Abstract
Immune checkpoints, including PD‐1/PD‐L1, play an important role in immunosuppression in various malignancies. Elevated levels of soluble programmed death ligand 1 (sPD‐L1) are associated with worse prognosis in multiple myeloma and diffuse large B cell lymphoma. Herein, the purpose of this study is to investigate the relationships between plasma sPD‐L1 levels and clinical response in peripheral T‐cell lymphoma (PTCL) patients.
A total of 37 PTCL patients and 20 healthy volunteers were enrolled. Peripheral blood from patients was collected prior to systemic therapy. Plasma levels of sPD‐L1 and IFN‐γ were measured by enzyme‐linked immunosorbent assay (ELISA). PD‐L1 expression in tissues was detected by immunohistochemistry (IHC). Clinical response for patients was evaluated.
ONCOMINE database analyses showed that PD‐L1 mRNA expression was significantly upregulated in PTCLs. The median sPD‐L1 level was 0.729 ng/mL for 20 healthy volunteers and 1.696 ng/mL for 37 PTCL patients which was significantly higher than that in healthy volunteers (0.000). The sPD‐L1 level was positively correlated with IFN‐γ level (0.000, r = 0.849) and was also positively associated with clinical staging (0.045), LDH level (0.003), and β2‐MG level (0.045). Patients with high sPD‐L1 level had lower overall response rate than those with low sPD‐L1 level (88.9% vs 50.0%, 0.022) and tended to have poorer PFS and OS. PD‐L1 expression in tissues matched very well with the sPD‐L1 level in PTCL patients. In conclusion, PTCL patients had higher sPD‐L1 level compared with healthy volunteers. High sPD‐L1 level was correlated with worse clinical response, suggesting that sPD‐L1 level was an underlying plasma biomarker to predict the prognosis for PTCL patients.
Keywords: clinical response, IFN‐γ, PTCLs, sPD‐L1
1. INTRODUCTION
Peripheral T‐cell lymphomas (PTCLs) are highly aggressive malignancies derived from post‐thymic T cells or natural killer (NK) cells, which comprise several subtypes, including PTCL not otherwise specified (PTCL‐NOS), angioimmunoblastic T‐cell lymphoma (AITL), anaplastic large‐cell lymphoma (ALCL), NK/T‐cell lymphoma (NKTCL), enteropathy‐associated T‐cell lymphoma (EATL), hepatosplenic T‐cell lymphoma (HSTL), and subcutaneous panniculitis‐like T‐cell lymphoma (αβ only) (SPTCL).1, 2, 3 PTCLs are also a group of rare disease, which accounts for only 5% to 10% of all non‐Hodgkin's lymphoma (NHL) in Western countries.1 However, PTCL patients are typically characterized by poor prognosis and high rate of relapse. Until now, there are no standard and effective treatments for PTCL patients. Therefore, it is urgent to find new treatment strategies as well as some new molecular markers for these patients. In recent years, immunotherapy has attracted worldwide attention as a new effective treatment method, which also provides new ideas for the treatment of PTCL patients.
Programmed death ligand 1 (PD‐L1) is one of the B7 superfamily members, which is expressed on the surface of antigen presenting cells (APC), tumor cells, and tumor‐infiltrating myeloid cells.4 Programmed cell death protein 1 (PD‐1), as the receptor of PD‐L1, is usually expressed on the surface of immune cells, including activated T cells and monocytes.5 Studies have reported that PD‐L1 is mainly induced by inflammatory cytokines, such as interferon gamma (IFN‐γ) or other.6, 7 Binding of PD‐L1 to PD‐1 can downregulate T‐cell responses, leading to immune suppression.8, 9, 10 Previous studies have found that blocking the PD‐1/PD‐L1 interaction using either PD‐1 or PD‐L1 antibodies can improve clinical responses and overall survival in various tumors.11, 12 The PD‐L1 expression has two forms, including membrane‐bound and soluble forms. Reports have shown that membrane‐PD‐L1 overexpression on tumor tissue is associated with poor prognosis in many malignancies.13, 14, 15, 16, 17 In recent years, soluble form of PD‐L1 (sPD‐L1) is also found to be involved in immune suppression and associated with poor prognosis in parts of malignancies.18, 19 In this study, we investigated the expression of sPD‐L1 in PTCL patients, and explored the value of sPD‐L1 levels to predict clinical response.
