Abstract
Cell surface transmembrane receptors often form nanometer to micrometer scale clusters to initiate signal transduction in response to environmental cues. Extracellular ligand oligomerization, domain-domain interactions, and binding to multivalent proteins all contribute to cluster formation. Here we review the current understanding of mechanisms driving cluster formation in a series of representative receptor systems: glycosylated receptors, immune receptors, cell adhesion receptors, Wnt receptors, and receptor tyrosine kinases. We suggest that these clusters share properties of systems that are known to undergo liquid-liquid phase separation, and could be investigated in this light.
Introduction
Cells are continually exposed to a variety of external signals that are sensed by diverse classes of transmembrane receptors. In response, these receptors initiate intracellular signaling cascades resulting in processes such as cell movement, differentiation, division, and apoptosis. Understanding mechanisms that propagate signals through transmembrane receptors is an important area of research that spans biophysics, biochemistry, cell biology, and organismic biology.
Many transmembrane receptors form dynamic assemblies that promote downstream signal transduction in cells (13, 132, 165). Diverse families of receptors including, but not limited to, receptor tyrosine kinases, cell adhesion receptors, and immune cell receptors undergo transitions from freely diffusing monomers or discrete oligomers to higher-order oligomers of indefinite stoichiometry following exposure to extracellular stimuli (3, 38, 120). In many cases, receptor organization into meso-scale (100s of nanometers) clusters, driven by higher-order oligomerization, appears to play an important role in downstream signaling (110, 113, 140, 144).
Recently, liquid-liquid phase separation (LLPS) driven by weak interactions between multivalent molecules was shown to be an important mechanism by which meso-scale structures can form in cells (5, 135). Such structures, termed biomolecular condensates, concentrate specific collections of proteins and nucleic acids without a surrounding membrane. In the cytoplasm and nucleoplasm, LLPS contributes to formation of condensates such as stress granules and the nucleolus. At membranes, LLPS has been shown to promote assembly of transmembrane proteins with their cytoplasmic binding partners into clusters (6, 35, 140, 171). LLPS has been shown in signaling pathways emanating from the intracellular domain of nephrin, a transmembrane adhesion molecule required to form the glomerular filtration barrier in kidneys, and linker for the activation of T cells (LAT), a transmembrane protein required for T cell activation. Both proteins undergo LLPS following phosphorylation of multiple tyrosine residues and subsequent binding by cytoplasmic, multivalent adaptor proteins. Similar to nephrin and LAT, many other transmembrane receptors become phosphorylated on multiple residues and bind multivalent adaptor proteins following activation (99). Many also interact extracellularly with multivalent ligands. It is therefore possible that LLPS represents a general cellular mechanism for clustering transmembrane receptors.
In this review, we briefly outline current thinking on the assembly and concomitant LLPS of multivalent proteins. We then discuss the properties and molecular interactions of a series of representative receptors known to form clusters upon stimulation: glycosylated receptors, immune receptors, cell adhesion receptors, Wnt receptors, and receptor tyrosine kinases (RTKs). We suggest that LLPS is potentially an important mechanism driving formation of transmembrane receptor clusters. Phase separation would result in receptor clusters that are organized in dynamic, but discrete, condensed phases that are orders of magnitude larger than the individual molecules and could have distinct biochemical and material properties that facilitate downstream signaling. We conclude with a discussion of future directions that will be useful for understanding how LLPS of both lipids and proteins on membranes might regulate signal transduction and cellular function.
Coupled Oligomerization and Phase Separation
Multivalent molecules have long been understood to play an important role in regulating receptor oligomerization (53, 54, 61, 81, 107). Drawing from ideas in polymer chemistry, numerous groups have shown that interactions between multivalent proteins can lead to formation of oligomeric assemblies whose size depends on the concentration of species and whose physical properties depend on the affinity and kinetics of binding and valency of the molecules (54, 81, 96, 104, 163). At high concentrations, multivalent systems can undergo sol-gel transitions when the fractional connectivity between units exceeds a critical threshold, forming infinitely-linked, macroscopic polymers (28, 46). To the best of our knowledge, however, until recently, studies of membrane proteins have not considered macroscopic phase separation, producing a density transition that is coupled to the sol-gel connectivity transition (or more generally, to oligomerization) (6, 58, 91, 130, 140). A key idea in this regard is that in poor solvent conditions, where molecules interact more strongly with themselves than with solvent, oligomerization decreases the inherent solubility of molecules, and hence promotes macroscopic phase separation (5, 46). Additionally, the notion of a phase separated compartment leads to the idea that interactions with the phase separating components can recruit additional molecules across the phase boundary (i.e. cause them to concentrate into the second phase) and generate a local chemical environment that is distinct from the surroundings. Below we describe recent considerations of oligomerization coupled to phase separation, as a prelude to discussion of membrane protein clustering.
