canihua grains spontaneous; CGL, Canihua grains with starter; CFS, Canihua flour spontaneous; CFL, Canihua flour with starter.
Figure 4.
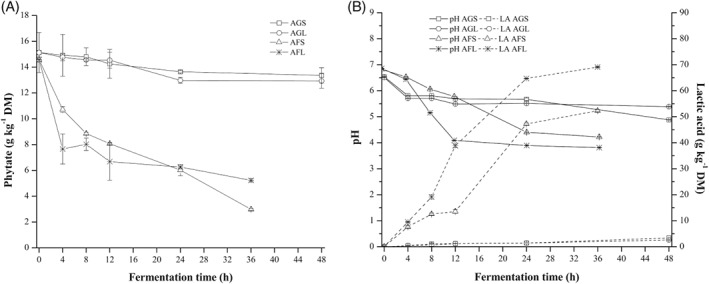
Fermentation of amaranth (grains and flour) – spontaneous fermentation and with starter culture Lp299v®. (A) Effect on phytate content. (B) Effect on pH and lactic acid content. AGS, Amaranth grains spontaneous; AGL, Amaranth grains with starter; AFS, Amaranth flour spontaneous; AFL, Amaranth flour with starter.
To evaluate the phytase activity of Lp299v®, the fermentation of quinoa flour was conducted after toasting quinoa grains. During fermentation process, the phytate content was degraded from 7.06 ± 0.20 to 6.28 ± 0.20 g kg−1 DM, the pH dropped from 6.45 ± 0.01 to 4.22 ± 0.04 and the lactic acid content was increased from 8.80 ± 0.10 to 37.8 ± 0.70 g kg−1 DM.
Part 2, Lp299v® fermentation of quinoa, canihua, and amaranth flours to evaluate mineral accessibility and to estimate mineral bioavailability
Part 2 of the study was focused on evaluating mineral accessibility (in vitro assay) and estimating mineral bioavailability (calculated with phytate : mineral molar ratios) only in flours fermented with Lp299v®. The fermentation conditions were selected based on the results from Part 1 of the study, which showed that: (a) phytate degradation rates resulting from fermentation of pseudocereals flours were higher than those for their grains; (b) Lp299v® fermentation can be better controlled than spontaneous fermentation (i.e. reduced fermentation time, dominant microorganism for fermentation, and higher production of lactic acid), and (c) fewer undesirable odors were perceived when using Lp299v® fermentation compared to spontaneous fermentation.
Table 1 shows the effects of fermentation with Lp299v® on pH, lactic acid, and phytate content in quinoa, canihua, and amaranth flours. Fermentation with Lp299v® significantly (P < 0.05) affected pH and lactic acid and phytate content was significantly reduced in all three pseudocereal flours. The reduction of pH was similar for all flours, reaching values that ranged between 3.86 and 3.90. The increase in lactic acid content was similar for quinoa and canihua flour, but amaranth flour had the lowest increase among them. Table 1 shows that there were no significant (P < 0.05) variations in the iron, zinc, and calcium content of the raw and fermented pseudocereal flours.
Table 1.
Effect of lactic acid fermentation with Lp299v® on pH, lactic acid, mineral, and phytate content and molar ratios of quinoa, canihua, and amaranth flour.1 All concentrations are expressed in dry matter
| Quinoa | Canihua | Amaranth | ||||
|---|---|---|---|---|---|---|
| Parameters | Raw | Fermented | Raw | Fermented | Raw | Fermented |
| Moisture (g kg−1)2 | 93.0 ± 3.0a | 90.6 ± 3.9a | 106 ± 5.2b | 67.0 ± 6.2a | 104 ± 3.5b | 55.0 ± 2.7a |
| pH | 6.20 ± 0.08b | 3.82 ± 0.11a | 6.27 ± 0.04b | 3.90 ± 0.02a | 6.42 ± 0.12b | 3.86 ± 0.04a |
| Lactic acid (g kg−1) | 14.1 ± 0.28a | 75.2 ± 3.6b | 16.3 ± 0.58a | 80.4 ± 1.2b | 6.20 ± 0.08a | 63.9 ± 1.8b |
| Iron (mg kg−1) | 50.8 ± 1.3a | 49.7 ± 4.1a | 117 ± 3.4a | 112 ± 3.2a | 70.0 ± 1.7a | 67.2 ± 1.7a |
| Zinc (mg kg−1) | 41.4 ± 0.40a | 40.2 ± 1.4a | 45.2 ± 0.60a | 42.3 ± 1.5a | 41.2 ± 0.49a | 39.2 ± 3.4a |
| Calcium (mg kg−1) | 0.63 ± 0.02a | 0.70 ± 0.13a | 1.57 ± 0.04a | 1.58 ± 0.14a | 1.52 ± 2.1a | 1.43 ± 1.6a |
| Phytate (g kg−1) | 8.80 ± 0.27b | 2.24 ± 0.25a | 9.21 ± 0.99b | 2.20 ± 0.08a | 11.9 ± 0.43b | 6.44 ± 0.33a |
| Phytate degradation (%) | — | 74.0 | — | 76.2 | — | 47.2 |
| Molar ratios 3 | ||||||
| Phy:Fe | 14.6 ± 0.62b | 3.90 ± 0.77a | 6.66 ± 0.71b | 1.66 ± 0.06a | 12.2 ± 0.87b | 8.12 ± 0.54a |
| Phy:Zn | 20.9 ± 1.1b | 5.55 ± 0.80a | 17.8 ± 2.9b | 6.32 ± 0.36a | 27.5 ± 1.8b | 18.3 ± 2.5a |
| Phy:Ca | 0.82 ± 0.04b | 0.20 ± 0.06a | 0.33 ± 0.05b | 0.09 ± 0.01a | 0.51 ± 0.07b | 0.28 ± 0.03a |
| Phy·Ca:Zn | 332 ± 18b | 94.9 ± 18a | 699 ± 125b | 249 ± 26a | 1005 ± 136b | 641 ± 121a |
Values are shown as mean ± standard deviation (n=6). Bivariate analysis was conducted for each pseudocereal (raw and fermented), different letters indicate significant differences in the parameters at P < 0.05.