2. MATERIALS AND METHODS
Data from three cohorts, including 11 ALCL patients, 28 PTCL‐NOS patients, and 81 matched normal control tissues, were obtained from the ONCOMINE database (https://www.oncomine.org) for PD‐L1 gene expression array. The comparison dataset analysis of PD‐L1 mRNA levels among diverse PTCL subtypes and matched normal control tissues was performed.
A total of 37 patients with PTCLs were enrolled in our study, and all patients were newly diagnosed, and diagnoses were further confirmed by two independent pathologists. All PTCL patients were treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP‐like regimens; besides, all NKTCL patients were treated with asparaginase‐based chemotherapies in Tianjin Medical University Cancer Institute and Hospital (TMUCIH, Tianjin, China) between January 2013 and January 2016. Peripheral blood from all patients prior to systemic therapy and 20 healthy volunteers was collected into tubes containing potassium EDTA and then was centrifuged at 1000 rpm at 4°C for 10 minutes. Plasma samples were stored at −80°C refrigerator until detection. Meanwhile, 11 matched formalin‐fixed paraffin‐embedded (FFPE) tissue from patients before treatment were collected to detect the expression of PD‐L1 using immunohistochemistry (IHC). The matched degree of PD‐L1 level between tissue and plasma was further evaluated. This study was approved by the Clinical Research Ethics Board of TMUCIH and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The written informed consent was obtained.
Levels of plasma sPD‐L1 and IFN‐γ were, respectively, tested using enzyme‐linked immunosorbent assay (ELISA) kits (Human PD‐L1 ELISA kit DB7H10, R&D Systems; Human IFN‐γ ELISA MAX Deluxe 430105, Biolegend) according to their manufacturer's instructions.
Immunohistochemical staining of PD‐L1 in FFPE tumor specimen was performed using the streptavidin‐peroxidase method (SP method). Firstly, the 4 to 5‐μm thickness FFPE slides were deparaffinized and rehydrated in three times of dimethylbenzene for 10 minutes each, two times of 100% ethanol for 5 minutes each, 95% alcohol for 5 minutes, and 70% alcohol for 1 minute. Antigen retrieval was carried out using EDTA buffer (pH 8.0) in 120°C for 3 minutes using microwave incubation; 0.3% hydrogen peroxide was used for blocking endogenous peroxidase activity in dark place at room temperature for 10 minutes. Then, they were incubated with anti‐PD‐L1 primary antibody (1:200, clone: E1L3N, Cell Signaling Technology, MA, USA) at 4°C overnight and were then incubated with secondary antibody rabbit IgG/HRP for 30 minutes at 37°C. The slides were counterstained with hematoxylin and covered under coverslips. If ≥5% of the total tissue cells showed membrane staining, it was defined as high PD‐L1 expression.
The status of Epstein‐Barr virus (EBV)‐encoded RNA (EBER) was evaluated to detect the EBV infection by in situ hybridization using a Novocastra ISH Kit (Novocastra Laboratories, Newcastle upon Tyne, UK) on 4 to 5‐μm thickness slides according to the manufacturer instructions.
2.1.1. Statistical analyses
SPSS 19.0 statistical software and Graphpad Prism v8.0 were utilized for statistical analyses. The correlations between sPD‐L1 and IFN‐γ levels were analyzed using Pearson's chi‐squared test. And the relationships between sPD‐L1 levels and clinicopathologic parameters were assessed using Fisher's exact test. Multivariate analyses for clinical response were performed using the binary logistic regression (LR) model. The cut‐off values for sPD‐L1 levels were initially set as the median level and were also optimized using the receiver operating characteristic (ROC) curve analyses. Overall response rate (ORR) was defined as the proportion of patients with confirmed complete response or partial response. Progression‐free survival (PFS) was defined as the time from diagnosis to disease progression or death without evidence of progression. Overall survival (OS) was defined as the time from diagnosis to death or date of last follow‐up. A log‐rank test was used for comparison, and P < .05 was considered statistically significant.