A brief introduction to phase separation
Phase separation occurs as a result of the intrinsic chemical properties of a macromolecule dissolved in a solvent (46). All macromolecules have varying degrees of weak, nonspecific interactions with each other and with the surrounding solvent. If the interactions between macromolecules are weaker than those with solvent, macromolecules will remain homogenously dissolved in solution. However, if interactions between macromolecules are stronger than those with solvent, a separate, dense phase can form (34, 45). At a given temperature, phase separation occurs at the concentration where the favorable energetics of macromolecule–macromolecule interactions are stronger than the unfavorable entropy of demixing. Above this threshold concentration, the macromolecular solution separates into two distinct phases, one dilute and a second more concentrated and denser. For flexible molecules both phases are typically liquids, and hence the process is termed liquid-liquid phase separation. The relative volumes of the two phases depend on the total macromolecule concentration. Just above the threshold, the dilute phase is much larger. As total macromolecule concentration increases, the concentrations of the dilute and dense phases remain constant, but the volume of the latter grows. When the total macromolecule concentration reaches (or exceeds) that of the dense phase, the solution again returns to homogeneity. The low- and high-concentration thresholds change smoothly as a function of temperature and map out the so-called binodal curve of the system (Figure 1a). Importantly, since between the two threshold concentrations the phase-separated state has the minimum free energy, the condensed phase will be maintained indefinitely with no input of energy.
Figure 1.
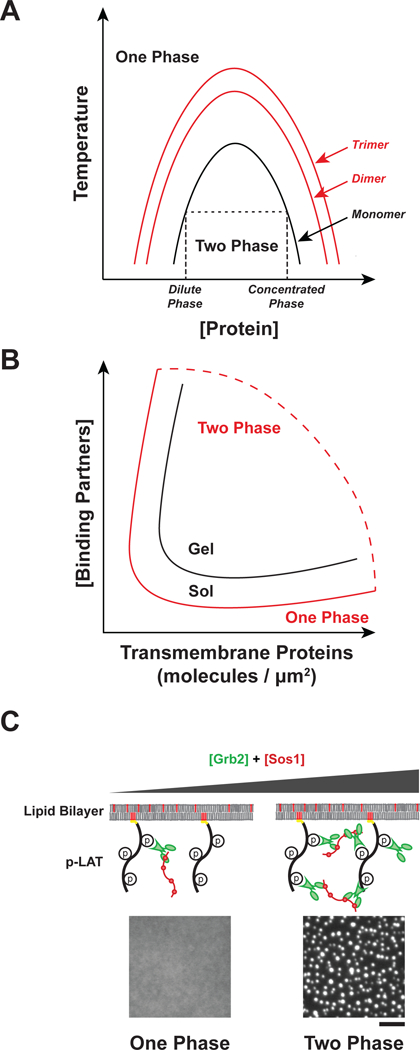
Regulation of sol-gel transition and phase separation. A) Oligomerization promotes phase separation. Phase diagram as a function of protein concentration and temperature for monomeric proteins (black line) or oligomers (red lines). Changes to temperature, pH, salt concentration, or crowding can also alter the shape of the binodal curve of the system. At low concentrations, molecules exist in a single dilute phase (One Phase). As concentration increases molecules separate into a dilute phase and a concentrated phase (Two Phase). At high concentrations, molecules exist in a single concentrated phase (One Phase). Phase separation is promoted by increasing the concentration of cytosolic binding partners. B) Phase diagram as a function of transmembrane protein density and cytosolic binding partner concentration. The binodal curve of the sol-gel transition (black) and the phase separation (red) represent distinct physical processes. C) Representative images showing in vitro phase separation of phospho-LAT, Grb2, and Sos1 on membranes. At low Grb2 and Sos1 concentrations the proteins exist in one dilute phase, but at higher concentrations phase separation occurs. Scale bar = 5 μm.
