Abstract
Background and Objectives: Lymphadenectomy during pulmonary metastasectomy (PM) is widely carried out. We assessed the potential benefit on patient survival and tumor recurrence of this practice.
Methods: One hundred eighty‐one patients undergoing a first PM were studied. Eighty‐six patients (47.5%) underwent lymphadenectomy (L+ group) whereas 95 (52.5%) did not undergo nodal harvesting (L−group). Main outcomes were overall survival (OS) and disease‐free survival (DFS). Median follow‐up was 25 months (interquartile range [IQR], 13‐49).
Results: At follow‐up 84 patients (46.4%) died, whereas 97 (53.6%) were still alive with recurrence in 78 patients (43%). There was no difference in 5‐year survival (L+ 30.0% vs L− 43.2%; P = .87) or in the 5‐year cumulative incidence of recurrence (L + 63.2% vs L−80%; P = .07) between the two groups. Multivariable analysis indicated that disease‐free interval (DFI) less than 29 months (P < .001) and lung comorbidities (P = .003) were significant predictors of death. Metastases from non‐small–cell lung cancer increased the risk of lung comorbidities by a factor of 19.8, whereas the risk of DFI less than 29 months was increased nearly 11‐fold. Competing risk regression identified multiple metastases (P = .004), head/neck primary tumor (P = .009), and age less than 67 years (P = .024) as independent risk factors for recurrence.
Conclusion: Associated lymphadenectomy showed not to give any additional advantage in terms of survival and recurrence after PM.
Keywords: lung metastases, lung resections, lymphadenectomy
1. INTRODUCTION
The oligometastatic disease was first defined by Hellman and Weichselbaum in 19951 and indicates that patients are at an intermediate state between a limited primary tumor and a poly‐metastatic disease.2 Pulmonary metastasectomy (PM) has become an acknowledged therapeutic option in the context of this disease.3, 4, 5 The previous series have identified several factors affecting survival3, 6, 7 but, in recent years, the attention has been increasingly focused on the role of intrathoracic lymph nodes status during PM.8, 9, 10, 11, 12 However, while it is widely accepted that systematic nodal dissection during surgery aids in prognostic stratification and identification of those patients with a higher risk of disease progression9, 11, 12 little is still known as to whether performing lymph nodes dissection during PM could influence patient survival as well as tumor recurrence.12
Therefore, the aim of our study was to investigate whether associated lymphadenectomy may affect mid‐term survival and tumor recurrence in patients undergoing PM. Furthermore, we aimed to identify predicting factor of these long‐term outcomes.
2. MATERIALS AND METHODS
This paper was structured according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.13
The approval was waived by Ethical Committee due to the retrospective analysis of the study according to National Laws regulating observational retrospective studies (Italian law no.11960, released on 13 July 2004). However, written consent for the use of clinical data for the scientific purpose was obtained from all patients.
2.1. Patient population
Clinical records from patients undergoing lung metastasectomy with curative intent between January 2005 and December 2017 in a single institution (Santa Maria Della Misericordia University Hospital, Udine, Italy) were reviewed. The population was divided into two groups: (a) Subjects undergoing associated lymphadenectomy and lymph node biopsy (L+) and (b) Patients who did not undergo lymphadenectomy (L−). Patients characteristics are reported in Table 1. One hundred eighty‐one patients underwent the first PM with a total of 260 lung nodules excised, accounting for a median number of metastases per patient of 1.43 (range, 1‐7). Eighty‐six patients (47.5%) belonged to the L+ group, whereas 95 (52.5%) were in the L− group. Lung comorbidities were mainly represented by chronic obstructive pulmonary disease (COPD). Twenty‐four patients were affected by (COPD) and one by interstitial lung disease.
Table 1.
Patients and tumor's characteristics
| All (n = 181) | L− (n=95) | L+ (n=86) | P | |
|---|---|---|---|---|
| Male sex | 105 (58) | 47 (49.5) | 58 (67.4) | .01 |
| Age at surgery | 66 (IQR 58‐70) | 65 (IQR 55‐69) | 67 (IQR 59‐73) | .05 |
| ASA | ||||
| 2 | 117 (64.7) | 64 (67.4) | 53 (61.6) | .41 |
| 3 | 64 (35.3) | 31 (32.6) | 33 (38.4) | |
| Comorbidities | ||||
| Coronaropathy | 14 (7.8) | 5 (5.3) | 9 (10.5) | .19 |
| Arrhythmia | 13 (7.2) | 7 (7.4) | 6 (7) | .91 |
| Hypertension | 70 (38.7) | 32 (33.7) | 38 (44.2) | .14 |
| Other cancers | 39 (21.5) | 16 (16.8) | 23 (26.7) | .10 |
| Lung disease | 25 (13.8) | 16 (16.8) | 23 (26.7) | .21 |
| Diabetes | 17 (9.3) | 9 (9.5) | 8 (9.3) | .96 |
| Chronic renal failure | 4 (2.2) | 2 (2.2) | 2 (2.3) | .91 |
| Liver disease | 8 (4.4) | 6 (6.3) | 2 (2.3) | .19 |
| Other | 40 (22.1) | 18 (18.9) | 22 (25.6) | .28 |
