Abstract
Introduction
We assessed the specific sonographic pattern of structural nerve abnormalities in immunoglobulin M (IgM) neuropathy and disease controls.
Methods
We enrolled 106 incident patients—32 patients with IgM neuropathy, 42 treatment‐naive patients with chronic inflammatory demyelinating polyneuropathy, and 32 patients with axonal neuropathies. All patients underwent standardized ancillary testing in addition to standardized sonography of the brachial plexus and the large arm and leg nerves bilaterally.
Results
We found widespread nerve enlargement in IgM neuropathy and chronic inflammatory demyelinating polyneuropathy (CIDP), with specific enlargement of brachial plexus and proximal segments of median nerve but not in axonal disease controls (P < .001). Sonographic nerve hypertrophy in IgM neuropathy was not associated with nerve conduction, clinical, or laboratory characteristics.
Discussion
Immunoglobulin M neuropathy is characterized by widespread nerve enlargement indistinguishable from CIDP. Our data provide evidence to confirm that the disease process is not confined to the more distal parts of nerves in either classical demyelinating or axonal variants of neuropathy with associated IgM.
Keywords: anti‐MAG, CIDP, EMG, IgM monoclonal gammopathy, IgM neuropathy, nerve ultrasound
Abbreviations
- CAP
chronic axonal polyneuropathy
- CB
conduction block
- CIDP
chronic inflammatory demyelinating polyneuropathy
- CSA
cross‐sectional area
- DML
distal motor latency
- IgM
immunoglobulin M
- IQR
interquartile range
- MAG
myelin‐associated glycoprotein
- MCV
motor conduction velocity
- MGUS
monoclonal gammopathy of unknown significance
- MRC
Medical Research Council
- NCS
nerve conduction study
- ODSS
overall disability sum‐score
- ROC
receiver operator characteristic
- SNAP
sensory nerve action potential
- TLI
terminal latency index
1. INTRODUCTION
Immunoglobulin M (IgM) monoclonal gammopathy of unknown significance (MGUS) is a rare cause of polyneuropathy (throughout the main text referred to as IgM neuropathy). In serum from most patients with IgM neuropathy, antibodies against myelin‐associated glycoprotein (anti‐MAG) or gangliosides can be detected.1, 2, 3, 4 Nerve conduction study (NCS) results often show abnormalities fulfilling criteria for demyelination, in particular predominant distal slowing that can be quantitated with the terminal latency index (TLI).5, 6 Insight into the underlying pathogenic mechanisms remains limited and hampers the design of treatment strategies.7, 8
Neuroimaging is a useful, novel, diagnostic approach and may also contribute to the clarification of the pathogenesis of neuropathies. It can be used to document specific patterns of nerve involvement and diffusivity that correspond with the integrity of myelin and axonal structures of the peripheral nerves.9, 10, 11, 12, 13 Using MRI, our group previously showed marked nerve (root) involvement in patients with IgM neuropathy, indicating that pathology is more widespread than the pattern of distal slowing that is suggested by NCS.10, 14, 15 Nerve ultrasound can be used to study nerve thickening in more detail because it allows assessment of multiple nerves in arms and legs in a short time.9, 16, 17, 18, 19, 20 We systematically investigated consecutive patients with IgM neuropathy and disease controls to determine whether there is a specific pattern of nerve involvement in this disorder.
2. MATERIALS AND METHODS
2.1. Study design, patients, and control participants
We performed an observational case‐control study in consecutive patients with IgM neuropathy and disease controls (treatment‐naive chronic inflammatory demyelinating polyneuropathy [CIDP] and chronic axonal polyneuropathies [CAP]).
All consecutive patients who visited the University Medical Center Utrecht, a tertiary center for neuromuscular disorders, between July 2013 and July 2016 with a confirmed diagnosis of IgM neuropathy, CIDP (definite or probable according to relevant diagnostic [consensus] criteria),4, 6, 21 or CAP were eligible for inclusion. Exclusion criteria were age < 18 years, unable to undergo nerve ultrasound investigation, and previous diagnosis of CIDP or CAP. We enrolled 106 patients (80 men and 26 women)—32 patients (17 prevalent and five incident) with a diagnosis of polyneuropathy and associated IgM MGUS (25 with demyelinating and seven with only axonal features according to NCS), 44 incident and treatment‐naive patients with CIDP, and a random sample of 32 incident patients with acquired CAP (18 cryptogenic axonal neuropathy, two diabetic neuropathy, and 12 other metabolic and toxic causes) without IgM monoclonal gammopathy. All patients underwent routine ancillary testing, including standardized grading of muscle strength, NCS, and laboratory testing (see Supporting Information Methods). Immunoglobulin M monoclonal gammopathy was determined by immunoelectrophoresis. Western blot and ELISA (enzyme‐linked immunosorbent assay) were used to assess the presence of antibodies against MAG and gangliosides.22, 23 We assessed muscle strength (S.G.), overall disability sum‐scores (ODSS) and Rankin scale in the patients with IgM neuropathy.24 Sensory and motor features were categorized into sensory dominant (exclusively sensory and sensory with subtle distal muscle weakness), sensorimotor (sensory with moderate distal muscle weakness), and motor dominant (pronounced muscle weakness).