3. RESULTS
3.1. Transcription levels of PD‐L1 in PTCL patients
We compared the transcriptional levels of PD‐L1 from three PTCLs cohorts with those in matched normal control tissue by using the ONCOMINE database (Figure 1). We found that the PD‐L1 mRNA expression was upregulated in PTCLs, which were significantly higher in PTCLs compared with matched normal control tissues (one cohort, 0.029; two cohorts, 0.020; and three cohorts, 0.021).
Figure 1.
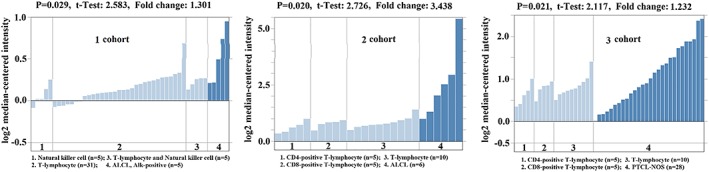
Transcription levels of PD‐L1 in three PTCLs cohorts and matched normal control tissue from the ONCOMINE database
3.2. Patient characteristics
The baseline characteristics of 37 PTCL patients were summarized in Table 1. The median age was 56 years (range: 16‐77 years). Patients consisted of 10 (27.3%) NKTCL, six (16.2%) ALCL, 10 (27.3%) AITL, six (16.2%) PTCL‐NOS, and five (13.0%) unknown subtype, who were only diagnosed as PTCL. Among these patients, 22 (59.5%) had advanced disease, 11 (29.7%) was middle‐high or high risk patients with International Prognostic Index (IPI) > 2, 21 (56.8%), and 22 (59.5%) accompanied with elevated LDH and β2‐MG level.
Table 1.
Patient's characteristics and sPD‐L1 level
| Parameters | No. (%) | sPD‐L1 | P Value | |
|---|---|---|---|---|
| Low | High | |||
| Gender | ||||
| Male | 20(54.1) | 10 | 10 | |
| Female | 17(45.9) | 9 | 8 | 1.000 |
| Age, median [year, range] | 56[16‐77] | |||
| ≤60 | 24(64.9) | 13 | 11 | |
| >60 | 13(35.1) | 6 | 7 | 0.737 |
| Stage | ||||
| I + II | 15(40.5) | 11 | 4 | |
| III + IV | 22(59.5) | 8 | 14 | 0.045 |
| IPI score | ||||
| ≤2 | 26(70.3) | 15 | 11 | |
| >2 | 11(29.7) | 4 | 7 | 0.295 |
| LDH | ||||
| Normal | 16(43.2) | 13 | 3 | |
| Elevated | 21(56.8) | 6 | 15 | 0.003 |
| β2‐MG | ||||
| Normal | 15(40.5) | 11 | 4 | |
| Elevated | 22(59.5) | 8 | 14 | 0.045 |
| Subtype | ||||
| NKTCL | 10(27.3) | 6 | 4 | |
| ALCL | 6(16.2) | 3 | 3 | |
| AITL | 10(27.3) | 4 | 6 | |
| PTCL‐NOS | 6(16.2) | 3 | 3 | |
| Unknown | 5(13.0) | 3 | 2 | 0.913 |
3.3. Plasma sPD‐L1 and IFN‐γ levels
As shown in Figure 2, the median level of sPD‐L1 of PTCL patients was 1.696 ng/mL, which was much higher than that of healthy volunteers (0.729 ng/mL, 0.000). The median level of IFN‐γ of PTCL patients was 4.555 pg/mL. Further analysis showed that the levels of sPD‐L1 were positively correlated with the level of IFN‐γ (0.000, r = 0.849).
Figure 2.