| Primary tumor | ||||
| Colorectal | 76 (42.0) | 34 (35.8) | 42 (48.8) | .07 |
| Gynecological | 5 (2.8) | 1 (1.0) | 4 (4.7) | .14 |
| Melanoma | 12 (6.6) | 7 (7.4) | 5 (5.8) | .67 |
| Breast | 7 (3.9) | 2 (2.2) | 5 (5.8) | .19 |
| Head/neck | 8 (4.4) | 3 (3.2) | 5 (5.8) | .38 |
| NSCLC | 15 (8.3) | 13 (13.6) | 2 (2.3) | .005 |
| Kidney | 25 (13.8) | 12 (12.6) | 13 (15.1) | .62 |
| Sarcoma | 18 (10.0) | 12 (12.6) | 6 (7.0) | .20 |
| Other | 15 (8.2) | 11 (11.6) | 4 (4.7) | .09 |
| Thyroid | 3 (1.7) | 3 (3.2) | 0 | |
| Parathyroid | 3 (1.7) | 2 (2.2) | 1 (1.2) | |
| Cholangiocarcinoma | 2 (1.1) | 2 (2.2) | 0 | |
| Pancreas | 2 (1.1) | 1 (1.0) | 1 (1.2) | |
| Liver | 2 (1.1) | 1 (1.0) | 1 (1.2) | |
| Bladder | 1 (0.5) | 1 (1.0) | 0 | |
| Small bowel | 1 (0.5) | 0 | 1 (1.2) | |
| Thoracic neurofibroma | 1 (0.5) | 1 (1.0) | 0 | |
| RT/CHT | ||||
| Neoadjuvant | 25 (13.8) | 13 (13.6) | 12 (13.9) | .95 |
| Adjuvant | 116 (64.1) | 58 (61.1) | 58 (67.4) | .37 |
| Number of lesions | ||||
| 1 | 136 (75.1) | 70 (73.8) | 66 (76.8) | .40 |
| 2 | 30 (16.6) | 15 (15.8) | 15 (17.4) | |
| 3 | 6 (3.3) | 4 (4.2) | 2 (2.3) | |
| 4 | 2 (1.1) | 0 | 2 (2.3) | |
| 5 | 5 (2.8) | 4 (4.2) | 1 (1.2) | |
| 6 | 1 (0.5) | 1 (1.0) | 0 | |
| 7 | 1 (0.5) | 1 (1.0) | 0 | |
| DFI | 29 (IQR 16‐54.5) | 29 (IQR 14‐57) | 29 (IQR 17‐40) | .89 |
Note: Values are expressed as n (%) or median (interquartile range).
Abbreviations: ASA, American Society of Anesthesiologist score; CHT, chemotherapy; DFI, disease‐free interval; NSCLC, non‐small–cell lung cancer; RT, radiotherapy.
A thoracic and abdominal computed tomography (CT) scan was performed preoperatively to evaluate the operability of lung lesions and to identify extra‐thoracic localizations of disease. When a CT scan showed enlargement of intrathoracic lymph nodes, a positron emission tomography (PET) scan was performed to assess metabolic activity at this site.14 In case of suspicious nodal uptake, a preoperative invasive mediastinal staging was carried out and patients with histologically proven nodal involvement were excluded from surgery. Fifty‐one patients had a PET scan done preoperatively and three had evidence of positive mediastinal nodal uptake. Two were investigated with cervical mediastinoscopy and one with endobronchial ultrasound.
The follow‐up after the treatment of the primary tumor included outpatient visit, thoracic and abdominal CT scan and blood test every 3 months for the first 2 year and every 6 months thereafter.
2.2. Main outcomes and definitions
Main outcomes were survival and freedom from the first recurrence of metastasis after surgery (disease‐free survival [DFS]).
Disease‐free interval (DFI) was defined as the time lapse between resection of the primary tumor and first diagnosis of pulmonary metastases whereas DFS was the period after PM without evidence of tumor re‐recurrence.
Neoadjuvant treatment was defined as the administration of chemotherapy or irradiation of a tumor before definitive surgical treatment, in this case, referred to the primary neoplasm whereas an adjuvant treatment was a systemic treatment administered after surgical resection.
Tumorectomy was defined as the removal of a nodule along with minimal surrounding normal pulmonary tissues. An “open approach” was a surgical procedure through thoracotomy or sternotomy.
2.3. Surgical indications
Indications for surgery were: (a) Controlled primary tumor; (b) Absence of extra‐thoracic disease or extra‐thoracic localizations judged amenable to local therapies. (c) Lung disease considered suitable for complete resection. (d) Absent proven intrathoracic lymph nodes involvement. (e) Predicted postoperative forced expiratory volume in 1 second and diffusing capacity of the lung for carbon monoxide more than 40%.15, 16
Bilateral metastases or number of nodules were not considered as exclusion criteria. Patients with evident macroscopic residual disease or where resection had been performed only for the diagnostic purpose were excluded from the analysis. Similarly, patients whose excised nodules did not confirm their metastatic nature were excluded. Finally, we included only patients who had not previously undergone previous PM or other ablative treatment for the same disease.
2.4. Surgery
Details on surgical procedures are reported in Table 2. Surgery was performed both by video‐assisted thoracic surgery (VATS) or standard thoracotomy based on anatomical considerations and surgeon's preferences. The extent of resection was minimal enough to guarantee complete excision with negative margins, in view of a lung‐sparing surgery. Therefore, single peripheral lesions were generally treated with wedge resections or segmentectomies, whereas larger or central nodules or multiple lesions located to the same lobe required major lung resections to be executed. Bilateral metastases were approached with staged/synchronous bilateral thoracotomy/VATS or median sternotomy.
