Abstract
Identifying the traits causing reproductive isolation and the order in which they evolve is fundamental to understanding speciation. Here, we quantify prezygotic and intrinsic postzygotic isolation among allopatric, parapatric, and sympatric populations of the butterflies Heliconius elevatus and Heliconius pardalinus. Sympatric populations from the Amazon (H. elevatus and H. p. butleri) exhibit strong prezygotic isolation and rarely mate in captivity; however, hybrids are fertile. Allopatric populations from the Amazon (H. p. butleri) and Andes (H. p. sergestus) mate freely when brought together in captivity, but the female F1 hybrids are sterile. Parapatric populations (H. elevatus and H. p. sergestus) exhibit both assortative mating and sterility of female F1s. Assortative mating in sympatric populations is consistent with reinforcement in the face of gene flow, where the driving force, selection against hybrids, is due to disruption of mimicry and other ecological traits rather than hybrid sterility. In contrast, the lack of assortative mating and hybrid sterility observed in allopatric populations suggests that geographic isolation enables the evolution of intrinsic postzygotic reproductive isolation. Our results show how the types of reproductive barriers that evolve between species may depend on geography.
Keywords: Butterflies, gene flow, hybrid sterility, prezygotic isolation, speciation
Under a biological species concept, understanding speciation requires identifying the reproductive barriers between taxa and the order that they evolve (Coyne and Orr 2004; Butlin et al. 2012). However, which kinds of barriers evolve first may depend on geography (Coyne and Orr 1997). In the absence of gene flow, no forces inhibit speciation and populations can diverge through any combination of deterministic or stochastic processes, such as selection or drift (Turelli et al. 2001). Allopatric populations may therefore exhibit various combinations of prezygotic and postzygotic barriers, and postzygotic isolation can be either extrinsic or intrinsic. In general, the conditions for speciation are thought to become more restrictive as gene flow increases (Nosil 2007; Kisel and Barraclough 2010). For example, an important class of intrinsic postzygotic barriers among species are deleterious epistatic interactions between two or more divergent loci, known as Dobzhansky–Muller incompatibilities (DMIs; Orr and Turelli 2001). DMIs have often been viewed as unlikely to arise in the face of gene flow, because hybridization among diverging lineages produces double heterozygous genotypes with reduced fitness (Coyne and Orr 2004).
However, the constraining effects of gene flow can be reduced or even eliminated by the genetic architecture of the traits driving speciation (Maynard Smith 1966; Felsenstein 1981; Gavrilets 2004). For instance, DMIs may evolve as pleiotropic by‐products of divergent selection, if it is strong enough to outweigh the production of hybrids with low fitness (Bank et al. 2012). When matings among populations involve a cost, gene flow may even promote the evolution of reproductive isolation, because selection directly favors increased mate discrimination (Dobzhansky 1940; Servedio and Noor 2003). This process, known as reinforcement, may occur during sympatric speciation, or following secondary contact between populations derived in allopatry.
Empirical tests of these theoretical predictions require characterizing the components of reproductive isolation between closely related taxa with different levels of gene flow (Coyne and Orr 1997; Funk 1998; Funk et al. 2006). For example, under reinforcement it is expected that sympatric populations should exhibit stronger sexual isolation than allopatric populations. The most extensive comparative data in this respect are from Drosophila, where prezygotic sexual isolation accumulates more rapidly between sympatric than between allopatric pairs of taxa (Coyne and Orr 1997). This pattern is likely due to reinforcement (Yukilevich 2012), but whether it is evolving in response to intrinsic or extrinsic postzygotic isolation remains unclear (Turelli et al. 2014). Here, we characterize the specific traits contributing to reproductive isolation in allopatric, parapatric and sympatric populations of mimetic Heliconius butterflies.
Heliconius (Nymphalidae) comprises an adaptive radiation of ∼48 known species and 300+ subspecies with relatively well understood ecology, and provides excellent opportunities to study reproductive isolation among diverging populations in different geographical contexts (Jiggins 2017; Mérot et al. 2017). Previous studies of Heliconius close to the species boundary have typically found evidence of prezygotic isolation and/or extrinsic postzygotic isolation (McMillan et al. 1997; Chamberlain et al. 2009; Merrill et al. 2011a). For example, shifts in mimetic pattern are often thought to initiate speciation (Bates 1862), because interspecific hybrids displaying intermediate color patterns are selected against by predators (Merrill et al. 2012). Furthermore, because color pattern is itself used as a mating cue (Jiggins et al. 2001b), Heliconius provides prime examples of speciation facilitated by pleiotropy among traits under divergent selection and those involved in mate choice (Servedio et al. 2011). Nonetheless, the existence of closely related, sympatric taxa that do not differ in mimetic pattern suggests that mating cues other than color pattern are also important (Giraldo et al. 2008). For example, recent studies have demonstrated a role for pheromones in mediating mate choice (Mérot et al. 2015; Darragh et al. 2017). Divergent host plant and habitat use have also been proposed as sources of reproductive isolation (Estrada and Jiggins 2002; Rosser et al. 2019), and adaptations to environmental gradients have been linked to speciation (Jiggins et al. 1996; Mérot et al. 2013).
Gene flow is thought to play an important role in Heliconius evolution and may have allowed the adaptive transfer of mimetic color pattern alleles among species, possibly even leading to speciation (Heliconius Genome Consortium 2012; Pardo‐Díaz et al. 2012; Zhang et al. 2016 but see Brower 2018). One example of this is Heliconius elevatus and Heliconius pardalinus. Heliconius elevatus is characterized by a red, black, and yellow “rayed” pattern, which it shares with Heliconius erato, Heliconius melpomene, and many other Heliconiini. In contrast, H. pardalinus exhibits a mottled brown, black, and orange “tiger” pattern that mimics similarly patterned Ithomiini, as well as other Heliconiini. Introgression of color pattern alleles between H. melpomene and the common ancestor of H. pardalinus and H. elevatus at two key loci appears to have triggered the switch to a rayed pattern (Heliconius Genome Consortium 2012; Wallbank et al. 2016). Contemporary gene flow between H. elevatus and H. pardalinus has yet to be estimated, although wild‐caught putative hybrids (Brower 2018) and the fertility of lab‐reared hybrids (see below) suggest that it does occur.
In the present paper, we characterize an extensive set of phenotypic traits potentially involved in prezygotic and intrinsic postzygotic isolation among populations of H. elevatus and H. pardalinus in northern Peru. Broad‐scale distribution maps show that H. elevatus overlaps with the subspecies H. p. butleri in the Amazonian lowlands (Rosser et al. 2012; Fig. 1). A different subspecies, H. p. sergestus, inhabits the upper Huallaga/Mayo valleys in the adjacent Andes, where H. elevatus is absent. The two H. pardalinus subspecies have diverged in their tiger color pattern to mimic different co‐occurring ithomiine butterflies (Fig. 2). Phylogenetic analysis of these populations using genome‐wide single nucleotide polymorphisms shows H. p. sergestus to be sister to a clade containing H. elevatus and H. p. butleri + H. p. dilatus (the latter two are closely related adjacent populations from the Peruvian Amazon, and are hereafter referred to collectively as H. p. butleri), thus rendering H. pardalinus as a whole paraphyletic (Heliconius Genome Consortium 2012 and Fig. 2A). In light of this paraphyly and geographic distribution of the taxa, in the present paper we address the following questions: (1) Which specific traits contribute to reproductive isolation? (2) Do the geographic patterns of prezygotic and postzygotic isolation suggest speciation with gene flow? (3) Where are the species boundaries in these taxa?
Figure 1.
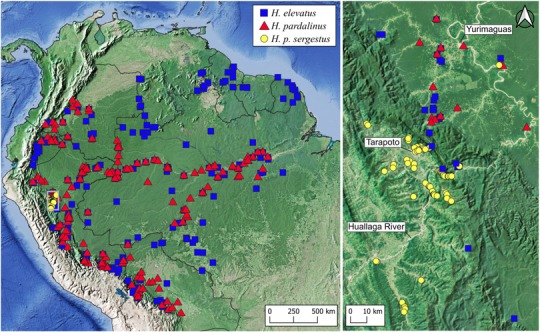
Left panel: The geographic distributions of H. elevatus and H. pardalinus (all Amazonian subspecies) at a continental scale, with the range of subspecies H. p. sergestus shown in yellow. Right panel: Local map showing the fine scale distributions in northern Peru, centered on the range of H. p. sergestus. In this map, the red triangles correspond to the subspecies H. p. butleri, which intergrades into other, similarly patterned subspecies in lowland Amazonia. Data are taken from Rosser et al. (2012) and supplemented with newer field collections made by the authors (see Methods and Results sections).
Figure 2.
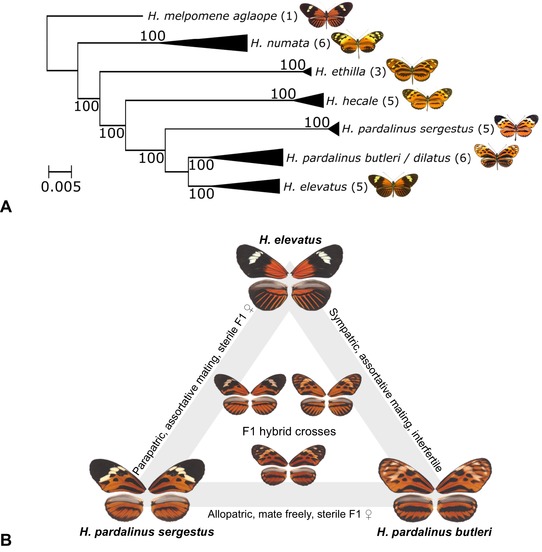
(A) Maximum‐likelihood phylogeny for the Peruvian silvaniform taxa, with H. melpomene aglaope as the outgroup, based on restriction site associated DNA (RAD) sequences (Supplementary Information S1). The scale bar refers to the number of substitutions per site, and node values are bootstrap support. Figures in brackets indicate the number of samples. Heliconius p. butleri clustered with the subspecies H. pardalinus dilatus from central Peru; the two are very similarly patterned and gradually intergrade. (B) Color patterns of the three parental taxa and their F1 hybrids, together with a summary of their relative geographic distributions and reproductive compatibility.
