Abstract
Atopic dermatitis negatively impacts work productivity. This study investigated the impact of nemolizumab on work productivity and activity impairment in adults with moderate to severe atopic dermatitis inadequately controlled by topical treatments in a two‐part, phase II, randomized control trial. The Work Productivity and Activity Impairment – Atopic Dermatitis questionnaire was an exploratory end‐point. Part A was a 12‐week, placebo‐controlled study in which patients received s.c. nemolizumab 0.1, 0.5 or 2.0 mg/kg every 4 weeks or 2.0 mg/kg every 8 weeks. Part B was a 52‐week extension in which all patients received active treatment. A total of 138 patients had Work Productivity and Activity Impairment – Atopic Dermatitis data; 104 were employed at baseline. At week 12, patients receiving nemolizumab every 4 weeks showed greater mean (standard error) Work Productivity and Activity Impairment – Atopic Dermatitis improvement (score reduction) from baseline versus placebo: Percent Work Time Missed (0.1, 0.5 or 2.0 mg/kg vs placebo): –4.0% (3.9%), –1.7% (4.2%) and –1.6% (4.2%) versus 4.9% (4.5%); Percent Impairment While Working, –15.8% (6.0%), –24.1% (6.5%) and –34.3% (6.4%) versus –16.5% (7.1%); Percent Overall Work Impairment, –16.3% (6.0%), –23.1% (6.5%) and –34.5% (6.3%) versus –16.6% (7.1%); and Percent Activity Impairment, –13.4% (5.3%), –23.5% (5.3%) and –41.9% (5.5%) versus –10.9% (5.7%). Improvements were sustained through week 64. Nemolizumab‐treated patients with moderate to severe atopic dermatitis reported improvements in Work Productivity and Activity Impairment through week 64.
Keywords: atopic dermatitis, clinical trial phase II, nemolizumab, pharmacotherapy, skin diseases
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory, T‐cell‐mediated skin disease associated with disseminated, intensely pruritic skin lesions. Itching, the dominant clinical feature of AD,1, 2, 3 is mediated by the binding of the pro‐inflammatory cytokine interleukin (IL)‐31 to IL‐31 receptor A on sensory neurones4, 5, 6 and chronic pruritus exacerbates disease through the itch–scratch cycle.7 Long‐term symptom alleviation is a treatment goal for this chronic condition; however, treatment options are limited for patients with AD unresponsive to topical therapies, such as the corticosteroids and calcineurin inhibitors that form the mainstay of therapy.3, 8, 9
Atopic dermatitis negatively impacts health‐related quality of life (HRQoL), sleep quality, mental health and work productivity,10, 11, 12 with greater disease severity associated with greater work and activity impairment.10, 12, 13 The effect of AD on mood and sleep disorders, HRQoL and work productivity has been reported to be similar to that of psoriasis;12 however, the burden of illness in AD is still a relatively understudied area. Pruritus is a key contributor to the burden of AD, and continuous itching leads to loss of sleep, reduced HRQoL and symptoms of depression, as well as impacting daily functioning including the ability to work and study.11, 14
Nemolizumab is an anti‐IL‐31 receptor A humanized monoclonal antibody that blocks IL‐31‐mediated signaling. In addition to stimulating pruritus, IL‐31 also has a role in perpetuation of the inflammatory response and dysregulation of the physical and functional properties of the skin barrier,5, 15, 16 both of which contribute to ongoing disease. Nemolizumab demonstrated improvements in pruritus, disease severity and sleep disturbance in a phase II, 12‐week, randomized, double‐blind, placebo‐controlled, dose‐finding study (Part A) in adults with moderate to severe AD inadequately controlled by topical treatments (XCIMA study; NCT 01986933).17 A double‐blind, 52‐week extension study (Part B) demonstrated that nemolizumab was associated with clinically meaningful reductions in pruritus and dermatitis when administrated for up to 64 weeks.18 Nemolizumab was also well tolerated, with no new safety concerns identified with long‐term use.17, 18 To date, work productivity in patients with AD has not been well studied. Therefore, the objective of the current post‐hoc analysis was to investigate the effects of nemolizumab on work productivity and activity impairment (WPAI) in patients with moderate to severe AD in the 12‐week, placebo‐controlled study (Part A) and subsequent long‐term extension (Part B) using the Work Productivity and Activity Impairment – Atopic Dermatitis (WPAI‐AD) questionnaire as the assessment index. We also evaluated the relationship between WPAI and indicators of disease severity to identify clinical outcomes associated with ability to work and daily activity.
Methods
Study design
Details of the study design have been reported previously.17, 18 Briefly, Part A was an evaluation of four nemolizumab dose regimens (0.1, 0.5 or 2.0 mg/kg s.c. every 4 weeks [Q4W] or 2.0 mg/kg s.c. every 8 weeks [Q8W]). In Part B, patients receiving nemolizumab in Part A continued the same dose and patients randomized to placebo in Part A were re‐randomized to nemolizumab (0.1, 0.5 or 2.0 mg/kg Q4W) in a 1:1:1 ratio (re‐randomized patients were not included in the current analysis).
The study was performed in accordance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. All study documents and procedures were approved by the appropriate ethics committee and institutional review board (IRB) at each study center (IRB protocol approval no. QUI1‐13‐404; dated 28 October 2013 for site of principal investigator), and written informed consent was provided by all patients. The trial registration number is NCT 01986933.