Table 2.
Operative approach and kind of resection
| All (n = 181) | L− (n = 95) | L+ (n = 86) | P | |
|---|---|---|---|---|
| Resection | ||||
| Tumorectomy | 5 (2.8) | 4 (4.2) | 1 (1.2) | 0.21 |
| Wedge resection | 105 (58.0) | 82 (86.3) | 23 (26.7) | <.0001 |
| Segmentectomy | 12 (6.7) | 6 (6.3) | 6 (7.0) | .85 |
| Lobectomy | 58 (32.0) | 3 (3.2) | 55 (64.0) | <.0001 |
| Bilobectomy | 1 (0.5) | 0 | 1 (1.1) | .29 |
| Pneumonectomy | 0 | 0 | 0 | |
| Approach | ||||
| VATS | 85 (47) | 55 (57.9) | 30 (34.9) | .002 |
| Open | 100 (55.2) | 42 (44.2) | 58 (67.4) | .0017 |
| Post‐resection status | ||||
| R0 | 174 (96.1) | 88 (92.6) | 86 (100) | .01 |
| R+ | 7 (3.9) | 7 (7.4) | 0 |
Note: Values are expressed as n (%).
Abbreviations: R0, no residual disease; R+, presence of residual disease; TH, thoracotomy; VATS, video‐assisted thoracic surgery.
Lymphadenectomy has been performed both as nodal sampling and lobe‐specific lymph node dissection. In detail, 62.8% of patients underwent both N1 and N2 nodal harvesting, 30.2% only N1 lymph nodes biopsy and 7% only N2. 41.9% of patients were submitted to a lobe‐specific nodal dissection (with excision of at least two N1 and two N2 nodal stations), whereas 58.1% underwent a nodal sampling with a median number of stations explored of 2 (IQR, 2‐3; range, 1‐5). Nodal sampling was defined as picking of a limited number of lymph nodes at specific N1‐N2 nodal stations, whereas a lobe‐specific node dissection is defined as the complete removal of all visible lymph nodes and surrounding fat tissue at level of the hylo‐mediastinal stations specific for the lobe where the resection has been performed, as defined by previous articles.17
In the L+ group, the lymph node specimens were histologically analyzed and categorized according to the International System for the staging of Lung Cancer.18
2.5. Statistical analysis
The normality of distribution was assessed using the Kolmogorov‐Smirnov test. Continuous data were summarized as mean and standard deviation or median and 25th to 75th percentiles in case of non‐normal distributions. Categorical variables were reported as counts and percentages. Comparisons were carried out using Fisher's exact test and McNemar test where appropriate.
The Kaplan‐Meier method and log‐rank test were used for survival analysis. A Cox regression model was used to estimate predictors of death. The proportional hazard assumption was confirmed by use of Schoenfeld residuals. Cumulative incidence curves were used to graphically depict tumor recurrence and statistical significance was tested with the Gray test. A competing risk analysis was used to avoid overestimation of the incidence of recurrence.
Cut‐offs were determined by receiver operating characteristic (ROC) curves analysis as the optimal threshold for predicting death and tumor recurrence. We validated the results using the bootstrap method (1000 iterations). Subgroup analyses were performed testing for interactions by entering interaction terms between each subgroup and main predictors, with an interaction P < .10 considered statistically significant. Furthermore, the effect of the main predictors in each subgroup was tested at multivariable analysis.
R, release 3.2.3 (R Foundation for Statistical Computing, Wien, Austria) software and “survival”, “cmprsk” and “forestplot” packages were utilized. Significance for hypothesis testing was set at the .05 two‐tailed level.
3. RESULTS
3.1. Main outcomes
Overall, 20 patients had bilateral resections. Seventeen patients (9.4%) were discovered with synchronous metastases at the time of diagnosis of their primary tumor and underwent pre or postoperative chemotherapy. Among the remainder 164 patients who developed metachronous metastases, 83 (50.6%) received a systemic treatment before or after PM. Administration of systemic treatment was established by medical oncologists, based on patients’ and tumor features.
Early results and postoperative complications are reported in Table 3. At median follow‐up (100% complete) of 25 months (IQR 13‐49 months, range 1‐155), 84 patients (46.4%) died, whereas 97 (53.6%) were still alive. Cancer progression was the cause of death in 67 patients (79.8%) whereas 17 (20.2%) died from other causes.
Table 3.
Complications
| All (n = 181) | L− (n = 95) | L+ (n = 86) | P | |
|---|---|---|---|---|
| Complications | ||||
| Total | 27 (14.9) | 10 (10.5) | 17 (19.8) | .08 |
| Hemorrhage | 4 (2.2) | 2 (2.1) | 2 (2.3) | .92 |
| Persistent air‐leak | 8 (4.4) | 2 (2.1) | 7 (8.1) | .02 |
| AF | 5 (2.8) | 0 | 5 (5.81) | .017 |
| ARDS | 2 (1.1) | 0 | 2 (2.3) | .13 |
| Pneumonia | 9 (5.0) | 3 (3.2) | 6 (7) | .23 |
| Other | 12 (6.6) | 4 (4.2) | 8 (9.3) | .17 |
Note: Values are expressed as n (%) or median (interquartile range).
Abbreviations: AF, atrial fibrillation; ARDS, acute respiratory distress syndrome.
