Abstract
Background
Approximately half of high‐grade serous ovarian carcinomas (HGSOCs) demonstrate homologous recombination repair (HR) pathway defects, resulting in a distinct clinical phenotype comprising hypersensitivity to platinum, superior clinical outcome, and greater sensitivity to poly(adenosine diphosphate‐ribose) polymerase (PARP) inhibitors. EMSY, which is known to be amplified in breast and ovarian cancers, encodes a protein reported to bind and inactivate BRCA2. Thus, EMSY overexpression may mimic BRCA2 mutation, resulting in HR deficiency. However, to our knowledge, the phenotypic consequences of EMSY overexpression in HGSOC patients has not been explored.
Methods
Here we investigate the impact of EMSY expression on clinical outcome and sensitivity to platinum‐based chemotherapy using available data from transcriptomically characterized HGSOC cohorts.
Results
High EMSY expression was associated with better clinical outcome in a cohort of 265 patients with HGSOC from Edinburgh (overall survival multivariable hazard ratio, 0.58 [95% CI, 0.38‐0.88; P = .011] and progression‐free survival multivariable hazard ratio, 0.62 [95% CI, 0.40‐0.96; P = .030]). Superior outcome also was demonstrated in the Medical Research Council ICON7 clinical trial and multiple publicly available data sets. Patients within the Edinburgh cohort who had high EMSY expression were found to demonstrate greater rates of complete response to multiple platinum‐containing chemotherapy regimens (radiological complete response rate of 44.4% vs 12.5% at second exposure; P = .035) and corresponding prolonged time to disease progression (median, 151.5 days vs 60.5 days after third platinum exposure; P = .004).
Conclusions
Patients with HGSOCs demonstrating high EMSY expression appear to experience prolonged survival and greater platinum sensitivity, reminiscent of BRCA‐mutant cases. These data are consistent with the notion that EMSY overexpression may render HGSOCs HR deficient.
Keywords: EMSY, homologous recombination, ovarian cancer, platinum response, survival
Short abstract
Patients with high‐grade serous ovarian carcinomas demonstrating high expression levels of EMSY appear to experience prolonged survival and hypersensitivity to multiple lines of platinum‐based chemotherapy. These data support the notion that overexpression of the gene product of EMSY, which is reported to bind and inactivate BRCA2, may mimic the phenotype conferred by BRCA mutation.
Introduction
Ovarian cancer is the most lethal gynecological malignancy, accounting for >14,000 deaths per year in the United States alone.1 High‐grade serous ovarian carcinoma (HGSOC) accounts for approximately 75% of cases, and is diagnosed at an advanced stage in the vast majority of patients.2, 3 Although HGSOC is typically sensitive to platinum‐based chemotherapy at the time of diagnosis, the majority of patients will experience disease recurrence, which accrues resistance to platinum resulting in sequentially shorter treatment‐free intervals before patients eventually succumb to disease.4
Over the last decade, a wealth of molecular data have been produced in an effort to better characterize HGSOC and to identify molecular subtypes of disease with biology that may be exploited therapeutically.5, 6, 7 However, currently only sequencing to detect mutations in the homologous recombination (HR) DNA repair genes BRCA1 and BRCA2 (BRCA) is routinely used to identify molecular subgroups that are clinically actionable. BRCA‐mutant patients experience prolonged survival, enhanced sensitivity to platinum even with multiple exposures, and a greater sensitivity to poly(adenosine diphosphate‐ribose) polymerase (PARP) inhibition by virtue of HR deficiency.8, 9, 10, 11, 12, 13
The EMSY gene, also known as C11orf30, encodes a nuclear protein that has been identified to bind and inactivate BRCA2 and is reportedly amplified in approximately 6% to 18% of HGSOC cases and 7% to 13% of sporadic breast cancer cases.5, 14, 15 EMSY colocalizes to sites of DNA damage, and overexpression of a truncated form of EMSY able to bind BRCA2 has been reported to induce genomic instability and sensitivity to the DNA‐damaging agent mitomycin C.14, 16 Overexpression of EMSY disrupts the BRCA2/RAD51 pathway after DNA damage and may override the HR players RPA and PALB2, which bind BRCA2 in the same region as EMSY.17 Thus, tumors with EMSY amplification may mimic those demonstrating mutational inactivation of BRCA2. Similar to BRCA mutation, EMSY amplification has been associated with poor prognosis in patients with breast cancer and is most common in the HGS histological subtype of OC.14, 15, 18, 19, 20, 21, 22
However, to our knowledge, no association between EMSY expression and clinical outcome in patients with HGSOC has been made to date. In the current study, we sought to perform in silico analysis of available transcriptomic data to investigate whether patients with HGSOCs demonstrating high expression of EMSY experience differential clinical outcome or sensitivity to platinum‐based chemotherapy.
Materials and Methods
Cohort Descriptions
The Edinburgh cohort comprised 265 HGSOC patients who were treated within the Edinburgh Cancer Centre between 1984 and 2006 and identified as part of a previous study of HGSOC.23 All patients received platinum‐containing first‐line chemotherapy subsequent to primary surgical debulking. The distribution of patient age at the time of diagnosis, extent of residual disease after primary debulking surgery, and International Federation of Gynecology and Obstetrics (FIGO) stage at the time of diagnosis are detailed in Table 1. The Medical Research Council (MRC) ICON7 cohort comprised 367 patients with HGSOC from the ICON7 clinical trial, 185 of whom received combination therapy with carboplatin and paclitaxel with bevacizumab and 182 of whom received the combination of carboplatin and paclitaxel alone. These specimens were from patients consenting to the translational research component of the study, and were collected across several international sites.
Table 1.