Oligomerization can be coupled to phase separation in multivalent proteins
A key feature of polymer systems is that interactions between molecules become stronger with increasing size, and hence the balance between self-self and self-solvent interactions changes, favoring the former. Thus, polymers become less soluble (have lower threshold concentration for phase separation) as they become larger (Figure 1a). The origins of this behavior are entropic, as the entropic penalty for segregating monomers into a second phase (demixing) is lower when those monomers are chemically connected into polymer chains. Analogous behavior has also been shown for folded proteins, including γ-crystallin (2), lysozyme, and albumin (157). These proteins all phase separate at high concentrations as monomers. But phase separation occurs at 10~100-fold lower concentrations when the proteins are crosslinked to dimers, trimers, and higher oligomers.
The oligomerization of multivalent proteins can be viewed in the same light. Multivalency enables formation of oligomeric assemblies, whose size increases with concentration (or, for a given concentration of monomeric units, with valency and affinity) (28, 46). As the size of such an assembly increases, its solubility decreases and phase separation becomes energetically favored (47). Thus, multivalency provides a mechanism to promote LLPS at much lower concentrations than would be possible with monomeric interaction elements. Within the condensed phase, the high concentration promotes further binding between molecules, and can produce sol-gel transitions (Figure 1b). In this way, oligomerization and phase separation are energetically coupled, with each favoring the other.
Although oligomerization (and sol-gel transitions) and phase separation are often coupled, it is important to note that they do represent distinct physical processes that can be experimentally separated (104). Thus, oligomerization can occur without phase separation, and phase separation can occur without oligomerization. For example, in polymerization of acrylamide gels for SDS PAGE, the molecules undergo a sol-gel transition (i.e. form a system-spanning network) but remain a homogeneous single phase. As described below, sol-gel transitions are often observed between multivalent carbohydrates and proteins, but this does not always coincide with phase separation (104, 163). In the opposite sense, phase separation is often observed for proteins in the absence of a sol-gel transition, for example with lysozyme (105) and numerous antibodies (118).
Computational and theoretical studies have suggested additional features of assembly and LLPS of cognate pairs of multivalent proteins. Using simulations, Harmon and colleagues predicted that the linkers between modular binding domains will strongly influence whether proteins assemble with or without concomitant phase separation (58). Very short linkers favor formation of dimers over large oligomers and therefore inhibit phase separation. But for longer linkers, the chemical properties of the linker determine whether sol-gel transitions occur with or without phase separation. Linkers with favorable interactions with solvent inhibit phase separation, since they are highly extended (corresponding to a large excluded volume), thus leading to sol-gel transitions in homogeneous solution. In contrast, linkers with unfavorable interactions with solvent will promote phase separation, since they tend to be compact (small excluded volume), thus leading to sol-gel transitions concomitant with LLPS.
Computational modeling has also shown that the relative stoichiometries of component modules can affect the propensity for phase separation (48). The pyrenoid is a carbon-fixing organelle in algae that undergoes LLPS due to multivalent interactions between Rubisco and EPYC1. Rubisco has eight binding sites for EPYC1, while EPYC1 has four binding sites for Rubisco. Modeling suggests that such systems will exhibit a “magic number” effect where certain numbers of particles form an unusually stable state. The magic number effect manifests when the valency of one partner is an integral multiple of the valency of the second and the binding sites of the two partners can be saturated. In this situation, LLPS is inhibited when the molecules are at the same module concentration due to formation of closed oligomers that do not further polymerize. This magic number effect could impact the phase diagram in many biological contexts and is predicted to give rise to unexpectedly sharp phase transitions.
Dimensionality considerations
In addition to concentration, affinity, valency, pH, and temperature, the dimensionality of a system is an important parameter that determines how a set of macromolecules will phase separate (123). Since the cytoplasm and nucleoplasm are three-dimensional environments (i.e. all molecules can diffuse in three dimensions), LLPS there produces liquid-like compartments that dynamically exchange components with their surroundings, can undergo fusion and fission, and maintain a spherical shape due to surface tension (73). Since the plasma membrane is a two-dimensional system (i.e. membrane-associated proteins only diffuse in two dimensions), LLPS there results in formation of dynamic, liquid like clusters of transmembrane receptors in the plane of the membrane (Figure 1c). It remains unknown the degree to which soluble interaction partners assemble in a third dimension (for a given concentration) when they localize to these membrane-associated clusters. Analogous to their 3D counterparts, membrane-associated clusters formed through LLPS are dynamic, can undergo fusion and fission, and are dependent on the concentration, affinity, and valency of the interacting components (6).