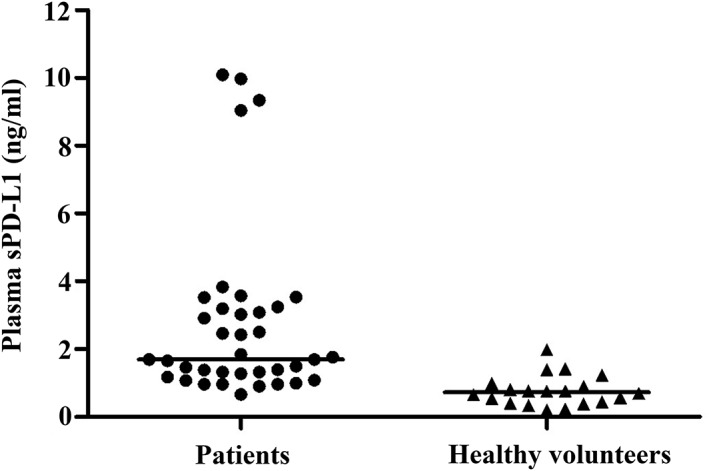
Level of plasma sPD‐L1 in PTCL patients and healthy volunteers
The cut‐off value of sPD‐L1 was initially set as the median level (1.696 ng/mL). According to this cut‐off value, 18 patients (48.6%) were classified into the high sPD‐L1 level group (>1.696 ng/mL), and the remaining 19 patients (51.4%) were classified into the low sPD‐L1 level group (≤1.696 ng/mL). The associations between sPD‐L1 levels and patient's clinical characteristics, including gender, age, stage, IPI score, LDH level, β2‐MG level, and subtypes, were explored in Table 1, suggesting that patients with elevated LDH level, advanced stage, and elevated β2‐MG level had higher sPD‐L1 levels than those with normal LDH level, early stage, and normal β2‐MG level, and that the sPD‐L1 level was also positively correlated with LDH levels (0.003), clinical staging (0.045), and β2‐MG level (0.045). However, there were no correlations between sPD‐L1 levels and other clinical characteristics.
3.4. Correlation between sPD‐L1 level and clinical response
After receiving at least four cycles treatment, five of all patients lost the clinical information, 11 patients showed a complete response (CR), 12 patients showed a partial response (PR), three patients showed a stable disease (SD), and six patients showed a progressive disease (PD). According to the cut‐off value of sPD‐L1 levels (median level, 1.696 ng/mL), as shown in Figure 3A, the ORR was 88.9% in low sPD‐L1 level group, which was significantly higher than that of high sPD‐L1 level group (50.0%, 0.022). Based on the ROC curve analyses, the optimal cut‐off value of sPD‐L1 levels for clinical response was 1.575 ng/mL, the area under the ROC curve was 0.773, and the sensitivity was 88.9% and specificity was 70.9%. According to the optimal cut‐off value of sPD‐L1 levels, the ORR was 93.3% in low sPD‐L1 level group, which was also significantly higher than that of high sPD‐L1 level group (52.9%, 0.018, Figure 3B). We further divided these patients into three cohorts according to sPD‐L1 level, including low, median, and high. We found that when the cut‐off values of sPD‐L1 levels fell within the levels in median cohort (Figure 4), the ORRs in low sPD‐L1 level group would be always significantly higher than that of high sPD‐L1 level group (cut‐off = 1.2, 0.386; cut‐off = 1.4, 0.05; cut‐off = 1.6, 0.018; cut‐off = 1.8, 0.022; cut‐off = 2.0, 0.049; cut‐off = 2.2, 0.049; cut‐off = 2.4, 0.049; and cut‐off = 2.6, 0.226). In addition, multivariate analyses based on the optimal cut‐off value of sPD‐L1 levels revealed that sPD‐L1 levels was associated with clinical response independently (OR:12.444, 95%CI [1.323‐117.032], 0.027), but not other clinical parameters including gender, age, stage, IPI score, LDH level, and β2‐MG level (P > .05, Table 2). These results displayed that high sPD‐L1 levels were correlated with worse clinical response, suggesting that sPD‐L1 levels were an underlying plasma biomarker to predict the clinical response in PTCL patients.
Figure 3.
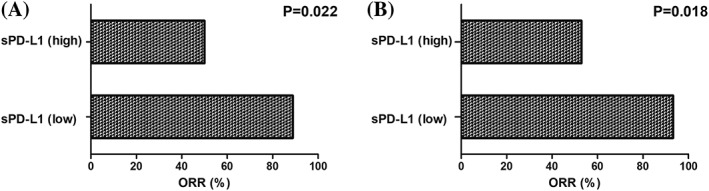
The overall response rate between high and low sPD‐L1 level groups according to the cut‐off value of median sPD‐L1 levels (A) and the optimal cut‐off value of sPD‐L1 levels (B)
Figure 4.
