Abstract
The Gag polyprotein is the building block of retroviral particles and its expression is sufficient for assembly in cells. In HIV-1, nucleic acid (NA) is required for recombinant Gag molecules to assemble in a defined system in vitro. Experiments performed by Barklis and co-workers suggested that NA contributes to assembly by promoting Gag oligomerization. Gag is composed of four main domains: the matrix (MA), capsid (CA), nucleocapsid (NC), and p6 domains. We have recently shown that the SP1 linker, which lies between the CA and NC domains, assumes a helical structure at high, but not low, concentrations. We suggested that Gag oligomerization mediates assembly via an SP1-dependent conformational switch that exposes new interfaces for assembly.
Although NA is required for assembly in vitro, deletion of NC, the main RNA-binding domain, does not eliminate particle formation in vivo; these particles lack NA. We hypothesized that alternative pathways that lead to Gag oligomerization or an increase in local Gag concentration, namely Gag–membrane or inter-protein interactions, rescue assembly in the absence of NC-RNA binding. We constructed mutants in which either Gag–membrane binding, the Gag dimer interface, or NC-RNA binding are disrupted. None of these mutants disables assembly. However, combined mutations in any two of these three classes render Gag completely unable to form virus-like particles. Thus, it seems, Gag utilizes at least three types of interactions to form oligomers and any two out of the three are sufficient for assembly.
Keywords: HIV-1, Gag, Assembly, In vivo, Oligomerization
Retroviral Gag proteins have been described as “assembly machines” (Wills and Craven, 1991). Thus, expression of the HIV-1 Gag protein in human cells is sufficient for efficient assembly and release of virus-like particles (VLPs).
A further, dramatic simplification of the experimental context for analysis of retroviral particle assembly mechanisms is the in vitro assembly system, in which recombinant Gag protein, purified from bacteria, is induced to assemble into virus-like particles in a fully defined system (Campbell and Rein, 1999; Campbell and Vogt, 1995). Obviously, the systematic application of in vitro analysis enables us to identify ligands and cofactors that contribute to or influence the assembly of Gag protein into virus-like particles.
The first in vitro studies on retroviral assembly (Campbell and Rein, 1999; Campbell and Vogt, 1995) found that recombinant Gag proteins were soluble in aqueous buffers, but instantly assembled into virus-like particles when single-stranded nucleic acids (NAs) were added to the protein. What is the mechanism underlying this effect, and what can it tell us about retrovirus assembly? Approximately 2–4 μg/ml of DNA or RNA was sufficient to cause HIV-1 Gag at 1 mg/ml to assemble into structures large enough to be pelleted in the microcentrifuge. This mass ratio represents about 5–7 nucleotides per Gag molecule. It is similar to the ratio of RNA to protein in a retrovirus particle, and also resembles most of the estimates of the “site size” of nucleocapsid (NC) proteins on NAs (Fisher et al., 2006; Karpel et al., 1987). These concordances imply that assembly under these conditions requires that each Gag molecule be bound to NA. Remarkably, even relatively short ssDNAs or ssRNAs, only ~15–40 nucleotides long, could support particle assembly (Campbell and Rein, 1999; Ma and Vogt, 2002, 2004). This striking result shows that the NA is not acting as a “string” upon which the Gag “beads” line up during assembly: clearly, the particles must be composed of very small Gag–NA complexes, and Gag molecules bound to one NA molecule must be interacting with Gag molecules bound to other NA molecules during the assembly of the particle.
In 1998, Zhang et al. reported that a chimeric protein, identical to HIV-1 Gag except that the NC domain (the principal NA-binding domain) was replaced by a leucine-zipper motif, assembled efficiently into virus-like particles in mammalian cells (Zhang et al., 1998). These particles are almost indistinguishable in their morphology from particles produced by wild-type Gag expression, but contain little or no RNA (Crist et al., 2009; Zennou et al., 2004). Thus, the presence of the leucine-zipper evidently eliminates the RNA-binding requirement for particle assembly. As pointed out by Vogt and colleagues, the zipper motif is really a dimerization domain (Johnson et al., 2002; Ma and Vogt, 2002, 2004). Taken together, these observations imply that the role of RNA-binding is to induce the formation of dimers or small oligomers of Gag; perhaps oligomerization somehow leads to the assembly of the full particle, even though virus particles contain thousands of Gag molecules.