In a 2D fluid bilayer transmembrane receptors exhibit density-dependent multivalency, which is a clear distinction from 3D phase separated droplets. At low density, the valency of a receptor is dictated only by its molecular structure. But at high densities, the valency of a receptor effectively increases as neighboring molecules can act together in engaging ligands. For example, at low membrane densities, diffusing membrane-attached monovalent proteins have a low affinity for soluble bi-valent antibodies, since the probability of two binding events is low (167). However, as the monovalent protein density increases, the apparent affinity for the bi-valent antibodies increases. The ability of increased receptor density to effectively increase valency is likely important in LLPS of membrane-associated systems.
Experimentally, we have observed that reducing the dimensionality of in vitro systems from three dimensions to two dimensions reduces the threshold concentration for phase separation as much as thirty-fold (6, 91). Because of this, if a multivalent cytoplasmic adaptor protein is at a concentration below the threshold for 3D phase separation but above the threshold for membrane-associated phase separation, the latter could be specifically triggered while constitutive phase separation in the cytoplasm is avoided.
In the following sections, we describe specific classes of transmembrane receptors that have either been shown to undergo LLPS, or are strong candidates for LLPS based on their known interactions, physical properties, and cellular behaviors.
Carbohydrate-lectin interactions in receptor oligomerization
Multivalent interactions between glycosylated cell surface receptors and different classes of lectins cause receptor oligomerization and initiation of downstream signaling (Figure 2) (16, 114). Glycoproteins are often modified with multiple saccharides, either as repeating units on a single oligosaccharide or as clustered repeats of the saccharide on the protein backbone (16, 106). Additionally, lectins often contain multiple carbohydrate binding domains or function as constitutive oligomers. Thus, carbohydrate-lectin interactions have the molecular features needed for oligomerization, sol-gel transitions and potentially LLPS of molecules on the cell surface.
Figure 2.
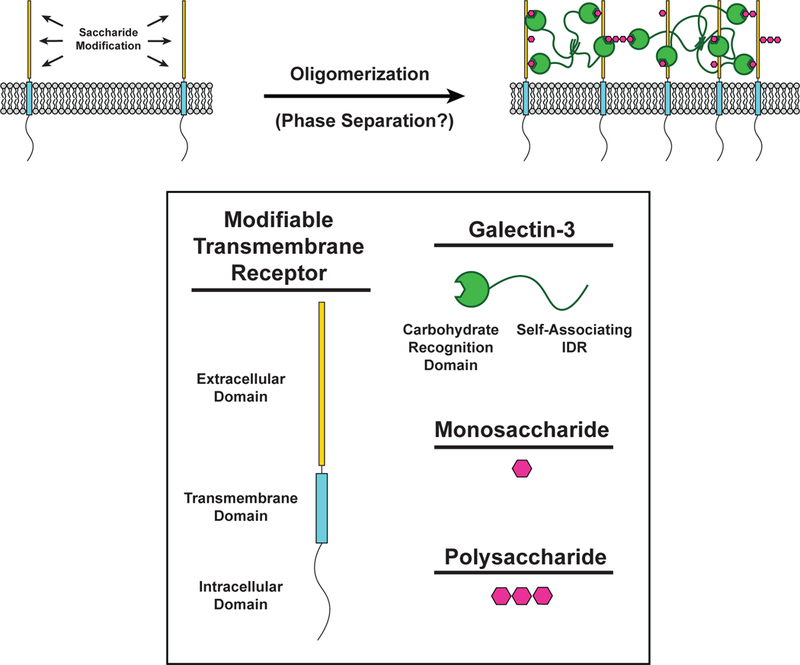
Carbohydrate-lectin interactions in receptor oligomerization. (LEFT) The extracellular domain of transmembrane receptors (Yellow) can be modified with monosaccharides and polysaccharides (Magenta) to create binding sites for the carbohydrate recognition domain (CRD) of Galectin-3 (Green). (RIGHT) Saccharide-modified transmembrane receptors are bound by the CRD of Galectin-3. The intrinsically disordered region (IDR) of Galectin-3 self-associates with other IDRs of neighboring Galectin-3 molecules to form a multivalent network of modified transmembrane receptors and Galectin-3.