2.2. Consent and approval of study protocols
All enrolled patients gave written informed consent. The study protocol was approved by the local medical ethics committee of the University Medical Center Utrecht.
2.3. Nerve conduction studies
We performed nerve conduction studies with a Nicolet VIKING IV electromyography machine (CareFusion Japan, Tokyo, Japan) after warming limbs in water at 37°C for at least 45 minutes.5, 25 Median, ulnar, fibular, tibial, and sural nerves were assessed in a standardized manner (see Supporting Information Methods). Criteria for demyelination have been published previously.26, 27 Terminal latency index was calculated for median and ulnar nerves5, 28 as
| (1) |
Nerve conduction studies were performed by one author (H.F.), who was not aware of the sonography results.
2.4. Nerve ultrasound studies
We used a Philips iU22 (Philips Medical Instruments, Bothell, Washington) with a 5‐17 MHz linear array transducer (L17‐5) to evaluate nerve and fascicle size and vascularization of a predefined set of nerves (see Supporting Information Methods).16, 29 We excluded the use of zoom function and limited change‐of‐depth settings to 0.5 cm to prevent inflation of nerve size. In addition to these standard assessments, nerve size of the median nerve in patients with IgM neuropathy was also assessed at the elbow. One author (S.G.), blinded to the electrophysiological measurements, performed all sonographic examinations. We also assessed whether there were differences between IgM neuropathy and CIDP controls in the distribution of enlargement along the length of nerves. To this end, we deployed nerve ultrasound ratios between sites (cross‐sectional areas [CSA] at known sites of nerve compression vs their proximal nerve segments) and sum‐scores (CSA at multiple sites within a nerve or brachial plexus).16, 17
2.5. Statistical analysis
We used nonparametric tests to compare and evaluate associations between variables. We used Spearman's ρ to test associations, Mann‐Whitney and Kruskal‐Wallis tests to compare continuous variables between groups, and χ2 testing to compare categorical variables between groups. Findings with a P‐value of < .05 after adjustment for multiple testing with the Benjamini‐Hochberg method when appropriate were considered significant. Multivariate logistic regression and receiver operating characteristics (ROC) analysis were applied to test for independent and discriminative sonographic variables. Statistical analysis was performed in SPSS 23.0 (IBM, Armonk, New York).
3. RESULTS
3.1. Clinical characteristics
Patient characteristics are summarized in Table 1. Patients with IgM neuropathy had longer disease duration compared with CIDP and CAP disease controls (Table 1). There was no difference in age or sex between the groups. In patients with IgM neuropathy, distal symmetric sensory dominant and sensorimotor involvement dominated the clinical presentation of their neuropathy, whereas only two of 32 (6%) displayed a proximal‐distal motor dominant phenotype that was more common in CIDP controls. Medical Research Council (MRC) sum‐score was lower in IgM neuropathy and in CIDP disease controls than in CAP disease controls.
Table 1.
Patient characteristics
| IgM neuropathy patients | ||||||
|---|---|---|---|---|---|---|
| Variables | Demyelinating, n = 25 | Axonal, n = 7 | Total, n = 32 | CIDP disease controls, n = 42 | CAP disease controls, n = 32 | P value |
| Age, median (IQR), y | 66 (61‐69) | 67 (60‐73) | 66 (61‐71) | 62 (51‐69) | 62 (54‐68) | .11 |
| Sex, men/women, n | 19/6 | 7/0 | 26/6 | 28/14 | 26/6 | .23 |
| Disease duration, median (IQR), mo | 26 (23‐90) | 48 (18‐73) | 36 (22‐81) | 12 (5‐29) | 18 (11‐48) | .002* |
| Clinical features, n (%) | ||||||
| Distal symmetric | 24/25 (96) | 6/7 (86) | 30/32 (94) | 20/42 (48) | 32/32 (100) | <.001* |
| Proximal and distal symmetric | 1/25 (4) | 1/7 (14) | 2/32 (6) | 22/42 (52) | 0 (0) | <.001* |
| Sensory dominant | 6/25 (24) | 3/7 (43) | 9/32 (28) | 8/42 (19) | 21/32 (66) | <.001* |
| Sensorimotor | 18/25 (72) | 3/7 (43) | 21/32 (66) | 11/42 (26) | 11/32 (34) | <.001* |
| Motor dominant | 1/25 (4) | 1/7 (14) | 2/32 (6) | 23/42 (55) | 0 (0) | <.001* |
| MRC sum‐score, median (IQR) | 117 (110‐118) | 117 (114‐120) | 117 (111‐119) | 112 (101‐118) | 120 (118‐120) | .009* |
Abbreviations: CAP, chronic axonal polyneuropathy; CIDP, treatment‐naive chronic inflammatory demyelinating polyneuropathy; IgM, immunoglobulin M; IgM neuropathy patients, patients with neuropathy and associated IgM monoclonal gammopathy of unknown significance; IQR, interquartile range; MRC sum‐score, Medical Research Council sum‐score of a predefined set of muscle groups bilaterally (range, 0‐120).
P < .05 significant difference.