Moisture content was determined for the fermented flours, which were dried at 60 °C for 4 h. The differences in the moisture content of the fermented samples (quinoa, canihua and amaranth) may be due to the fact that, during the drying process (60 °C for 4 h), the amount of wet suspension that was placed on the trays was not weighed.
Table 2 shows iron, zinc, and calcium accessibility in raw and Lp299v® fermented flours. Mineral accessibility was significantly improved in all three fermented flours. The highest increases in iron, zinc, and calcium accessibility were observed in fermented quinoa flour (3.6, 4.0, and 3.5‐fold, respectively). The phytate : mineral molar ratios values were reduced in all fermented flours, but almost all of them were still above threshold values. Thus, in quinoa and canihua flour, estimated zinc bioavailability improved from low (Phy:Zn 20.9 and 17.8, respectively) to moderate (Phy:Zn 5.55 and 6.32, respectively). Estimated calcium bioavailability was significantly improved in canihua flour (Phy:Ca 0.09) but was still above the threshold in quinoa and amaranth flours.
Table 2.
In vitro iron, zinc, and calcium accessibility of raw and fermented quinoa, canihua, and amaranth flours
| Sample | Iron accessibility (%) | Zinc accessibility (%) | Calcium accessibility (%) |
|---|---|---|---|
| Quinoa | |||
| Raw | 9.10 ± 1.9 | 14.1 ± 0.9 | 7.35 ± 1.2 |
| Fermenteda | 32.6 ± 3.8 | 56.9 ± 8.1 | 25.5 ± 5.5 |
| Canihua | |||
| Raw | 10.6 ± 0.9 | 9.96 ± 1.9 | 12.3 ± 2.1 |
| Fermentedb | 25.3 ± 2.3 | 46.2 ± 3.5 | 25.3 ± 1.6 |
| Amaranth | |||
| Raw | 9.80 ± 1.2 | 14.9 ± 3.1 | 7.93 ± 2.5 |
| Fermentedb | 26.4 ± 1.7 | 25.6 ± 1.1 | 19.1 ± 3.3 |
Values are shown as mean ± standard deviation (n = 3).
Means of raw and fermented samples are significantly different (P < 0.05).
Quinoa flour fermented with Lp299v® at 30 °C for 24 h.
Canihua or amaranth flour fermented with Lp299v® at 30 °C for 12 h.
Discussion
In the present study it was found that phytate degradation in quinoa, canihua, and amaranth depended on the type of pseudocereal, physical state (i.e. grain or flour), and type of fermentation (spontaneous or with Lp299v®). The study also showed a significant increase in mineral accessibility and estimated bioavailability of iron, zinc, and calcium after Lp299v® fermentation of flours.
Lactic acid fermentation was used to reduce the amount of phytate in a variety of cereals such as wheat, maize, oat, sorghum, and finger millet.26 Studies regarding the fermentation of pseudocereals, especially canihua and amaranth, however, are still limited. In our study, it was found that the fermentation of pseudocereal flours, whether spontaneous or with Lp299v®, achieved a significantly higher degradation of phytate than fermentation of the grains (Figs 2(A)–4(A)). There were significant differences in phytate degradation among fermented grains of quinoa, canihua, and amaranth. Spontaneous fermentation and fermentation with Lp299v® of grains reduced phytate content by 47–51% in quinoa, 25–27% in canihua, and 12–14% in amaranth after 48 h. The higher level of phytate degradation in quinoa may be due to the fact that quinoa grains are subjected to an abrasion process to eliminate saponins6, and this may result in easier diffusion of the nutrients and water necessary for LAB growth and the activation of endogenous phytase.27 Canihua and amaranth grains, on the other hand, do not undergo any process that could strongly modify the seed coat. It is therefore likely that phytate in quinoa, canihua, and amaranth grains is not only located in the seed coat, and may occur throughout the entire seed matrix.28, 29 Konishi et al.28, 29 reported that phosphorous in quinoa and amaranth grains is located in the embryo and the pericarp, where it is associated with the pectic compounds of the cell wall.