Patients
Key inclusion criteria for Parts A and B have been described previously.17, 18 Eligible patients were 18–65 years of age with moderate to severe AD inadequately controlled by topical corticosteroids or topical calcineurin inhibitors, with an Eczema Area and Severity Index (EASI) score of 10 or more, pruritus visual analog scale (VAS) score of 50 mm or more and static Investigator's Global Assessment (sIGA) score of 3 or more. The WPAI‐AD questionnaire was administrated to patients in the USA and Japan only, owing to limited availability of local language versions of the WPAI‐AD questionnaire.19 Patients were required to have available WPAI‐AD data from baseline and subsequent visits to be eligible for the current analysis.
Study assessments
The WPAI – Specific Health Problem (WPAI‐SHP) questionnaire,19, 20 developed to assess the impact of specific conditions on the ability to work and perform regular daily activities, was adapted for use in AD. The questionnaire has four domains: (i) Work Time Missed (absenteeism); (ii) Percent Impairment While Working (presenteeism); (iii) Percent Overall Work Impairment; and (iv) Percent Activity Impairment.19, 20 Percent overall work productivity loss is calculated based on absenteeism and presenteeism using the formula: absenteeism + (% of time worked × presenteeism). Notably, percent overall work impairment only targets the working population, while activity impairment targets both the working and non‐working population.
When completing the WPAI‐AD questionnaire, patients were asked to consider the last 7 days prior to the study visit. WPAI‐AD (expressed as percentage of impairment, with higher numbers indicating greater impairment and lesser productivity) and Dermatology Life Quality Index (DLQI) (measured on a scale of 0–30, with higher scores representing greater impairment) were completed by patients every 4 weeks throughout the total study period (Parts A and B). Pruritus VAS (which ranges from 0 [no itch] to 100 mm [worst imaginable itch]) and sleep disturbance VAS (which ranges from 0 [no sleep loss] to 100 mm [inability to sleep at all]) were completed by patients once daily during Part A, and every 7 days during Part B. EASI (which ranges from 0–72, with higher scores indicating worse disease severity) was measured at each study visit during Parts A and B. On days with study visits, patient‐reported outcome assessments were completed prior to other study assessments. Patients were evaluated by the same assessor at all visits for consistency (when possible) and assessor training was employed to minimize variation across study sites.
Study end‐points
Work Productivity and Activity Impairment ‐ Atopic Dermatitis was an exploratory end‐point in the study; primary and secondary efficacy end‐points have been previously described.17, 18 Briefly, the primary end‐point was percentage improvement from baseline in pruritus VAS score, and secondary end‐points included changes from baseline in EASI score, SCORing Atopic Dermatitis score, sIGA, body surface area affected by AD and sleep disturbance VAS.
Statistics
Analyses were performed in the WPAI‐AD subgroup of the intent‐to‐treat (ITT) population (all randomized patients who received at least one dose of placebo or nemolizumab in Part A or B and had ≥1 post‐dose efficacy assessment). All WPAI analyses were performed as described for the primary and secondary end‐point analyses.17 Patients enrolled into the exploratory arm (nemolizumab 2.0 mg/kg Q8W) and all data collected during or after rescue therapy were excluded from analyses of Part A. Missing data were imputed using the last observation carried forward method for analyses from Part A (placebo controlled). In analyses of the total study period (Parts A and B), all data during and after administration of rescue therapy were included and no data imputation was applied. Patients receiving nemolizumab 2.0 mg/kg Q8W were included in analyses of total study period only. Patients randomized to placebo in Part A were excluded from analyses of the total study period (Parts A and B). ancova for each domain of WPAI individually, adjusted for baseline WPAI‐AD score and region, was performed to estimate the least squares mean change from baseline in WPAI‐AD scores at week 12 in the nemolizumab 0.1, 0.5 and 2.0 mg/kg Q4W groups versus the placebo group. As the analyses were exploratory, adjustments for multiple testing were not applied. To identify clinical outcomes which may correspond with work productivity in AD, Pearson correlation analysis was performed in patients receiving nemolizumab (0.1, 0.5 or 2.0 mg/kg Q4W) to assess the relationship between improvement from baseline at week 12 in WPAI‐AD scores and improvement from baseline at week 12 in dermatitis score (EASI) or patient‐reported outcomes (pruritus VAS, sleep disturbance VAS and DLQI). To further assess the impact of pruritus on work and activity impairment, WPAI‐AD scores were evaluated in patients receiving nemolizumab Q4W (0.1, 0.5 or 2.0 mg/kg) with 50% or more improvement from baseline at week 12 in pruritus VAS score (high‐responders) versus patients with less than 50% improvement from baseline at week 12 in pruritus VAS score (low‐responders and non‐responders). Statistical analyses were performed using SAS software, version 9.2 (TS2M3; SAS Institute, Cary, NC, USA).