High valency interactions between polysaccharides and members of the galectin family of lectins often produce ordered, cross-linked lattices visible by electron microscopy (16). Galectin-3 is unique in its ability to form heterogeneous, disorganized complexes with multivalent carbohydrates (1). When mixed with multivalent carbohydrates in vitro, galectin-3 rapidly precipitates into micrometer-sized irregularly shaped clusters, as determined by solution-based turbidity assays and visualized by EM. This behavior is seen over a narrow range of carbohydrate concentrations and was suggested to represent a solid phase (1). In cells, galectin-3 organizes transmembrane receptors in clusters and slows their diffusion, consistent with more solid-like material properties (15). Recently, the disordered N-terminal domain of galectin-3 was shown to undergo LLPS through multivalent interactions with other galectin-3 carbohydrate recognition domains (92). Whether other galectins undergo similar phase transitions via multivalent self-association has not, to our knowledge, been investigated.
Concanavalin A (Con A), a tetrameric plant lectin also oligomerizes and precipitates when mixed with multivalent carbohydrates in vitro (30, 54). Engineered ligands have been used to determine features that promote Con A oligomerization. Linear oligomeric ligands most effectively promote clustering of Con A, and the shape of the ligands is an important factor for determining the rate of oligomerization and density of Con A in clusters (54). Furthermore, properties of the linkers between the saccharides, such as flexibility and length, also affect oligomerization (30, 39).
These multivalent saccharide-lectin interactions share many parallels with the multivalent protein-protein interactions described later in this review. The valency-dependent oligomerization and the crystalline organization of lectin-saccharide complexes suggest that glycosylated receptors could undergo sol-gel transitions at the membrane to form a uniform solid-like gel. Indeed, lectin clustering often reduces the local mobility of membrane receptors (15, 106). With the recent discovery that galectin-3 can undergo LLPS, more research is needed to determine whether phase separation plays a role in saccharide-lectin oligomer formation.
Immune Cell Receptors
Clusters of transmembrane receptors on immune cells have been observed for decades. Early studies showed that the receptors in both T cells (160, 168) and B cells (31) formed clusters on membranes in response to external stimuli. More recently, the transmembrane adaptor protein, LAT, was also observed to assemble into clusters following TCR activation (18). Below, we discuss how LLPS of LAT promotes downstream signaling and end with a brief discussion about the potential role of LLPS in other immune receptor clusters (Figure 3).
Figure 3.
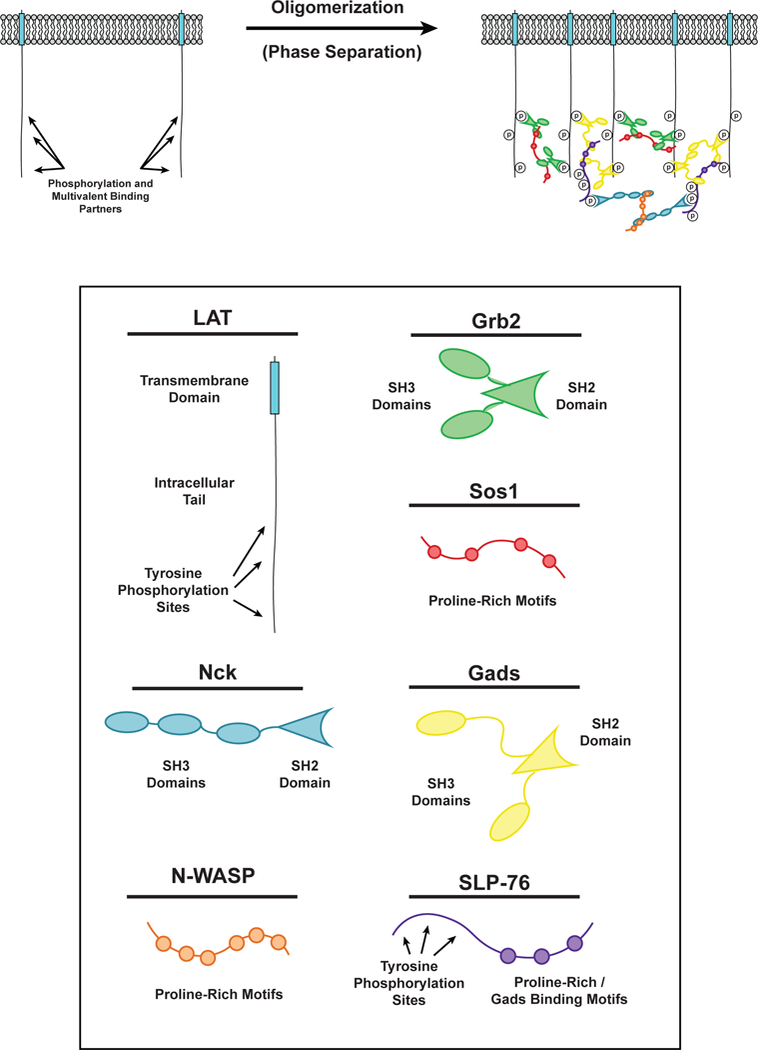