Demographics of HGSOC Patients With High and Low EMSY Expression in the Edinburgh Cohort
| Low EMSY Expression | High EMSY Expression | |||||
|---|---|---|---|---|---|---|
| Demographics | No. | % | No. | % | P | |
| HGSOC | Patients | 228 | 37 | |||
| FIGO stage of disease at time of diagnosis | I | 13 | 5.9% | 1 | 2.8% | NSa |
| II | 15 | 6.8% | 5 | 13.9% | ||
| III | 153 | 68.9% | 22 | 61.1% | ||
| IV | 41 | 18.5% | 8 | 22.2% | ||
| NA | 6 | 1 | ||||
| Residual disease after debulking surgery | <2 cmb | 81 | 40.5% | 17 | 48.6% | NSc |
| 2‐5 cm | 51 | 25.5% | 8 | 22.9% | ||
| >5 cm | 68 | 34.0% | 10 | 28.6% | ||
| NA | 28 | 2 | ||||
| Median age at diagnosis, years | 61 (range, 32‐86) | 61 (range, 43‐81) | NSd | |||
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; HGSOC, high‐grade serous ovarian carcinoma; NA, not available; NS, not significant.
Determined by the Fisher's exact test: early‐stage (stage I‐II) versus late‐stage (stage III‐IV) disease at the time of diagnosis.
Due to the retrospective nature of these data and the historical classification of optimal surgical resection as <2 cm residual disease in older cases, optimal surgical success could not be resolved beyond <2 cm within the Edinburgh cohort.
Determined by the chi‐square test: <2 cm versus ≥2 cm.
Determined by the Student t test.
Edinburgh and MRC ICON7 Cohort Gene Expression Data
Gene expression data for the Edinburgh and MRC ICON7 study cohorts were generated as part of a previous study identifying transcriptomically‐defined molecular subtypes of HGSOC.23 Briefly, for each cohort, RNA was extracted from macrodissected, formalin‐fixed, paraffin‐embedded tumor material (High Pure kit; Roche Life Science, Indianapolis, Indiana), cDNA amplification was performed (FFPE WTA System V2; NuGEN, Leek, the Netherlands), and fragmentation and labeling was performed (NuGEN Encore Biotin Module) followed by hybridization to the Ovarian DSA cDNA microarray platform (Almac Diagnostics, Craigavon, United Kingdom). Each cohort was preprocessed using the Robust Multi‐Array Average (RMA)24 method prior to a comprehensive quality control analysis, including assessments of sample quality via the Affymetrix percentage present metric (Affymetrix, Santa Clara, California)25 and cohort metrics using principal components analysis and Kolmogorov‐Smirnov distributions analysis. Probe sets that were informative for EMSY gene expression were extracted and per‐sample EMSY expression was calculated as the mean expression between probe sets (see Supporting Table S1 and Table S2) (see Supporting Fig. S1).
Identification of the Threshold for High EMSY Expression Within the Edinburgh Cohort
The optimal threshold for dichotomization of the Edinburgh cohort into high and low EMSY expression was identified using cutpoint analysis of univariable survival (see Supporting Fig. S2). This approach identified 14% as the optimal threshold, which was subsequently validated by application to independent transcriptomic data sets.
Publicly Available Gene Expression Data Sets
Gene expression data from the studies by Pils et al,26 Tothill et al,7 Mateescu et al,27 and The Cancer Genome Atlas (TCGA)5 were accessed using the curatedOvarianData R package.28 EMSY gene expression data were extracted for samples of serous histology within their respective studies. Samples documented as serous grade 1 were excluded as possible low‐grade SOC.
Survival Data
Clinical annotation for the Edinburgh cohort was retrieved from the Edinburgh Ovarian Cancer Database, in which data are entered prospectively in an unselected manner by a single individual as part of routine care. Survival data for the Pils et al,26 Tothill et al,7 Mateescu et al,27 and TCGA5 data sets were accessed using the curatedOvarianData R package.28
Platinum Response Data
Detailed response data to each cytotoxic therapy regimen for patients in the Edinburgh cohort were collected retrospectively from the Edinburgh Ovarian Cancer Database in conjunction with archived patient notes. Radiological responses were reported as per World Health Organization or Response Evaluation Criteria in Solid Tumors (RECIST) criteria, with the exception of the need for confirmatory scans. CA 125 tumor marker responses were reported according to Gynecological Cancer InterGroup (GCIG) guidelines.29
Statistical Analyses
Statistical analyses were performed using R (version 3.5.1; R Foundation, Vienna, Austria). Survival analyses were conducted using Cox proportional hazards models for progression‐free survival (PFS) and overall survival (OS). Survival differences were visualized using the Kaplan‐Meier method. Multivariable survival analyses accounted for the success of primary surgical debulking, FIGO stage at the time of diagnosis, and patient age at the time of diagnosis, with the exception of the Mateescu et al data set, in which data regarding surgical debulking and patient age were not available. Within the MRC ICON7 data set, chemotherapy regimen (bevacizumab treatment vs placebo) was also accounted for in multivariable analyses where patients from both treatment arms were analyzed together. Survival differences are presented as univariable or multivariable hazard ratios (uniHR or multiHR) alongside their corresponding 95% CIs and P values. Comparisons of categorical variables were performed using the chi‐square or Fisher's exact tests as appropriate. Differences in time to disease progression from receipt of platinum were evaluated using the Mann‐Whitney U test. Adjustments for multiple testing were made using the Bonferroni correction when specified.