Recent studies have suggested a possible mechanism by which oligomerization of Gag could trigger particle assembly. HIV-1 Gag contains a short “spacer” region, termed “SP1”, between its CA and NC domains (see Fig. 1). Several lines of evidence indicate that the major Gag–Gag interactions in particle assembly are in the capsid (CA) or SP1 domain (Ako-Adjei et al., 2005; Briggs et al., 2009; Crist et al., 2009; Krishna et al., 1998). If RNA-binding promotes assembly by promoting oligomerization, then it would presumably induce a change in CA, exposing or activating new interfaces for interaction with the CA domains of other Gag molecules. Perhaps RNA-binding by the NC domain, and the resulting oligomerization, sends a signal to the CA domain triggering these changes. Because of its location between NC and CA, SP1 might function in transmitting this signal.
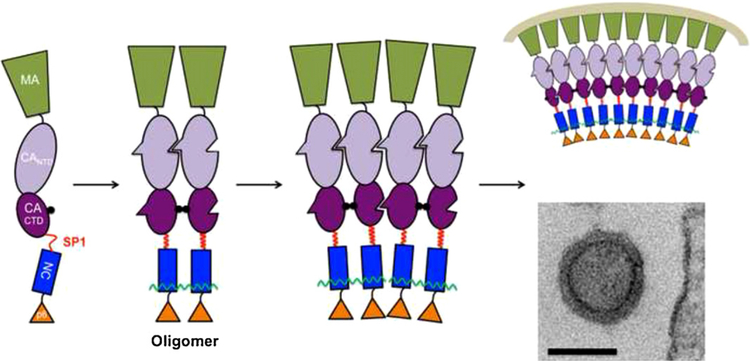
Fig. 1.
Suggested model for oligomerization-driven assembly. Binding of free Gag molecules to RNA promotes oligomerization, increasing local Gag concentration. In our model, the SP1 linker assumes a helical conformation at a sufficiently high Gag concentration; this in turn triggers a conformational change leading to the appearance of new interfaces for assembly. The figure shows, from left to right, a Gag molecule free in solution; an oligomer of Gag bound to a short RNA molecule (wavy line); a hypothetical assembly intermediate consisting of two such oligomers joined together solely via Gag–Gag interaction; and (top) a schematic of a portion of an immature virus-like particle, and (bottom) a typical transmission electron micrograph of an authentic immature virus-like particle. Bar = 100 nm.
New results on SP1 are fully consistent with these speculations. Several reports have shown that the ability of Gag to assemble into normal particles is exquisitely sensitive to changes in the sequence of SP1 (Accola et al., 1998; Datta et al., 2011; Krausslich et al., 1995). While SP1 is largely unstructured in aqueous buffers (Newman et al., 2004), it adopts an α-helical structure in 30% trifluoroethanol (Morellet et al., 2005). Using circular dichroism spectroscopy (Datta et al., 2011), we found that a peptide representing the sequence spanning the CA-SP1 junction is unstructured in dilute aqueous solution (e.g., 0.05 μM), but adopts a helical conformation when the dielectric constant of the medium is reduced by addition of other organic solvents such as ethanol, in addition to trifluoroethanol. Remarkably, it also undergoes a similar conformational change when its concentration in water is raised from 0.05 μM to ~5 μM. The sequence of the peptide is such that if it were to form an α-helix, the helix would be amphipathic, with a polar face and a hydrophobic face. It seems very plausible that raising the concentration of the peptide shifts an equilibrium toward a new peptide conformation, in which the individual molecules form helices whose hydrophobic faces are buried in a bundle of helices. It can be easily imagined that the same shift occurs in the SP1 region of Gag when the local Gag concentration is increased, and that the change in SP1 conformation can then be “propagated” into the CA domain, priming the molecule for particle assembly (See Fig. 1).