Median survival was 24.5 months (IQR, 13‐49) in the L+ group and 25 months (IQR, 13‐48 months) in the L− group (P = .98). Median DFS was 11 months (IQR, 5‐29 months) in both groups (P = .79). Overall five‐year survival (Figure 1A) was 41.2% and there was no difference in survival between the two groups (Figure 1B; P = .87). Without considering patients with NSCLC, 5‐year survival was still comparable (39.7% [28.2%‐54.8%] vs 43.8% [31.7%‐60.6%] in L+ and L−, respectively, P > .9).
Figure 1.
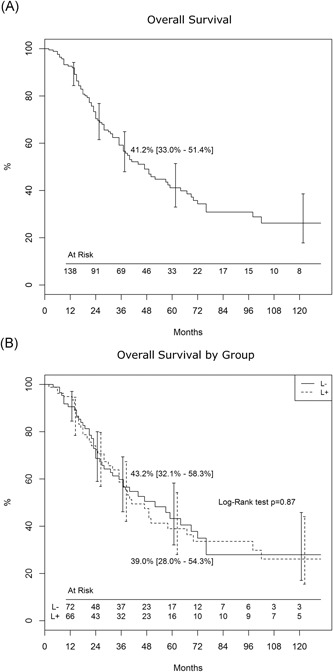
A, Actuarial survival in the whole population. B, Overall survival by groups (Lymphadenectomy, L+ and no‐ lymphadenectomy, L−)
Recurrence of metastasis occurred in 78 (43%) patients (Table 4): localized pulmonary recurrence was encountered in 34 (43.6%) patients, whereas 44 (56.4%) patients developed lung and extra‐pulmonary (n = 18) or isolated extra‐pulmonary (n = 26) metastases. Treatment of recurrent metastases included further surgical excision or radiotherapy (RT) for limited disease or systemic therapies for widespread tumor diffusion or patients unfit or unwilling to undergo surgical treatment. Of note, 64.7% of patients who had limited lung recurrence were submitted for further surgical resection, whereas systemic treatment has been proposed for 61.1% of patients with the concomitant extra‐pulmonary disease.
Table 4.
Pattern of recurrence and treatment
| All (n = 78) | Lung (n = 34, 43.6) | Lung+extra pulmonary (n = 18, 23.1) | Extra pulmonary only (n = 26, 33.3) | |
|---|---|---|---|---|
| Surgery | 34 (44.1) | 22 (64.7) | 3 (16.7) | 9 (34.6) |
| Radiotherapy | 7 (9.1) | 1 (2.9) | 1 (5.5) | 5 (19.2) |
| Chemotherapy | 29 (37.7) | 10 (29.4) | 11 (61.1) | 8 (30.8) |
| No treatment | 6 (7.8) | 1 (2.9) | 2 (11.1) | 3 (11.5) |
| Other local treatment a | 1 (1.3) | 0 | 0 | 1 (3.8) |
Note: Values are expressed as n (%).
Transarterial liver chemoembolization (TACE).
Overall, 5‐year cumulative incidence of recurrence (Figure 2A) was 72.5% (0.15% variance) and there was no statistically significant difference between groups (Figure 2B; 80.0% [0.23% variance] vs 63.2% [0.37% variance], in l− and L+, respectively P = .073). Corrected by NSCLC, these figures were 62.8% (0.38%) vs 80.8% (0.29%) in L+ and L−, respectively, P = .06.
Figure 2.
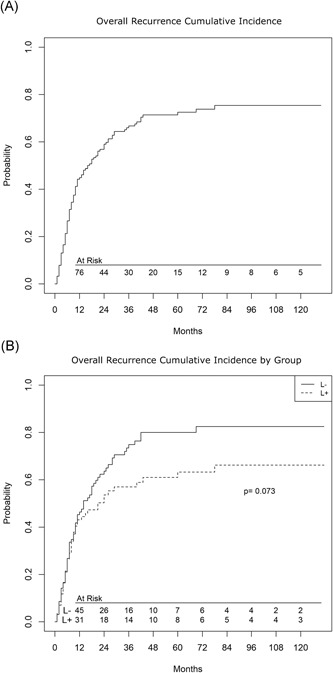
A, Cumulative incidence of recurrence in the whole population. B, Cumulative incidence of recurrence by groups (lymphadenectomy, L+ and no‐ lymphadenectomy, L−)
3.2. Predictors of outcomes
Multivariable analysis indicated that DFI (P < .001) and lung comorbidities (P = .003) were significant predictors of death (Figure 3 A). Using a ROC curve the DFI cutoff was less than 29 months (AUC 0.71 [0.62‐0.79]). At competing risk regression (Figure 3 B), multiple metastases (P = .004), head/neck primary tumor (P = .009) and younger age (0.024) were identified as independent risk factors for recurrence. At ROC curve the age cutoff was less than 67 years (AUC 0.73 [0.65‐0.82]). Pathological lymph node involvement did not result in a significant predictor of recurrence (P = .21).
Figure 3 .