Results
Patients
Participant flow through study Part A and Part B has been previously reported.18 Of the 264 patients in the ITT population, 138 who received placebo or nemolizumab in Part A had available WPAI‐AD data and were included in the current analysis (Table 1). Of these, 111 patients completed Part A, 95 participated in Part B and 59 completed Part B. Patient baseline demographics and clinical characteristics in the ITT WPAI‐AD subgroup were similar to those of the overall study population,17 and 104 patients were employed at baseline. While patients reported low baseline absenteeism (mean Percent Work Time Missed, range 1–12%), which was comparable with patients with moderate to severe AD and patients with other moderate to severe diseases (plaque psoriasis, rheumatoid arthritis and asthma) reported elsewhere,10, 21, 22, 23 they reported greater baseline presenteeism (mean Percent Overall Work Impairment, range 53–62%) and impairment when performing daily activities (mean Percent Activity Impairment, range 59–69%), suggesting a high burden of disease in this patient population.
Table 1.
Baseline demographics and disease characteristics (ITT population, WPAI‐AD subgroup)
| Characteristic | Placebo (n = 28) | Nemolizumab | ||||
|---|---|---|---|---|---|---|
| 0.1 mg/kg Q4W (n = 28) | 0.5 mg/kg Q4W (n = 28) | 2.0 mg/kg Q4W (n = 27) | 2.0 mg/kg Q8W (n = 27) | Nemolizumab total (n = 110) | ||
| Patients, n (%) | ||||||
| USA | 12 (43) | 12 (43) | 12 (43) | 11 (41) | 12 (44) | 47 (43) |
| Japan | 16 (57) | 16 (57) | 16 (57) | 16 (59) | 15 (56) | 63 (57) |
| Male sex, n (%) | 14 (50) | 13 (46) | 14 (50) | 18 (67) | 11 (41) | 56 (51) |
| Age, years | 39 ± 13.0 | 32 ± 10.9 | 35 ± 11.1 | 38 ± 11.3 | 35 ± 12.5 | 35 ± 11.5 |
| Weight, kg | 73 ± 24.3 | 73 ± 25.2 | 73 ± 22.4 | 72 ± 17.0 | 70 ± 22.1 | 72 ± 21.6 |
| Pruritus VAS, mm | 78 ± 12.9 | 78 ± 11.2 | 77 ± 12.1 | 78 ± 11.1 | 78 ± 11.8 | 78 ± 11.4 |
| EASI score | 31 ± 16.1 | 35 ± 17.5 | 31 ± 18.5 | 31 ± 12.6 | 30 ± 16.1 | 32 ± 16.2 |
| Body surface area affected, % | 45 ± 31.5 | 57 ± 29.5 | 50 ± 30.9 | 59 ± 24.2 | 51 ± 29.3 | 54 ± 28.5 |
| sIGA score, n (%) | ||||||
| 3 | 13 (46) | 9 (32) | 12 (43) | 10 (37) | 14 (52) | 45 (41) |
| 4 | 13 (46) | 11 (39) | 12 (43) | 14 (52) | 9 (33) | 46 (42) |
| 5 | 2 (7) | 8 (29) | 4 (14) | 3 (11) | 4 (15) | 19 (17) |
| DLQI | 15 ± 5 | 15 ± 6 | 14 ± 6 | 15 ± 6 | 15 ± 8 | 15 ± 6 |
| Sleep disturbance VAS | 65 ± 23 | 68 ± 21 | 65 ± 22 | 65 ± 23 | 66 ± 22 | 66 ± 22 |
| WPAI‐AD | ||||||
| Employed, n (%) | 20 (71) | 20 (71) | 22 (79) | 19 (70) | 23 (85) | 84 (76) |
| Percent Work Time Missed† | 1 ± 4.6 | 5 ± 8.8 | 12 ± 29.4 | 2 ± 5.8 | 11 ± 25.0 | 8 ± 20.6 |
| Percent Impairment While Working† | 52 ± 27.6 | 61 ± 26.8 | 53 ± 28.3 | 55 ± 25.3 | 51 ± 28.9 | 55 ± 27.1 |
| Percent Overall Work Impairment† | 53 ± 27.2 | 62 ± 26.0 | 53 ± 28.6 | 56 ± 25.1 | 53 ± 30.2 | 56 ± 27.4 |
| Percent Activity Impairment | 63 ± 23.6 | 69 ± 25.7 | 59 ± 27.5 | 62 ± 28.0 | 65 ± 29.0 | 64 ± 27.5 |
Data are reported as mean ± standard deviation, unless otherwise stated. †Patients employed at baseline. DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; ITT, intent‐to‐treat; Q4W, every 4 weeks; Q8W, every 8 weeks; sIGA, static Investigator's Global Assessment; VAS, visual analog scale; WPAI‐AD, Work Productivity and Activity Impairment – Atopic Dermatitis.
Improvement from baseline in WPAI‐AD scores
At week 12, patients receiving nemolizumab Q4W demonstrated greater least squares mean (standard error) decrease from baseline (i.e. improvement) in WPAI‐AD scores compared with patients receiving placebo (Table 2). Improvement from baseline in WPAI‐AD scores was observed from week 4 (Fig. 1). Improvements in WPAI‐AD work and activity impairment domain scores observed at week 12 were sustained up to week 64 in patients receiving nemolizumab Q4W and Q8W (Fig. 2).
Table 2.