Results
High EMSY Expression Was Associated With Superior Survival in the Edinburgh HGSOC Cohort
Gene expression data for 265 HGSOCs in the Edinburgh cohort were probed for EMSY expression. 14% of HGSOC patients with the highest levels of EMSY expression (high‐EMSY) demonstrated prolonged OS compared with the remainder of the cohort (low‐EMSY) (uniHR, 0.63 [95% CI, 0.43‐0.93; P = .020]) (Fig. 1A) (Table 2).5, 7, 26, 27 A multivariable model accounting for FIGO stage at diagnosis, residual disease after primary debulking surgery, and patient age demonstrated an OS benefit for patients within the high‐EMSY group (multiHR, 0.58 [95% CI, 0.38‐0.88; P = .011]) (see Supporting Table S3.1). A multivariable model also demonstrated that the high‐EMSY group had prolonged PFS versus the low‐EMSY group (multiHR, 0.62 [95% CI, 0.40‐0.96; P = .030]) (Fig. 1B) (see Supporting Table S3.2).
Figure 1.
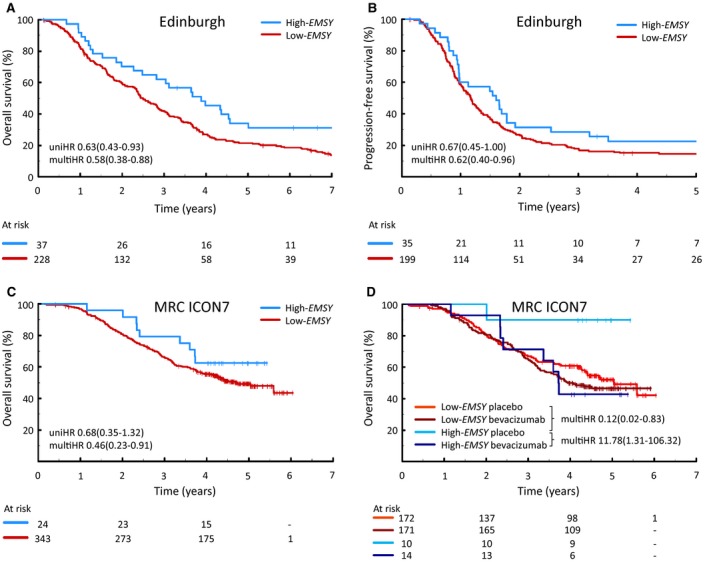
Clinical outcome in patients with high‐grade serous ovarian carcinomas (HGSOCs) demonstrating high EMSY expression. (A) Overall survival (OS) in the Edinburgh cohort. (B) Progression‐free survival (PFS) in the Edinburgh cohort. (C) OS in the Medical Research Council (MRC) ICON7 clinical trial cohort. (D) OS in the MRC ICON7 clinical trial cohort stratified by trial arm. multiHR indicates multivariable hazard ratio; uniHR, univariable hazard ratio.
Table 2.
Univariable and Multivariable Analyses of Clinical Outcome in Patients With HGSOCs Demonstrating High EMSY Expression across Multiple Data Sets
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Data Set | Event Type | High EMSY Expression No. of Cases | Low EMSY Expression No. of Cases | HR | 95% CI | P | HR | 95% CI | P |
| Edinburgh | OS | 37 | 228 | 0.63 | 0.43‐0.93 | .020 | 0.58 | 0.38‐0.88 | .011 |
| PFS | 0.67 | 0.45‐1.00 | .052 | 0.62 | 0.40‐0.96 | .030 | |||
| MRC ICON7 cohort | OS | 24 | 343 | 0.68 | 0.35‐1.32 | .254 | 0.46 | 0.23‐0.91 | .025 |
| PFS | 1.27 | 0.82‐1.97 | .280 | 0.89 | 0.57‐1.38 | .599 | |||
| Pils et al26 cohort | OS | 24 | 146 | 0.31 | 0.10‐1.02 | .053 | 0.27 | 0.08‐0.87 | .028 |
| PFS | 0.70 | 0.41‐1.22 | .210 | 0.52 | 0.29‐0.92 | .026 | |||
| Mateescu et al27cohort | OS | 11 | 64 | 0.40 | 0.17‐0.94 | .035 | 0.43 | 0.18‐0.99 | .048 |
| PFS | 0.51 | 0.24‐1.09 | .084 | 0.59 | 0.27‐1.25 | .168 | |||
| Tothill et al7 cohort | OS | 35 | 210 | 0.50 | 0.27‐0.93 | .029 | 0.60 | 0.32‐1.13 | .112 |
| PFS | 0.70 | 0.44‐1.09 | .114 | 0.63 | 0.39‐1.04 | .072 | |||
| TCGA5 cohort | OS | 77 | 472 | 0.95 | 0.68‐1.34 | .789 | 1.18 | 0.83‐1.66 | .358 |
| TCGA stage III/IV | PFS | 71 | 435 | 0.62 | 0.41‐0.94 | .046a | 0.68 | 0.45‐1.04 | .076 |
Abbreviations: HGSOC, high‐grade serous ovarian carcinoma; HR, hazard ratio; MRC, Medical Research Council; OS, overall survival; PFS, progression‐free survival; TCGA, The Cancer Genome Atlas.
Bonferroni‐adjusted P value.
Impact of High EMSY Expression Within the MRC ICON7 Cohort
To validate the association between superior clinical outcome and high EMSY expression, the gene expression cutoff value from the Edinburgh data set was applied directly to the MRC ICON7 cohort characterized on the same gene expression platform.23 Patients in the high‐EMSY group within this cohort demonstrated prolonged OS when accounting for FIGO stage at diagnosis, residual disease after primary debulking surgery, trial arm (bevacizumab‐treated patients vs control arm), and age at diagnosis (multiHR, 0.46 [95% CI, 0.23‐0.91; P = .025]) (Fig. 1C) (see Supporting Table S3.3), but did not demonstrate superior PFS (multiHR, 0.89 [95% CI, 0.57‐1.38; P = .599]) (see Supporting Fig. S3).