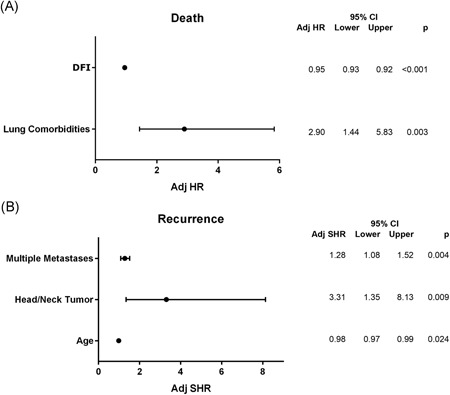
A, Predictors of death at Cox Regression. B, Predictors of recurrence at competing‐risk analysis. CI, confidence interval; HR, hazard risk; SHR, sub‐hazard risk. DFI, disease‐free interval
We found a significant interaction for death between lung comorbidities and NSCLC (P = .025) and DFI less than 29 (P = .014). At sub‐analysis, for death (Figure 4) NSCLC increased by 19.8 times the risk of lung comorbidities and 10.7 times the risk of DFI less than 29 months.
Figure 4.
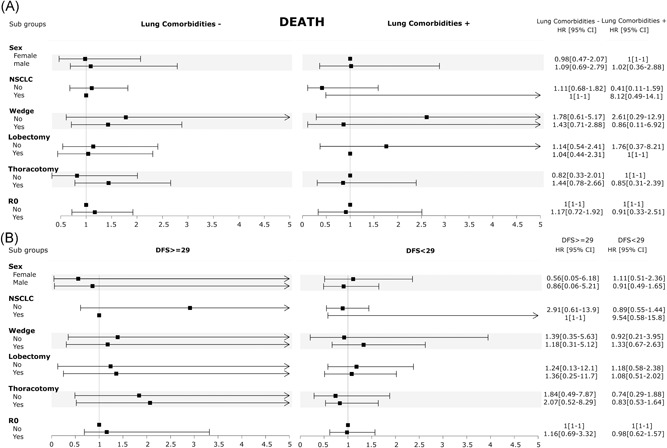
Sub‐analysis for death. A, Interaction between lung comorbidities and potential influencing factors. B, Interaction between disease‐free survival (DFS) and potential influencing factors. CI, confidence interval; HR, hazard risk; NSCLC, non‐small–cell lung cancer
Any significant interaction was also found for recurrence and sub‐analysis. In addition, Figure 5 shows that the risk of recurrence was lower when potential interacting variables were added.
Figure 5.
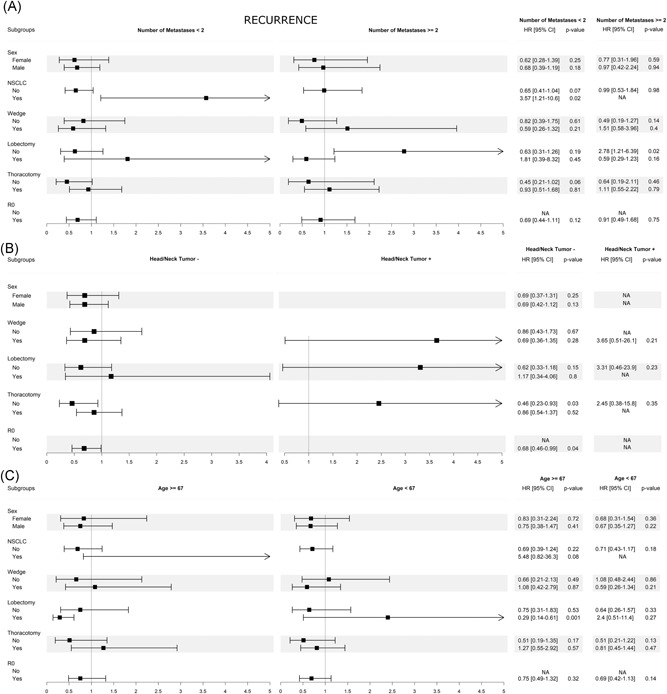
Sub‐analysis for recurrence. A, Interaction between multiple metastases and potential influencing factors. B, Interaction between head/neck tumor and potential influencing factors. C, Interaction between age and potential influencing factors. CI, confidence interval; SHR, sub‐hazard risk
Therefore, since adding the co‐factors hazard risk did not increase, a number of metastases more than 2, head‐neck tumor and age less than 67 were independent factors of recurrence.
4. COMMENT
The present study was undertaken to investigate whether associated lymphadenectomy could influence the midterm survival and tumor recurrence in patients undergoing PM.
The main finding of our study is the absence of any significant difference neither in midterm survival nor recurrence between patients who underwent associated lymphadenectomy or not. However, although not statistically significant, a trend towards a lower re‐recurrence risk (80.0% vs 63.2%, P = .073) was observed in the L+ group. The lack of benefits of lymphadenectomy has already been reported by other groups.12 In addition, stereotactic body radiotherapy (SBRT) is gaining increasing popularity and some trials are ongoing comparing outcomes between surgery an SBRT.19 If the results will confirm a non‐inferiority of SBRT over surgery, the former might become an attractive option for this patients due to its lower invasiveness and reduced overall risks.20 However, it must be considered that, although the reported incidence of involved lymph nodes is not high, the CT scan has a high incidence of false‐negative and the positive predictive value of PET in detecting lymph node involvement is low,21 thus rendering histological confirmation necessary, even if the patient is offered a nonsurgical approach.
In our practice, we do not routinely use PET scan in the preoperative workup of patients who are candidates to lung metastasectomy, unless an intrathoracic nodal involvement is suspected based on CT scan images. In accordance with our practice, recent investigations showed how the number of metastases was correctly identified both by CT and PET scan in only about 60% of cases. Agreement between clinical and pathological nodal involvement is quite low for both techniques, however with a little advantage for PET scan.21 Therefore, given the impact of nodal involvement on the prognosis of these patients we usually prefer to reserve this investigation and delay surgical treatment only for patients with high suspicion of more advanced disease.