What mechanisms might result in oligomerization, or increases in the local concentration, of Gag in the mammalian cell? As discussed above, binding of NA molecules might be one such mechanism. However, it is also known that HIV-1 Gag is in monomer–dimer equilibrium in free solution; this dimeric interaction has been localized to a specific site in the C-terminal domain (“CTD”) within the CA domain (Datta et al., 2007b; Gamble et al., 1997). This interface, which was previously described for free CA protein (Gamble et al., 1997), centers on Gag residues W316 and M317, and replacement of these two residues with alanine practically eliminates the dimeric interaction. It seems likely that this dimerization reaction contributes to oligomerization of Gag in vivo. Direct evidence for this contribution in cultured cells was obtained in quantitative fluorescence resonance energy transfer studies (Hogue et al., 2009).
Yet another possible way that the local Gag concentration might be raised in vivo is by binding to the plasma membrane, reducing the “space” within which the protein is confined from the three-dimensional volume of the cytoplasm to a two-dimensional surface. The binding of HIV-1 Gag to the plasma membrane is completely dependent upon its N-terminal myristic acid modification (Bryant and Ratner, 1990; Gottlinger et al., 1989). In fact, it has been reported that the aliphatic chain of the myristate is partially buried when recombinant N-terminal fragments of Gag are at low concentration in solution, but are exposed when the concentration is raised (Tang et al., 2004); this observation raises the possibility of a positive feedback loop, in which oligomerization of Gag results in myristate exposure, leading to an increase in its membrane affinity, which would presumably raise the local Gag concentration still more.
We have investigated the contributions of these three elements to particle assembly. 293 T cells were transiently transfected with an expression plasmid containing a codon-optimized HIV-1 Gag gene (Schneider et al., 1997), allowing Rev-independent expression of Gag. Mutations were introduced that disrupt NC–RNA binding, the CA dimer interface, or plasma membrane binding. Assembly was assessed by transmission electron microscopy (TEM) of the transfected cells. As expected, expression of the wild-type Gag sequence led to formation of rather regular, nearly round structures budding from the plasma membrane; these virus-like particles had diameters of approximately 110–120 nm (see Fig. 1).
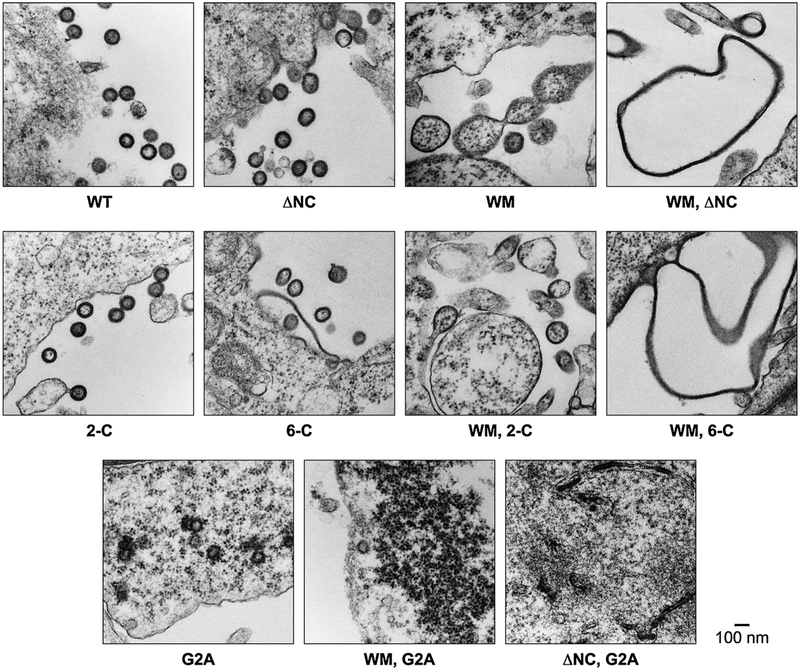
We found (Fig. 2) that mutations in any one of the three functional regions of Gag tended to degrade the quality of particle assembly: in other words, the mutant proteins assembled into large, darkly staining structures in the cells, but these structures were somewhat less uniform and regular than the virus-like particles formed by wild-type Gag (see Fig. 2). Images of the particles formed by W316M317/AA (“WM”) mutant Gag, in which the dimer interface in the C-terminal domain of the CA domain has been almost entirely ablated by mutation, have previously been published (Datta et al., 2007a), and show that the cells still release nearly round structures whose size is considerably more variable than those formed by wild-type Gag. They also appear to contain ribosomes, suggesting that cytoplasm is engulfed during their formation. Thus, the mutation in this Gag protein reduces the regularity of particle assembly. Nevertheless, the mutant protein does retain the ability to assemble into structures with an overall appearance roughly similar to that of wild-type VLPs; specifically, the fact that they resemble the latter in size and shape implies that the radius of curvature with which they assemble, while more variable than in the wild type, has not been drastically altered by the mutation. It should be noted that biophysical measurements (Datta et al., 2007b) indicated that the affinity of WM mutant monomers for each other is ~1/100 that of wild-type monomers; we do not know whether this residual tendency to dimerize contributes to assembly of the mutant in vivo.