Despite severely limited power, analysis of the high‐EMSY population demonstrated that patients in the high‐EMSY group within the bevacizumab treatment arm (14 patients) had inferior survival compared with those patients who received chemotherapy alone (10 patients) (multiHR, 11.78 [95% CI, 1.31‐106.32; P = .027]) (Fig. 1D) (see Supporting Table S3.4). Within the control arm specifically, patients in the high‐EMSY group demonstrated markedly superior OS compared with patients in the low‐EMSY group (multiHR, 0.12 [95% CI, 0.02‐0.083; P = .032) (see Supporting Table S3.5).
Validation of Superior Outcomes in Patients With HGSOCs With High EMSY Expression
To further validate the association between high EMSY expression and superior clinical outcome, publicly available gene expression data sets were accessed using the curatedOvarianData package.28 These data sets were characterized on a variety of platforms, thereby preventing direct application of the cutoff value from the Edinburgh cohort. Therefore, the use of thresholds at the 14th percentile of EMSY expression within each data set was proposed. The validity of this approach was assessed using the MRC ICON7 control cohort arm and, consistent with results using the absolute threshold derived from the Edinburgh cohort, analysis of the 14% of HGSOCs with the highest EMSY expression demonstrated prolonged OS (multiHR, 0.23 [95% CI, 0.07‐0.72; P = .012]) (see Supporting Fig. S4).
EMSY expression data were extracted for data sets from studies by Tothill et al,7 Pils et al,26 Mateescu et al,27 and TCGA.5 Superior OS for patients in the high‐EMSY group was demonstrated in the cohorts from the Pils et al26 (multiHR, 0.27 [95% CI, 0.08‐0.87; P = .028]) (Fig. 2A) (see Supporting Table S3.6) and Mateescu et al27 (multiHR, 0.43 [95% CI, 0.18‐0.99; P = .048]) (Fig. 2C) (see Supporting Table S3.7) studies. The cohort from the study by Pils et al demonstrated superior PFS within the high‐EMSY population (multiHR, 0.52 [95% CI, 0.29‐0.92; P = .026]) (Fig. 2B) (see Supporting Table S3.8).26 The corresponding PFS difference in the Mateescu et al27 cohort was not found to be statistically significant (uniHR, 0.51 [95% CI, 0.24‐1.09; P = .084]; and multiHR, 0.59 [95% CI, 0.27‐1.25; P = .168]) (Fig. 2D) (see Supporting Table S3.9). Notably, data regarding residual disease after primary debulking surgery were not available for the Mateescu et al27 study cohort, thereby precluding the ability to account for this variable.
Figure 2.
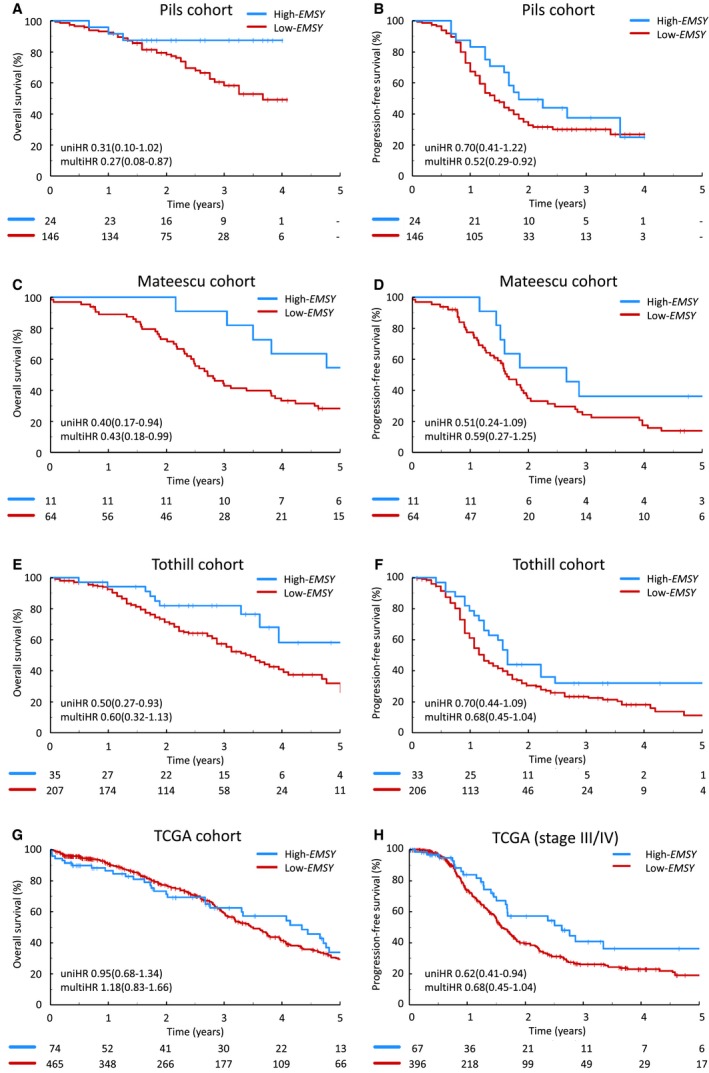
Clinical outcome of patients with high‐grade serous ovarian carcinomas (HGSOCs) demonstrating high EMSY expression across multiple data sets. (A) Overall survival (OS) within the Pils et al cohort.26 (B) Progression‐free survival (PFS) within the Pils et al cohort.26 (C) OS within the Mateescu et al cohort.27 (D) PFS within the Mateescu et al cohort.27 (E) OS within the Tothill et al cohort.7 (F) PFS within the Tothill et al cohort.7 (G) OS within The Cancer Genome Atlas (TCGA) cohort5 and (H) PFS of patients with advanced stage disease within the TCGA cohort.5 multiHR indicates multivariable hazard ratio; uniHR, univariable hazard ratio.