Change from baseline in WPAI at week 12
| Parameter | Placebo (n = 28) | Nemolizumab | ||
|---|---|---|---|---|
| 0.1 mg/kg Q4W (n = 28) | 0.5 mg/kg Q4W (n = 28) | 2.0 mg/kg Q4W (n = 27) | ||
| Percent Work Time Missed | ||||
| n | 14 | 18 | 17 | 16 |
| LSmean | 4.93 | −3.98 | −1.72 | −1.62 |
| SE | 4.48 | 3.91 | 4.15 | 4.17 |
| P † | 0.1387 | 0.2875 | 0.2892 | |
| Percent Impairment While Working | ||||
| n | 13 | 18 | 15 | 16 |
| LSmean | −16.48 | −15.82 | −24.12 | −34.32 |
| SE | 7.08 | 5.99 | 6.51 | 6.35 |
| P † | 0.9438 | 0.4309 | 0.0666 | |
| Percent Overall Work Impairment | ||||
| n | 13 | 18 | 15 | 16 |
| LSmean | −16.58 | −16.32 | −23.14 | −34.50 |
| SE | 7.07 | 5.97 | 6.49 | 6.32 |
| P † | 0.9778 | 0.4974 | 0.0646 | |
| Percent Activity Impairment | ||||
| n | 22 | 26 | 25 | 24 |
| LSmean | −10.86 | −13.40 | −23.50 | −41.88 |
| SE | 5.69 | 5.27 | 5.34 | 5.46 |
| P † | 0.7446 | 0.1089 | 0.0002 | |
† P‐value compared with placebo. LSmean, least square mean; Q4W, every 4 weeks; Q8W, every 8 weeks; SE, standard error; WPAI, Work Productivity and Activity Impairment.
Figure 1.
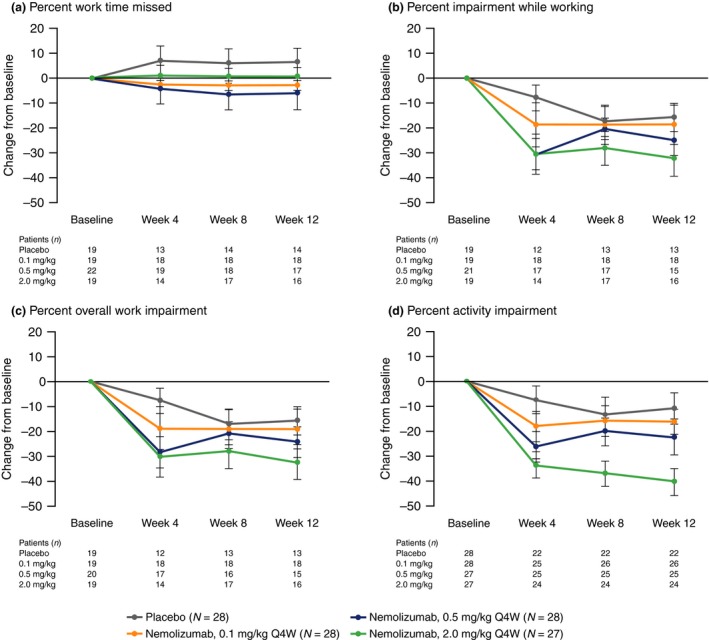
Change from baseline in WPAI‐AD scores at weeks 4, 8 and 12 for nemolizumab compared with placebo. (a) Percent Work Time Missed. (b) Percent Impairment While Working. (c) Percent Overall Work Impairment. (d) Percent Activity Impairment. Data show mean ± standard error. Q4W, every 4 weeks; WPAI‐AD, Work Productivity and Activity Impairment – Atopic Dermatitis.
Figure 2.
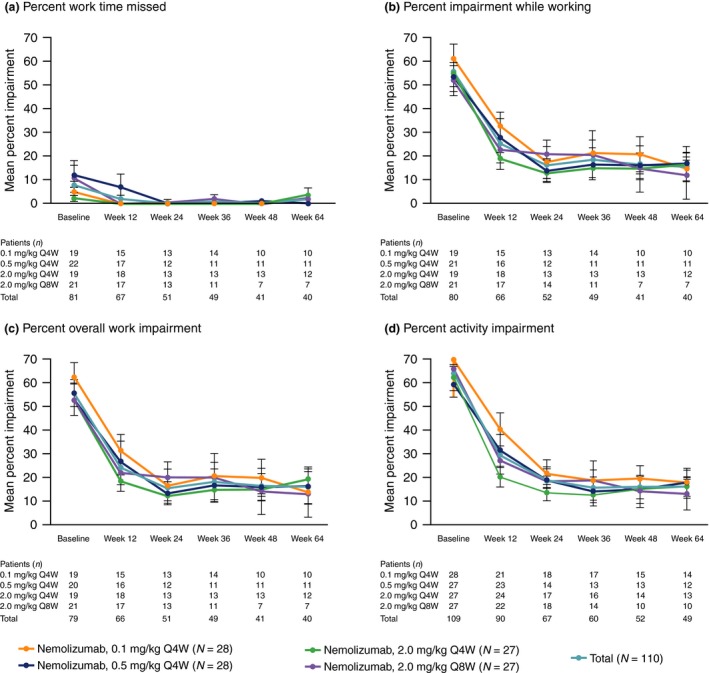
Absolute WPAI‐AD scores at baseline and weeks 12, 24, 36, 48 and 64 for nemolizumab. Patients who were randomized to placebo in Part A of the study were excluded from analysis of the total period (Part A and B). (a) Percent Work Time Missed. (b) Percent Impairment While Working. (c) Percent Overall Work Impairment. (d) Percent Activity Impairment. Data show mean ± standard error. Q4W, every 4 weeks; Q8W, every 8 weeks; WPAI‐AD, Work Productivity and Activity Impairment – Atopic Dermatitis.