Methods
Live butterflies collected in the Peruvian departments of San Martín, Loreto, and Ucayali were used to establish butterfly stocks in outdoor insectaries in Tarapoto, Peru, and heated indoor insectaries in York, UK. Adult butterflies were fed sugar/pollen solution and provided with additional pollen sources such as Lantana camara (Verbenaceae), Gurania sp. (Cucurbitaceae), and Polianthes tuberosa (Asparagaceae). Larvae were fed primarily using Passiflora caerulea, P. edulis, P. riparia, and P. serrato‐digitata (Passifloraceae). Experiments involving H. elevatus, H. p. butleri, and H. p. sergestus used a mixture of wild and captive‐bred butterflies. Crosses between the taxa were produced in the insectaries, through either natural matings or handpairing (Clarke and Sheppard 1956, and see Supplementary Information S2). Statistical analyses were carried out in R (R Core Team 2018) using base functions, unless otherwise stated.
PREZYGOTIC ISOLATION: GEOGRAPHY, HABITAT, AND CLIMATE
Geographic barriers or divergent adaptations that prevent populations from encountering one another can be important sources of reproductive isolation (Kirkpatrick and Ravigné 2002; Coyne and Orr 2004; Sobel et al. 2010). To determine the local distributions and habitats of H. elevatus, H. p. butleri, and H. p. sergestus near Tarapoto, we made extensive collections during 2009–2016. To quantify the climatic niche of each taxon, we obtained 30 arcsec (1 km2 resolution) gridded climate data (WorldClim version 1; Hijmans et al. 2005). We then used ArcGIS 10 to extract mean annual temperature values and annual precipitation values for each collection locality.
PREZYGOTIC ISOLATION: FEMALE HOST PLANT PREFERENCE
Host plant shifts have long been recognized as holding the potential to create reproductive isolation in phytophagous insects, especially when mating occurs on or near the host plant (Ehrlich and Raven 1964; Bush 1969). To investigate whether H. elevatus, H. p. butleri, and H. p. sergestus differ in host plant use, we recorded field observations in Peru, Bolivia, Brazil, Suriname, and French Guiana, and supplemented these with records from the literature. However, such data are hard to obtain, and furthermore, may simply reflect which host plants are available to local populations of butterflies, rather than divergent adaptations between them. We therefore conducted laboratory experiments in Peru to test for differences in host plant preference. Reared and wild caught females of a single taxon were released into a large cage (2.5 m [W] × 5 m [L] × 2 m [H]) containing 21 species of Passiflora (Table S1) commonly found near Tarapoto and representing potential host plants. Groups of 3–33 females from a single taxon were taken at random from stocks and left to oviposit in this cage for up to 7 days. At the end of each day, the number of eggs laid on each plant species was recorded, and the eggs removed. To reduce the effects of individual variation in female preference and host plant quality, each butterfly taxon was tested repeatedly over several months. To measure similarity in host plant use, we calculated pairwise values of Pianka's (1973) niche overlap index for the three taxa, using the number of eggs laid across the 21 host plants. The index varies from zero (when no resources are shared) to one (when resource use is identical).
We also conducted a second experiment to test for differences in host plant preference while directly controlling for variation in individual preference and host plant size/quality. Single females were introduced into a cage measuring 1 m (W) × 2 m (L) × 1.7 m (H), with four approximately equally sized shoots of potential host plants (P. edulis, P. laurifolia, P. riparia, and P. serrato‐digitata) placed in each corner of the cage. At the end of each day, the number of eggs laid on each plant species was recorded and the eggs removed. For each pairwise comparison of taxa, we used Generalized Linear Mixed Effect Models (GLMM) with negative binomial errors to test for differences in the number of eggs laid on each plant, using the R package lme4 (Bates et al. 2015). Host plant species and butterfly taxon were specified as fixed effects, and individual as a random factor. Two nested models were fit for each of the three pairwise comparisons, one including the interaction among fixed effects (i.e., evidence of a difference in species preference) and one without. Models were tested against one another using ANOVA, and the Akaike information criterion (AIC) was used for model selection.
PREZYGOTIC ISOLATION: MALE COLOR PATTERN PREFERENCE
An important mating cue for male Heliconius is female wing color pattern (Jiggins et al. 2001b; Merrill et al. 2011a). To test whether H. elevatus, H. p. butleri, and H. p. sergestus males exhibit a preference for their own color pattern phenotype, we measured courtship effort by males when given a choice of female wings, one bearing their own phenotype and the other bearing an alternative phenotype. We then used Generalized Linear Models (GLMs) with binomial errors to estimate the predicted probability of a male courting its own phenotype or the alternative, with a categorical predictor indicating the six pairwise comparisons.
Experiments were conducted in Peru and the UK using the experimental setup shown in Figure S1. In the experiments in the UK, male preference data were collected only for H. elevatus and H. p. butleri. Groups of five males (either H. elevatus or H. p. butleri) were presented with a pair of model wings (one H. elevatus and one H. p. butleri), and trials lasted for 25 min. The number of approaches (clear, directed flights to within 10 cm of a model), hovers (sustained flight 5–15 cm over a model), and alightings (landing on or next to a model) by the males directed toward each of the model wings was recorded (Klein and de Araújo 2010). After a courtship event, the male was caught and its identity recorded. In Peru, we used pairs of males (representing two of the three taxa) to avoid having to catch individuals after each courtship. Males were presented with two female wing models exhibiting the corresponding color patterns and placed in the experimental cage 1 day before testing to allow acclimatization. Courtship trials lasted 15–30 min.
PREZYGOTIC ISOLATION: MALE SEX PHEROMONES
Sex pheromones are a potentially important source of sexual/behavioral prezygotic reproductive isolation because they can be used as a cue for mate choice (Smadja and Butlin 2009). In butterflies, male sex pheromones are mostly emitted from specialized scales on the wings (Rutowski 1980), known as androconia. In male Heliconius, the androconia are most strongly concentrated on the anterior margin of the dorsal hind wing (Emsley 1965), and the volatiles they produce are involved in female mate choice (Darragh et al. 2017). We therefore tested the male androconia of H. elevatus, H. p. butleri, and H. p. sergestus for differences in the putative pheromone compounds. Dichloromethane extracts from the androconial region were taken from males of 10 H. elevatus, 13 H. p. butleri, and 5 H. p. sergestus (Fig. S2). Control samples of the non‐androconial region on the posterior margin of the hind wing were also taken from five of the males of each taxon. In addition, control samples of the anterior margin of the hind wing were taken from two H. elevatus and two H. p. butleri females (no H. p. sergestus females were sampled). All butterflies were ∼21 days old.
Samples were analyzed by gas chromatography–mass spectrometry (GC–MS). Tridecyl acetate was used as internal standard so the amount (nmoles) of each compound in each sample could be calculated. Compounds produced by butterflies were identified through comparison of mass spectra and gas chromatographic retention indices with synthetic samples and mass spectrometric databases (see Mann et al. 2017 for full details). One H. elevatus non‐androconial control showing signs of contamination was discarded. Compounds were classed as putative male sex pheromone components if they were present in greater amounts in the male androconial region than either male nonandroconial controls (significance determined using Wilcoxon signed rank tests), female controls (significance determined using Mann–Whitney U‐tests), or both. This putative male sex pheromone dataset was reduced to two dimensions by nonmetric multidimensional scaling (NMDS) ordination with a Bray–Curtis similarity matrix, using the vegan R package (Oksanen et al. 2017). For this, we used the proportion of compounds found for each individual. Finally, we carried out an analysis of similarities with the nonparametric ANOSIM to test whether the three taxa exhibited different pheromone profiles.
PREZYGOTIC ISOLATION: ASSORTATIVE MATING
To test for the presence of prezygotic barriers that prevent H. elevatus, H. p. butleri, and H. p. sergestus from mating in the event that they encounter one another, we presented single virgin females to groups of males of the three taxa. The experiments are not intended as an accurate simulation of the butterflies mating behavior in the wild (in fact, H. p. butleri and H. p. sergestus very rarely encounter each other, see below). Moreover, the strength of assortative mating is the product of male and/or female choice, and represents the sum effect of multiple potential barriers (e.g., pheromones and color pattern preference).
Males comprised groups of three (one of each taxon) or 15 (five of each taxon) individuals, and were at least 1‐week old to ensure sexual maturity. Experiments were monitored hourly to catch mating pairs and lasted up to 5 days, although most matings occurred in the first few hours. Females were also checked regularly for the presence of spermatophores in case a mating had occurred but not observed. In the event of an observed mating, the mating pair was replaced and not reused. In the event a mating occurred but was not observed, all the butterflies were replaced. The log likelihood of a female of a given taxon mating with an H. elevatus, H. p. butleri, or H. p. sergestus male was calculated as
where Pi is the probability of a type i mating, yi is the number of type i matings, n is the number of total matings, and k is the number of different mating types (3). Support limits for Pi were obtained by finding all sets of parameter values with loge likelihoods within two units of the maximum likelihood estimate (Edwards 1972). To test for an effect of the number of males in the experiment, we used a likelihood ratio test to compare the mating probabilities estimated separately for experiments with three and 15 males (four parameters) with those estimated combining the experiments (two parameters).
PREZYGOTIC ISOLATION: MALE COURTSHIP BEHAVIOURS
To test for taxon‐specific differences in male preference alone, we counted stereotyped courtship behaviors (Klein and de Araújo 2010) exhibited by males toward females during the assortative mating trials involving 15 males (five of each taxon) and a single virgin female. For 15 min every hour between 10 a.m. and 3 p.m., we recorded the numbers of approaches, hovers, and alightings of males toward the female. To obtain estimates of the number of courtship events per female for the three behaviors, for each of the three taxa we fitted GLMMs with negative binomial errors to account for overdispersion and with number of courtship events by males as the dependent variable (for each type of courtship, giving nine models in total) using the R package lme4 (Bates et al. 2015). Male taxon was included as the independent variable and individual female as a random effect.
POSTZYGOTIC ISOLATION: EGG HATCH RATE AND PUPAL SURVIVORSHIP
An important source of intrinsic postzygotic isolation resulting from genetic incompatibilities among taxa is sterility and reduced viability of hybrids. To test for this effect, we measured egg hatch rate and pupal survivorship of crosses within and among the three taxa, including backcrosses and F1 × F1 crosses (Tables 4 and 5). Experiments were conducted in our Peruvian insectaries. For egg hatch rate, eggs were initially collected at the end of each day, and those from a single female housed together in plastic containers. However, egg parasitism by Ooencyrtus sp. near marcelloi (det. John Noyes, May 2015) and possibly cannibalism resulted in lower measured hatch rates for within species crosses than found in previously published studies (McMillan et al. 1997; Jiggins et al. 2001a; Naisbit et al. 2002). Subsequently, egg collection was carried out every 2 hours between 9 a.m. and 5 p.m., and eggs housed in individual plastic containers. If an egg did not hatch after 7 days from the date of collection, it was inspected under a microscope for the presence of parasitoids. If an egg parasitoid was found, the egg was excluded from hatch rate calculation.