Lymph node involvement is generally considered as the invasion of tumor cells into local lymphatic vessels9 and a positive nodal status indicates a marked aggressive behavior of some tumors with an increased propensity to spread to lympho‐vascular structures9, 12 resulting in a higher trend to further metastatic recurrence and shorter overall survival. Lymphadenectomy has therefore been advocated to allow a more accurate prognostic stratification as well as a more accurate evaluation of the patients for potential adjuvant treatments.11
Promoters of lymphadenectomy base their conviction on the following postulates: (a) The identification of metastatically involved lymph nodes undoubtedly carries a worse prognosis.22 (b) Mediastinal lymphadenectomy has a recognized low mortality and morbidity.23 (c) In case of metastatic lymph nodes, lymphadenectomy might increase the chance of removing all tumor deposits and a potential source of further spreading.9
In our series and in accordance with what has been reported by the International Registry,3 only 6.9% of patients who underwent lymphadenectomy had histologically proven lymph nodal involvement although other authors have described this figure to range between 5% and 32%.3, 8, 12, 21, 24, 25 We might hypothesize that the low prevalence of nodal metastases might be due to our close follow‐up protocol and the consequent early referral of patients and to the strict preoperative work‐up to select candidates for surgical treatment.
Another finding of our analysis, in accordance with large retrospective studies,7, 26 is that a short interval between primary tumor resection and development of metastases (DFI < 29 months) had a significant impact on survival with a 5% risk reduction per 1‐month DFI increase. In addition, we found also that the presence of lung comorbidities was associated with a 2.9‐fold rise of the risk of death. In our series lung disease were mainly represented by obstructive pulmonary disease (24 of 25, 96%). We might assume that patients with lung comorbidities, and mainly those who already underwent major lung resection for their primary tumor, may be compromised from a functional point of view to safely undergo any further surgical treatment, and therefore less effective systemic treatments might have been undertaken under these circumstances. This observation, from our point of view, confirms the current indication for a lung‐sparing resection for pulmonary metastases, resulting in adequate postoperative pulmonary function which reflects in the good postoperative quality of life, both main pre‐requisites when a surgical treatment option is offered to this subset of patients.27, 28 Therefore, wedge resection might be advisable when both anatomical conditions and tumor extension allow a limited pulmonary resection.28
Interestingly, whereas primary NSCLC alone was not an independent risk factor of death and neither survival (P > .9) nor recurrence (P = .06) was different between the two groups even corrected by this factor, NSCLC increased the risk of death by about 20 times when associated with lung disease and by more than 10 times if associated to DFI less than 29 months. Whether this subset of patients is representative of a particularly aggressive lung cancer histotype is unknown and, however, beyond the scope of this investigation. However, in case of patients with short DFI/or lung comorbidities, when concomitant signs of potential NSCLC aggressiveness are present, such as the spreading of tumor cells through the bronchial tree,29 both indication and surgical resection extension might be questionable. Indeed, aerogenous tumor spread is an acknowledged negative prognostic factor gaining increasing attention in recent years.30 Under these circumstances, we believe that indication for surgery should be carefully discussed by a multidisciplinary team and tailored to the individual patient. Unfortunately, we currently have no information to perform an analysis on this subset of patients, but this will be the subject of further research.
Finally, multivariable analysis revealed three independent predictors of tumor recurrence:
-
(a)
Multiple metastases increased the risk of developing a further recurrence after surgery by more than 28% and this represented something new since, whereas there was already evidence of its influence on survival,3, 25, 31 its impact on recurrence had never specifically investigated before. (b) Metastases arising from tumors of the head and neck district were significantly associated with 3.3‐time higher risk of recurrence. Also, this is a new finding since this factor was explored only in small series with short follow and however only in relation to survival.32, 33 (c) Patients aged less than 67 years had a significantly higher risk of tumor recurrence and this risk lowered by 2% per year increase. This is in contrast with the trend of results in literature33, 34 and it could be justified by the supposed tendency of tumor cells to be more indolent in older hosts, although this has only been demonstrated in murine experimental studies35 and still not been confirmed in human clinical investigations.36
5. LIMITATIONS
This study has some limitations: first, the retrospective design and the relatively small number of patients impose some caution in interpreting our results.
Furthermore, the study does not compare patients with pathologic lymph node involvement in the setting of metastasectomy and this might have introduced a bias to the study design. Nonetheless, this was beyond the principal aim of the study mainly focused on surgical lymphadenectomy that could help surgeons in referring patients for potential adjuvant treatments.
Moreover, pathologists from our institution do not routinely count the number of lymph nodes, therefore the median number of lymph nodes excised cannot be defined.
Additionally, the small number within histotype‐subgroups did not allow us to carry out an analysis by tumor type. We are collecting data for ongoing research that will be focused on this subject. However, many previous publications reported on pulmonary metastases arising from different primitive tumors,3, 37, 38, 39, 40 and survival estimates do not differ consistently between populations with single or multiple histotypes of origin.41, 42, 43, 44, 45
6. CONCLUSION
In our experience, associated lymphadenectomy did not give any additional advantage in terms of survival and recurrence after metastasectomy and it was not a predictive factor of these main outcomes. Larger cohort prospective studies are necessary to confirm our findings and to define the true prognostic impact of lymphadenectomy during PM.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
SYNOPSIS
Lymphadenectomy during pulmonary metastasectomy is widely performed to stage patients and identify those at higher risk of disease progression. We assessed major prognostic outcomes of patients who underwent lymphadenectomy or not during pulmonary metastasectomy. No differences in overall survival and rates of recurrence were found between the two groups.