Prolonged OS was demonstrated in high‐EMSY patients from the study by Tothill et al7 at the univariable level (uniHR, 0.50 [95% CI, 0.27‐0.93; P = .029]) (Fig. 2E), but did not reach statistical significance in a multivariable model accounting for patient age, FIGO stage at diagnosis, and residual disease after debulking surgery (multiHR, 0.60 [95% CI, 0.32‐1.13; P = .112]) (see Supporting Table S3.10). The apparent prolonged PFS noted on multivariable analysis in patients in the high‐EMSY group in the cohort of patients from the study by Tothill et al7 did not cross the threshold for statistical significance (multiHR, 0.63 [95% CI, 0.39‐1.04; P = .072]) (Fig. 2F) (see Supporting Table S3.11).
The TCGA cohort5 did not demonstrate significantly prolonged PFS within the high‐EMSY group (uniHR, 0.74 [95% CI, 0.50‐1.09; P = .122]) (see Supporting Fig. S5). PFS analysis restricted to patients diagnosed at an advanced stage of disease demonstrated prolonged PFS in this subset (uniHR, 0.62 [95% CI, 0.41‐0.94; Bonferroni‐adjusted P = .046]) (Fig. 2H) (see Supporting Table S3.12). High EMSY expression was not found to be associated with superior OS within the TCGA cohort5 (uniHR, 0.95 [95% CI, 0.68‐1.35]) (Fig. 2G).
Impact of Sampling Site on the Identification of the High‐EMSY Subgroup With a Superior Clinical Outcome
Data regarding the sampling site of arrayed specimens were available for the cohort from the study by Tothill et al.7 Patients in the high‐EMSY group with sampling of the primary adnexal mass (ovary or fallopian tube) were found to demonstrate significantly superior OS (multiHR for OS, 0.28 [95% CI, 0.09‐0.90; P = .032]; and multiHR for PFS, 0.55 [95% CI, 0.28‐1.10; P = .091]) (see Supporting Figs S6A and S6C), whereas those from other sampling sites demonstrated no apparent survival benefit (multiHR for OS, 0.85 [95% CI, 0.37‐1.97; P = .710]; and multiHR for PFS, 0.91 [95% CI, 0.43‐1.89; P = .794]) (see Supporting Figs. S6B and S6D). The vast majority of samples from the Edinburgh and MRC ICON7 data sets were derived from primary adnexal masses and the sampling site data were not available for the data sets from the studies by Pils et al,26 Mateescu et al,27 and TCGA,5 thereby precluding investigation of the potential impact of extra‐adnexal sampling in these data sets.
High EMSY Expression Was Associated With Greater Platinum Sensitivity in the Edinburgh Cohort
Detailed response data regarding cytotoxic therapy regimens were collected for the Edinburgh cohort. Patients in the high‐EMSY group demonstrated a superior radiological complete response rate at the time of second platinum exposure (44.4% [4 of 9 patients] vs 12.5% [8 of 64 patients]; Fisher's exact P = .035) (Fig. 3A). Similarly, patients in the high‐EMSY group were found to have superior rates of complete CA 125 tumor marker response at the time of first (88.0% [22 of 25 patients] vs 55.0% [82 of 149 patients]; P = .002) and second (53.3% [8 of 15 patients] vs 21.3% [17 of 80 patients]; P = .021) platinum exposure. At the time of fourth platinum exposure, patients in the high‐EMSY group demonstrated a significantly greater objective CA 125 response rate (100% [3 of 3 patients] vs 0% [0 of 4 patients]; Fisher's exact P = .029) (see Supporting Fig. S7). Response data stratified by type of platinum‐containing regimen are detailed in Supporting Table S4.
Figure 3.
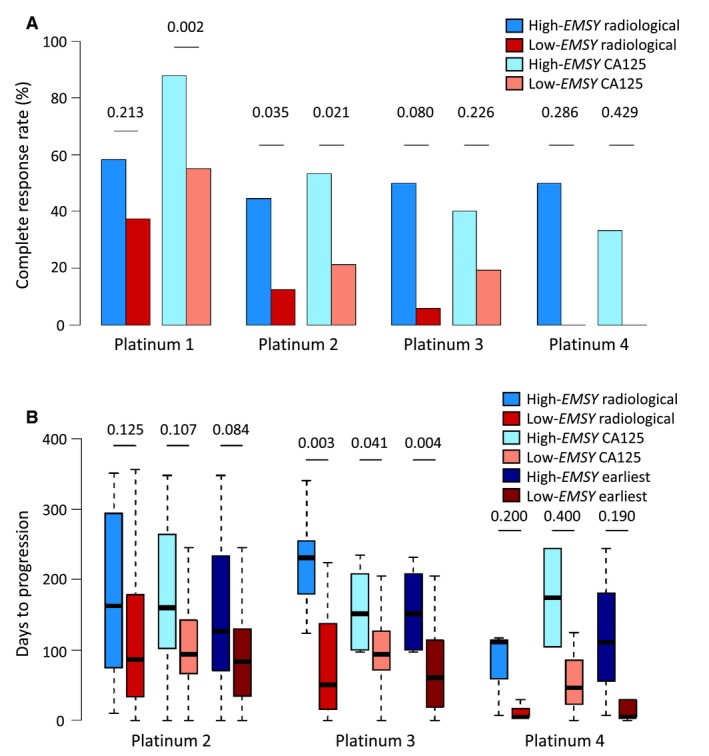
Platinum sensitivity of patients with high‐grade serous ovarian carcinomas (HGSOCs) with high EMSY expression within the Edinburgh cohort. (A) Rate of complete radiological and CA 125 tumor marker response to platinum‐containing chemotherapy. (B) Time to radiological, CA 125, and earliest disease progression from receipt of platinum‐containing chemotherapy.