The degradation of phytate in pseudocereal flours may depend on the activation of endogenous phytase and on the production of exogenous phytase by starter culture. To follow up the effect of spontaneous fermentation or fermentation with Lp299v® on the degradation of phytate we analyzed the kinetics of the decrease in phytate content and the increase in total acidity, expressed as lactic acid content.
The phytate degradation curves of the flours in Figs 2(A)–4(A) appear to indicate a first‐order process. This is also the most likely reaction kinetic mechanism because it is a single‐component reaction catalyzed by enzymes (assuming that the enzyme concentration is constant).
| (6) |
The degradation rate of a first‐order reaction is proportional to the concentration; the availability of the phytate for the degradation process, however, depends on the level of destruction of the tissue. Thus, it was assumed that a fraction of the phytate (phy ∞) is unavailable for the degradation processes. We assume that this is the concentration at the end‐point of the experiment:
| (7) |
Solving the differential Eqn (7) leads to:
| (8) |
where [phy] is the concentration of phytate, [phy t] is the concentration of phytate as a function of time, time 0 or infinity, k phy is the phytate degradation rate constant (h−1) and t is time in hours. The phytate degradation rate constant is obtained from the slope of against t.
To distinguish between the endogenous degradation and exogenous degradation resulting from the bacterial fermentation, we compared the rate of phytate degradation with the rate of lactic acid formation. The lactic acid formation in Figs 2(B)–4(B) appears to be a linear function with time. The corresponding kinetic equations will be:
| (9) |
| (10) |
| (11) |
where ch refers to the carbohydrates, la the lactic acid concentration, and k la is the lactic acid formation rate constant in g kg−1 DM h−1.
The results for the degradation rate of phytate and formation rate of lactic acid are shown in Table 3. The degradation rate differs between the systems with rapid degradation in canihua flour (0.26 h−1) and the much slower degradation of phytate in amaranth flour (0.05 h−1).
Table 3.
Phytate degradation rate constant (k phy ) and lactic acid formation rate constant (k la) during spontaneous fermentation and fermentation with Lp299v® of quinoa, canihua, and amaranth flours
| Samples |
phy
∞
(g kg−1 DM) |
k phy (h−1) |
k
la
(g kg−1 DM h−1) |
|---|---|---|---|
| QFS | 1.27 ± 0.46 | −0.16 ± 0.03 | 1.40 ± 0.10 |
| QFL | 1.46 ± 0.05 | −0.25 ± 0.01 | 1.63 ± 0.30 |
| CFS | 6.10 ± 0.02 | −0.26 ± 0.01 | 1.62 ± 0.40 |
| CFL | 6.00 ± 0.22 | −0.26 ± 0.04 | 2.29 ± 0.40 |
| AFS | 29.9 ± 0.80 | −0.05 ± 0.00 | 1.57 ± 0. 20 |
| AFL | 52.4 ± 1.20 | −0.07 ± 0.02 | 2.04 ± 0.30 |
QFS: Quinoa flour spontaneous; QFL: Quinoa flour with starter. CFS: Canihua flour spontaneous; CFL: Canihua flour with starter; AFS: Amaranth flour spontaneous; AFL: Amaranth flour with starter.
In Fig. 5, the phytate degradation rate constant is plotted against the lactic acid formation rate constant. A large difference between the lactic acid formation rates for inoculated and spontaneously fermented samples of quinoa and amaranth is visible. A comparison of the two types of fermentation, however, shows only a slight increase in the phytate degradation rates. The canihua samples show a quite similar rate of lactic acid formation, with only a small impact of inoculation, but a very rapid rate of phytate degradation. Assuming that the lactic acid formation rate is a good measure of total fermentation activity, it appears that the main effect is the difference in the endogenous enzyme activities of the different pseudocereals, and to a lesser extent, the contribution from the exogenous phytase activity likely to have been produced during fermentation.
Figure 5.