In patients with IgM neuropathy, the median serum IgM concentration was 3.9 g/L (2.9‐7.1); in patients with demyelinating features it was 4.1 g/L (3.1‐6.6), and in patients with only axonal features it was 3.0 g/L (2.7‐11). Anti‐myelin–associated glycoprotein antibodies were present only in patients with IgM neuropathy (21/32 [66%]), particularly in those with demyelinating electrodiagnostic characteristics (18/25 [72%] vs 3/7 [43%] in IgM neuropathy with only axonal features). Anti‐GM1 (2/32 [6%]), anti‐GM2 (3/32 [9%]), and anti‐GD1a (3/32 [9%]) antibodies were found in IgM neuropathy patients both with and without demyelinating features. Median ODSS in the IgM neuropathy patients was 4 (interquartile range [IQR], 3‐5), and median Rankin scale was 2. We found no association between presence of anti‐MAG antibodies or IgM MGUS serum concentration and age, disease duration, MRC sum‐score, ODSS, or Rankin scale. Seventeen patients with IgM neuropathy had been previously treated a median of 3 years (IQR, 2‐5) prior to study inclusion, 11 with rituximab and six with short courses of intravenous immunoglobulins. Treatment‐naive patients had a shorter disease duration compared with those with previous treatment (P = .04). However, there were no differences in other clinical characteristics or nerve size between treatment‐naive patients and those who had been treated previously.
3.2. Sonographic studies
We found multiple nerve sites with focal enlargements in all patients with IgM neuropathy. Results of CSA measurements (Table S1) are summarized in Figures 1, 2, 3. Enlargement of nerves at common sites of nerve compression (ie, median nerve at carpal tunnel, ulnar nerve at cubital sulcus, and fibular nerve at fibular head) was present in all 32 patients with IgM neuropathy, in 40 of 42 (95%) CIDP disease controls, and in 31 of 32 (97%) CAP disease controls. Hence, all 32 IgM MGUS neuropathy patients showed enlargement in nerve segments proximal to common compression sites in median (forearm, upper arm) and ulnar (forearm and upper arm, outside the sulcus) nerves. In contrast, we found only mild enlargement in these more proximal nerve segments in CAP disease controls: eight of 32 (25%, upper arm) in median and 17 of 32 (44%, distal and proximal sulcus) in ulnar. Neither the number of enlarged nerves nor nerve size in the IgM neuropathy group correlated with age, disease duration, MRC sum‐score, ODSS, Rankin scale, presence of anti‐MAG antibodies, or IgM MGUS serum concentration.
Figure 1.
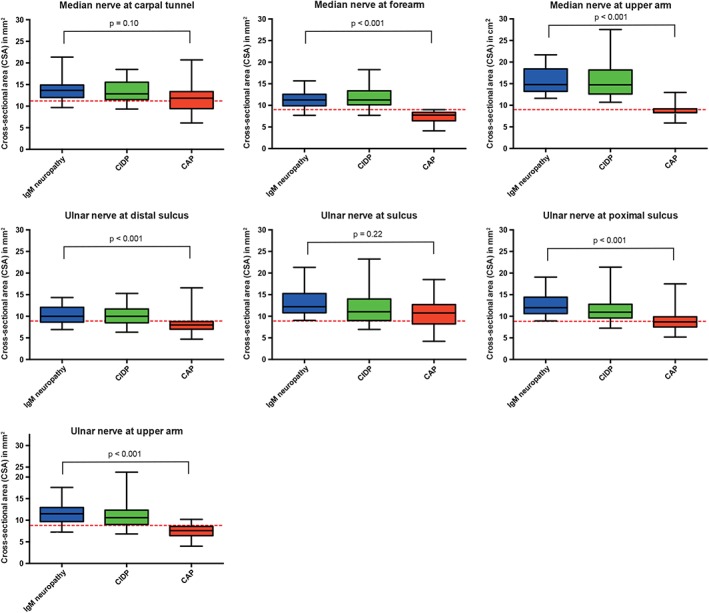
Nerve size in arm nerves in immunoglobulin M (IgM) neuropathy and disease controls. Patients with neuropathy and associated IgM monoclonal gammopathy of unknown significance (IgM neuropathy), treatment‐naive chronic inflammatory neuropathy (CIDP), and chronic axonal neuropathy (CAP) disease controls. Red dashed lines represent cutoff values for abnormal nerve size (CSA), as published previously. CSA, cross‐sectional area, error bars represent 2SD (i.e. 95%) [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
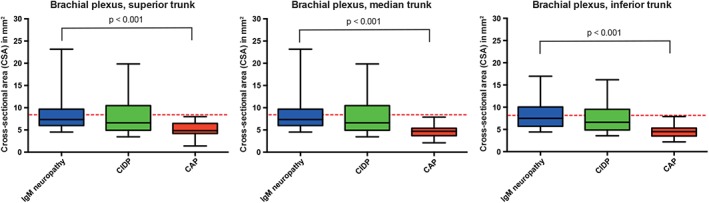
Nerve size in brachial plexus in immunoglobulin M (IgM) neuropathy and disease controls. Patients with neuropathy and associated IgM monoclonal gammopathy of unknown significance (IgM neuropathy), treatment‐naive chronic inflammatory neuropathy (CIDP), and chronic axonal neuropathy (CAP) disease controls. Red dashed lines represent cutoff values for abnormal nerve size (CSA), as published previously. CSA, cross‐sectional area, error bars represent 2SD (i.e. 95%) [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.