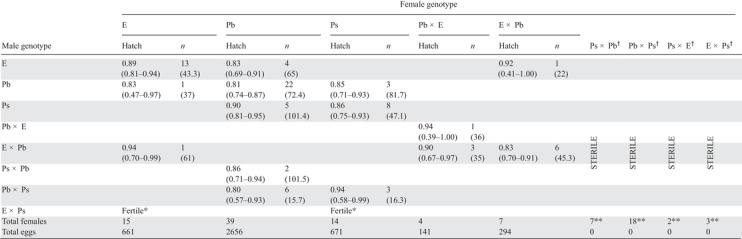
Table 4.
Estimated egg hatch rates for crosses within and between taxa; E = H. elevatus, Pb = H. p. butleri, Ps = H. p. sergestus. F1 genotypes comprise the mother's identify followed by the father's, i.e. female “Pb × E” had a H. p. butleri mother and a H. elevatus father. Hatch rates (hatch) are estimated assuming no parasitism, and include 95% confidence intervals (in brackets). n = the number of broods per cross and mean brood size (in brackets). Fertile? * indicates that the crosses appear to be fertile on the basis of crosses made outside of controlled experiments.† sterile crosses; female F1s with genotypes Ps × Pb, Pb × Ps, Ps × E and E × Ps were found to be sterile with undeveloped ovaries. ** the number of females dissected to determine the status of ovary development
|
Table 5.
Survival of pupae from seven cross types. See Table 4 legend for the codes of each cross type; n is the number of pupae; is the proportion of individuals, with 95% confidence intervals provided for the proportion of successful emergences
| E × E | Pb × Pb | Ps × Ps | E × Pb | (E × Pb) × (E × Pb) | Pb × Ps | Pb × (Pb × Ps) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n |
|
n |
|
n |
|
n |
|
n |
|
n |
|
n |
|
||||||||
| Failed to form pupae | 12 | 0.07 | 20 | 0.07 | 0 | 0 | 2 | 0.07 | 7 | 0.03 | 0 | 0 | 2 | 0.02 | |||||||
| Never emerged | 18 | 0.1 | 4 | 0.01 | 4 | 0.13 | 3 | 0.1 | 17 | 0.08 | 1 | 0.03 | 1 | 0.01 | |||||||
| Failed to emerge | 14 | 0.08 | 9 | 0.03 | 3 | 0.1 | 1 | 0.03 | 8 | 0.04 | 0 | 0 | 1 | 0.01 | |||||||
| Total failures | 44 | 0.25 | 33 | 0.11 | 7 | 0.23 | 6 | 0.2 | 32 | 0.16 | 1 | 0.03 | 4 | 0.05 | |||||||
| Emerged successfully | 131 | 0.75 | 255 | 0.89 | 24 | 0.77 | 24 | 0.8 | 173 | 0.84 | 31 | 0.97 | 79 | 0.95 | |||||||
| (0.68, 0.81) | (0.84, 0.92) | (0.60, 0.89) | (0.63, 0.90) | (0.79, 0.89) | (0.84, 1.00) | (0.88, 0.98) | |||||||||||||||
| Total pupations | 175 | 288 | 31 | 30 | 205 | 32 | 83 | ||||||||||||||
To test for variation in hatch rate, logistic regression was used to model the proportion of eggs hatching. We began by testing for an association between hatch rate and a binary predictor indicating whether eggs were collected before or after the change in protocol. We then added cross type as a predictive factor to this model and tested whether its inclusion significantly improved the fit, using likelihood ratio tests. Differences in survival between replicate broods due to unaccounted genetic or environmental variation led to higher variance than can be explained by a binomial distribution. Therefore, the variance was specified as , where μ is the mean and Φ the dispersion parameter.
We also tested for hybrid inviability in pupae by recording the survival of pupae of seven cross types (Table 5). Survival was recorded as either (1) successful emergence of a butterfly, (2) failed emergence from the pupa, (3) nothing emerged from the pupa, or (4) prepupa failed to form a pupa. Information on brood identity was not available, and therefore we were not able to account for between‐brood variance as was done for egg hatch rate.
QUANTIFYING ISOLATION
We followed the method presented by Sobel and Chen (2014) to quantify the level of reproductive isolation (Ri) caused by each trait, using the formula:
where x is the probability of gene flow, which can be calculated for each trait. Ri is a relative measure where Ri = 0 implies random mating, Ri = 1 represents complete assortative mating, and Ri = –1 complete disassortative mating. The calculation of x depends on the trait being considered and is detailed in Supplementary Information S6.
Results
PREZYGOTIC ISOLATION: GEOGRAPHY, HABITAT AND CLIMATE
Geographic data show the lowland subspecies of H. pardalinus are sympatric with H. elevatus at a broad scale across Amazonia (Fig. 1). However, at a fine scale our field collections suggest that the two exhibit habitat segregation, with H. elevatus typically encountered in tall, well‐drained, ridge‐top forest, and H. pardalinus more commonly found in swampy, low‐lying areas with scrubby vegetation (see also Brown 1976). Nonetheless, we have observed the two flying together at three sites (Muniches and Micaela Bastidas, both near Yurimaguas, in Peru and Careiro Castanho, south of Manaus in Brazil). The ranges of H. p. butleri and H. p. sergestus are separated by the Cordillera Escalera, which lies between the upper Huallaga/Mayo valley and Amazonian lowlands (Fig. 1). The two occupy very different habitat types, with H. p. sergestus primarily occurring in tropical dry forest created by the rain shadow of the cordillera. Heliconius p. sergestus is notable for exhibiting extreme temporal variations in abundance. In 2016, the H. p. sergestus population increased to such a degree that specimens were collected in the Amazon lowlands, flying together with H. p. butleri (Fig. 1). Even including this extreme event, the known distribution of H. p. sergestus is highly restricted, with a maximum linear extent of 160 km. Unlike H. p. butleri, H. elevatus inhabits the Cordillera Escalera up to 1000 meters and reaches the ecotone to the dry forests where H. p. sergestus occurs. The climatic niches of the three taxa are shown in Figure 3 and reflect these geographic distributions; H. elevatus and H. p. butleri overlap in their climatic niches, but H. elevatus also inhabits cooler and drier environments than H. p. butleri. Heliconius p. sergestus and H. p. butleri exhibit more marked divergence in their climatic envelopes, with segregation along the rainfall gradient.
Figure 3.
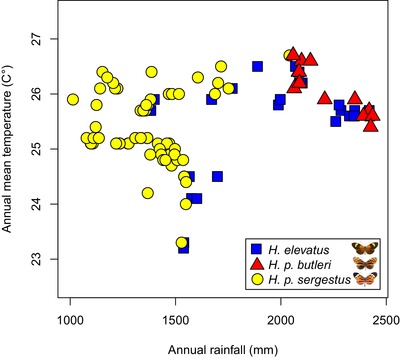
Observed climatic niches of H. elevatus (blue squares), H. p. butleri (red triangles), and H. p. sergestus (yellow circles) along rainfall (mm) and temperature (°C) gradients.
PREZYGOTIC ISOLATION: FEMALE HOST PLANT PREFERENCE
Near Tarapoto, we have recorded H. elevatus ovipositing on P. laurifolia, P. coccinea, and P. vitifolia. However, its most important host plant is a large, canopy growing species in the Laurifoliae group, from here on referred to as P. (Laurifoliae) sp. We have also recorded H. p. sergestus and H. p. butleri ovipositing on P. laurifolia (and closely related variants of it). Elsewhere in the Amazon basin, H. elevatus has been recorded ovipositing on P. laurifolia and P. longiracemosa, and populations of H. pardalinus have been recorded ovipositing on P. coccinea, P. spinosa, and P. nitida (NR and JLBM, pers. obs.; Benson et al. 1975). Detailed notes summarizing what is known about the wild host plant use of these taxa are given in Supplementary Information S4.
In the first host plant experiment, 51 H. p. butleri females laid 425 eggs on 16 species of host plant; 37 H. p. sergestus females laid 162 eggs on 10 species of host plant; and 34 H. elevatus females laid 173 eggs on 14 species of host plant. The plant most frequently used by H. p. butleri was P. edulis, on which it laid 24% of its eggs. Passiflora edulis was also the plant most frequently used by H. p. sergestus (38% of eggs laid). Consistent with our observations in the wild, the plant most frequently used by H. elevatus was P. (Laurifoliae) sp. (41% of eggs laid). The full results of this experiment are shown in Figure 4 and Table S1. Pianka's niche overlap coefficient showed high similarity in host plant use between H. p. butleri and H. p. sergestus (O = 0.81), whereas host plant overlap was less between H. elevatus and H. p. butleri (O = 0.47) and least between H. elevatus and H. p. sergestus (O = 0.38).
Figure 4.
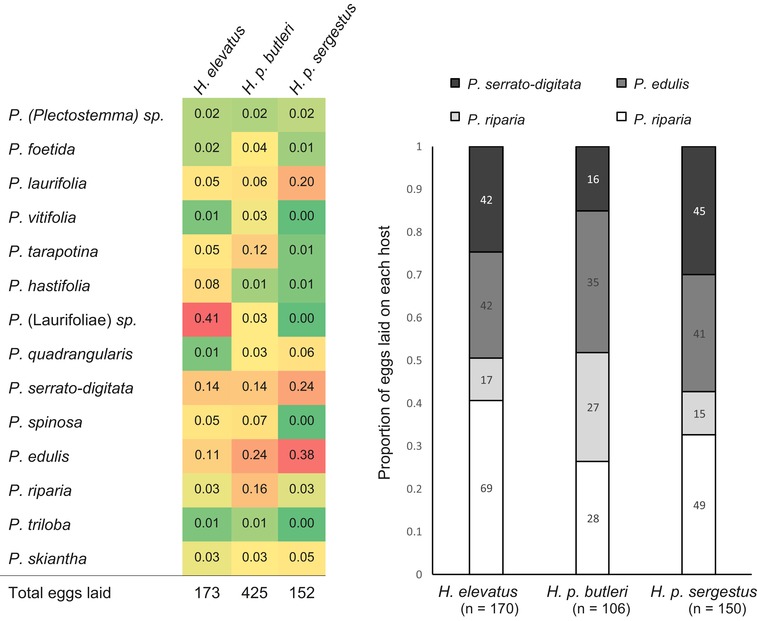
Host plant preference of the three taxa. (A) Preference measured as the proportion of eggs laid by multiple females on 21 species of Passiflora (Table S1) commonly occurring near Tarapoto and representing potential host plants. Seven plant species were not oviposited on and are not shown. (B) Preference measured as the proportion of eggs laid on size/quality matched shoots of four Passiflora species. In brackets is the total number of eggs laid by each taxon. 12 H. elevatus, 12 H. p. butleri, and 10 H. p. sergestus females were tested. Numbers within each column show the number of eggs laid on each host plant.