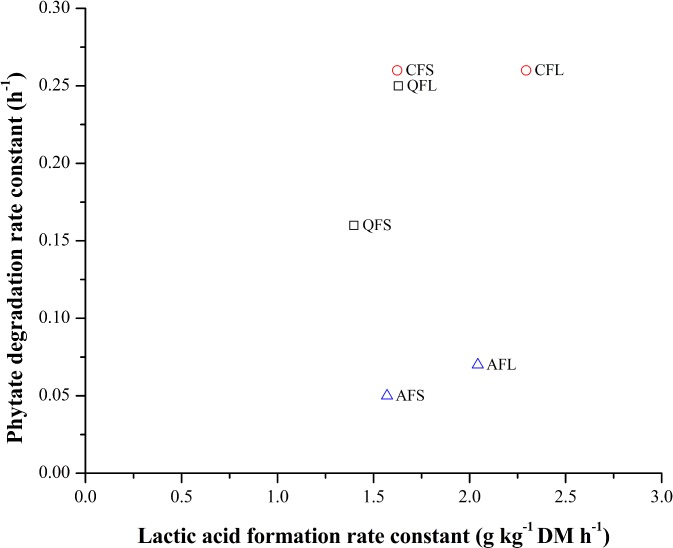
Effect of lactic acid formation rate constant (k la) of spontaneous fermentation and with Lp299v® fermentation on the degradation rate constant of phytate (k phy). QFS, Quinoa flour spontaneous; QFL, Quinoa flour with starter; CFS, Canihua flour spontaneous; CFL, Canihua flour with starter; AFS, Amaranth flour spontaneous; AFL, Amaranth flour with starter.
The pH is an important factor in phytate degradation. Although the reduction in pH follows the same pattern in all three pseudocereals, canihua and amaranth flours fermented with Lp299v® reached pH ≤ 4 in less time than quinoa flour. The reduction in pH is related to the formation of organic acids, mainly lactic acid,30, 31 during the metabolism of the microorganisms. The differences in the availability of nutrients such amino acids and sugars1 in pseudocereals can affect microorganism growth; it has been reported that canihua has a higher free sugar content, which could favor microorganism growth during fermentation.32 It has also been reported that saponins in quinoa may have an inhibitory effect on microorganisms; thus, they need more time to adapt to the new environment.33 A comparison of the pH reductions during spontaneous fermentation and fermentation with Lp299v® in the three pseudocereal flours showed that a lower pH and a higher content of lactic acid were reached after fermentation with Lp299v®. This can be explained by the dominance of lactic microorganisms from the beginning of the fermentation process. In contrast, during spontaneous fermentation of quinoa, canihua, and amaranth flours there may be different microorganisms naturally present in the flour, which compete for nutrients.34 Valencia et al.18 reported a reduction in pH from 6.3 to 3.8 and increase in lactic acid from 70 to 120 g kg−1 DM after quinoa flour was fermented with Lactobacillus plantarum for 18 h. Dallagnol et al.30 reported that, in a quinoa flour suspension fermented with Lactobacillus plantarum CRL 778 for 24 h at 30 °C, the pH dropped from 6.0 to 3.6. The final pH of a control (spontaneous) fermentation without the addition of starter culture was 4.9 after the same time. The researchers reported that the acidity content of fermentation with starter was 2.5‐fold higher than with spontaneous fermentation.30 A limitation of the present study is that spontaneous fermentation was stopped when pH reached 4 in Lp299v® fermentation; the actual time when the spontaneous fermentation reached pH 4 was not recorded.
The changes in pH provide the optimum conditions for the activation of endogenous plant phytase and microbial phytase.34 Egli et al.35 reported that apparent phytase activity in raw quinoa (0.6 PU g−1 DM) and amaranth grains (1.3 PU g−1 DM) at pH 5.0 and 45 °C was lower than for wheat (3.08 PU g−1 DM) and rye grains (6.92 PU g−1 DM), but higher than the phytase activity in oats (0.14 PU g−1 DM). Previous studies have also reported that some strains of LAB release phytase enzymes to hydrolyze phytate but other strains produce phytase to release phosphorous when the only source of this compound is phytate.34 In the present study, the phytate degradation during the spontaneous fermentation may be due mainly to the activation of endogenous phytase. In quinoa, the optimum phytase activity may be at a pH range of 6.36–6.53 (12 h at 30 °C) where 73% of phytate content was reduced during spontaneous fermentation. The phytate degradation during fermentation with Lp299v® might be due to a combined effect of endogenous phytase activity and microbial phytase. It seems that the phytate degradation during the first 8 h (pH range 5.51–6.53) was mainly due to the activated endogenous phytase of quinoa flour, but further phytate degradation between 12 and 48 h (with a pH reduction to 3.88) could be due to an exogenous phytase activity produced by the microorganism rather than the endogenous phytase. Our results agree with Valencia et al.,18 who reported an 82–88% reduction of phytate content in quinoa flours fermented with Lactobacillus plantarum for 16–18 h. Another microorganism used to reduce phytate in quinoa was Rhizopus oligosporus; fermentation of cooked quinoa grains with Rhizopus oligosporus for 40 h reduced the phytate content by 72% from the initial levels.36 In canihua flour, it is likely that the degradation was mainly dominated by the endogenous phytase present in the flour, because the phytate content was similar after spontaneous fermentation and fermentation with Lp299v®. It appears that the pH for optimum activity of endogenous phytase in canihua flour at 30 °C is in the range of 5.93–6.86. At this pH range, the phytate content was degraded by 55% in 4 h in both types of fermentation. At the end of fermentation (pH 3.78, 36 h), the total degradation was 93% of the initial phytate content in both types of fermentation. To date there are no published reports on either spontaneous fermentation of canihua or fermentation with LAB fermentation. So far, only toasting, extrusion, and cooking methods have been investigated as means of phytate degradation in this crop.37 The fermentation of amaranth flour was the least effective for phytate degradation within the first 12 h. According to García‐Mantrana et al.,38 the low phytate degradation during amaranth fermentation can be attributed to the elevated phytate concentration, the presence of endogenous phytase inhibitory compounds in amaranth flour, or the inhibition of phytase activity by phosphate released to the substrate. The addition of the starter culture had a positive effect on the degradation of phytate during the first 4 h of fermentation with Lp299v® with a reduction in pH from 6.82% to 6.50% and 