Correlation between WPAI‐AD and disease severity
Patients with available WPAI‐AD data demonstrated improvement from baseline at week 12 in pruritus VAS, EASI, sleep disturbance VAS and DLQI, which are indicators of disease severity (Fig. 3). Pearson correlation analysis revealed a strong positive relationship between percent change from baseline at week 12 in WPAI‐AD scores and that of pruritus VAS (WPAI‐AD activity impairment and work domains, r = 0.53–0.75) and sleep disturbance VAS (WPAI‐AD work and activity impairment domains, r = 0.64–0.70) (Fig. 4). Moderate positive correlations were also observed between percent change from baseline at week 12 in WPAI‐AD scores and that of EASI (WPAI‐AD work and activity impairment domains, r = 0.37–0.41) and DLQI (WPAI‐AD activity impairment and work domains, r = 0.29–0.34). Pearson correlation analysis of absolute change from baseline in WPAI‐AD scores demonstrated a similar trend (Fig. S1).
Figure 3.
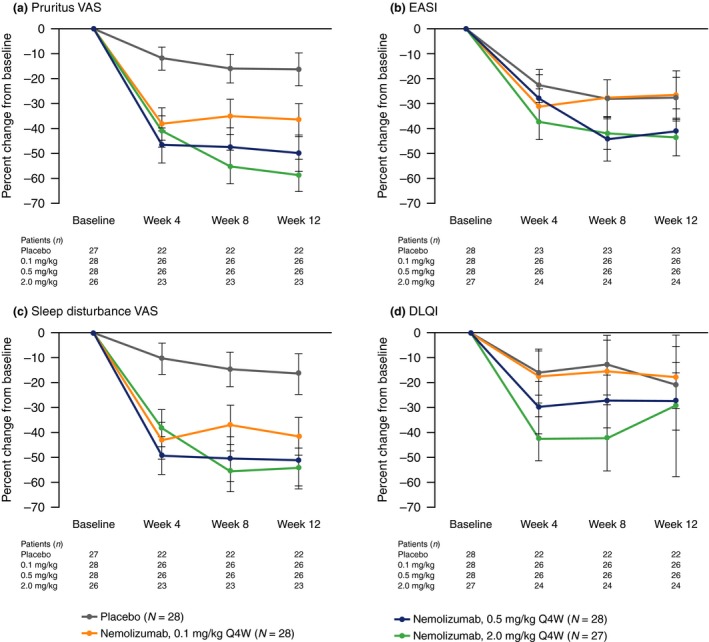
Percent change from baseline in pruritus VAS, EASI, sleep disturbance VAS and DLQI at weeks 4, 8 and 12 in patients with available WPAI data. (a) Pruritus VAS. (b) EASI. (c) Sleep Disturbance VAS. (d) DLQI. Data show mean ± standard error. DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; Q4W, every 4 weeks; VAS, visual analog scale; WPAI, Work Productivity and Activity Impairment.
Figure 4.
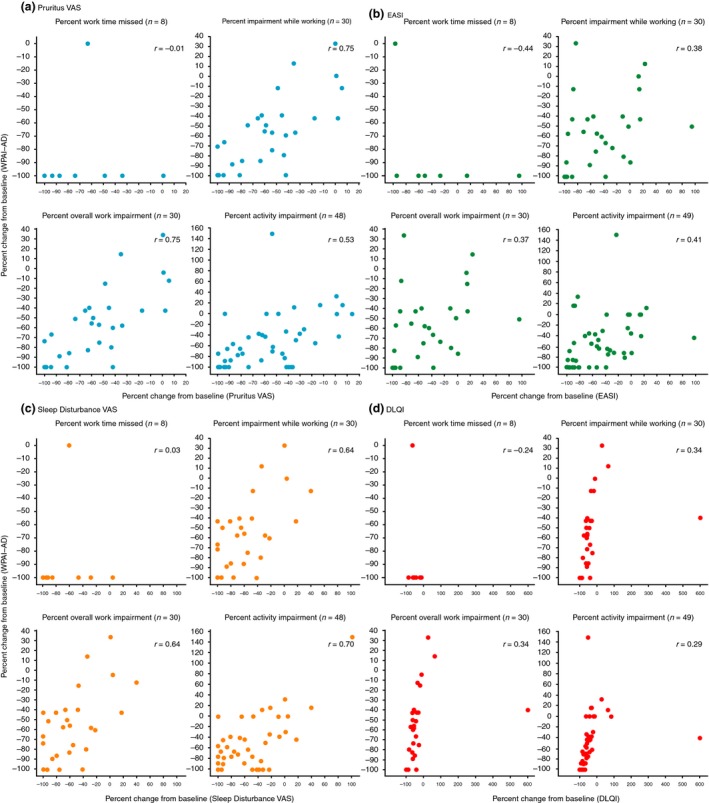
Pearson correlation to assess percent change from baseline at week 12 in WPAI‐AD scores and percent change from baseline at week 12 in pruritus VAS, EASI, sleep disturbance VAS and DLQI scores in patients receiving nemolizumab 0.1, 0.5 or 2.0 mg/kg Q4W. (a) Pruritus VAS. (b) EASI. (c) Sleep Disturbance VAS. (d) DLQI. DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; Q4W, every 4 weeks; VAS, visual analog scale; WPAI‐AD, Work Productivity and Activity Impairment – Atopic Dermatitis.