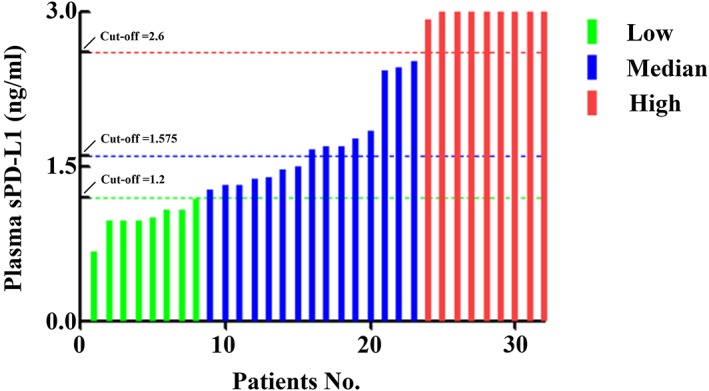
Three cohorts of PTCL patients with low (green), median (blue), and high (red) sPD‐L1 level. The threshold of cut‐off values between low and median cohorts was 1.2, and the threshold of cut‐off values between median and high cohorts was 2.6. The optimal cut‐off value was 1.575
Table 2.
Multivariate binary logistic regression (LR) analyses for overall response rate in PTCL patients
| Parameters | Overall Response Rate | ||
|---|---|---|---|
| OR | 95% confidence interval | P value | |
| Gender (male vs female) | 0.269 | ||
| Age (≤60 vs >60) | 0.477 | ||
| Stage (I + II vs III + IV) | 0.084 | ||
| IPI score (≤2 vs >2) | 0.933 | ||
| LDH (Normal vs elevated) | 0.647 | ||
| β2‐MG (Normal vs elevated) | 0.914 | ||
| sPD‐L1 (Low vs high) | 12.444 | 1.323‐117.032 | 0.027 |
3.5. Correlation between sPD‐L1 level and survival
The survival analysis revealed that the median PFS for high and low sPD‐L1 level groups was 42.7 months (95% CI, 27.9‐57.6) and 53 months (95% CI, 33.7‐72.3), respectively. As well, the median OS for high and low sPD‐L1 level groups was 48.3 months (95% CI, 35.2‐61.2) and 71 months (95% CI, 51.0‐90.9), respectively. However, there were too few patients for a statistical significance for PFS (log‐rank test, 0.904) and OS (log‐rank test, 0.896). But these results suggested at least that patients with high sPD‐L1 levels tended to have shorter PFS and OS than those with low sPD‐L1 levels.
3.6. Association between sPD‐L1 and tissue PD‐L1 expression
Eleven matched FFPE specimen from patient tumor biopsies, including three NKTCL, one ALCL, three AITL, three PTCL‐NOS, and one unknown PTCL subtype, were available. IHC staining results displayed that five patients had low tissue PD‐L1 expression, which matched with the patients with low sPD‐L1 level (Figure 5A). These patients included one AITL (Patient No. P012), one unknown PTCL subtype (Patient No. P026), one ALCL (Patient No. P031), and two PTCL‐NOS (Patient No. P036 and P041). And other five patients had high tissue PD‐L1 expression, which matched with the patients with high sPD‐L1 level (Figure 5B). These patients included one PTCL‐NOS (Patient No. P009), two NKTCL (Patient No. P038 and P039), and two AITL (Patient No. P013 and P022). Only one patient had no good matched result, who had low tissue PD‐L1 expression and high plasma sPD‐L1 level (Figure 3C). This patients was one NKTCL (Patient No. P008). These results showed that plasma sPD‐L1 level appeared to have a positive relationship with tissue PD‐L1 expression in PTCL patients, and both of them had a high matched rate (90.9%). We also performed the EBER detection and found that three NKTCL patients (Patient No. P008, P038, and P039), two AITL patients (Patient No. P013 and P022), and one unknown PTCL subtype patient (Patient No. P026) were EBV‐positive. Four (66.7%) of six EBV‐positive patients showed high tissue PD‐L1 expression, and five (83.3%) showed high sPD‐L1 level, verifying that PD‐L1 expression was associated with EBV infection in PTCL patients. These findings further suggested that plasma sPD‐L1 levels were an underlying biomarker to predict the worse prognosis instead of tissue PD‐L1 expression for PTCL patients.
Figure 5.