47% of phytate content degradation; in spontaneous fermentation, the pH reduction from 6.86 to 6.44 reduced phytate by 26%. However, when the fermentation was performed for a total of 36 h, spontaneous fermentation was more effective in reducing phytate content (80% reduction) than fermentation with Lp299v® (64% phytate degradation). This higher phytate degradation in the late phase of spontaneously fermented amaranth flour may be explained by the fact that microorganisms naturally present in the flour may have had further phytase activity at a lower pH. Amare et al.39 reported a 77% reduction in phytate after spontaneous fermentation of amaranth flour for 48 h at 22 °C; this reduction may be due to the activation of endogenous phytase or the action of exogenous phytase released by microorganisms naturally present in flours when a favorable pH was reached.
The fermentation of toasted quinoa flour was performed to evaluate the effect of Lp299v® on phytate degradation. The thermal treatment to which quinoa grains were subjected might have inactivated the endogenous phytase and other enzymes and microorganism in these grains. According to Greiner and Konietzny,40 a temperature above 65 °C may inactivate the endogenous phytase of crops. Although there is no information about endogenous phytase activity in toasted quinoa, Brejnholt et al.41 reported that the endogenous phytase activity of wheat was reduced in 93% after heat treatment (95 °C, 10 min). Our results showed that degradation of phytate during fermentation was 11%; this degradation might be due to the phytase activity of the added microorganism rather than endogenous phytase. Thus, it seems that Lp299v® has low phytase activity at pH range 4.22–6.49 at 30 °C. Bering et al.31 also reported low phytase activity of Lp299v® when the substrate was heat‐treated oat gruel.
The fermentation of pseudocereal flours with Lp299v® was further investigated, in Part 2, to evaluate iron, zinc, and calcium accessibility and to estimate their bioavailability. Fermentation has been used as an effective method to improve mineral bioavailability in many staple foods: in cereals such as wheat, rice, and sorghum,42 legumes such as soybeans, chickpea, green gram and black gram,42 as well as cassava roots.43 In the present study, fermentation of flours with Lp299v® proved to be effective in increasing mineral accessibility and reducing the values of phytate : mineral molar ratios in fermented flours. The bioavailability of iron, zinc, and calcium has been estimated using phytate : mineral molar ratios as suggested by the International Zinc Nutrition Consultative Group (IZiNCG).11 In quinoa and canihua fermented flour, the Phy:Zn and Phy·Ca:Zn molar ratios were below the critical values (< 15 and < 200, respectively), indicating that estimated zinc bioavailability increased during the fermentation process. The Phy:Ca molar ratio in canihua flour was under the threshold (0.17). The Phy:Fe molar ratios were also reduced after fermentation, but were still above the critical value for quinoa (3.90), canihua (1.66), and amaranth (8.12). Our findings showed that fermentation of pseudocereals flours with Lp299v® resulted in significant (P < 0.05) improvement in accessibility of all studied minerals. The increased accessibility (a 3.5 to 4.0‐fold increase) of minerals in fermented quinoa is in agreement with the reduction of phytate content (3.9‐fold reduction) and consequently with the reduction of Phy:Fe, Phy:Zn, and Phy:Ca molar ratios (3.7–4.1‐fold reduction). Valencia et al.18 also reported that iron solubility increased three to five times after fermentation of quinoa flour with Lactobacillus plantarum. In fermented canihua flour, the increase in zinc accessibility (4.6‐fold) corresponds with phytate content reduction (4.2‐fold) and Phy:Zn molar ratio reduction (3.8‐fold). But the increase in iron and calcium accessibility (2.4 and 2.0‐fold, respectively) was lower than the decrease in the corresponding molar ratios (Phy:Fe 4.2‐fold, Phy:Ca 3.7‐fold decrease). In the case of fermented amaranth flour, the increase in mineral accessibility (1.7–2.5‐fold) was somehow similar to phytate reduction (1.8‐fold) and slightly higher than the reduction of the phytate : mineral molar ratios (1.5–1.8‐fold). The discrepancies between the accessibility results and estimated bioavailability can be explained by the presence of other mineral inhibitors, such as phenolic compounds,44, 45 in these grains. Quinoa and canihua are reported to have higher phenolic compound content;46, 47, 48 the high content might explain the differences in iron accessibility in regards to estimated mineral bioavailability in these flours. However, in fermented amaranth the low mineral accessibility can be due to the combined effect of phenolic compounds and the high content of phytate that remains after fermentation. Baye et al.44 reported that the molar ratios are not always good predictors of mineral bioavailability; however, our findings, particularly good for quinoa, showed good agreement between minerals' in vitro accessibility and estimated mineral bioavailability through molar ratios, as has been suggested by IZiNCG.49
In general, fermentation was effective in reducing phytate in all three pseudocereals, with higher phytate degradation in canihua and quinoa. Accessibility was increased for iron, zinc, and calcium in all pseudocereal flours. However, the estimated bioavailability of mainly iron indicates that the remaining phytate content may still impair its bioavailability in these three pseudocereals. It is therefore necessary to seek further strategies for phytate degradation and improving the bioavailability of these minerals.