Londero F, Morelli A, Parise O, et al. Lymphadenectomy during pulmonary metastasectomy: Impact on survival and recurrence. J Surg Oncol. 2019;120:768‐778. 10.1002/jso.25635
Francesco Londero and Angelo Morelli equally contributed to this work.
References
REFERENCES
- 1. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8‐10. [DOI] [PubMed] [Google Scholar]
- 2. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8(6):378‐382. [DOI] [PubMed] [Google Scholar]
- 3. Pastorino U, Buyse M, Friedel G, et al. Long‐term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113(1):37‐49. [DOI] [PubMed] [Google Scholar]
- 4. Friedel G, Pastorino U, Ginsberg RJ, et al. Results of lung metastasectomy from breast cancer: prognostic criteria on the basis of 467 cases of the international registry of lung metastases. Eur J Cardiothorac Surg. 2002;22(3):335‐344. [DOI] [PubMed] [Google Scholar]
- 5. Robert JH, Ambrogi V, Mermillod B, Dahabreh D, Goldstraw P. Factors influencing long‐term survival after lung metastasectomy. Ann Thorac Surg. 1997;63(3):777‐784. [DOI] [PubMed] [Google Scholar]
- 6. Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Prauer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91(8):1066‐1071. [DOI] [PubMed] [Google Scholar]
- 7. Smith R, Pak Y, Kraybill W, Kane JM 3rd. Factors associated with actual long‐term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol. 2009;35(4):356‐361. [DOI] [PubMed] [Google Scholar]
- 8. Loehe F, Kobinger S, Hatz RA, Helmberger T, Loehrs U, Fuerst H. Value of systematic mediastinal lymph node dissection during pulmonary metastasectomy. Ann Thorac Surg. 2001;72(1):225‐229. [DOI] [PubMed] [Google Scholar]
- 9. Pfannschmidt J, Klode J, Muley T, Dienemann H, Hoffmann H. Nodal involvement at the time of pulmonary metastasectomy: experiences in 245 patients. Ann Thorac Surg. 2006;81(2):448‐454. [DOI] [PubMed] [Google Scholar]
- 10. Welter S, Jacobs J, Krbek T, Poettgen C, Stamatis G. Prognostic impact of lymph node involvement in pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg. 2007;31(2):167‐172. [DOI] [PubMed] [Google Scholar]
- 11. Veronesi G, Petrella F, Leo F, et al. Prognostic role of lymph node involvement in lung metastasectomy. J Thorac Cardiovasc Surg. 2007;133(4):967‐972. [DOI] [PubMed] [Google Scholar]
- 12. Hamaji M, Cassivi SD, Shen KR, et al. Is lymph node dissection required in pulmonary metastasectomy for colorectal adenocarcinoma? Ann Thorac Surg. 2012;94(6):1796‐1800. [DOI] [PubMed] [Google Scholar]
- 13. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18(6):805‐835. [DOI] [PubMed] [Google Scholar]
- 14. Shreve P, Faasse T. Role of positron emission tomography‐computed tomography in pulmonary neoplasms. Radiol Clin North Am. 2013;51(5):767‐779. [DOI] [PubMed] [Google Scholar]
- 15. Beckles MA, Spiro SG, Colice GL, Rudd RM American College of Chest Physicians . The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest. 2003;123(1 Suppl):105S‐114S. [DOI] [PubMed] [Google Scholar]
- 16. Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest. 2003;123(6):2096‐2103. [DOI] [PubMed] [Google Scholar]
- 17. Okada M, Sakamoto T, Yuki T, Mimura T, Miyoshi K, Tsubota N. Selective mediastinal lymphadenectomy for clinico‐surgical stage I non–small cell lung cancer. Ann Thorac Surg. 2006;81(3):1028‐1032. [DOI] [PubMed] [Google Scholar]
- 18. Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111(6):1710‐1717. [DOI] [PubMed] [Google Scholar]
- 19. Treasure T. Surgery and ablative techniques for lung metastases in the pulmonary metastasectomy in colorectal cancer (PulMiCC) trial: is there equivalence? J Thorac Dis. 2016;8(Suppl 9):S649‐S651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shultz DB, Filippi AR, Thariat J, Mornex F, Loo BW Jr, Ricardi U. Stereotactic ablative radiotherapy for pulmonary oligometastases and oligometastatic lung cancer. J Thorac Oncol. 2014;9(10):1426‐1433. [DOI] [PubMed] [Google Scholar]
- 21. Guerrera F, Renaud S, Schaeffer M, et al. Low accuracy of computed tomography and positron emission tomography to detect lung and lymph node metastases of colorectal cancer. Ann Thorac Surg. 2017;104(4):1194‐1199. [DOI] [PubMed] [Google Scholar]
- 22. Dominguez‐Ventura A, Nichols FC 3rd. Lymphadenectomy in metastasectomy. Thorac Surg Clin. 2006;16(2):139‐143. [DOI] [PubMed] [Google Scholar]
- 23. Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early‐stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81(3):1013‐1019. [DOI] [PubMed] [Google Scholar]