In the second host plant experiment, we tested the preferences of females across four host plant species using a mixed effect model to account for variation in individual preference, and using shoots of equal size/quality. A total of 170, 106, and 150 eggs were laid by 14 H. elevatus, 13 H. p. butleri, and 10 H. p. sergestus females, respectively. Heliconius elevatus laid 41% of its eggs on a single host (P. laurifolia), and H. p. butleri and H. p. sergestus both laid 33% of their eggs on their preferred hosts, P. edulis and P. serrato‐digitata, respectively (Fig. 4). We found a significant interaction between butterfly taxon and host plant species when comparing H. elevatus and H. p. butleri, indicating different host plant preferences (P = 0.02; ∆AIC = 3.7), and a marginally nonsignificant interaction when comparing H. p. sergestus and H. p. butleri (P = 0.06; ∆AIC = 1.4). We found no significant interaction when comparing H. elevatus and H. p. sergestus (P = 0.94; ∆AIC = 5.6).
PREZYGOTIC ISOLATION: MALE COLOR PATTERN PREFERENCE
One hundred and sixty‐seven males were tested for color pattern preference. Data for all courtship events are given in Figure S3. Here, we restrict our results to hovers (591 events, performed by 119 individuals) as this behavior is the most unambiguous sign of courtship. For each taxon, the estimated probabilities and 95% confidence intervals of courting the conspecific model are given in Table 1. Initially, we included a binary predictor corresponding to whether data were collected in Peru or the UK; however, as no significant difference was found (P = 0.91), these datasets were combined. Heliconius elevatus showed a significant preference for its own phenotype when presented with models of itself and either H. p. butleri or H. p. sergestus. Heliconius p. butleri also showed a significant preference for its own phenotype when presented with models of itself and H. p. sergestus, but courted its own phenotype and the H. elevatus phenotype about equally. Heliconius p. sergestus showed no statistically significant preference for any color pattern phenotype.
Table 1.
Male color pattern preference. Male butterflies were presented with conspecific and heterospecific models of female butterflies. The table shows the estimated probabilities (±95% confidence intervals) from GLMs of a male showing hovering courtship behavior toward its own color pattern relative to the other. Predicted probabilities significantly different to 0.5 (i.e., showing significant preference) are shown in bold; n is the number of hovers performed, and the number of individuals tested is shown in brackets

|
PREZYGOTIC ISOLATION: MALE SEX PHEROMONES
GC–MS analysis detected 53 compounds from samples representing 28 male individuals of H. elevatus, H. p. butleri, and H. p. sergestus (10, 13, and 5, respectively) and two female controls of H. elevatus and H. p. butleri each. Thirteen compounds were excluded because they were likely contaminants or because they only appeared once in the dataset. Male androconia were found to contain more compounds, and in larger quantities, than both male hind wing and female controls (Table 2). Thirty‐three of the 40 retained compounds were present in significantly different amounts in at least one of the pairwise comparisons between taxa (Table S2; details of the species‐specific chemical blends are provided in the Supplementary Information S5). Finally, 25 of the compounds were found in significantly higher concentrations in the male androconia compared to either the male hind wing control or the female control (or both). When plotted, the results from the NMDS analysis of these 25 compounds show that the three taxa form nonoverlapping groups along NMDS axis 1 (Fig. 5); H. elevatus and H. p. butleri individuals cluster at opposite ends of this axis and H. p. sergestus individuals cluster in between. The chemical composition of the taxa was significantly different (ANOSIM R = 0.97, P = 0.001).
Table 2.
Summary of putative pheromone compounds detected by GC–MS analysis of wing extracts. Values shown are the median amount (interquartile range) in nmol of total compounds found in extracts from male androconia, male hind wing controls, and female controls of all three taxa, and n is the average number of detectable compounds in each extract. Mann–Whitney U‐test results are presented for each male androconia/control comparison of total concentration of compounds; male androconia have significantly higher total concentrations. No female control samples were analyzed for H. p. sergestus
| Male androconia | Control regions | |||||
|---|---|---|---|---|---|---|
| Taxon | Total (nmol) | n | Total (nmol) | n | P‐value | |
| H. elevatus | 18.6 (15.6–22.4) | 21.9 | Male hind wing control | 3.9 (1.4–1.8) | 15.7 | 0.0002 |
| Female control | 4.4 (4.1–4.7) | 17 | 0.03 | |||
| H. p. butleri | 39.9 (37.3–49.0) | 30.7 | Male hind wing control | 1.7 (1.4–1.8) | 12.6 | 3 × 10–5 |
| Female control | 1.5 (1.4–1.6) | 9 | 0.02 | |||
| H. p. sergestus | 8.1 (7.9–10.2) | 23.8 | Male hind wing control | 2.2 (2.1–2.5) | 13.9 | 0.008 |
| Female control | – | – | – | |||
Figure 5.
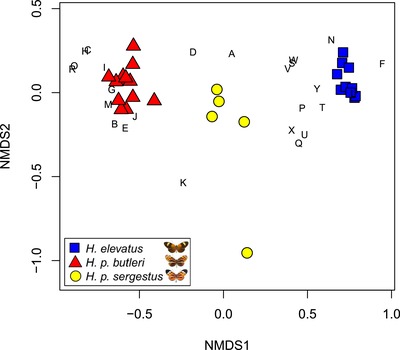
Taxa (represented by symbols) and compounds (represented by letters) along the first two dimensions of the NMDS ordination of 25 putative sex pheromone compounds found in hind‐wing androconia of males. Axes represent gradients of similarity among samples (similarity in compound composition) and among compounds (similarity in relative abundance across samples). A, homovanillyl alcohol; B, hexahydrofarnesylacetone; C, ?‐eicosene; D, ??‐heneicosadiene; E, (Z)‐9‐heneicosen; F, heneicosane; G, ?‐docosene; H, oleyl acetate; I, octadecyl acetate; J, phytol; K, (Z)‐9‐tricosene; L, tricosane; M, (Z)‐11‐eicosenyl acetate; N, tetracosane; O, (Z)‐11‐eicosenyl propionate; P, pentacosane; Q, 11‐methylpentacosane; R, (Z)‐13‐docosenyl acetate; S, hexacosane; T, 11‐methylhexacosane; U, heptacosane; V, 11‐methylheptacosane; W, octacosane; X, nonacosane; Y, octacosanal (? indicates unknown position of double bond).
PREZYGOTIC ISOLATION: ASSORTATIVE MATING
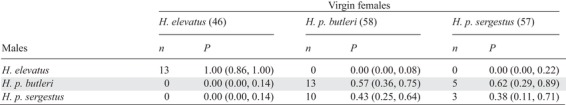
We presented 161 virgin females to males of H. elevatus, H. p. butleri, and H. p. sergestus, resulting in 44 matings over 253 trials (Table 3). Heliconius elevatus females mated only with H. elevatus males (n = 13). Heliconius p. butleri females mated with both H. p. butleri (n = 13) and H. p. sergestus (n = 10) males, but not with H. elevatus. Similarly, H. p. sergestus females mated with both H. p. butleri (n = 5) and H. p. sergestus (n = 3), but not with H. elevatus. The maximum likelihood parameter estimates for mating probabilities with associated support limits are given in Table 3. Analyzing experiments using three males and 15 males separately did not significantly improve the likelihood estimates, and hence data from the two experiments were combined (likelihood ratio tests: for H. elevatus females χ² = 0, df = 2, P = NS; for H. p. butleri females χ² = 0.44, df = 2, P = NS; for H. p. sergestus females χ² = 0, df = 2, P = NS).
Table 3.
Results from assortative mating trials between the three taxa. Single virgin females were presented to equal numbers of H. elevatus, H. p. butleri, and H. p. sergestus males, and the number of matings (n) recorded. Numbers in brackets after the female taxa give the total number of virgin females tested (not all of which mated). The P column is the maximum likelihood estimate of the probability of mating with a male of each taxa, with support limits in brackets
|
PREZYGOTIC ISOLATION: INTRA‐ AND INTERTAXON COURTSHIP BEHAVIOURS
During behavioral assays of male courtship, we recorded a total 388 approaches, 616 hovers, and 105 alightings. Data for all courtship events are given in Table S3 and Figure S4. Figure 6 shows the expected numbers of hovers per trial received by females of each taxon from males of the three taxa, as output from the GLMMs. Heliconius elevatus males hovered over H. elevatus females significantly more than H. p. sergestus and H. p. butleri males (although this was no longer significant for the latter after using the conservative Bonferroni correction method, see Table S3). Heliconius p. butleri males hovered over H. p. butleri females significantly more than H. elevatus and H. p. sergestus males. In contrast, H. p. sergestus males did not hover over H. p. sergestus females significantly more than the males of the other taxa.
Figure 6.
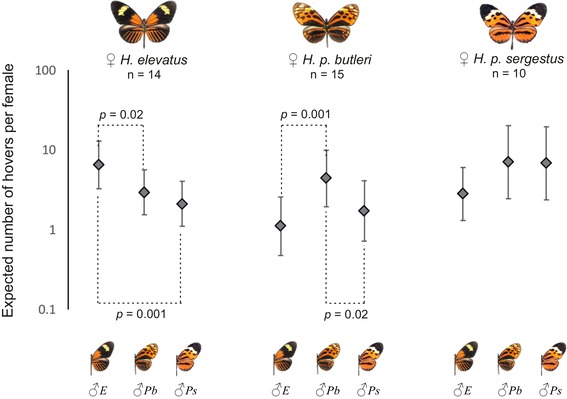
Assay of hovering courtship behavior within and between taxa. Single female virgins were presented to groups of 15 males (five of each taxon) and hover courtship toward the female was recorded. The expected number of hover courtship behaviors per trial by males toward the female taxa and the statistical significance of any differences were obtained from GLMM model outputs. Error bars are 95% Wald confidence intervals; n is the number of virgin females tested of each taxon; E, H. elevatus; Pb, H. p. butleri; Ps, H. p. sergestus. Results of the other courtship behaviors measured (approaches and alightings) are shown in Figure S4, and details of the significance values following Bonferroni correction are provided in Table S3.