Conclusion
The present study showed that the phytate content in quinoa, canihua, and amaranth is degraded during fermentation. Fermentation proved to be more effective when pseudocereal flours were fermented rather than the grains. The production of lactic acid during fermentation resulted in a variation in pH, which provided the necessary conditions for the activation of endogenous phytase and microbial phytase. The phytate degradation seems to be mainly due to endogenous phytase activity in the different pseudocereals rather than the phytase produced by the added microorganism. A more controlled fermentation resulted when the starter culture Lp299v® was used. After fermentation of pseudocereal flours, mineral accessibility, and estimated mineral bioavailability was significantly improved. In addition to phytate degradation, this improvement was also due to the preservation of the mineral contents during the processing. The inclusion of fermented pseudocereal flour with enhanced mineral bioavailability may increase the nutritional quality of plant‐based diets. Further studies are proposed to optimize the fermentation process, to achieve greater mineral bioavailability, and to evaluate the sensory properties of fermented flours.
Acknowledgements
This work has been funded by the Swedish International Development Agency (SIDA).
REFERENCES
- 1. Békés F, Schoenlechner R and Tömösközi S, Chapter 14 ‐ Ancient Wheats and Pseudocereals for Possible use in Cereal‐Grain Dietary Intolerances, in Cereal Grains, 2nd edn, ed. by Wrigley C, Batey I. and Miskelly D. Woodhead Publishing, Cambridge, pp. 353–389 (2017). [Google Scholar]
- 2. Schoenlechner R, Siebenhandl S and Berghofer E, 7 ‐ Pseudocereals, in Gluten‐Free Cereal Products and Beverages, ed. by Arendt EK. and Dal Bello F. Academic Press, San Diego, p. 149‐VI (2008). [Google Scholar]
- 3. Perez‐Rea D and Antezana‐Gomez R, Chapter 12 ‐ The Functionality of Pseudocereal Starches, in Starch in Food, 2nd edn, ed. by Sjöö M. and Nilsson L. Woodhead Publishing, Cambridge, pp. 509–542 (2018). [Google Scholar]
- 4. Reguera M and Haros CM, Structure and composition kernels, in Pseudocereals Chemistry and Technology, ed. by Haros CM. and Schoenlechner R. Wiley‐Blackwell, West Sussex, pp. 28–48 (2017). [Google Scholar]
- 5. Lazarte CE, Carlsson N‐G, Almgren A, Sandberg A‐S and Granfeldt Y, Phytate, zinc, iron and calcium content of common Bolivian food, and implications for mineral bioavailability. J Food Compost Anal 39:111–119 (2015). [Google Scholar]
- 6. Ruales J and Nair BM, Saponins, phytic acid, tannins and protease inhibitors in quinoa (Chenopodium quinoa, Willd) seeds. Food Chem 48:137–143 (1993). [Google Scholar]
- 7. Reddy NR and Sathe SK, Food phytates. CRC Press, Boca Raton, (2001). [Google Scholar]
- 8. Harland BF and Morris ER, Phytate: a good or a bad food component? Nutr Res 15:733–754 (1995). [Google Scholar]
- 9. Schlemmer U, Frolich W, Prieto RM and Grases F, Phytate in foods and significance for humans: food sources, intake, processing, bioavailability, protective role and analysis. Mol Nutr Food Res 53:S330–S375 (2009). [DOI] [PubMed] [Google Scholar]
- 10. Bailey RL, West KP Jr and Black RE, The epidemiology of global micronutrient deficiencies. Ann Nutr Metab 66:22–33 (2015). [DOI] [PubMed] [Google Scholar]
- 11. Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lonnerdal B et al, International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 25:S99–S203 (2004). [PubMed] [Google Scholar]
- 12. Haros M, Carlsson NG, Almgren A, Larsson‐Alminger M, Sandberg AS and Andlid T, Phytate degradation by human gut isolated Bifidobacterium pseudocatenulatum ATCC27919 and its probiotic potential. Int J Food Microbiol 135:7–14 (2009). [DOI] [PubMed] [Google Scholar]
- 13. Reale A, Mannina L, Tremonte P, Sobolev AP, Succi M, Sorrentino E et al, Phytate degradation by lactic acid bacteria and yeasts during the wholemeal dough fermentation: a 31P NMR study. J Agric Food Chem 52:6300–6305 (2004). [DOI] [PubMed] [Google Scholar]