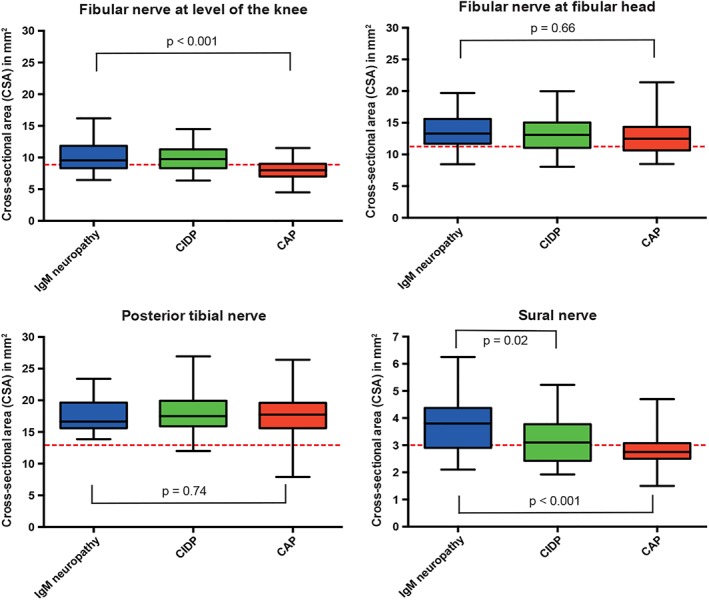
Nerve size in leg nerves in immunoglobulin M (IgM) neuropathy and disease controls. Patients with neuropathy and associated IgM monoclonal gammopathy of unknown significance (IgM neuropathy), treatment‐naive chronic inflammatory neuropathy (CIDP), and chronic axonal neuropathy (CAP) disease controls. The red dashed lines represent cutoff values for abnormal nerve size (CSA), as published previously. CSA, cross‐sectional area, error bars represent 2SD (i.e. 95%) [Color figure can be viewed at wileyonlinelibrary.com]
Nerve size was larger in IgM neuropathy patients than in CAP disease controls in all nerves except for the median nerve at the carpal tunnel, ulnar nerve at the sulcus, fibular nerve at the fibular head, and posterior tibial nerve (Figures 1 and 3, Table S1). We found enlargement of the brachial plexus in patients with IgM neuropathy—in 20 of 25 (80%) with demyelinating features and in six of seven (86%) with axonal features. Furthermore, we also found hypertrophy of brachial plexus in 35 of 44 (80%) CIDP disease controls but not in any of the CAP disease controls. Only sural nerve hypertrophy was more pronounced in IgM neuropathy than in CIDP (Figure 3, Table S1). When we evaluated the nerve ultrasound ratios and sum‐scores, only the sum of nerve size in median nerves (forearm and upper arm segments in both arms) was significantly higher in IgM neuropathy than in the CIDP disease controls (Figure 4).
Figure 4.
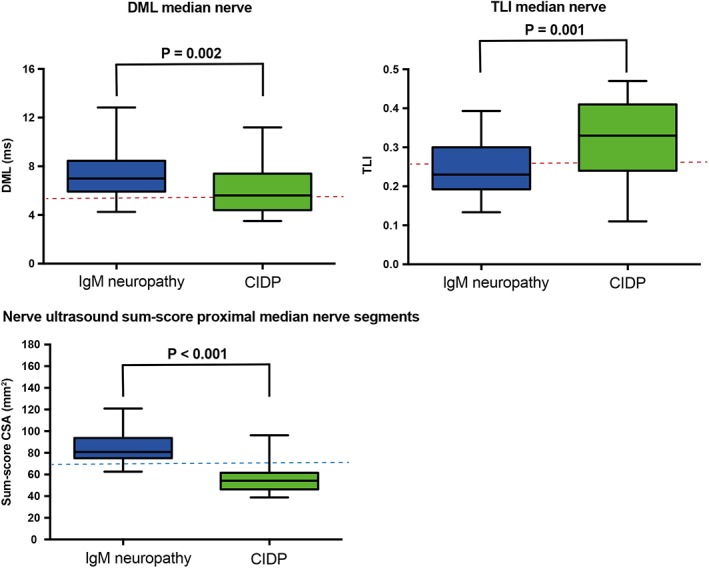
Nerve conduction studies and nerve ultrasound variables in immunoglobulin M (IgM) neuropathy and chronic inflammatory demyelinating polyneuropathy (CIDP). Variables of nerve conduction studies and nerve ultrasound differ between demyelinating neuropathy associated with IgM monoclonal gammopathy of unknown significance (IgM neuropathy) and CIDP. Upper panels show distal motor latency (DML) and terminal latency index (TLI) in median nerve. Red dashed lines represent cutoff values for disproportionate prolongation of DML.6 Lower panel shows nerve ultrasound sum‐score of proximal median nerve segments: sum of nerve size of median nerve in forearm and upper arm in both arms (cross‐sectional area [CSA]). The blue dashed line represents proposed nerve ultrasound cutoff value after multivariate logistic regression and receiver operating characteristics analysis to discern IgM neuropathy from CIDP, error bars represent 2SD (i.e. 95%) [Color figure can be viewed at wileyonlinelibrary.com]
Hypervascularization was seen in one patient with IgM neuropathy in the fibular nerve at the level of the knee and in two CIDP and two CAP disease controls at common sites of nerve compression (median nerve at carpal tunnel, ulnar nerve at cubital sulcus, and fibular nerve at fibular head).