POSTZYGOTIC ISOLATION: EGG HATCH RATE AND PUPAL SURVIVORSHIP
Data on egg hatch rate were collected from 4,423 eggs from 110 broods (Table 4). We observed taxon‐specific differences in fecundity between the taxa, with female H. elevatus and H. p. sergestus laying fewer eggs per day on average than H. p. butleri (Supplementary Information S6). Female F1s (n = 25) produced by crossing H. p. butleri and H. p. sergestus in either direction laid no eggs, and dissection of female gonads confirmed them to be sterile, with ovaries lacking maturing eggs. F1 females produced by crossing either H. p. sergestus males with H. elevatus females (n = 3) or H. elevatus males with H. p. sergestus females (n = 2) were also sterile. Female F1s produced by crossing H. p. butleri and H. elevatus were found to be fully fertile in both directions, with intermediate fecundity and with no significant differences in egg hatch rate when compared with pure females. Table 4 shows the predicted hatch rates and confidence intervals for each possible cross without parasitism.
We also tested pupal survivorship of within taxon (pure) and between taxon (hybrid) crosses from a total of 844 pupae (Table 5). Although we found some evidence for variation in pupal survivorship across cross types (test for equality of proportions; χ² = 29.21, df = 6, P < 0.001), F1 and F2 individuals had equivalent or higher survivorship than pure crosses. This suggests there are no strong reductions in pupal survivorship attributable to hybrid incompatibilities. Therefore, aside from sterility in female F1s between H. p. sergestus and either H. p. butleri or H. elevatus, we found no evidence for sterility or reductions in viability of between‐taxon crosses. Additional observations on the life history and immature stages of H. elevatus, H. p. butleri, and H. p. sergestus are given in Supplementary Information S6.
QUANTIFYING ISOLATION
We used Sobel and Chen's (2014) method of quantifying reproductive isolation to summarize under a single measure the strength of isolation caused by each of the barriers studied in our experiments. The results presented in Table 6 show strong sexual prezygotic isolation in the sympatric and parapatric pairs, whereas the allopatric pair shows weak sexual prezygotic isolation. Although the sympatric pair displays no postzygotic isolation, both allopatric and parapatric pairs show intermediate to high levels of postzygotic isolation mediated by female F1 sterility.
Table 6.
Reproductive isolation, Ri, caused by different traits for each taxon pair. Pheromones, color pattern preference, and live courtship (underlined) each contribute to the overall “Mating” reproductive isolation. * Values of 1 assigned due to female hybrid sterility; ‐ no data available
| Prezygotic | ||||||
|---|---|---|---|---|---|---|
| Geography | Host plant | Pheromones | Color pattern preference | Live courtship | Mating | |
| H. elevatus to H. p. butleri | −0.17 | 0.06 | 1 | 0.39 | 0.48 | 1 |
| H. elevatus to H. p. sergestus | >0.99 | 0.24 | 1 | 0.21 | 0.5 | 1 |
| H. p. butleri to H. p. sergestus | >0.99 | −0.62 | 1 | 0.34 | 0.20 | 0.03 |
| Postzygotic | ||||||
|---|---|---|---|---|---|---|
| F1 hatch rate | F2 hatch rate | BC hatch rate | F1 pupal success | F2 pupal success | BC pupal success | |
| H. elevatus to H. p. butleri | 0.01 | −0.02 | −0.04 | 0.01 | −0.01 | – |
| H. elevatus to H. p. sergestus | – | 1* | – | – | 1* | – |
| H. p. butleri to H. p. sergestus | −0.02 | 1* | 0.31 | ‐0.08 | 1* | 0.27 |
*Values of 1 assigned due to female hybrid sterility.
Discussion
Diverging populations in geographic contact typically exhibit prezygotic isolation and/or extrinsic postzygotic isolation, with no intrinsic postzygotic isolation (Coyne and Orr 2004, and see appendix of Chamberlain et al. (2009) for additional examples). Indeed, theory predicts that it is difficult for DMIs to evolve in the face of gene flow (Turelli et al. 2001; Bank et al. 2012), even leading some authors to claim that finding a lack of hybrid interfertility or inviability is a “litmus test” of sympatric speciation (Coyne and Orr 2004, p. 177). Here, we show that sympatric taxa (H. elevatus and H. p. butleri) show strong prezygotic isolation and that they rarely hybridize in captivity. Nonetheless, we also show, via forced matings, that the hybrids are completely fertile. In contrast, allopatric taxa separated by a narrow cordillera (H. p. butleri and H. p. sergestus) mate freely when brought together in captivity, even though female F1 hybrids are sterile. The parapatric taxa (H. elevatus and H. p. sergestus) exhibit both assortative mating and sterility of F1 female hybrids. These findings are summarized in Figure 2B. We now discuss each reproductive barrier in detail, before discussing the geography of divergence and species boundaries in these taxa.
PREZYGOTIC ISOLATION
Heliconius elevatus and H. p. butleri exhibit fine scale habitat divergence and are only occasionally found together; populations of each are relatively scarce and patchy, inhabiting well‐drained forest versus seasonally flooded forest, respectively. Such habitat divergence is expected among sympatric species to prevent competitive exclusion (Hardin 1960), and consequently is also a requirement of models of sympatric speciation (van Doorn et al. 1998). Heliconius p. sergestus, meanwhile, inhabits the dry forests within its narrow endemic range, making it parapatric with H. elevatus and allopatric with H. p. butleri (although the two are usually separated by as little as 20 km near Tarapoto). Because we know H. p. sergestus is capable of crossing the cordillera separating it from H. p. butleri, it seems likely that its geographic isolation is maintained by divergent adaptations, rather than the simple barrier effect of the mountains alone (Sobel et al. 2010). Abiotic gradients may be one of the most common drivers of speciation across all the domains of life (Li et al. 2016), and aridity gradients in particular have been associated with divergence among other Heliconius species (Jiggins et al. 1996; Jiggins and Davies 1998; Arias et al. 2008).
Heliconius elevatus and H. p. butleri also exhibit divergent host plant use; while they use the same suite of Passiflora, they do so at different frequencies, with H. elevatus more specialized and favoring canopy vines. Divergence between H. p. butleri and H. p. sergestus seems much less, although still likely significant. Our experiments produced conflicting results regarding host plant use in H. elevatus and H. p. sergestus, with one experiment indicating them to be very different, and the other failing to find a difference. This contradictory result likely stems from different sets of host plants tested in each experiment (one being a small subset of the other). In particular, H. elevatus’ preferred host P. (Laurifoliae) sp. was not included in the second experiment. The result also hints that the genetic basis for host plant differences among these taxa may involve multiple loci or alleles. Because Heliconius inhabit and often mate in the vicinity of their host plants (Mallet 1984, 1986; Estrada and Gilbert 2010), host plant divergence between sympatric and parapatric divergence may contribute to speciation, as with other phytophagous insects (Bush 1969; Berlocher and Feder 2002). Furthermore, because these host plant and habitat‐based prezygotic barriers act earlier in the sequence of reproductive barriers, they may more strongly reduce gene flow than later‐acting barriers such as pheromones (Ramsey et al. 2003).
Males of H. elevatus show a strong preference for their own wing color pattern phenotype, confirming a role of color pattern in mate choice, as with other Heliconius species (Jiggins et al. 2001b; Chamberlain et al. 2009; Merrill et al. 2011a) and butterflies in general (Silberglied and Taylor 1973; Papke et al. 2007). This barrier appears unidirectional, because courting male H. p. butleri and H. p. sergestus do not discriminate between models of their own taxon and those of H. elevatus (Table 1; note that H. p. butleri does show a preference for its own phenotype over H. p. sergestus). Despite this, in controlled experiments neither H. p. butleri nor H. p. sergestus males ever mated with H. elevatus females (Table 3). Mating in butterflies is typically thought to involve long‐range visual searching by males, with females then responding to male pheromones at close range (Vane‐Wright and Boppré 1993). Female choice for male pheromones has been shown in Heliconius (Mérot et al. 2015; Darragh et al. 2017; Southcott and Kronforst 2018), and we found marked differences between the male sex pheromones of all three taxa (Fig. 5). However, the lack of matings among taxa may also be the result of males responding to species‐specific female sex pheromones, and we note that although H. elevatus, H. p. butleri, and H. p. sergestus males all approached live H. elevatus females at similar rates, H. p. butleri and H. p. sergestus males actively courted them less (Table S3, Fig. 6). Overall, our data suggest that prezygotic isolation is very strong among all three of our study taxa (Table 6), but the relative contributions of sexual or habitat‐related barriers depends on the geography of the taxa (Ramsey et al. 2003; Sobel et al. 2010; Sobel and Chen 2014).
INTRINSIC POSTZYGOTIC ISOLATION
Despite strong prezygotic sexual isolation, sympatric H. elevatus and H. p. butleri have no detectable intrinsic postzygotic isolation (Table 6) and produce fertile hybrids. Prezygotic isolation without intrinsic incompatibilities is also found in several other closely related pairs of Heliconius species (McMillan et al. 1997; Kronforst et al. 2006; Chamberlain et al. 2009; Merrill et al. 2011a, 2015; Jiggins 2017).
In contrast, female hybrids from crosses between H. p. sergestus and either H. elevatus or H. p. butleri are sterile. In Heliconius, most previously documented cases of hybrid sterility are from crosses between the relatively divergent H. melpomene and H. cydno lineages, which show strong prezygotic isolation (Naisbit et al. 2002; Mérot et al. 2017). However, female hybrid sterility has also been documented among geographically distant populations of H. melpomene from Panama and French Guiana (Jiggins et al. 2001b). As the heterogametic sex in most Lepidoptera is the female (ZW), sex‐biased hybrid sterility is in accordance with Haldane's (1922) rule. This kind of intrinsic barrier is most readily explained via “dominance theory,” in which one or more epistatic partner loci in a DMI is recessive and found on the Z chromosome, and is thus exposed only in the heterogametic sex (Turelli and Orr 1995; Turelli and Moyle 2007). Z‐linked hybrid sterility has previously been confirmed in Heliconius (Jiggins et al. 2001a; Naisbit et al. 2002). We found no evidence for a reduction in hybrid viability, thus our results also conform to the general finding that hybrid sterility evolves before hybrid lethality (Presgraves 2002).