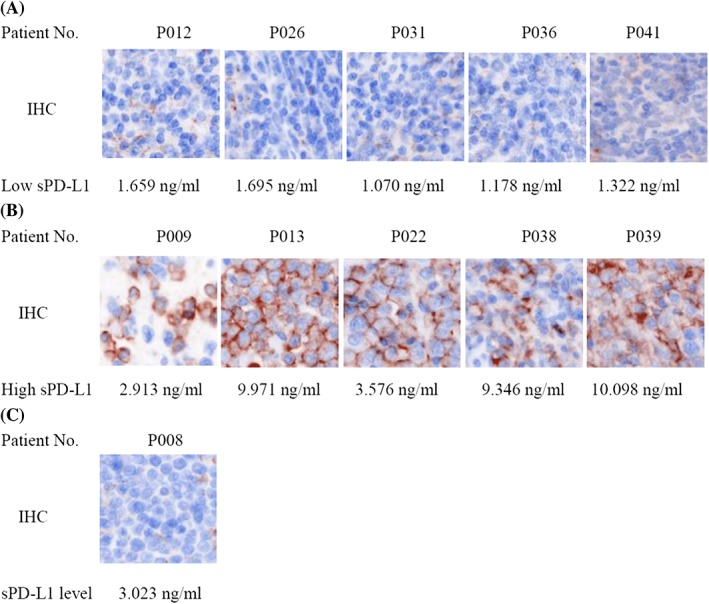
The matched rate between plasma sPD‐L1 level and tissue PD‐L1 expression in patients. A, Low expression of plasma sPD‐L1 and tissue PD‐L1; B, high expression of plasma sPD‐L1 and tissue PD‐L1; C, high expression of plasma sPD‐L1 and low expression of tissue PD‐L1
4. DISCUSSION
Blockade of PD1/PD‐L1 pathway is a new and promising therapeutic approach in many cancers, such as melanoma, Hodgkin's lymphoma, and some subtypes of non‐Hodgkin lymphoma.20, 21, 22 PD‐1/PD‐L1 antibody has manifested an underlying clinical benefit for PTCL patients,23 and their redeployment into PTCLs is just occurring now (NCT numbers: NCT03493451, NCT03075553, NCT03021057). However, whether plasma sPD‐L1 could become a biomarker for PTCLs still remains unknown.
Previous report showed that the sPD‐L1 was produced by matrix metalloproteinases from immune and tumor cells.24 However, the accurate sources of sPD‐L1 remain unclear. Generally, soluble forms of receptors or ligands are usually produced through proteolytic cleavage of membrane‐bound proteins, such as sTNF and sB7‐H3,24, 25 or by translation of alternative spliced mRNA, such as sCD86.26 Report has suggested that IFN‐γ can induce upregulation of PD‐L1 expression.7, 27 In this study, we indeed found that the levels of sPD‐L1 were positively correlated with IFN‐γ level in PTCL patients. It further suggested that IFN‐γ could upregulate the sPD‐L1 level in PTCLs. Studies have reported that elevated sPD‐L1 level is associated with poor prognosis in renal cell carcinoma and DLBCL.19 Among the patients with renal cell carcinoma, higher sPD‐L1 levels are associated with larger tumors, tumors with necrosis, and advanced stage and high grade. High sPD‐L1 levels are also reported to be an independent prognostic factor for poorer PFS in multiple myeloma.28 These results suggest that sPD‐L1 levels have a potentiality instead of tissue PD‐L1 level to predict the anti‐immune response for some subtypes of tumors. In our study, we found that sPD‐L1 levels in PTCL patients were much higher than that in healthy volunteers. We also found that patients with lower sPD‐L1 level always had good clinical response (high ORR) and tended to have an excellent survival. However, a statistical significance was not found for OS and PFS analysis. The reason may be that the sample size is too small. A study of enlarged sample size needs to be developed to further verify our findings. In addition, our study revealed that patients with elevated LDH level, advanced stage, and elevated β2‐MG level had higher sPD‐L1 levels, which also suggested that sPD‐L1 levels had a potential effect to predict PTCL progression. Studies have also confirmed that PD‐L1 expression in tumor tissue could affect the patient prognosis by tumor‐intrinsic signaling and adaptive immunosuppression.29, 30, 31 In order to reveal the association between sPD‐L1 level and tissue PD‐L1 expression in PTCLs, we detected the expression of tissue PD‐L1 in some matched patients by IHC. Our results showed that it exhibited a high matched rate between sPD‐L1 level and tissue PD‐L1 expression for these patients. However, because of the heterogeneity among tumors, the matched rate between sPD‐L1 level and tissue PD‐L1 expression can be different in diverse malignancies. Previous study has reported that no association is found between sPD‐L1 level and tissue PD‐L1 expression in DLBCL.19 But another study reported that sPD‐L1 level positively corrected with tissue PD‐L1 expression, suggesting a high matched rate, in NKTCL,32 which is similar to our results in PTCLs. Except for the heterogeneity among tumors, another reason for these different results may also be due to different sources of PD‐L1 antibodies. Another important study has identified that different sources of PD‐L1 antibodies will raise some questions about their similarity and the potential interchangeability of the tests, and they found that three of four PD‐L1 antibodies, including 22c3, 28‐8, and E1L3N, were concordant and reproducible as read by pathologists; however, SP142 was an outlier.33 The same sourced PD‐L1 antibody (E1L3N) is used in PTCLs in our study and in NKTCL,32 which may be a potential reason for the concordant results in the two studies. However, another sourced PD‐L1 antibody (ab58810) is selected in DLBCL.19