3.3. Nerve conduction studies
Distal motor latency was longer in median and ulnar nerves, and TLI was lower in IgM neuropathy than in CIDP controls (Figure 4, Table S2). The sensory nerve action potential (SNAP) of sural nerve was more often absent in IgM neuropathy than in CIDP (Table S2) and showed an inverse relation with disease duration (P = .03). Conduction block (CB) was present in only a few nerve segments of the patients with IgM neuropathy (one definite CB in median and one in ulnar nerves, three possible CB in median and four in ulnar nerves). In contrast, we found CB to be a more frequent finding in CIDP (14 definite CB in median and 21 in ulnar nerves, 17 possible CB in median and 25 in ulnar nerves). We found a comparable distribution of most other NCS variables in the IgM neuropathy group and CIDP disease controls (Table S2). Hence, these NCS variables showed no association with clinical and demographic data.
3.4. Correlation of sonography with nerve conduction studies
In patients with IgM neuropathy, there was no difference in nerve size or number of enlarged nerves or nerve segments between those with exclusively axonal and those with (distal) demyelinating features. Furthermore, we found no difference in IgM neuropathy between the axonal/demyelinating subgroups with respect to age, sex, disease duration, MRC sum‐score, IgM plasma concentration, or presence of anti‐MAG antibodies. Loss of sensory or motor axons and distal conduction slowing in motor axons (that fulfilled the criteria for demyelination) were not related to nerve size or number of enlarged nerve segments.
3.5. Combined ultrasound and NCS to distinguish IgM neuropathy from CIDP
Although IgM neuropathy diagnosis is based primarily on the presence of IgM MGUS and (distal) demyelination according to NCS results, the European Federation of Neurological Societies/Peripheral Nerve Society criteria also address the electrodiagnostic distinction of IgM neuropathy from CIDP based on TLI (≤0.25 for median and ulnar nerves). We found only moderate sensitivity of this TLI variable that correctly identified 14 of 25 (56%) patients with IgM neuropathy (Figure 4). However, specificity was poor because 13 of 42 (31%) CIDP controls also fulfilled this criterion. To investigate whether nerve ultrasound may further aid their distinction, we applied logistic regression and ROC analysis to test combinations on measurements of nerve size at multiple sites. Only the nerve ultrasound sum‐score of median nerve size over four anatomical sites >70 mm2 showed considerable improvement and discerned 23 of 25 (92%) IgM neuropathy patients with demyelinating features compared with four of 42 (10%) CIDP controls (Figure 4). In addition, this nerve ultrasound score was also abnormal in five of seven (71%) IgM neuropathy patients with axonal features but none of the CAP disease controls.
4. DISCUSSION
Morphological changes in patients with IgM neuropathy are widespread, both in proximal and distal nerve segments. Disproportionate enlargement in proximal median nerve segments and sural nerves are specific findings in IgM neuropathy. This pattern of nerve enlargement resembles that of CIDP, but is distinct from the one observed in chronic axonal neuropathies.
Four other studies previously compared sonographic findings in patients with paraproteinemic neuropathies.18, 19, 20, 30 All of these studies used different sonographic protocols and scoring systems (without predefined normal values for nerve size) and showed considerable heterogeneity of patient characteristics, including control groups with and without IgM. These studies all consequently reported variable nerve size in small samples of patients with IgM neuropathy. In our study, we used an elaborate standardized nerve ultrasound protocol and previously published cutoff values for abnormality in a fairly large sample of patients with IgM neuropathy and disease controls without IgM MGUS.16, 29 Moreover, our patient group was homogeneous because we included only participants with IgM MGUS and related neuropathy as patients.21, 31, 32 In contrast, the association of IgA and IgG MGUS is coincidental rather than causative.21, 31, 32 We found widespread and homogeneous enlargement of multiple nerves in both IgM neuropathy and CIDP.10 Only the nerve ultrasound sum of nerve size in median nerves (forearm and upper arm segments in both arms) showed promise in distinction between IgM neuropathy and CIDP, but additional studies are required to validate this finding and evaluate whether it can complement NCS. Although we investigated a relatively large number of patients, we did not find differences in nerve size between patients with antibodies to MAG and those without antibodies to MAG, as has been reported in previous studies.18, 19 Contrary to a previous MRI study in which there were no abnormalities at the cervical nerve roots in two patients with exclusively axonal NCS features,10 our study showed widespread nerve enlargement in patients with IgM neuropathy irrespective of NCS results (ie, with both axonal and demyelinating features). This may be explained by the elaborate sonographic protocol that evaluated more sites and by the lack of objective cutoff values for abnormality in MRI.