The median time to first (radiological or CA 125 tumor marker) disease progression after second platinum exposure was 127 days in the high‐EMSY group compared with 83.5 days in the low‐EMSY group, but this did not reach statistical significance (P = .084) (Fig. 3B). Patients in the high‐EMSY group demonstrated a significantly longer time to first disease progression after third platinum exposure (median, 151.5 days vs 60.5 days; P = .004), which was significant when considering only progression by radiology (median, 231 days vs 50 days; P = .003) and CA 125 tumor marker (median, 151.5 days vs 94 days; P = .041) specifically.
High EMSY Expression Was Associated With Superior Outcome in Patients With High‐Risk HGSOC
Patients with advanced stage (FIGO stage III/IV) HGSOC with gross macroscopic residual disease after debulking surgery have particularly poor prognosis (“high‐risk” patients).30 Within the Edinburgh cohort, a greater percentage of patients in the high‐EMSY group remained alive without disease recurrence within the context of high‐risk disease at 2 years (25.0% [4 of 16 patients] vs 9.2% [10 of 109 patients]; P = .081), 3 years (18.8% [3 of 16 patients] vs 3.6% [4 of 110 patients]; P = .043), 5 years (17.6% [3 of 17 patients] vs 2.7% [3 of 111 patients]; P = .031), and 10 years (12.5% [2 of 16 patients] vs 0.9% [1 of 112 patients]; P = .041) from diagnosis (see Supporting Fig. S8A), suggesting that high‐risk patients with high EMSY expression are more likely to achieve favorable long‐term clinical outcome.
A similar effect was observed within the cohort from the study by Pils et al26 at 12 months (100% [9 of 9 patients] vs 61.5% [24 of 39 patients]; P = .041), 18 months (88.9% [8 of 9 patients] vs 17.6% [6 of 34 patients]; P < .001), and 2 years (50.0% [4 of 8 patients] vs 3.1% [1 of 32 patients]; P = .004) (see Supporting Fig. S8B). Although similar trends were observed in the high‐risk patients with adnexal specimens from the study by Tothill et al7 at 12 months from diagnosis, these did not approach statistical significance (100% [5 of 5 patients] vs 62.5% [20 of32 patients]; P = .152) (see Supporting Fig. S9). However, patients with late‐stage disease in the Tothill et al7 study cohort (irrespective of residual disease after debulking surgery) demonstrated the same effect at 12 months (95% [19 of 20 patients] vs 67.8% [78 of 115 patients]; P = .014) and 18 months (70% [14 of 20 patients] vs 42.3% [47 of 111 patients]; P = .029) from diagnosis (see Supporting Fig. S8C).
Discussion
Approximately one‐half of HGSOCs are described as having identifiable defects in the HR pathway, with the archetypal defects being germline or somatic BRCA inactivation.3 Surprisingly, the EMSY gene, the product of which has been shown to bind and inactivate BRCA2, has received relatively little attention in HGSOC, despite being associated with a poor prognosis in individuals with breast cancer.14, 15, 18, 19, 20, 21, 22
Amplification of 11q13 has been identified as a common event in patients with breast cancer and OC, and previous investigations have pointed toward EMSY as the critical gene in this amplicon.14, 15, 22 To our knowledge, only a single study to date has reported on 11q13 amplification and prognosis in patients with OC, and reported no survival difference in patients with SOC upon multivariable analysis,21 with other studies not investigating the impact on patient outcome.5, 15 Notably, this study did not distinguish HGSOC from low‐grade SOC, which now is recognized as a distinct clinical and molecular disease entity.31, 32, 33 Moreover, given the mixed reports of correlation strength between EMSY copy number and expression,14, 15, 34 investigation of the association between EMSY expression and survival may prove more fruitful than associations with copy number alone.
Through in silico analysis of local and publicly available transcriptomic data, we identify a subgroup of HGSOCs defined by high levels of EMSY expression. The threshold for EMSY overexpression was defined within the Edinburgh cohort and validated directly within the MRC ICON7 cohort characterized on the same platform. To identify the high‐EMSY population within independent cohorts characterized by heterogeneous methodologies, we used a percentile‐based expression threshold after validating this approach in the MRC ICON7 cohort. Although a significant difference in outcome within the TCGA cohort was evident only upon exploratory analysis limited to patients with advanced stage disease at the time of diagnosis, multivariable analysis restricted to patients with late‐stage disease in the other evaluable cohorts confirmed prolonged survival in their respective populations (data not shown).
Similar to BRCA‐mutant HGSOC, high‐EMSY HGSOCs appear to demonstrate prolonged survival across multiple independent data sets, and demonstrate a greater benefit from platinum‐based chemotherapy than their low‐EMSY counterparts. Patients in the high‐EMSY group within the Edinburgh cohort demonstrated a >3‐fold radiological complete response rate to second platinum exposure compared with patients in the low‐EMSY group.
Intriguingly, within the MRC ICON7 cohort, we demonstrated that the benefit conferred by high EMSY expression may be abrogated upon addition of the antiangiogenic agent bevacizumab to first‐line therapy, although the power of these analyses was severely limited. Clearly, overinterpretation of these data must be avoided in light of the low numbers of patients in the high‐EMSY group between the two treatment arms, and the presented analyses do not fulfil the REporting recommendations for tumor MARKer prognostic studies (REMARK) criteria for biomarker studies.35 However, these data do suggest that analysis of the differential impact of bevacizumab treatment between HR‐intact and HR‐deficient HGSOCs may now be warranted. Robust evaluation of the impact of HR status on bevacizumab efficacy has the potential to better define those patients who are most likely to derive benefit from the addition of antiangiogenic agents to first‐line care. These data will be of particular interest in light of ongoing trials combining antiangiogenics with PARP inhibitors.36
Advanced stage of disease at the time of diagnosis and suboptimal surgical resection both are associated with markedly inferior survival in patients with HGSOC.30 Patients with both of these features represent those with particularly high‐risk disease. We observed that within these high‐risk patients, those with high EMSY expression have a greater chance of remaining free of disease recurrence (12.5% vs <1% at 10 years from diagnosis within the Edinburgh cohort), suggesting that patients in the high‐EMSY group could represent a group with favorable long‐term clinical outcome even in the face of otherwise poor prognostic markers.