Improvement from baseline in WPAI‐AD scores in high‐responders versus low‐responders or non‐responders
Of the 83 patients receiving nemolizumab Q4W (0.1, 0.5 or 2.0 mg/kg), 29 with work‐related WPAI‐AD data and 36 with general activity impairment data were defined as high‐responders. A similar number of patients were considered low‐responders or non‐responders (26 and 38, respectively). High‐responders demonstrated greater improvement in WPAI‐AD scores across all four domains, compared with low‐responders or non‐responders (Fig. 5). Of the 28 patients receiving placebo, the number of patients considered high‐responders was too low for meaningful comparison: four or less patients defined as high‐responders; and 18 or less defined as low‐responders or non‐responders.
Figure 5.
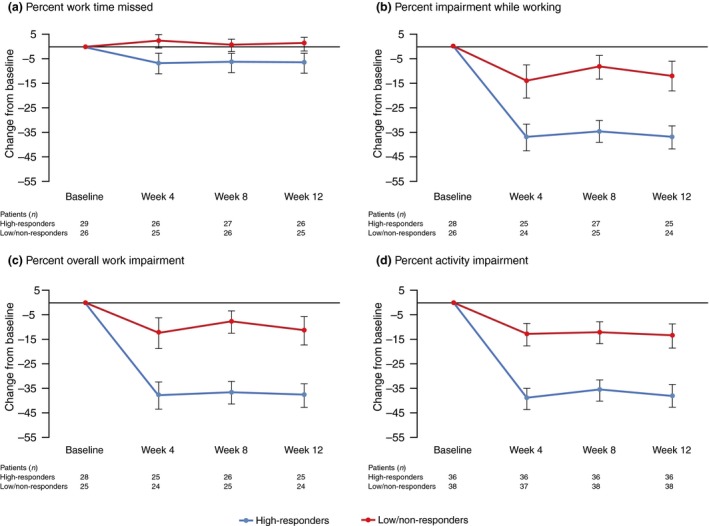
Change from baseline in WPAI‐AD scores at weeks 4, 8 and 12 in high‐responders (patients receiving nemolizumab Q4W with 50% or more improvement from baseline at week 12 in pruritus VAS score) and low‐responders or non‐responders. (a) Percent Work Time Missed. (b) Percent Impairment While Working. (c) Percent Overall Work Impairment. (d) Percent Activity Impairment. Data show mean ± standard error. Q4W, every 4 weeks; VAS, visual analog scale; WPAI‐AD, Work Productivity and Activity Impairment – Atopic Dermatitis.
Discussion
Nemolizumab‐treated patients with moderate to severe AD reported numerically greater improvement in work productivity and ability to perform daily activities compared with patients receiving placebo in this 12‐week, randomized phase II study. Improvement in the ability to perform non‐work‐related regular activities was most markedly observed for patients receiving nemolizumab 2.0 mg/kg Q4W versus placebo, although it should be emphasized that this was an exploratory analysis with no formal testing planned to compare groups; therefore, no inferences can be drawn from the statistical analyses. Overall, improvements in WPAI‐AD were observed after 4 weeks of therapy and sustained for up to 64 weeks and our findings suggest that, of the AD symptoms evaluated here, pruritus and sleep disturbance most negatively impacted WPAI‐AD. Disease severity and HRQoL also impaired WPAI‐AD but to a lesser extent. These findings build on prior studies which demonstrate that pruritus, as the dominant symptom of AD, contributes to sleeplessness and reduced HRQoL in patients with AD.11, 14, 24, 25 As pruritus is a subjective symptom, it is difficult to distinguish the effect on other clinical outcomes, even in patients with significant improvement. Therefore, it is important to assess the effect on functional outcomes such as work productivity and daily activity. Our analysis identified significant improvement in high‐responders in WPAI‐AD scores across all four domains compared with low‐responders which suggests that improvement in pruritus by nemolizumab can lead to preferable effects on other functional outcomes. This reinforces the importance of alleviating pruritus in AD and highlights the broader impact of the disease on patient functioning in the workplace and daily life.
In addition to the impact on HRQoL, skin disorders are associated with substantial economic burden. A comprehensive study into the economic impact of skin diseases in the USA estimated the overall annual cost of skin disorders in 2004 at $US39.3 billion.26 This total included $US10.2 billion in lost productivity costs accounting for missed time from work to seek medical care, impaired ability to work and lost future earnings owing to premature death.26 Patients with AD incur significantly greater health‐care resource use and direct costs versus individuals without AD, with the cost burden rising with increasing disease severity.27, 28 While calculating the total direct and indirect costs of AD is challenging as it is a common disease with a broad spectrum of severity, the total annual financial burden associated with AD in the USA in 2004 was estimated at $US4.2 billion, the equivalent of $US5.3 billion in 2015.26, 29 This value does not include costs associated with presenteeism or work time missed due to reasons other than medical visits that are commonly associated with AD.26, 29 The same study reported the corresponding financial burden in 2004 associated with psoriasis at the lower cost of $US3.7 billion.26, 29 Using WPAI data from a phase III study investigating the impact of adalimumab on moderate to severe psoriasis, Kimball et al.30 estimated the potential annual indirect cost savings for employers associated with reduction in work productivity impairment owing to psoriasis at approximately $US4500 per full‐time employed patient, corresponding to an 11.1% improvement in work productivity impairment versus placebo, indicating the potential productivity benefits for patients with AD.