REINFORCEMENT AND SPECIATION WITH GENE FLOW
A key prediction of reinforcement is that populations with the potential for gene flow should show higher sexual isolation than allopatric populations, and our data are broadly consistent with this (but see Noor 1999). We observed strong assortative mating between the sympatric H. elevatus and H. p. butleri, but not between the allopatric H. p. sergestus and H. p. butleri. Accordingly, the most divergent male sex pheromone profiles are also those of H. p. butleri and H. elevatus, with H. p. sergestus intermediate. In addition, H. p. butleri and H. elevatus males show a preference for courting females of their own taxon, whether presented with model wings or live females, whereas H. p. sergestus does not (Table 1, Fig. 6). Given the parapatric contact between H. elevatus and H. p. sergestus (with potential for intermediate levels of gene flow), we might expect that matings between H. elevatus and H. p. sergestus should be more common than matings Between H. elevatus and H. p. butleri. Unfortunately, the difficulty of achieving matings among the taxa mean we are unable to draw any conclusions in this respect.
If the strong reproductive isolation between H. p. butleri and H. elevatus in sympatry is indeed due to reinforcement, it is curious that the pair exhibits no apparent hybrid sterility. Instead, reinforcement is presumably driven by ecological postzygotic barriers and other ecological differences that cannot be measured using methods employed here. For example, in Heliconius, hybrids among taxa from different mimicry rings may suffer because they have intermediate, nonmimetic phenotypes (Figure 2B) that are vulnerable to predators (Merrill et al. 2012; Arias et al. 2016). A similar lack of correlation between sexual barriers and intrinsic postzygotic barriers is also found among sympatric species pairs of Drosophila, and is likely explained by unmeasured ecological factors (Turelli et al. 2014).
Formal estimates of gene flow between H. elevatus and H. p. butleri have yet to be calculated, but their interfertility and the existence of putative wild hybrids (Brower 2018, M. Joron, pers. comm.) suggest that at least part of the speciation process is taking place in the face of gene flow. However, while the pair are now unambiguously sympatric, it is unclear whether this has been the case throughout divergence (Losos and Glor 2003). Prezygotic isolation is presently very strong because multiple traits act in concert to reduce gene flow. If hybrid speciation was triggered by exchange of the rayed phenotype between H. melpomene and the ancestor of H. elevatus (Heliconius Genome Consortium 2012; Wallbank et al. 2016), rapid attainment of tight linkage disequilibrium among these traits would have been necessary to prevent erosion of mimicry and other species differences (Felsenstein 1981; Duenez‐Guzman et al. 2009; Butlin and Smadja 2018). One of the introgressed color pattern loci, cortex, is trapped in a fixed ∼400 kb inversion in H. pardalinus, with H. elevatus having apparently receiving its uninverted copy of the cortex color locus from a rayed form of H. melpomene (Jay et al. 2018). Reduced recombination between the inverted and uninverted chromosome could have aided rapid achievement of such tight linkage disequilibrium during putative hybrid speciation of H. elevatus (Noor et al. 2001; Feder et al. 2003). Moreover, tight linkage among color pattern, mating preference, and host plant use has been demonstrated in other Heliconius species (Kronforst et al. 2006; Merrill et al. 2011b, 2013, 2019). Nonetheless, it is perhaps more plausible that H. elevatus initially established itself in allopatry or parapatry (Duenez‐Guzman et al. 2009; Rosser et al. 2015). Conceivably, hybrid sterility might also have evolved between H. elevatus and H. pardalinus during this initial period, before being lost as a result of gene flow after secondary contact, leaving the peripherally distributed H. p. sergestus as an older relict of the ancestral H. pardalinus. This postspeciation introgression scenario would also explain the current genomic paraphyly of H. pardalinus relative to H. elevatus (Fig. 2A).
SPECIES BOUNDARIES
Are H. p. butleri, H. p. sergestus, and H. elevatus two species? Or three? Or one? In the relaxed biological concept of many of today's ornithologists (Gill 2014), three species would almost certainly be recognized on the grounds that all the taxa display some sort of reproductive isolation from one another. We would agree that the sympatric H. elevatus and H. p. butleri are separate species because in nature, multiple prezygotic barriers allow them to maintain separate identities in sympatry across almost the entire Amazon drainage. Whether H. p. sergestus is a third distinct species is a more arbitrary decision. On the one hand, it seems likely that H. p. sergestus would merge with H. p. butleri if the two were to become sympatric, despite the sterility of hybrid females. On the other hand, their largely allopatric distributions appear to be maintained by adaptations to different habitats, and so they could be also be seen as reproductively isolated, and good species under the biological species concept (Sobel et al. 2010). For the moment, we follow the conservative species concept of most lepidopterists (and of Heliconius taxonomists, in particular [G. Lamas, in Jiggins 2017]) and continue to recognize H. p. sergestus and H. p. butleri as geographic subspecies within H. pardalinus. This accords with our treatment of other species of Heliconius, for example, H. melpomene, which also shows hybrid sterility among distant populations (Jiggins et al. 2001a). Although others may disagree with these standards, it is important to note that the current study is not biased by the particular species delimitation we have adopted here.
Conclusions
In the 20th century, both reinforcement and sympatric speciation were often considered unlikely for theoretical and empirical reasons (Mayr 1963; Felsenstein 1981; Barraclough and Vogler 2000). Concurrently, much speciation research focused on hybrid incompatibilities and sterility, perhaps because Drosophila offers such a tractable system with which to address such questions (Orr 2005). The present century has seen a change in attitudes toward speciation with gene flow (Bolnick and Fitzpatrick 2007), and a large body of research has developed focusing on prezygotic and extrinsic postzygotic isolation, and the role of ecology in speciation (Schluter 2009; Nosil 2012). Here, we present evidence suggesting important roles for both geographic isolation and gene flow during speciation, and furthermore our results highlight how the evolution of assortative mating and intrinsic postzygotic isolation may depend on geography.
Associate Editor: C. M. Smadja
Handling Editor: Mohamed A. F. Noor
Supporting information
Figure S1. Experimental setup for the male colour pattern preference experiment.
Figure S2. Dotted lines illustrate the areas sampled for pheromone analysis for both the androconial and hind wing control regions in the three taxa.
Figure S3. Likelihood of courtship behaviors toward a) the H. elevatus color pattern by males of the two sympatric species, H. elevatus and H. p. butleri; b) the H. elevatus pattern by the two parapatric species, H. elevatus and H. p. sergestus; and c) the H. p. butleri pattern by the two allopatric sub‐species, H. p. butleri and H. p. sergestus.
Figure S4. Assay of courtship behaviors within and between taxa. Single virgin females were presented to groups of 15 males (5 of each taxon) and courtship behaviors (approach, hover or alighting) toward the females were recorded.
Table S1. Proportion of eggs laid on different host plants by H. elevatus, H. p. butleri and H. p. sergestus during the host plant experiment with 21 Passiflora species.
Table S2. Results from 1) the Wilcoxon signed rank test comparing the androconial region at the anterior margin of the hind wing and the non‐androconial region at the posterior margin of the hind wing. 2) Mann‐Whitney U test comparing the androconial region of males with the anterior margin of the hind wing in females. 3) Kruskal‐Wallis testing for differences in the amount of each compound in pairwise comparisons of taxa (not used for the determination of “putative pheromone” list).
Table S3. Statistical significance of pairwise comparisons between the numbers of courtship behaviors (Figures 6 and S4) made by males of each taxon towards females of a given taxon (top three columns, with the number of individual females tested in brackets).
Table S4. Pupation duration in days of the three taxa.
AUTHOR CONTRIBUTIONS
NR, LMQ, and KKD conceived the study. LMQ and JM conducted male color pattern preference experiments. NR, CS, and PV conducted assortative mating experiments. NE, CS, PV, and NR conducted hybrid sterility and inviability experiments. BC, JM, FM, and SS conducted pheromone analysis. NR and LMQ conducted host plant experiments. NR and RMP made field collections and recorded habitat/host plant observations in the wild. LMQ did the Sobel and Chen analysis. NR, LMQ, JLBM, and KKD wrote the paper. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was funded by NERC grant NE/K012886/1 to KKD, grant Schu 984/13‐1 from the DFG to SS, and Harvard University. We also thank SERFOR, the Peruvian Ministry of Agriculture, and the Área de Conservación Regional Cordillera Escalera for collecting permits (0289‐2014‐MINAGRI‐DGFFS/DGEFFS, 020‐014/GRSM/PEHCBM/DMA/ACR‐CE, 040–2015/GRSM/PEHCBM/DMA/ACR‐CE). We are extremely grateful to the following people for help and support with field work in Peru: C. Cordova, M. Tuanama, M. Chouteau, M. McClure, M. Arias, J. Caldwell, C. Perez, C. Lopez, G. Lamas, and A. Burns. We also thank horticultural technicians P. Scott and C. Lancaster for their support when rearing at the University of York. Finally, we thank three anonymous reviewers and the Associate Editor, Dr. C. Smadja, for their insightful comments.
Contributor Information
Neil Rosser, Email: neil.rosser@york.ac.uk.
Lucie M. Queste, Email: lmq500@york.ac.uk.