Fig. 2.
Transmission electron micrographs of structures formed in 293 T cells by mutant and wild-type Gag proteins.
The site of myristylation of retroviral Gag proteins is always the N-terminal glycine. Therefore, one way to prevent membrane association of HIV-1 Gag is to mutate the glycine codon to an alanine codon. When this G2A mutant Gag is expressed in 293 T cells, release of pelletable structures containing the protein is almost completely eliminated (Bryant and Ratner, 1990; Gottlinger et al., 1989). Examination of the cells by TEM reveals that there are no particles budding from the plasma membrane, unlike what is seen with wild-type Gag. However, the cytoplasm of these cells does contain darkly staining structures not seen in mock-transfected cells (Datta et al., 2011). These structures are round and similar in size to authentic VLPs, but are somewhat variable in appearance and are frequently not closed. They are also studded with ribosomes, unlike wild-type VLPs. Thus, here again, the mutation has altered the assembly behavior of the Gag protein, but has little effect on the radius of curvature of the assembled structure.
We also tested mutations that might affect the ability of Gag to interact with RNA. These included a mutation replacing the first two cysteines of the N-terminal zinc finger within NC (“2-C”) and another replacing all six cysteines in both zinc fingers (“6-C”). In fact, as previously reported by Ott et al. (2003), deletion of the entire NC domain from HIV-1 Gag does not significantly reduce virus-like particle assembly and release, at least in transiently transfected 293 T cells. The size and shape of the particles assembled in transfected cells is variable, but in all of these cases includes particles very similar to those produced with wild-type Gag. Our results indicate that the particles lacking the NC domain contain very little, if any, RNA (O’Carroll et al., 2012).
We also tested Gag proteins containing combinations of the mutations described above. In every case, Gag protein with two, rather than one, of these mutations had completely lost the ability to assemble into structures with a regular radius of curvature. Thus, when Gag with the WM mutation and a deletion of its NC domain was transfected into 293 T cells, it gave rise to extremely aberrant structures, including sheets or string-like structures and very large, empty spheres (“balloons”) with no characteristic curvature and no obvious resemblance to virus-like particles. Gag containing both G2A and WM mutations formed darkly stained stretches and clusters of darkly stained intracellular material, frequently near the plasma membrane. Finally, G2A Gag lacking its NC domain gave rise to darkly stained arcs and incomplete spheres within the cytoplasm. None of these double mutants showed a detectable tendency to generate structures with even an approximately uniform radius of curvature (Fig. 2).
It should be noted that our experimental system expresses these Gag proteins at significantly higher levels than in natural infections. As the efficiency of assembly is a function of the level of Gag in the cell (Yadav et al., 2012), overexpression will enhance assembly by Gag mutants with impaired assembly capability. This is presumably why Gag proteins lacking their NC domain assemble in our hands, while Gag proteins with NC defects fail to do so under other experimental conditions (Dawson and Yu, 1998; Grigorov et al., 2007).