Presenteeism is a major contributor to productivity loss, with evidence to suggest it has a greater economic impact than absenteeism.31, 32, 33 Our patients reported high levels of presenteeism at baseline; more than half of their work productivity and daily activities were impaired compared with those in prior studies of moderate to severe AD and other moderate to severe skin disorders,10, 21, 34 suggesting a high burden of disease. Patients with high levels of impairment may therefore have the potential to derive economic benefits associated with nemolizumab therapy and the alleviation of pruritus.
The study has a number of limitations to consider when evaluating the findings. These include the small sample size and the absence of a placebo arm in Part B, which might have introduced bias owing to administration of only active drug. The WPAI‐AD was only assessed in the USA and Japan and may not be generalizable to patients from other countries. A difference in work culture between Japan and the USA may have also impacted study findings. Patient‐reported absenteeism and presenteeism were not validated against employment records, and information regarding employment status (full‐time, part‐time, job type) was unavailable.
In summary, nemolizumab‐treated patients with moderate to severe AD inadequately controlled by topical treatments reported sustained improvements in work productivity and daily activities. Nemolizumab‐associated improvement in pruritus and pruritus‐associated sleep disturbance could lead to reduced productivity‐related burden in AD.
Conflict of Interest
R. M. is an employee of Chugai Pharmaceutical Co. Ltd and owns stock in that company. K. K., M. F. and T. R. are consultants for Chugai Pharmaceutical Co. Ltd and have received honoraria. M. N. is an employee of Chugai Pharmaceutical Co. Ltd. M. F. is the Editor of the Journal of Dermatology.
Supporting information
Figure S1. Pearson correlation to assess absolute change from baseline at week 12 in WPAI‐AD scores and percent change from baseline at week 12 in pruritus VAS, EASI, sleep disturbance VAS and DLQI scores in patients receiving nemolizumab 0.1, 0.5 or 2.0 mg/kg Q4W. (a) Pruritus VAS. (b) EASI. (c) Sleep disturbance VAS. (d) DLQI. DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; Q4W, every 4 weeks; VAS, visual analog scale; WPAI‐AD, Work Productivity and Activity Impairment – Atopic Dermatitis.
Table S1. List of investigators in the USA and Japan
Acknowledgments
We thank the patients for participating in the trial, and study investigators from Japan and the USA (Table S1). We thank the project team members at Chugai Pharmaceutical, Keiko Hirokawa, Misako Makishima and Michiaki Tanaka, for their expert contribution to analysis planning and data interpretation; Miho Yuki for statistical analysis; and Nobuhiko Ishizuka for project management. Medical writing assistance was provided by Alyson Bexfield, Ph.D., of Caudex, Oxford, UK, funded by Chugai Pharmaceutical Co. Ltd. This study was supported by Chugai Pharmaceutical Co. Ltd. The study was designed jointly by the sponsor and the investigators. The sponsors were responsible for the collection and maintenance of the data. All authors had input into manuscript development and approved the manuscript before submission, and the authors made the decision to submit the manuscript for publication.
Prior presentation: Data were presented at the 27th European Academy of Dermatology and Venerology Congress, 12–16 September 2018.
Data sharing statement: We provide qualified researchers access to individual patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details of Chugai's Data Sharing Policy are available here (www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html).
References
- 1. Eichenfield LF, Tom WL, Chamlin SL et al Guidelines of care for the management of atopic dermatitis: Part 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014; 70: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saeki H, Nakahara T, Tanaka A et al Clinical practice guidelines for the management of atopic dermatitis 2016. J Dermatol 2016; 43: 1117–1145. [DOI] [PubMed] [Google Scholar]
- 3. Wollenberg A, Oranje A, Deleuran M et al ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol 2016; 30: 729–747. [DOI] [PubMed] [Google Scholar]
- 4. Cevikbas F, Wang X, Akiyama T et al A sensory neuron‐expressed IL‐31 receptor mediates T helper cell‐dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 2014; 133: 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dillon SR, Sprecher C, Hammond A et al Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol 2004; 5: 752–760. [DOI] [PubMed] [Google Scholar]
- 6. Feld M, Garcia R, Buddenkotte J et al The pruritus‐ and TH2‐associated cytokine IL‐31 promotes growth of sensory nerves. J Allergy Clin Immunol 2016; 138: 500–508. [DOI] [PubMed] [Google Scholar]
- 7. Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol 2006; 118: 178–189. [DOI] [PubMed] [Google Scholar]
- 8. Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs 2013; 15: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eichenfield LF, Tom WL, Berger TG et al Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whiteley J, Emir B, Seitzman R, Makinson G. The burden of atopic dermatitis in US adults: results from the 2013 National Health and Wellness Survey. Curr Med Res Opin 2016; 32: 1645–1651. [DOI] [PubMed] [Google Scholar]