LITERATURE CITED
- Arias, C. F. , Muñoz A. G., Jiggins C. D., Mavárez J., Bermingham E., and Linares M.. 2008. A hybrid zone provides evidence for incipient ecological speciation in Heliconius butterflies. Mol. Ecol. 17:4699–4712. [DOI] [PubMed] [Google Scholar]
- Arias, M. , le Poul Y., Chouteau M., Boisseau R., Rosser N., Théry M., and Llaurens V.. 2016. Crossing fitness valleys: empirical estimation of a fitness landscape associated with polymorphic mimicry. Proc. R. Soc. B Biol. Sci. 283:20160391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank, C. , Bürger R., and Hermisson J.. 2012. The limits to parapatric speciation: Dobzhansky–Muller incompatibilities in a continent–island model. Genetics 191:845–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough, T. G. , and Vogler A. P.. 2000. Detecting the geographical pattern of speciation from species‐level phylogenies. Am. Nat. 155:419–434. [DOI] [PubMed] [Google Scholar]
- Bates, D. , Maechler M., Bolker B., and Walker S.. 2015. Fitting linear mixed‐effects models using lme4. J. Stat. Softw. 67:1–48. [Google Scholar]
- Bates, H. W. 1862. XXXII. Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidae. Trans. Linn. Soc. Lond. 23:495–566. [Google Scholar]
- Benson, W. W. , Brown K. S., and Gilbert L. E.. 1975. Coevolution of plants and herbivores: passion flower butterflies. Evolution 29:659–680. [DOI] [PubMed] [Google Scholar]
- Berlocher, S. H. , and Feder J. L.. 2002. Sympatric speciation in phytophagous insects: moving beyond controversy? Annu. Rev. Entomol. 47:773–815. [DOI] [PubMed] [Google Scholar]
- Bolnick, D. I. , and Fitzpatrick B. M.. 2007. Sympatric speciation: models and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 38:459–487. [Google Scholar]
- Brower, A. V. Z. 2018. Alternative facts: a reconsideration of putatively natural interspecific hybrid specimens in the genus Heliconius (Lepidoptera: Nymphalidae). Zootaxa 4499:1–87. [DOI] [PubMed] [Google Scholar]
- Brown, K. S. 1976. An illustrated key to the silvaniform Heliconius (Lepidoptera: Nymphalidae) with descriptions of new subspecies. Trans. Am. Entomol. Soc. 102:373–484. [Google Scholar]
- Bush, G. L. 1969. Sympatric host race formation and speciation in frugivorous flies of the genus Rhagoletis (Diptera, Tephritidae). Evolution 23:237–251. [DOI] [PubMed] [Google Scholar]
- Butlin, R. , Debelle A., Kerth C., Snook R. R., Beukeboom L. W., Castillo Cajas R. F., Diao W., Maan M. E., Paolucci S., Weissing F. J., et al. 2012. What do we need to know about speciation? Trends Ecol. Evol. 27:27–39. [DOI] [PubMed] [Google Scholar]
- Butlin, R. K. , and Smadja C. M.. 2018. Coupling, reinforcement, and speciation. Am. Nat. 191:155–172. [DOI] [PubMed] [Google Scholar]
- Chamberlain, N. L. , Hill R. I., Kapan D. D., Gilbert L. E., and Kronforst M. R.. 2009. Polymorphic butterfly reveals the missing link in ecological speciation. Science 326:847–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, C. A. , and Sheppard P. M.. 1956. Handpairing of butterflies. Lepidoptera News 10:47–53. [Google Scholar]
- Coyne, J. A. , and Orr H. A.. 1997. “Patterns of speciation in Drosophila” revisited. Evolution 51:295–303. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A. , and Orr H. A.. 2004. Speciation. Sinauer Associates Inc., Sunderland, MA. [Google Scholar]
- Darragh, K. , Vanjari S., Mann F., Gonzalez‐Rojas M. F., Morrison C. R., Salazar C., Pardo‐Diaz C., Merrill R. M., McMillan W. O., Schulz S., et al. 2017. Male sex pheromone components in Heliconius butterflies released by the androconia affect female choice. PeerJ 5:e3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky, T. 1940. Speciation as a stage in evolutionary divergence. Am. Nat. 74:312–321. [Google Scholar]
- Duenez‐Guzman, E. A. , Mavárez J., Vose M. D., and Gavrilets S.. 2009. Case studies and mathematical models of ecological speciation. 4. Hybrid speciation in butterflies in a jungle. Evolution 63:2611–2626. [DOI] [PubMed] [Google Scholar]
- Edwards, A. W. F. 1972. Likelihood. Cambridge Univ. Press, Cambridge, U.K. [Google Scholar]
- Ehrlich, P. R. , and Raven P. H.. 1964. Butterflies and plants: a study in coevolution. Evolution 18:586–608. [Google Scholar]
- Emsley, M. G. 1965. Speciation in Heliconius (Lep., Nymphalidae): morphology and geographic distribution. Zoologica 50:191–254. [Google Scholar]
- Estrada, C. , and Gilbert L. E.. 2010. Host plants and immatures as mate‐searching cues in Heliconius butterflies. Anim. Behav. 80:231–239. [Google Scholar]
- Estrada, C. , and Jiggins C. D.. 2002. Patterns of pollen feeding and habitat preference among Heliconius species. Ecol. Entomol. 27:448–456. [Google Scholar]
- Feder, J. L. , Roethele J. B., Filchak K., Niedbalski J., and Romero‐Severson J.. 2003. Evidence for inversion polymorphism related to sympatric host race formation in the apple maggot fly, Rhagoletis pomonella . Genetics 163:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein, J. 1981. Skepticism towards Santa Rosalia, or why are there so few kinds of animals? Evolution 35:124–138. [DOI] [PubMed] [Google Scholar]
- Funk, D. J. 1998. Isolating a role for natural selection in speciation: host adaptation and sexual isolation in Neochlamisus bebbianae leaf beetles. Evolution 52:1744–1759. [DOI] [PubMed] [Google Scholar]
- Funk, D. J. , Nosil P., and Etges W. J.. 2006. Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc. Natl. Acad. Sci. 103:3209–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets, S. 2004. Fitness landscapes and the origin of species. Princeton Univ. Press, Princeton, NJ. [Google Scholar]
- Gill, F. B. 2014. Species taxonomy of birds: which null hypothesis? Auk 131:150–161. [Google Scholar]
- Giraldo, N. , Salazar C., Jiggins C. D., Bermingham E., and Linares M.. 2008. Two sisters in the same dress: Heliconius cryptic species. BMC Evol. Biol. 8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane, J. B. S. 1922. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12:101–109. [Google Scholar]
- Hardin, G. 1960. The competitive exclusion principle. Science 131:1292–1297. [DOI] [PubMed] [Google Scholar]
- Heliconius Genome Consortium . 2012. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans, R. J. , Cameron S. E., Parra J. L., Jones P. G., and Jarvis A.. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25:1965–1978. [Google Scholar]
- Jay, P. , Whibley A., Frézal L., Á. Rodríguez de Cara M., Nowell R. W., Mallet J., Dasmahapatra K. K., and Joron M.. 2018. Supergene evolution triggered by the introgression of a chromosomal inversion. Curr. Biol. 28:1839–1845. [DOI] [PubMed] [Google Scholar]
- Jiggins, C. D. 2017. The ecology and evolution of Heliconius butterflies. Oxford Univ. Press, Oxford, U.K. [Google Scholar]
- Jiggins, C. D. , and Davies N.. 1998. Genetic evidence for a sibling species of Heliconius charithonia (Lepidoptera; Nymphalidae). Biol. J. Linn. Soc. 64:57–67. [Google Scholar]
- Jiggins, C. D. , McMillan O., Neukirchen W., and Mallet J.. 1996. What can hybrid zones tell us about speciation? The case of Heliconius erato and H. himera (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. 59:221–242. [Google Scholar]
- Jiggins, C. D. , Linares M., Naisbit R. E., Salazar C., Yang Z. H., and Mallet J.. 2001a. Sex‐linked hybrid sterility in a butterfly. Evolution 55:1631–1638. [DOI] [PubMed] [Google Scholar]
- Jiggins, C. D. , Naisbit R. E., Coe R. L., and Mallet J.. 2001b. Reproductive isolation caused by colour pattern mimicry. Nature 411:302–305. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, M. , and Ravigné V.. 2002. Speciation by natural and sexual selection: models and experiments. Am. Nat. 159:S22–35. [DOI] [PubMed] [Google Scholar]
- Kisel, Y. , and Barraclough T. G.. 2010. Speciation has a spatial scale that depends on levels of gene flow. Am. Nat. 175:316–334. [DOI] [PubMed] [Google Scholar]
- Klein, A. L. , and de Araújo A. M.. 2010. Courtship behavior of Heliconius erato phyllis (Lepidoptera, Nymphalidae) towards virgin and mated females: conflict between attraction and repulsion signals? J. Ethol. 28:409–420. [Google Scholar]
- Kronforst, M. R. , Young L. G., Kapan D. D., McNeely C., O'Neill R. J., and Gilbert L. E.. 2006. Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. Proc. Natl. Acad. Sci. 103:6575–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K. , Wang H., Cai Z., Wang L., Xu Q., Lövy M., Wang Z., and Nevo E.. 2016. Sympatric speciation of spiny mice, Acomys, unfolded transcriptomically at Evolution Canyon, Israel. Proc. Natl. Acad. Sci. 113:8254–8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos, J. B. , and Glor R. E.. 2003. Phylogenetic comparative methods and the geography of speciation. Trends Ecol. Evol. 18:220–227. [Google Scholar]
- Mallet, J. 1984. Population structure and evolution in Heliconius butterflies. Ph.D. thesis, University of Texas at Austin, Austin, TX. [Google Scholar]
- Mallet, J. 1986. Dispersal and gene flow in a butterfly with home range behavior: Heliconius erato (Lepidoptera: Nymphalidae). Oecologia 68:210–217. [DOI] [PubMed] [Google Scholar]
- Mann, F. , Vanjari S., Rosser N., Mann S., Dasmahapatra K. K., Corbin C., Linares M., Pardo‐Diaz C., Salazar C., Jiggins C., et al. 2017. The scent chemistry of Heliconius wing androconia. J. Chem. Ecol. 43:843–857. [DOI] [PubMed] [Google Scholar]
- Maynard Smith, J. 1966. Sympatric speciation. Am. Nat. 100:637–650. [Google Scholar]
- Mayr, E. 1963. Animal species and evolution. Harvard Univ. Press, Cambridge, MA. [Google Scholar]
- McMillan, W. O. , Jiggins C. D., and Mallet J.. 1997. What initiates speciation in passion‐vine butterflies? Proc. Natl. Acad. Sci. 94:8628–8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérot, C. , Mavárez J., Evin A., Dasmahapatra K. K., Mallet J., Lamas G., and Joron M.. 2013. Genetic differentiation without mimicry shift in a pair of hybridizing Heliconius species (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. 109:830–847. [Google Scholar]
- Mérot, C. , Frérot B., Leppik E., and Joron M.. 2015. Beyond magic traits: multimodal mating cues in Heliconius butterflies. Evolution 69:2891–2904. [DOI] [PubMed] [Google Scholar]