In conclusion, high sPD‐L1 level was associated with poor clinical response and tended to have shorter PFS and OS for PTCL patients. This is the first time to demonstrate the relationship between plasma sPD‐L1 level and prognosis in PTCLs. Plasma sPD‐L1 level may serve as a meaningful and less invasive biomarker to predict the clinical response for PTCL patients.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (81770213), the National Key New Drug Creation Special Programs (2017ZX09304‐021, 2018ZX09201015), the Key Research Projects of Tianjin Municipal Health Bureau (15KG145, 2015KZ081), the Tianjin Medical University Cancer Institute and Hospital Foundation (1504), and the National Human Genetic Resources Sharing Service Platform (2005DKA21300)/Cancer Biobank of Tianjin Medical University Cancer Institute and Hospital.
Zhang X, Liu L, Zhou S, et al. Plasma soluble programmed death ligand 1 levels predict clinical response in peripheral T‐cell lymphomas. Hematological Oncology. 2019;37:270–276. 10.1002/hon.2636
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/hon.2635
Contributor Information
Xianhuo Wang, Email: tjzlyy_xianhuow@163.com.
Huilai Zhang, Email: zhlwgq@126.com.
Kai Fu, Email: kfu@unmc.edu.
REFERENCES
- 1. Vose J, Armitage J, Weisenburger D. International T‐cell lymphoma project. International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124‐4130. [DOI] [PubMed] [Google Scholar]
- 2. Dearden CE, Johnson R, Pettengell R, et al. Guidelines for the management of mature T‐cell and NK‐cell neoplasms (excluding cutaneous T‐cell lymphoma). Br J Haematol. 2011;153(4):451‐485. [DOI] [PubMed] [Google Scholar]
- 3. Ellin F, Jerkeman M, Törnqvist J, Brudin L, Relander T. Impact of comorbidity on survival in peripheral T‐cell lymphomas: a Swedish Lymphoma Registry study. Hematol Oncol. 2018;36(1):159‐165. [DOI] [PubMed] [Google Scholar]
- 4. Korehisa S, Oki E, Iimori M, et al. Clinical significance of programmed cell death‐ligand 1 expression and the immune microenvironment at the invasive front of colorectal cancers with high microsatellite instability. Int J Cancer. 2018;142(4):822‐832. [DOI] [PubMed] [Google Scholar]
- 5. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26(1):677‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou W, Chen L. Inhibitory B7‐family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467‐477. [DOI] [PubMed] [Google Scholar]
- 7. Gao Y, Yang J, Cai Y, et al. IFN‐γ‐mediated inhibition of lung cancer correlates with PD‐L1 expression and is regulated by PI3K‐AKT signaling. Int J Cancer. 2018;143(4):931‐943. [DOI] [PubMed] [Google Scholar]
- 8. Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119(2):317‐327. [DOI] [PubMed] [Google Scholar]
- 9. Quezada SA, Peggs KS. Exploiting CTLA‐4, PD‐1 and PD‐L1 to reactivate the host immune response against cancer. Br J Cancer. 2013;108(8):1560‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Radvanyi L, Pilon‐Thomas S, Peng W, et al. Antagonist antibodies to PD‐1 and B7‐H1 (PD‐L1) in the treatment of advanced human cancer‐‐letter. Clin Cancer Res. 2013;19(19):5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ansell SM, Lesokhin AM, Borrello I, et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366(26):2443‐2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7‐H1 is associated with poor prognosis in renal cell carcinoma patients with long‐term follow‐up. Cancer Res. 2006;66(7):3381‐3385. [DOI] [PubMed] [Google Scholar]