There was no association between nerve conduction and sonographic variables. We could not corroborate the previously found negative correlation between nerve size and motor conduction velocity in the of median and ulnar nerves of the forearm.18 This lack of association between NCS and nerve ultrasound is a more general observation in inflammatory neuropathies. For example, we have not been able to find a correlation between nerve thickening and focal NSC abnormalities (eg, CB).17, 33 The disproportionate distal slowing that characterizes IgM neuropathy was not reflected by predominant nerve thickening in distal nerve segments. The only possible exception was the sural nerve. We found larger size (CSA) and absent SNAP of sural nerves more frequently in IgM neuropathy than in CIDP.5 Furthermore, both conduction slowing and sonographic nerve enlargement at entrapment sites are frequent findings in IgM neuropathy and indicate increased sensitivity for nerve entrapment that adds to additional mechanisms of nerve injury in IgM neuropathy.34
Sonographic findings show more similarities between patients with IgM neuropathy and those with CIDP compared with NCS findings and immune biology. Predominant distal slowing is the hallmark of IgM neuropathy, while multifocal abnormalities (conduction slowing or block) are more common in CIDP. Furthermore, the underlying immune‐mediated mechanisms probably differ with little or no contribution of T lymphocytes in addition to antibodies in IgM neuropathy, in contrast to CIDP in which activity of T cells is more important.35, 36 We could speculate that IgM deposits are more pronounced distally, for example, due to local differences in MAG expression or accessibility and that this is reflected in differential vulnerability of nerve segments with predominantly distal changes in myelin and paranodal morphology and axonal loss.37, 38 The involvement of proximal nerve segments in IgM neuropathy may alternatively predispose to (axonal) dysfunction that accumulates at the most distal segments of the nerves.
How can we explain comparable sonographic findings in IgM neuropathy and CIDP when other features differ? It is possible that both humoral (IgM neuropathy and CIDP) and cellular (CIDP) immune‐mediated mechanisms can cause disruption of the myelin sheath that results in nerve thickening. In IgM neuropathy, IgM deposits in the myelin sheath, and binding to MAG and other glycoproteins in myelin lamellae causes characteristic widening of uncompacted myelin sheaths and segmental demyelination‐remyelination.15, 39, 40, 41, 42 In CIDP, intricate and only partially elucidated cellular and humoral responses are the main driving forces behind onion bulb formation, neural infiltrates, and edema.43 All these processes apparently generate focal thickenings in both disorders44 that can be detected with ultrasound in nerves throughout their length.
Limitations of our study are the cross‐sectional design and the inclusion of a subset of patients with IgM neuropathy who had been treated for an average of 3 years prior to investigation. Nerve size may vary with disease activity and duration,45, 46 which is why we chose to include only treatment‐naive CIDP and chronic axonal neuropathies without IgM MGUS as controls. Our study provided no evidence of differences in nerve size between IgM neuropathy patients with short and longer disease duration or treatment status, so these factors are unlikely to have influenced sonographic findings between groups.
In conclusion, IgM neuropathy is characterized by widespread nerve thickening similar but not completely identical to CIDP. Additional studies are required to evaluate whether nerve ultrasound may be a useful biomarker to monitor experimental treatment effects in IgM neuropathy.
CONFLICT OF INTEREST
The authors report no disclosures relevant to the study reported here.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Appendix S1: Supplementary Information
Supplement table 1 Summary of results of nerve size measurements
Supplement table 2 Summary nerve conduction studies IgM neuropathy and CIDP disease controls.
Funding information Prinses Beatrix Spierfonds: W.OR14‐08.
Funding information Prinses Beatrix Spierfonds, Grant/Award Number: W.OR14‐08
REFERENCES
- 1. Visser NA, Notermans NC, Linssen RS, van den Berg LH, Vrancken AF. Incidence of polyneuropathy in Utrecht, the Netherlands. Neurology. 2015; 84(3):259‐264. [DOI] [PubMed] [Google Scholar]
- 2. Nobile‐Orazio E, Manfredini E, Carpo M, et al. Frequency and clinical correlates of anti‐neural IgM antibodies in neuropathy associated with IgM monoclonal gammopathy. Ann Neurol. 1994;36(3):416‐424. [DOI] [PubMed] [Google Scholar]
- 3. Stork AC, van der Pol WL, Franssen H, Jacobs BC, Notermans NC. Clinical phenotype of patients with neuropathy associated with monoclonal gammopathy: a comparative study and a review of the literature. J Neurol. 2014;261(7):1398‐1404. [DOI] [PubMed] [Google Scholar]