In summary, we have identified three functional elements within the HIV-1 Gag protein. No one of these elements is required for assembly of particles with a roughly consistent, correct radius of curvature, as long as the other two elements are present. This relationship implies that the three elements are functionally redundant with each other, and suggests that they all contribute to assembly via a common pathway. As discussed above, we would propose that membrane-binding, Gag protein dimerization, and cooperative NA-binding all contribute by raising the local Gag concentration, thus promoting the initial oligomerization step and triggering a conformational change in the SP1 and CA regions of Gag. Once this has occurred, Gag molecules are added to the assembling particle in the correct spatial orientation needed for assembly with the correct radius of curvature. Studies on the effects of oligomerization upon the properties of Gag are now under way.
Acknowledgements
We thank Demetria Harvin and Jane Mirro for technical assistance and Rachael Crist for participation in the project. We thank David Ott and Robert Gorelick for helpful discussions. This work was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, in part by the NIH Intramural AIDS Targeted Antiviral Program, and part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E.
References
- Accola MA, Hoglund S, Gottlinger HG, 1998. A putative alpha-helical structure which overlaps the capsid-p2 boundary in the human immunodeficiency virus type 1 Gag precursor is crucial for viral particle assembly. Journal of Virology 72 (3), 2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ako-Adjei D, Johnson MC, Vogt VM, 2005. The retroviral capsid domain dictates virion size, morphology, and coassembly of gag into virus-like particles. Journal of Virology 79 (21), 13463–13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs JA, Riches JD, Glass B, Bartonova V, Zanetti G, Krausslich HG, 2009. Structure and assembly of immature HIV. Proceedings of the National Academy of Sciences of the United States of America 106 (27), 11090–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M, Ratner L, 1990. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proceedings of the National Academy of Sciences of the United States of America 87 (2), 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Rein A, 1999. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. Journal of Virology 73 (3), 2270–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Vogt VM, 1995. Self-assembly in vitro of purified CA–NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. Journal of Virology 69 (10), 6487–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RM, Datta SA, Stephen AG, Soheilian F, Mirro J, Fisher RJ, Nagashima K, Rein A, 2009. Assembly properties of human immunodeficiency virus type 1 Gag-leucine zipper chimeras: implications for retrovirus assembly. Journal of Virology 83 (5), 2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SA, Temeselew LG, Crist RM, Soheilian F, Kamata A, Mirro J, Harvin D, Nagashima K, Cachau RE, Rein A, 2011. On the role of the SP1 domain in HIV-1 particle assembly: a molecular switch? Journal of Virology 85 (9), 4111–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SAK, Curtis JE, Ratcliff W, Clark PK, Crist RM, Lebowitz J, Krueger S, Rein A, 2007a. Conformation of the HIV-1 Gag protein in solution. Journal of Molecular Biology 365 (3), 812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SAK, Zhao Z, Clark PK, Tarasov S, Alexandratos JN, Campbell SJ, Kvaratskhelia M, Lebowitz J, Rein A, 2007b. Interactions between HIV-1 Gag molecules in solution: an inositol phosphate-mediated switch. Journal of Molecular Biology 365 (3), 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson L, Yu XF, 1998. The role of nucleocapsid of HIV-1 in virus assembly. Virology 251 (1), 141–157. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Fivash MJ, Stephen AG, Hagan NA, Shenoy SR, Medaglia MV, Smith LR, Worthy KM, Simpson JT, Shoemaker R, McNitt KL, Johnson DG, Hixson CV, Gorelick RJ, Fabris D, Henderson LE, Rein A, 2006. Complex interactions of HIV-1 nucleocapsid protein with oligonucleotides. Nucleic Acids Research 34 (2), 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble TR, Yoo S, Vajdos FF, von Schwedler UK, Worthylake DK, Wang H, McCutcheon JP, Sundquist WI, Hill CP, 1997. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science 278 (5339), 849–853. [DOI] [PubMed] [Google Scholar]