- 14. Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD‐L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146(1):15‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu P, Wu D, Li L, Chai Y, Huang J. PD‐L1 and survival in solid tumors: a meta‐analysis. PLoS One. 2015;10(6):e0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B‐cell lymphoma. Blood. 2015;126(19):2193‐2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xing W, Dresser K, Zhang R, et al. PD‐L1 expression in EBV‐negative diffuse large B‐cell lymphoma: clinicopathologic features and prognostic implications. Oncotarget. 2016;7(37):59976‐59986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frigola X, Inman BA, Lohse CM, et al. Identification of a soluble form of B7‐H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17(7):1915‐1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B‐cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28(12):2367‐2375. [DOI] [PubMed] [Google Scholar]
- 20. Tie Y, Ma X, Zhu C, et al. Safety and efficacy of nivolumab in the treatment of cancers: a meta‐analysis of 27 prospective clinical trials. Int J Cancer. 2017;140(4):948‐958. [DOI] [PubMed] [Google Scholar]
- 21. Matsuki E, Younes A. Checkpoint inhibitors and other immune therapies for Hodgkin and non‐Hodgkin lymphoma. Curr Treat Options Oncol. 2016;17(6):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA‐4, PD‐1/PD‐L1, and HLA‐G. Eur Urol. 2015;68(2):267‐279. [DOI] [PubMed] [Google Scholar]
- 23. Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698‐2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Wang Q, Shi B, et al. Development of a sandwich ELISA for evaluating soluble PD‐L1 (CD274) in human sera of different ages as well as supernatants of PD‐L1+ cell lines. Cytokine. 2011;56(2):231‐238. [DOI] [PubMed] [Google Scholar]
- 25. Hikita A, Tanaka N, Yamane S, et al. Involvement of a disintegrin and metalloproteinase 10 and 17 in shedding of tumor necrosis factor‐alpha. Biochem Cell Biol. 2009;87(4):581‐593. [DOI] [PubMed] [Google Scholar]
- 26. Jeannin P, Magistrelli G, Aubry JP, et al. Soluble CD86 is a costimulatory molecule for human T lymphocytes. Immunity. 2000;13(3):303‐312. [DOI] [PubMed] [Google Scholar]
- 27. Lee SJ, Jang BC, Lee SW, et al. Interferon regulatory factor‐1 is prerequisite to the constitutive expression and IFN‐gamma‐induced upregulation of B7‐H1 (CD274). FEBS Lett. 2006;580(3):755‐762. [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Wang H, Chen H, et al. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget. 2015;6(38):41228‐41236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deng C, Li Z, Guo S, et al. Tumor PD‐L1 expression is correlated with increased TILs and poor prognosis in penile squamous cell carcinoma. Oncoimmunology. 2016;6(2):e1269047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou C, Tang J, Sun H, et al. PD‐L1 expression as poor prognostic factor in patients with non‐squamous non‐small cell lung cancer. Oncotarget. 2017;8(35):58457‐58468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brogden KA, Vali S, Abbasi T. PD‐L1 is a diverse molecule regulating both tumor‐intrinsic signaling and adaptive immunosuppression. Transl Cancer Res. 2016;5(S7):S1396‐S1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagato T, Ohkuri T, Ohara K, et al. Programmed death‐ligand 1 and its soluble form are highly expressed in nasal natural killer/T‐cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol Immunother. 2017;66(7):877‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rimm DL, Han G, Taube JM, et al. A prospective, multi‐institutional, pathologist‐based assessment of 4 immunohistochemistry assays for PD‐L1 expression in non‐small cell lung cancer. JAMA Oncol. 2017;3(8):1051‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]