- 4. Notermans NC, Franssen H, Eurelings M, Van der Graaf Y, Wokke JH. Diagnostic criteria for demyelinating polyneuropathy associated with monoclonal gammopathy. Muscle Nerve. 2000;23(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 5. Franssen H, Notermans NC. Length dependence in polyneuropathy associated with IgM gammopathy. Ann Neurol. 2006;59(2):365‐371. [DOI] [PubMed] [Google Scholar]
- 6. Joint Task Force of the European Federation of Neurological Societies/Peripheral Nerve Society . Guideline on management of paraproteinemic demyelinating neuropathies. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15(3):185‐195. [DOI] [PubMed] [Google Scholar]
- 7. Lunn MP, Nobile‐Orazio E. Immunotherapy for IgM anti‐myelin‐associated glycoprotein paraprotein‐associated peripheral neuropathies. Cochrane Database Syst Rev. 2012;5(5):CD002827. [DOI] [PubMed] [Google Scholar]
- 8. Niermeijer JM, Fischer K, Eurelings M, Franssen H, Wokke JH, Notermans NC. Prognosis of polyneuropathy due to IgM monoclonal gammopathy: a prospective cohort study. Neurology. 2010;74(5):406‐412. [DOI] [PubMed] [Google Scholar]
- 9. Goedee HS, Brekelmans GJ, van Asseldonk JT, Beekman R, Mess WH, Visser LH. High resolution sonography in the evaluation of the peripheral nervous system in polyneuropathy—a review of the literature. Eur J Neurol. 2013;20(10):1342‐1351. [DOI] [PubMed] [Google Scholar]
- 10. Eurelings M, Notermans NC, Franssen H, et al. MRI of the brachial plexus in polyneuropathy associated with monoclonal gammopathy. Muscle Nerve. 2001;24(10):1312‐1318. [DOI] [PubMed] [Google Scholar]
- 11. Haakma W, Jongbloed BA, Froeling M, et al. MRI shows thickening and altered diffusion in the median and ulnar nerves in multifocal motor neuropathy. Eur Radiol. 2017;27(5):2216‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jongbloed BA, Haakma W, Goedee HS, et al. Comparative study of peripheral nerve MRI and ultrasound in multifocal motor neuropathy and amyotrophic lateral sclerosis. Muscle Nerve. 2016;54(6):1133‐1135. [DOI] [PubMed] [Google Scholar]
- 13. Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. 2015;86(9):973‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barnett MH, Barnett Y, Burke D, Willison H. Neurological picture. Spinal nerve root hypertrophy in chronic ataxic neuropathy with antiglycolipid IgM antibodies. J Neurol Neurosurg Psychiatry. 2011;82(1):97. [DOI] [PubMed] [Google Scholar]
- 15. Stefansson K, et al. Neuropathy accompanying IgM lambda monoclonal gammopathy. Acta Neuropathol. 1983;59(4):255‐261. [DOI] [PubMed] [Google Scholar]
- 16. Goedee HS, Brekelmans GJ, van den Berg LH, Visser LH. Distinctive patterns of sonographic nerve enlargement in Charcot‐Marie‐Tooth type 1A and hereditary neuropathy with pressure palsies. Clin Neurophysiol. 2015;126(7):1413‐1420. [DOI] [PubMed] [Google Scholar]
- 17. Goedee HS, van der Pol WL, van Asseldonk JH, et al. Diagnostic value of sonography in treatment‐naive chronic inflammatory neuropathies. Neurology. 2016;88(2):143‐151. [DOI] [PubMed] [Google Scholar]
- 18. Athanasopoulou IM, Rasenack M, Grimm C, et al. Ultrasound of the nerves—an appropriate addition to nerve conduction studies to differentiate paraproteinemic neuropathies. J Neurol Sci. 2016;362:188‐195. [DOI] [PubMed] [Google Scholar]
- 19. Lucchetta M, Padua L, Granata G, et al. Nerve ultrasound findings in neuropathy associated with anti‐myelin‐associated glycoprotein antibodies. Eur J Neurol. 2015;22(1):193‐202. [DOI] [PubMed] [Google Scholar]
- 20. Padua L, Martinoli C, Pazzaglia C, et al. Intra‐ and internerve cross‐sectional area variability: new ultrasound measures. Muscle Nerve. 2012;45(5):730‐733. [DOI] [PubMed] [Google Scholar]
- 21. Joint Task Force of European Federation of Neurological Societies/Peripheral Nerve Society. Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. J Peripher Nerv Syst. 2010;15(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 22. Eurelings M, Ang CW, Notermans NC, Van Doorn PA, Jacobs BC, Van den Berg LH. Antiganglioside antibodies in polyneuropathy associated with monoclonal gammopathy. Neurology. 2001;57(10):1909‐1912. [DOI] [PubMed] [Google Scholar]
- 23. Stork AC, Jacobs BC, Tio‐Gillen AP, et al. Prevalence, specificity and functionality of anti‐ganglioside antibodies in neuropathy associated with IgM monoclonal gammopathy. J Neuroimmunol. 2014;268(1‐2):89‐94. [DOI] [PubMed] [Google Scholar]
- 24. Merkies IS, Schmitz PI, van der Meche FG, Samijn JP, van Doorn PA, Inflammatory Neuropathy C, Treatment g . Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry. 2002;72(5):596‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Notermans NC, Franssen H, Wieneke GH, Wokke JH. Temperature dependence of nerve conduction and EMG in neuropathy associated with gammopathy. Muscle Nerve. 1994;17(5):516‐522. [DOI] [PubMed] [Google Scholar]