- 24. Ercan S, Nichols FC, Trastek VF, et al. Prognostic significance of lymph node metastasis found during pulmonary metastasectomy for extrapulmonary carcinoma. Ann Thorac Surg. 2004;77(5):1786‐1791. [DOI] [PubMed] [Google Scholar]
- 25. Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg. 2007;84(1):324‐338. [DOI] [PubMed] [Google Scholar]
- 26. Harting MT, Blakely ML, Jaffe N, et al. Long‐term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg. 2006;41(1):194‐199. [DOI] [PubMed] [Google Scholar]
- 27. Rusch VW. Pulmonary metastasectomy. Current indications. Chest. 1995;107(6 Suppl):322s‐331s. [DOI] [PubMed] [Google Scholar]
- 28. Welter S, Cheufou D, Sommerwerck U, Maletzki F, Stamatis G. Changes in lung function parameters after wedge resections: a prospective evaluation of patients undergoing metastasectomy. Chest. 2012;141(6):1482‐1489. [DOI] [PubMed] [Google Scholar]
- 29. Jin Y, Sun PL, Park SY, et al. Frequent aerogenous spread with decreased E‐cadherin expression of ROS1‐rearranged lung cancer predicts poor disease‐free survival. Lung Cancer. 2015;89(3):343‐349. [DOI] [PubMed] [Google Scholar]
- 30. Gaikwad A, Souza CA, Inacio JR, et al. Aerogenous metastases: a potential game changer in the diagnosis and management of primary lung adenocarcinoma. AJR Am J Roentgenol. 2014;203(6):W570‐W582. [DOI] [PubMed] [Google Scholar]
- 31. Groeger AM, Kandioler D, Mueller MR, End A, Eckersberger F, Wolner E. Survival after surgical treatment of recurrent pulmonary metastases. Eur J Cardiothorac Surg. 1997;12(5):703‐705. [DOI] [PubMed] [Google Scholar]
- 32. Younes RN, Gross JL, Silva JF, Fernandez JA, Kowalski LP. Surgical treatment of lung metastases of head and neck tumors. Am J Surg. 1997;174(5):499‐502. [DOI] [PubMed] [Google Scholar]
- 33. Yotsukura M, Kinoshita T, Kohno M, et al. Survival predictors after resection of lung metastases of head or neck cancers. Thorac Cancer. 2015;6(5):579‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wedman J, Balm AJ, Hart AA, et al. Value of resection of pulmonary metastases in head and neck cancer patients. Head and Neck. 1996;18(4):311‐316. [DOI] [PubMed] [Google Scholar]
- 35. Beheshti A, Benzekry S, McDonald JT, et al. Host age is a systemic regulator of gene expression impacting cancer progression. Cancer Res. 2015;75(6):1134‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taccoen X, Valeri A, Descotes JL, et al. Renal cell carcinoma in adults 40 years old or less: young age is an independent prognostic factor for cancer‐specific survival. Eur Urol. 2007;51(4):980‐987. [DOI] [PubMed] [Google Scholar]
- 37. Seebacher G, Decker S, Fischer JR, Held M, Schafers HJ, Graeter TP. Unexpected lymph node disease in resections for pulmonary metastases. Ann Thorac Surg. 2015;99(1):231‐236. [DOI] [PubMed] [Google Scholar]
- 38. Lo Faso F, Solaini L, Lembo R, et al. Thoracoscopic lung metastasectomies: a 10‐year, single‐center experience. Surg Endosc. 2013;27(6):1938‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Long H, Zheng Y, Situ D, Ma G, Lin Z, Wang J. Hand‐assisted thoracoscopic surgery for bilateral lung metastasectomy through sternocostal triangle access. Ann Thorac Surg. 2011;91(3):852‐858. [DOI] [PubMed] [Google Scholar]
- 40. Casiraghi M, De Pas T, Maisonneuve P, et al. A 10‐year single‐center experience on 708 lung metastasectomies: the evidence of the “international registry of lung metastases”. J Thorac Oncol. 2011;6(8):1373‐1378. [DOI] [PubMed] [Google Scholar]
- 41. Guerrini GP, Lo Faso F, Vagliasindi A, et al. The role of minimally invasive surgery in the treatment of lung metastases. J Invest Surg. 2017;30(2):110‐115. [DOI] [PubMed] [Google Scholar]
- 42. Tacconi F, Ambrogi V, Pompeo E, Sellitri F, Mineo TC. Substernal hand‐assisted videothoracoscopic lung metastasectomy: long term results in a selected patient cohort. Thorac Cancer. 2011;2(2):45‐53. [DOI] [PubMed] [Google Scholar]
- 43. McCormack PM, Burt ME, Bains MS, Martini N, Rusch VW, Ginsberg RJ. Lung resection for colorectal metastases. 10‐year results. Arch Surg. 1992;127(12):1403‐1406. [DOI] [PubMed] [Google Scholar]
- 44. Cerfolio RJ, Allen MS, Deschamps C, et al. Pulmonary resection of metastatic renal cell carcinoma. Ann Thorac Surg. 1994;57(2):339‐344. [DOI] [PubMed] [Google Scholar]
- 45. Girelli L, Locati L, Galeone C, et al. Lung metastasectomy in adenoid cystic cancer: is it worth it? Oral Oncol. 2017;65:114‐118. [DOI] [PubMed] [Google Scholar]