- 14. Sanz‐Penella MJ, Tamayo‐Ramos JA and Haros CM, Application of Bifidobacteria as starter culture in whole wheat sourdough breadmaking. Food Bioproc Tech 5:2370–2380 (2012). [Google Scholar]
- 15. Sandberg A‐S and Andlid T, Phytogenic and microbial phytases in human nutrition. Int J Food Sci Technol 37:823–833 (2002). [Google Scholar]
- 16. Hammes WP, Brandt MJ, Francis KL, Rosenheim J, Seitter MFH and Vogelmann SA, Microbial ecology of cereal fermentations. Trends Food Sci Technol 16:4–11 (2005). [Google Scholar]
- 17. Sharma A and Kapoor AC, Levels of antinutritional factors in pearl millet as affected by processing treatments and various types of fermentation. Plant Foods Hum Nutr 49:241–252 (1996). [DOI] [PubMed] [Google Scholar]
- 18. Valencia S, Svanberg U, Sandberg AS and Ruales J, Processing of quinoa (Chenopodium quinoa, Willd): effects on in vitro iron availability and phytate hydrolysis. Int J Food Sci Nutr 50:203–211 (1999). [DOI] [PubMed] [Google Scholar]
- 19. Alvarez‐Jubete L, Arendt EK and Gallagher E, Nutritive value of pseudocereals and their increasing use as functional gluten‐free ingredients. Trends Food Sci Technol 21:106–113 (2010). [Google Scholar]
- 20. Molin G, Ahrne S, Bengmark S and Jeppson B, Intestine colonizing lactobacilli Patent application number SE9102238A (1992).
- 21. Ulmius M, Johansson‐Persson A, Nordén TI, Bergenståhl B and Önning G, Gastrointestinal release of β‐glucan and pectin psing an in vitro method. Cereal Chem 88:385–390 (2011). [Google Scholar]
- 22. Sandberg A‐S and Svanberg U, Phytate hydrolysis by phytase in cereals; effects on in vitro estimation of iron availability. J Food Sci 56:1330–1333 (1991). [DOI] [PubMed] [Google Scholar]
- 23. AOAC , Official Methods of Analysis of AOAC International. AOAC International, Gaithersburg, MD: (2000). [Google Scholar]
- 24. Nuobariene L, Cizeikiene D, Gradzeviciute E, Hansen ÅS, Rasmussen SK, Juodeikiene G et al, Phytase‐active lactic acid bacteria from sourdoughs: isolation and identification. LWT ‐ Food Sci Technol 63:766–772 (2015). [Google Scholar]
- 25. Carlsson N‐G, Bergman E‐L, Skoglund E, Hasselblad K and Sandberg A‐S, Rapid analysis of inositol phosphates. J Agric Food Chem 49:1695–1701 (2001). [DOI] [PubMed] [Google Scholar]
- 26. Charalampopoulos D, Wang R, Pandiella SS and Webb C, Application of cereals and cereal components in functional foods: a review. Int J Food Microbiol 79:131–141 (2002). [DOI] [PubMed] [Google Scholar]
- 27. Eklund‐Jonsson C, Sandberg AS and Alminger ML, Reduction of phytate content while preserving minerals during whole grain cereal tempe fermentation. J Cereal Sci 44:154–160 (2006). [Google Scholar]
- 28. Konishi Y, Hirano S, Tsuboi H and Wada M, Distribution of minerals in quinoa (Chenopodium quinoa Willd.) seeds. Biosci Biotechnol Biochem 68:231–234 (2004). [DOI] [PubMed] [Google Scholar]
- 29. Konishi Y, Takezoe R and Murase J, Energy dispersive X‐ray microanalysis of element distribution in amaranth seed. Biosci Biotechnol Biochem 62:2288–2290 (1998). [DOI] [PubMed] [Google Scholar]
- 30. Dallagnol A, Pescuma M, De Valdez G and Rollán G, Fermentation of quinoa and wheat slurries by Lactobacillus plantarum CRL 778: proteolytic activity. Appl Microbiol Biotechnol 97:3129–3140 (2013). [DOI] [PubMed] [Google Scholar]
- 31. Bering S, Suchdev S, Sjøltov L, Berggren A, Tetens I and Bukhave K, A lactic acid‐fermented oat gruel increases non‐haem iron absorption from a phytate‐rich meal in healthy women of childbearing age. Br J Nutr 96:80–85 (2007). [DOI] [PubMed] [Google Scholar]
- 32. Repo‐Carrasco R, Espinoza C and Jacobsen SE, Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev Int 19:179–189 (2003). [Google Scholar]
- 33. Vogelmann SA, Seitter M, Singer U, Brandt MJ and Hertel C, Adaptability of lactic acid bacteria and yeasts to sourdoughs prepared from cereals, pseudocereals and cassava and use of competitive strains as starters. Int J Food Microbiol 130:205–212 (2009). [DOI] [PubMed] [Google Scholar]