Within the data set from the study by Tothill et al,7 we observed that although high EMSY expression was associated with prolonged survival, this phenomenon was not apparent in patients in whom expression data were generated using samples that were not taken from the primary adnexal mass. There are several plausible explanations for this observation. First, differences in the tumor microenvironment at different anatomic sites may well have impacted EMSY expression. Second, the contamination of tumor samples with different nonmalignant cell types has the potential to alter bulk EMSY expression. Third, intratumor heterogeneity as a result of tumor evolution may have resulted in differential tumor cell EMSY expression between the primary tumor mass and more distant disease sites. In any respect, differential gene expression between sampling sites has clear implications for the identification of pertinent molecular events in both the clinical and research setting. In particular, the frequency of extra‐adnexal sampling may account for weakened trends between EMSY expression and clinical outcome in some data sets. Transcriptomic characterization of the Edinburgh23 and MRC ICON7 cohorts was performed on specimens that were nearly exclusively taken from the primary adnexal mass, and in both of these data sets samples were macrodissected prior to RNA extraction to minimize stromal contamination. Both these cohorts demonstrated a marked OS benefit in patients in the high‐EMSY group.
These data demonstrate the power of in silico analysis of preexisting data sets to identify novel, clinically meaningful subtypes of disease. Collectively, the current study findings allude to a subgroup of HGSOC patients defined by high levels of EMSY expression who appear to demonstrate improved clinical outcome and greater sensitivity to platinum‐based chemotherapy, consistent with the notion that EMSY overexpression renders HGSOCs HR deficient. Given the role of EMSY in disrupting the BRCA2/RAD51 HR pathway demonstrated by previous studies14, 16, 17 and the apparent BRCA‐like clinical phenotype described here in patients with high‐EMSY HGSOC, investigation of the sensitivity of EMSY‐overexpressing tumors to PARP inhibition has the potential to improve our understanding of which patients benefit the most from these agents. The correlation of EMSY expression with existing HR deficiency signatures and scores should also be explored. Moreover, the impact of differential EMSY expression in other disease settings, most notably breast and prostate cancer, should now be investigated.
Funding Support
Funded by a Medical Research Council PhD Studentship and Medical Research Council‐funded Research Fellowship awarded to Robert L. Hollis, and by charitable donation from The Nicola Murray Foundation.
Conflict of Interest Disclosures
Laura Knight, Andrena McCavigan, D. Paul Harkin, and Richard Kennedy are employees of Almac Diagnostics, a precision medicine company focused on the discovery, validation, and commercialization of novel diagnostic and companion diagnostic tests. Gordon C. Jayson has received grants from Roche and AstraZeneca for work performed outside of the current study. Charlie Gourley has received honoraria from AstraZeneca, Tesaro, Cor2Ed, Medscape, and Sierra Oncology; has acted as a paid consultant for AstraZeneca, Clovis, NuCana, Tesaro, Roche, Foundation One, and Cor2Ed; and is named on 1 patent issued (patent PCT/US2012/040805) and 4 pending patents (patent PCT/GB2013/053202, patent 1409479.1, patent 1409476.7, and patent 1409478.3) related to gene expression signatures predicting treatment response in ovarian cancer and his institution receives research funding from AstraZeneca, Aprea AB, NuCana, and Tesaro for work performed outside of the current study. The other authors made no disclosures.
Author Contributions
Robert L. Hollis: Conceptualization, data curation, formal analysis, investigation, visualization, writing–original draft, and writing–review and editing. Michael Churchman: Conceptualization and writing–review and editing. Caroline O. Michie: Investigation. Tzyvia Rye: Data curation. Laura Knight and Andrena McCavigan: Methodology. Timothy Perren, Gordon C. Jayson, Richard S. Kaplan, Amit Oza, D. Paul Harkin, and Richard Kennedy: Resources and writing–review and editing. Alistair Williams and W. Glenn McCluggage: Investigation and writing–review and editing. C. Simon Herrington: Investigation, supervision, conceptualization, and writing–review and editing. Charlie Gourley: Conceptualization, supervision, funding acquisition, writing–original draft, and writing–review and editing.
Supporting information
We would like to extend our thanks to the Edinburgh Ovarian Cancer Database, to all of the patients who contributed to this study, and to the Nicola Murray Foundation for their generous support of the Nicola Murray Centre for Ovarian Cancer Research.