- Mérot, C. , Salazar C., Merrill R. M., Jiggins C. D., and Joron M.. 2017. What shapes the continuum of reproductive isolation? Lessons from Heliconius butterflies. Proc. R. Soc. Lond. B Biol. Sci. 284:20170335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, R. M. , Gompert Z., Dembeck L. M., Kronforst M. R., McMillan O. W., and Jiggins C. D.. 2011a. Mate preference across the speciation continuum in a clade of mimetic butterflies. Evolution 65:1489–1500. [DOI] [PubMed] [Google Scholar]
- Merrill, R. M. , Van Schooten B., Scott J. A., and Jiggins C. D.. 2011b. Pervasive genetic associations between traits causing reproductive isolation in Heliconius butterflies. Proc. R. Soc. B Biol. Sci. 278:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, R. M. , Wallbank R. W. R., Bull V., Salazar P. C. A., Mallet J., Stevens M., and Jiggins C. D.. 2012. Disruptive ecological selection on a mating cue. Proc. R. Soc. B Biol. Sci. 279:4907–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, R. M. , Naisbit R. E., Mallet J., and Jiggins C. D.. 2013. Ecological and genetic factors influencing the transition between host‐use strategies in sympatric Heliconius butterflies. J. Evol. Biol. 26:1959–1967. [DOI] [PubMed] [Google Scholar]
- Merrill, R. M. , Dasmahapatra K. K., Davey J. W., Dell'Aglio D. D., Hanly J. J., Huber B., Jiggins C. D., Joron M., Kozak K. M., Llaurens V., et al. 2015. The diversification of Heliconius butterflies: what have we learned in 150 years? J. Evol. Biol. 28:1417–1438. [DOI] [PubMed] [Google Scholar]
- Merrill, R. M. , Rastas P., Martin S. H., Melo M. C., Barker S., Davey J., McMillan W. O., and Jiggins C. D.. 2019. Genetic dissection of assortative mating behavior. PLoS Biol. 17:e2005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbit, R. E. , Jiggins C. D., Linares M., Salazar C., and Mallet J.. 2002. Hybrid sterility, Haldane's rule and speciation in Heliconius cydno and H. melpomene . Genetics 161:1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor, M. A. F. 1999. Reinforcement and other consequences of sympatry. Heredity 83:503–508. [DOI] [PubMed] [Google Scholar]
- Noor, M. A. F. , Grams K. L., Bertucci L. A., and Reiland J.. 2001. Chromosomal inversions and the reproductive isolation of species. Proc. Natl. Acad. Sci. 98:12084–12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil, P. 2007. Divergent host plant adaptation and reproductive isolation between ecotypes of Timema cristinae walking sticks. Am. Nat. 169:151–162. [DOI] [PubMed] [Google Scholar]
- Nosil, P . 2012. Ecological speciation. Oxford Univ. Press, Oxford, U.K. [Google Scholar]
- Oksanen, J. , Blanchet F. G., Kindt R., Legendre P., Minchin P. R., O'Hara R. B., Simpson G. L., Solymos P., Stevens M. H. H., and Wagner H.. 2017. vegan: community ecology package. R package version 2.3‐5. 2016.
- Orr, H. A. 2005. The genetic basis of reproductive isolation: insights from Drosophila . Proc. Natl. Acad. Sci. 102:6522–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H. A. , and Turelli M.. 2001. The evolution of postzygotic isolation: accumulating Dobzhansky‐Muller incompatibilities. Evolution 55:1085–1094. [DOI] [PubMed] [Google Scholar]
- Papke, R. S. , Kemp D. J., and Rutowski R. L.. 2007. Multimodal signalling: structural ultraviolet reflectance predicts male mating success better than pheromones in the butterfly Colias eurytheme L. (Pieridae). Anim. Behav. 73:47–54. [Google Scholar]
- Pardo‐Díaz, C. , Salazar C., Baxter S. W., Mérot C., Figueiredo‐Ready W., Joron M., McMillan W. O., and Jiggins C. D.. 2012. Adaptive introgression across species boundaries in Heliconius butterflies. PLoS Genet. 8:e1002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianka, E. R. 1973. The structure of lizard communities. Annu. Rev. Ecol. Syst. 4:53–74. [Google Scholar]
- Presgraves, D. C. 2002. Patterns of postzygotic isolation in Lepidoptera. Evolution 56:1168–1183. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2018. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Ramsey, J. , Bradshaw H. D., Schemske D. W., and Morgan M.. 2003. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57:1520–1534. [DOI] [PubMed] [Google Scholar]
- Rosser, N. , Phillimore A. B., Huertas B., Willmott K. R., and Mallet J.. 2012. Testing historical explanations for gradients in species richness in heliconiine butterflies of tropical America. Biol. J. Linn. Soc. 105:479–497. [Google Scholar]
- Rosser, N. , Kozak K. M., Phillimore A. B., and Mallet J.. 2015. Extensive range overlap between heliconiine sister species: evidence for sympatric speciation in butterflies? BMC Evol. Biol. 15:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser, N. , Freitas A. V. L., Huertas B., Joron M., Lamas G., Mérot C., Simpson F., Willmott K. R., Mallet J., and Dasmahapatra K. K.. 2019. Cryptic speciation associated with geographic and ecological divergence in two Amazonian Heliconius butterflies. Zool. J. Linn. Soc. 186:233–249. [Google Scholar]
- Rutowski, R. L. 1980. Male scent‐producing structures in Colias butterflies. J. Chem. Ecol. 6:13–26. [Google Scholar]
- Schluter, D. 2009. Evidence for ecological speciation and its alternative. Science 323:737–741. [DOI] [PubMed] [Google Scholar]
- Servedio, M. R. , and Noor M. A. F.. 2003. The role of reinforcement in speciation: theory and data. Annu. Rev. Ecol. Evol. Syst. 34:339–364. [Google Scholar]
- Servedio, M. R. , Doorn G. S. V., Kopp M., Frame A. M., and Nosil P.. 2011. Magic traits in speciation: ‘magic’ but not rare? Trends Ecol. Evol. 26:389–397. [DOI] [PubMed] [Google Scholar]
- Silberglied, R. E. , and Taylor O. R.. 1973. Ultraviolet differences between the sulphur butterflies, Colias eurytheme and C. philodice, and a possible isolating mechanism. Nature 241:406–408. [DOI] [PubMed] [Google Scholar]
- Smadja, C. , and Butlin R. K.. 2009. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102:77–97. [DOI] [PubMed] [Google Scholar]
- Sobel, J. M. , and Chen G. F.. 2014. Unification of methods for estimating the strength of reproductive isolation. Evolution 68:1511–1522. [DOI] [PubMed] [Google Scholar]
- Sobel, J. M. , Chen G. F., Watt L. R., and Schemske D. W.. 2010. The biology of speciation. Evolution 64:295–315. [DOI] [PubMed] [Google Scholar]
- Southcott, L. , and Kronforst M. R.. 2018. Female mate choice is a reproductive isolating barrier in Heliconius butterflies. Ethology 124:862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M. , and Orr H. A.. 1995. The dominance theory of Haldane's rule. Genetics 140:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M. , and Moyle L. C.. 2007. Asymmetric postmating isolation: Darwin's corollary to Haldane's rule. Genetics 176:1059–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli, M. , Barton N. H., and Coyne J. A.. 2001. Theory and speciation. Trends Ecol. Evol. 16:330–343. [DOI] [PubMed] [Google Scholar]
- Turelli, M. , Lipkowitz J. R., and Brandvain Y.. 2014. On the Coyne and Orr‐igin of species: effects of intrinsic postzygotic isolation, ecological differentiation, X chromosome size, and sympatry on Drosophila speciation. Evolution 68:1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn, G. S. , Noest A. J., and Hogeweg P.. 1998. Sympatric speciation and extinction driven by environment dependent sexual selection. Proc. R. Soc. Lond. B Biol. Sci. 265:1915–1919. [Google Scholar]
- Vane‐Wright, R. I. , and Boppré M.. 1993. Visual and chemical signalling in butterflies: functional and phylogenetic perspectives. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 340:197–205. [Google Scholar]
- Wallbank, R. W. R. , Baxter S. W., Pardo‐Diaz C., Hanly J. J., Martin S. H., Mallet J., Dasmahapatra K. K., Salazar C., Joron M., Nadeau N., et al. 2016. Evolutionary novelty in a butterfly wing pattern through enhancer shuffling. PLoS Biol. 14:e1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukilevich, R. 2012. Asymmetrical patterns of speciation uniquely support reinforcement in drosophila. Evolution 66:1430–1446. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Dasmahapatra K. K., Mallet J., Moreira G. R. P., and Kronforst M. R.. 2016. Genome‐wide introgression among distantly related Heliconius butterfly species. Genome Biol. 17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Experimental setup for the male colour pattern preference experiment.
Figure S2. Dotted lines illustrate the areas sampled for pheromone analysis for both the androconial and hind wing control regions in the three taxa.
Figure S3. Likelihood of courtship behaviors toward a) the H. elevatus color pattern by males of the two sympatric species, H. elevatus and H. p. butleri; b) the H. elevatus pattern by the two parapatric species, H. elevatus and H. p. sergestus; and c) the H. p. butleri pattern by the two allopatric sub‐species, H. p. butleri and H. p. sergestus.
Figure S4. Assay of courtship behaviors within and between taxa. Single virgin females were presented to groups of 15 males (5 of each taxon) and courtship behaviors (approach, hover or alighting) toward the females were recorded.
Table S1. Proportion of eggs laid on different host plants by H. elevatus, H. p. butleri and H. p. sergestus during the host plant experiment with 21 Passiflora species.
Table S2. Results from 1) the Wilcoxon signed rank test comparing the androconial region at the anterior margin of the hind wing and the non‐androconial region at the posterior margin of the hind wing. 2) Mann‐Whitney U test comparing the androconial region of males with the anterior margin of the hind wing in females. 3) Kruskal‐Wallis testing for differences in the amount of each compound in pairwise comparisons of taxa (not used for the determination of “putative pheromone” list).
Table S3. Statistical significance of pairwise comparisons between the numbers of courtship behaviors (Figures 6 and S4) made by males of each taxon towards females of a given taxon (top three columns, with the number of individual females tested in brackets).
Table S4. Pupation duration in days of the three taxa.