- Gottlinger HG, Sodroski JG, Haseltine WA, 1989. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proceedings of the National Academy of Sciences of the United States of America 86 (15), 5781–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorov B, Decimo D, Smagulova F, Pechoux C, Mougel M, Muriaux D, Darlix JL, 2007. Intracellular HIV-1 Gag localization is impaired by mutations in the nucleocapsid zinc fingers. Retrovirology 4, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue IB, Hoppe A, Ono A, 2009. Quantitative fluorescence resonance energy transfer microscopy analysis of the human immunodeficiency virus type 1 Gag–Gag interaction: relative contributions of the CA and NC domains and membrane binding. Journal of Virology 83 (14), 7322–7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MC, Scobie HM, Ma YM, Vogt VM, 2002. Nucleic acid-independent retrovirus assembly can be driven by dimerization. Journal of Virology 76 (22), 11177–11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpel RL, Henderson LE, Oroszlan S, 1987. Interaction of retroviral structural proteins with single-stranded nucleic acids. Journal of Biological Chemistry 262, 4961–4967. [PubMed] [Google Scholar]
- Krausslich HG, Facke M, Heuser AM, Konvalinka J, Zentgraf H, 1995. The spacer peptide between human immunodeficiency virus capsid and nucleocapsid proteins is essential for ordered assembly and viral infectivity. Journal of Virology 69 (6), 3407–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna NK, Campbell S, Vogt VM, Wills JW, 1998. Genetic determinants of Rous sarcoma virus particle size. Journal of Virology 72 (1), 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YM, Vogt VM, 2002. Rous sarcoma virus Gag protein-oligonucleotide interaction suggests a critical role for protein dimer formation in assembly. Journal of Virology 76 (11), 5452–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YM, Vogt VM, 2004. Nucleic acid binding-induced Gag dimerization in the assembly of Rous sarcoma virus particles in vitro. Journal of Virology 78 (1), 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morellet N, Druillennec S, Lenoir C, Bouaziz S, Roques BP, 2005. Helical structure determined by NMR of the HIV-1 (345–392)Gag sequence, surrounding p2: implications for particle assembly and RNA packaging. Protein Science 14 (2), 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JL, Butcher EW, Patel DT, Mikhaylenko Y, Summers MF, 2004. Flexibility in the P2 domain of the HIV-1 Gag polyprotein. Protein Science 13 (8), 2101–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll IP, Crist RM, Mirro J, Harvin D, Soheilian F, Kamata A, Nagashima K, Rein A, 2012. Functional redundancy in HIV-1 virus particle assembly. Journal of Virology, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott DE, Coren LV, Chertova EN, Gagliardi TD, Nagashima K, Sowder RC 2nd, Poon DT, Gorelick RJ, 2003. Elimination of protease activity restores efficient virion production to a human immunodeficiency virus type 1 nucleocapsid deletion mutant. Journal of Virology 77 (10), 5547–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN, 1997. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. Journal of Virology 71 (7), 4892–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Loeliger E, Luncsford P, Kinde I, Beckett D, Summers MF, 2004. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proceedings of the National Academy of Sciences of the United States of America 101 (2), 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills JW, Craven RC, 1991. Form, function, and use of retroviral gag proteins. AIDS 5 (6), 639–654 (Editorial). [DOI] [PubMed] [Google Scholar]
- Yadav SS, Wilson SJ, Bieniasz PD, 2012. A facile quantitative assay for viral particle genesis reveals cooperativity in virion assembly and saturation of an antiviral protein. Virology 429 (2), 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zennou V, Perez-Caballero D, Gottlinger H, Bieniasz PD, 2004. APOBEC3G incorporation into human immunodeficiency virus type 1 particles. Journal of Virology 78 (21), 12058–12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qian H, Love Z, Barklis E, 1998. Analysis of the assembly function of the human immunodeficiency virus type 1 gag protein nucleocapsid domain. Journal of Virology 72 (3), 1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]