See referenced original article on pages 2720‐4, this issue.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Kurman RJ. Origin and molecular pathogenesis of ovarian high‐grade serous carcinoma. Ann Oncol. 2013;24(suppl 10):x16‐x21. [DOI] [PubMed] [Google Scholar]
- 3. Bowtell DD, Bohm S, Ahmed AA, et al. Rethinking ovarian cancer II: reducing mortality from high‐grade serous ovarian cancer. Nat Rev Cancer. 2015;15:668‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooke SL, Brenton JD. Evolution of platinum resistance in high‐grade serous ovarian cancer. Lancet Oncol. 2011;12:1169‐1174. [DOI] [PubMed] [Google Scholar]
- 5. Cancer Genome Atlas Research Network . Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patch AM, Christie EL, Etemadmoghadam D, et al. Whole‐genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489‐494. [DOI] [PubMed] [Google Scholar]
- 7. Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198‐5208. [DOI] [PubMed] [Google Scholar]
- 8. Tan DS, Rothermundt C, Thomas K, et al. “BRCAness” syndrome in ovarian cancer: a case‐control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26:5530‐5536. [DOI] [PubMed] [Google Scholar]
- 9. Candido‐dos‐Reis FJ, Song H, Goode EL, et al; for EMBRACE ; kConFab Investigators ; Australian Ovarian Cancer Study Group . Germline mutation in BRCA1 or BRCA2 and ten‐year survival for women diagnosed with epithelial ovarian cancer. Clin Cancer Res. 2015;21:652‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum‐sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382‐1392. [DOI] [PubMed] [Google Scholar]
- 11. Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP‐ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123‐134. [DOI] [PubMed] [Google Scholar]
- 12. Bolton KL, Chenevix‐Trench G, Goh C, et al;EMBRACE ; kConFab Investigators ; Cancer Genome Atlas Research Network . Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917‐921. [DOI] [PubMed] [Google Scholar]
- 14. Hughes‐Davies L, Huntsman D, Ruas M, et al. EMSY links the BRCA2 pathway to sporadic breast and ovarian cancer. Cell. 2003;115:523‐535. [DOI] [PubMed] [Google Scholar]
- 15. Brown LA, Irving J, Parker R, et al. Amplification of EMSY, a novel oncogene on 11q13, in high grade ovarian surface epithelial carcinomas. Gynecol Oncol. 2006;100:264‐270. [DOI] [PubMed] [Google Scholar]
- 16. Raouf A, Brown L, Vrcelj N, et al. Genomic instability of human mammary epithelial cells overexpressing a truncated form of EMSY. J Natl Cancer Inst. 2005;97:1302‐1306. [DOI] [PubMed] [Google Scholar]
- 17. Cousineau I, Belmaaza A. EMSY overexpression disrupts the BRCA2/RAD51 pathway in the DNA‐damage response: implications for chromosomal instability/recombination syndromes as checkpoint diseases. Mol Genet Genomics. 2011;285:325‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown LA, Johnson K, Leung S, et al. Co‐amplification of CCND1 and EMSY is associated with an adverse outcome in ER‐positive tamoxifen‐treated breast cancers. Breast Cancer Res Treat. 2010;121:347‐354. [DOI] [PubMed] [Google Scholar]
- 19. Kirkegaard T, Nielsen KV, Jensen LB, et al. Genetic alterations of CCND1 and EMSY in breast cancers. Histopathology. 2008;52:698‐705. [DOI] [PubMed] [Google Scholar]
- 20. Zhu Y, Wu J, Zhang C, et al. BRCA mutations and survival in breast cancer: an updated systematic review and meta‐analysis. Oncotarget. 2016;7:70113‐70127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown LA, Kalloger SE, Miller MA, et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer. 2008;47:481‐489. [DOI] [PubMed] [Google Scholar]
- 22. Rodriguez C, Hughes‐Davies L, Valles H, et al. Amplification of the BRCA2 pathway gene EMSY in sporadic breast cancer is related to negative outcome. Clin Cancer Res. 2004;10:5785‐5791. [DOI] [PubMed] [Google Scholar]
- 23. Gourley C, McCavigan A, Perren T, et al. Molecular subgroup of high‐grade serous ovarian cancer (HGSOC) as a predictor of outcome following bevacizumab. J Clin Oncol. 2014;32:5502. [Google Scholar]
- 24. Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCall MN, Murakami PN, Lukk M, Huber W, Irizarry RA. Assessing affymetrix GeneChip microarray quality. BMC Bioinformatics. 2011;12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pils D, Hager G, Tong D, et al. Validating the impact of a molecular subtype in ovarian cancer on outcomes: a study of the OVCAD Consortium. Cancer Sci. 2012;103:1334‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mateescu B, Batista L, Cardon M, et al. miR‐141 and miR‐200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med. 2011;17:1627‐1635. [DOI] [PubMed] [Google Scholar]
- 28. Ganzfried BF, Riester M, Haibe‐Kains B, et al. curatedOvarianData: clinically annotated data for the ovarian cancer transcriptome. Database (Oxford). 2013;2013:bat013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rustin GJ, Vergote I, Eisenhauer E, et al; Gynecological Cancer Intergroup . Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer. 2011;21:419‐423. [DOI] [PubMed] [Google Scholar]
- 30. Hoppenot C, Eckert MA, Tienda SM, Lengyel E. Who are the long‐term survivors of high grade serous ovarian cancer? Gynecol Oncol. 2018;148:204‐212. [DOI] [PubMed] [Google Scholar]
- 31. Gockley A, Melamed A, Bregar AJ, et al. Outcomes of women with high‐grade and low‐grade advanced‐stage serous epithelial ovarian cancer. Obstet Gynecol. 2017;129:439‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23(suppl 10):x111‐x117. [DOI] [PubMed] [Google Scholar]
- 33. Hollis RL, Gourley C. Genetic and molecular changes in ovarian cancer. Cancer Biol Med. 2016;13:236‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilkerson PM, Dedes KJ, Wetterskog D, et al. Functional characterization of EMSY gene amplification in human cancers. J Pathol. 2011;225:29‐42. [DOI] [PubMed] [Google Scholar]
- 35. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM; Statistics Subcommittee of the NCI‐EORTC Working Group on Cancer Diagnostics . REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93:387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mirza MR, Mortensen CE, Avall‐Lundqvist E, et al. ENGOT‐OV24‐NSGO/AVANOVA: niraparib versus bevacizumab‐niraparib combination versus bevacizumab and niraparib as sequential therapy in women with platinum‐sensitive epithelial ovarian, fallopian tube, or peritoneal cancer. J Clin Oncol. 2015;33:TPS5607. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