- 26. Van Asseldonk JT, Van den Berg LH, Kalmijn S, Wokke JH, Franssen H. Criteria for demyelination based on the maximum slowing due to axonal degeneration, determined after warming in water at 37 degrees C: diagnostic yield in chronic inflammatory demyelinating polyneuropathy. Brain. 2005;128(Pt 4):880‐891. [DOI] [PubMed] [Google Scholar]
- 27. Van Asseldonk JT, Van den Berg LH, Wieneke GH, Wokke JH, Franssen H. Criteria for conduction block based on computer simulation studies of nerve conduction with human data obtained in the forearm segment of the median nerve. Brain. 2006;129(Pt 9):2447‐2460. [DOI] [PubMed] [Google Scholar]
- 28. Kaku DA, England JD, Sumner AJ. Distal accentuation of conduction slowing in polyneuropathy associated with antibodies to myelin‐associated glycoprotein and sulphated glucuronyl paragloboside. Brain. 1994;117(Pt 5):941‐947. [DOI] [PubMed] [Google Scholar]
- 29. Goedee HS, Brekelmans GJ, Visser LH. Multifocal enlargement and increased vascularization of peripheral nerves detected by sonography in CIDP: a pilot study. Clin Neurophysiol. 2014;125(1):154‐159. [DOI] [PubMed] [Google Scholar]
- 30. Garg N, Park SB, Howells J, et al. Anti‐MAG neuropathy: role of IgM antibodies, the paranodal junction and juxtaparanodal potassium channels. Clin Neurophysiol. 2018;129(10):2162‐2169. [DOI] [PubMed] [Google Scholar]
- 31. Magy L, Chassande B, Maisonobe T, Bouche P, Vallat JM, Leger JM. Polyneuropathy associated with IgG/IgA monoclonal gammopathy: a clinical and electrophysiological study of 15 cases. Eur J Neurol. 2003;10(6):677‐685. [DOI] [PubMed] [Google Scholar]
- 32. Nobile‐Orazio E, Casellato C, Di Troia A. Neuropathies associated with IgG and IgA monoclonal gammopathy. Rev Neurol (Paris). 2002;158(10 Pt 1):979‐987. [PubMed] [Google Scholar]
- 33. Goedee HS, van der Pol WL, van Asseldonk JH, et al. Nerve sonography to detect peripheral nerve involvement in vasculitis syndromes. Neurol Clin Pract. 2016;6(4):293‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faber CG, Notermans NC, Wokke JH, Franssen H. Entrapment in anti myelin‐associated glycoprotein neuropathy. J Neurol. 2009;256(4):620‐624. [DOI] [PubMed] [Google Scholar]
- 35. Eurelings M, Notermans NC, Wokke JH, Bosboom WM, Van den Berg LH. Sural nerve T cells in demyelinating polyneuropathy associated with monoclonal gammopathy. Acta Neuropathol. 2002;103(2):107‐114. [DOI] [PubMed] [Google Scholar]
- 36. Van Rhijn I, Van den Berg LH, Bosboom WM, Otten HG, Logtenberg T. Expression of accessory molecules for T‐cell activation in peripheral nerve of patients with CIDP and vasculitic neuropathy. Brain. 2000;123(Pt 10):2020‐2029. [DOI] [PubMed] [Google Scholar]
- 37. Lombardi R, Erne B, Lauria G, et al. IgM deposits on skin nerves in anti‐myelin‐associated glycoprotein neuropathy. Ann Neurol. 2005;57(2):180‐187. [DOI] [PubMed] [Google Scholar]
- 38. Franssen H, Straver DC. Pathophysiology of immune‐mediated demyelinating neuropathies—part II: Neurology. Muscle Nerve. 2014;49(1):4‐20. [DOI] [PubMed] [Google Scholar]
- 39. Jacobs JM. Morphological changes at paranodes in IgM paraproteinaemic neuropathy. Microsc Res Tech. 1996;34(6):544‐553. [DOI] [PubMed] [Google Scholar]
- 40. Vital A. Paraproteinemic neuropathies. Brain Pathol. 2001;11(4):399‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Willison HJ, Trapp BD, Bacher JD, Dalakas MC, Griffin JW, Quarles RH. Demyelination induced by intraneural injection of human antimyelin‐associated glycoprotein antibodies. Muscle Nerve. 1988;11(11):1169‐1176. [DOI] [PubMed] [Google Scholar]
- 42. Tatum AH. Experimental paraprotein neuropathy, demyelination by passive transfer of human IgM anti‐myelin‐associated glycoprotein. Ann Neurol. 1993;33(5):502‐506. [DOI] [PubMed] [Google Scholar]
- 43. Vital C, Vital A, Lagueny A, et al. Chronic inflammatory demyelinating polyneuropathy: immunopathological and ultrastructural study of peripheral nerve biopsy in 42 cases. Ultrastruct Pathol. 2000;24(6):363‐369. [DOI] [PubMed] [Google Scholar]
- 44. Rebai T, Mhiri C, Heine P, Charfi H, Meyrignac C, Gherardi R. Focal myelin thickenings in a peripheral neuropathy associated with IgM monoclonal gammopathy. Acta Neuropathol. 1989;79(2):226‐232. [DOI] [PubMed] [Google Scholar]
- 45. Grimm A, Vittore D, Schubert V, et al. Ultrasound aspects in therapy‐naive CIDP compared to long‐term treated CIDP. J Neurol. 2016;263(6):1074‐1082. [DOI] [PubMed] [Google Scholar]
- 46. Zaidman CM, Pestronk A. Nerve size in chronic inflammatory demyelinating neuropathy varies with disease activity and therapy response over time: a retrospective ultrasound study. Muscle Nerve. 2014;50(5):733‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Supplement table 1 Summary of results of nerve size measurements
Supplement table 2 Summary nerve conduction studies IgM neuropathy and CIDP disease controls.