- 11. Simpson EL, Bieber T, Eckert L et al Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol 2016; 74: 491–498. [DOI] [PubMed] [Google Scholar]
- 12. Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. Impact of atopic dermatitis on health‐related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol 2017; 77: 274–279. [DOI] [PubMed] [Google Scholar]
- 13. Yano C, Saeki H, Ishiji T et al Impact of disease severity on work productivity and activity impairment in Japanese patients with atopic dermatitis. J Dermatol 2013; 40: 736–739. [DOI] [PubMed] [Google Scholar]
- 14. Chrostowska‐Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol 2013; 27: e239–e242. [DOI] [PubMed] [Google Scholar]
- 15. Hänel KH, Pfaff CM, Cornelissen C et al Control of the physical and antimicrobial skin barrier by an IL‐31‐IL‐1 signaling network. J Immunol 2016; 196: 3233–3244. [DOI] [PubMed] [Google Scholar]
- 16. Bagci IS, Ruzicka T. IL‐31: a new key player in dermatology and beyond. J Allergy Clin Immunol 2018; 141: 858–866. [DOI] [PubMed] [Google Scholar]
- 17. Ruzicka T, Hanifin JM, Furue M et al Anti‐interleukin‐31 receptor A antibody for atopic dermatitis. N Engl J Med 2017; 376: 826–835. [DOI] [PubMed] [Google Scholar]
- 18. Kabashima K, Furue M, Hanifin JM et al Nemolizumab in moderate‐to‐severe atopic dermatitis: randomized, phase II, long‐term study. J Allergy Clin Immunol 2018; 142(4): 1121–1130. [DOI] [PubMed] [Google Scholar]
- 19. Reilly Associates Health Outcomes Research . Work productivity and activity impairment. Available at: http://www.reillyassociates.net/index.html. Accessed 11/24/2017, 2013.
- 20. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4: 353–365. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong AW, Lynde CW, McBride SR et al Effect of ixekizumab treatment on work productivity for patients with moderate‐to‐severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol 2016; 152: 661–669. [DOI] [PubMed] [Google Scholar]
- 22. Chen H, Blanc PD, Hayden ML, Bleecker ER, Chawla A, Lee JH. Assessing productivity loss and activity impairment in severe or difficult‐to‐treat asthma. Value Health 2008; 11: 231–239. [DOI] [PubMed] [Google Scholar]
- 23. Takeuchi T, Nakajima R, Komatsu S et al Impact of adalimumab on work productivity and activity impairment in Japanese patients with rheumatoid arthritis: large‐scale, prospective, single‐cohort ANOUVEAU study. Adv Ther 2017; 34: 686–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murota H, Kitaba S, Tani M et al Impact of sedative and non‐sedative antihistamines on the impaired productivity and quality of life in patients with pruritic skin diseases. Allergol Int 2010; 59: 345–354. [DOI] [PubMed] [Google Scholar]
- 25. Murota H, Kitaba S, Tani M, Wataya‐Kaneda M, Katayama I. Effects of nonsedative antihistamines on productivity of patients with pruritic skin diseases. Allergy 2010; 65: 929–930. [DOI] [PubMed] [Google Scholar]
- 26. Bickers DR, Lim HW, Margolis D et al The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 2006; 55: 490–500. [DOI] [PubMed] [Google Scholar]
- 27. Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J Am Acad Dermatol 2018; 78: 54–61. e51. [DOI] [PubMed] [Google Scholar]
- 28. Shrestha S, Miao R, Wang L, Chao J, Yuce H, Wei W. Burden of atopic dermatitis in the United States: analysis of healthcare claims data in the Commercial, Medicare, and Medi‐Cal Databases. Adv Ther 2017; 34: 1989–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol 2017; 137: 26–30. [DOI] [PubMed] [Google Scholar]
- 30. Kimball AB, Yu AP, Signorovitch J et al The effects of adalimumab treatment and psoriasis severity on self‐reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol 2012; 66: e67–e76. [DOI] [PubMed] [Google Scholar]
- 31. Goetzel RZ, Long SR, Ozminkowski RJ, Hawkins K, Wang S, Lynch W. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med 2004; 46: 398–412. [DOI] [PubMed] [Google Scholar]
- 32. Kigozi J, Jowett S, Lewis M, Barton P, Coast J. The estimation and inclusion of presenteeism costs in applied economic evaluation: a systematic review. Value Health 2017; 20: 496–506. [DOI] [PubMed] [Google Scholar]
- 33. Ricci JA, Stewart WF, Chee E, Leotta A, Foley K, Hochberg MC. Pain exacerbation as a major source of lost productive time in US workers with arthritis. Arthritis Rheum 2005; 53: 673–681. [DOI] [PubMed] [Google Scholar]
- 34. Kimball AB, Edson‐Heredia E, Zhu B et al Understanding the relationship between pruritus severity and work productivity in patients with moderate‐to‐severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol 2016; 15: 183–188. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Pearson correlation to assess absolute change from baseline at week 12 in WPAI‐AD scores and percent change from baseline at week 12 in pruritus VAS, EASI, sleep disturbance VAS and DLQI scores in patients receiving nemolizumab 0.1, 0.5 or 2.0 mg/kg Q4W. (a) Pruritus VAS. (b) EASI. (c) Sleep disturbance VAS. (d) DLQI. DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; Q4W, every 4 weeks; VAS, visual analog scale; WPAI‐AD, Work Productivity and Activity Impairment – Atopic Dermatitis.
Table S1. List of investigators in the USA and Japan