- 34. Coulibaly A, Kouakou B and Chen J, Phytic acid in cereal grains: structure, healthy or harmful ways to reduce phytic acid in cereal grains and their effects on nutritional quality. Am J Plant Nutr Fert Technol 1:1–22 (2011). [Google Scholar]
- 35. Egli I, Davidsson L, Juillerat MA, Barclay D and Hurrell RF, The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. J Food Sci 67:3484–3488 (2002). [Google Scholar]
- 36. Duliński R, Starzyńska‐Janiszewska A, Byczyński Ł and Błaszczyk U, Myo‐inositol phosphates profile of buckwheat and quinoa seeds: effects of hydrothermal processing and solid‐state fermentation with Rhizopus oligosporus . Int J Food Prop 20:2088–2095 (2017). [Google Scholar]
- 37. Repo‐Carrasco‐Valencia R, Encina CR, Binaghi MJ, Greco CB and Ronayne de Ferrer PA, Effects of roasting and boiling of quinoa, kiwicha and kaniwa on composition and availability of minerals in vitro . J Sci Food Agric 90:2068–2073 (2010). [DOI] [PubMed] [Google Scholar]
- 38. García‐Mantrana I, Monedero V and Haros M, Application of phytases from bifidobacteria in the development of cereal‐based products with amaranth. Eur Food Res Technol 238:853–862 (2014). [Google Scholar]
- 39. Amare E, Mouquet‐Rivier C, Rochette I, Adish A and Haki GD, Effect of popping and fermentation on proximate composition, minerals and absorption inhibitors, and mineral bioavailability of Amaranthus caudatus grain cultivated in Ethiopia. J Food Sci Technol 53:2987–2994 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Greiner R and Konietzny U, Endogenous phytate‐degrading enzymes are responsible for phytate reduction while preparing beans (Phaseolus vulgaris). J Food Process Preserv 22:321–331 (1998). [Google Scholar]
- 41. Brejnholt SM, Dionisio G, Glitsoe V, Skov LK and Brinch‐Pedersen H, The degradation of phytate by microbial and wheat phytases is dependent on the phytate matrix and the phytase origin. J Sci Food Agric 91:1398–1405 (2011). [DOI] [PubMed] [Google Scholar]
- 42. Humer E and Schedle K, Fermentation of food and feed: a technology for efficient utilization of macro and trace elements in monogastrics. J Trace Elem Med Biol 37:69–77 (2016). [DOI] [PubMed] [Google Scholar]
- 43. Lazarte CE, Vargas M and Granfeldt Y, Zinc bioavailability in rats fed a plant‐based diet: a study of fermentation and zinc supplementation. Food Nutr Res 59:27796 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baye K, Mouquet‐Rivier C, Icard‐Vernière C, Picq C and Guyot J‐P, Changes in mineral absorption inhibitors consequent to fermentation of Ethiopian injera: implications for predicted iron bioavailability and bioaccessibility. Int J Food Sci Technol 49:174–180 (2014). [Google Scholar]
- 45. Gabaza M, Shumoy H, Muchuweti M, Vandamme P and Raes K, Iron and zinc bioaccessibility of fermented maize, sorghum and millets from five locations in Zimbabwe. Food Res Int 103:361–370 (2018). [DOI] [PubMed] [Google Scholar]
- 46. Repo‐Carrasco‐Valencia R, Hellström JK, Pihlava J‐M and Mattila PH, Flavonoids and other phenolic compounds in Andean indigenous grains: quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem 120:128–133 (2010). [Google Scholar]
- 47. Carciochi RA, Galván‐D'Alessandro L, Vandendriessche P and Chollet S, Effect of germination and fermentation process on the antioxidant compounds of quinoa seeds. Plant Foods Hum Nutr 71:361–367 (2016). [DOI] [PubMed] [Google Scholar]
- 48. Paśko P, Bartoń H, Zagrodzki P, Gorinstein S, Fołta M and Zachwieja Z, Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem 115:994–998 (2009). [Google Scholar]
- 49. Dahdouh S, Grande F, Espinosa SN, Vincent A, Gibson R, Bailey K et al, Development of the FAO/INFOODS/IZINCG global food composition database for phytate. J Food Compost Anal 78:42–48 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