Phase separation of LAT in T cell signaling. (LEFT) LAT transmembrane adaptor proteins can be phosphorylated at three tyrosine residues in the disordered tail to created binding sites for the multivalent adaptor proteins Grb2 (Green) and Gads (Yellow). (RIGHT) Phosphorylated tyrosine residues on LAT can be bound by the SH2 domain of Grb2. The SH3 domains of Grb2 bind to proline-rich motifs of Sos1 (Red) to form a phase-separated clusters of LAT, Grb2, and Sos1. Similarly, phosphorylated tyrosine residues on LAT can be bound by the SH2 domain of Gads. The SH3 domains of Gads bind to proline-rich motifs and a RxxK motif in SLP-76 (Purple) to form a phase-separated cluster of LAT, Gads, and SLP-76. Phosphorylated tyrosine residues on SLP-76 can be bound by the SH2 domain of Nck (Cyan). The SH3 domains of Nck bind to the proline-rich motifs of N-WASP (Orange) to create another level of multivalent interactions that can contribute to the phase separation of LAT on the membrane of T cells.
LAT is a transmembrane adaptor protein that coordinates most of the proximal signals transmitted downstream of the T cell receptor at the immune synapse (the interface between an immune cell bound to a target cell, e.g. a T cell bound to an antigen presenting cell (APC)) (37). LAT is composed of a short extracellular element, a transmembrane helix, and a disordered intracellular region that contains nine tyrosine residues. Upon activation of the TCR, these tyrosines are phosphorylated by the Syk family kinase, ZAP-70 (175). Phosphorylation of the distal tyrosine residues of LAT is necessary for downstream signaling (4) and LAT cluster formation (Figure 3) (19, 140). These phospho-tyrosine (pTyr) residues are docking sites for multivalent Src Homology (SH) 2 / SH3 domain containing proteins, including PLC-γ, Grb2, and Gads (122). The proline-rich motifs (PRMs) of Sos1, a guanine nucleotide exchange factor (GEF) for the Ras GTPase, bind to Grb2 resulting in Ras activation (70, 75). The PRMs of SLP-76, an adaptor protein, bind to Gads, and SLP-76 phosphorylation by Zap-70 results in recruitment of the actin effectors Nck, WASP, and the Arp2/3 complex to polymerize actin filaments (9, 86, 140). Together, these molecules generate the main proximal outputs of TCR activation, stimulation of the Map kinase cascade (from Ras), calcium release and protein kinase C activation (from PLC-γ), and actin assembly (from the Arp2/3 complex).
The multivalency of the molecules enriched in LAT clusters suggested that their interactions could lead to LLPS and that this process might contribute to TCR signaling. Consistent with this idea, clustering of LAT at the T cell-APC interface requires multivalent interactions between LAT, Grb2, and Sos1 (68). Clustering is driven by interactions between phospho-LAT (pLAT), Grb2, and the proline-rich motifs (PRMs) of Sos1, independent of Sos1 RasGEF activity (83). Recent in vitro reconstitutions have demonstrated that, indeed, multivalent interactions between pLAT, Grb2, and Sos1 promote their LLPS, resulting in the formation of micron-scale clusters on membranes (Figure 1c) (70, 140). Multivalent interactions of pLAT with either Grb2 and Sos1, or Gads and SLP-76 are sufficient to promote this effect. Su and colleagues biochemically reconstituted the signaling pathway from the ζ-chain of the TCR to the Arp2/3 complex, including formation of clusters, and found that the clusters potently assembled actin filaments on the bilayer, similar to observations in activated T cells (86, 140).
In this work, clustering was shown to have three functional consequences. First, it increased the specific actin assembly activity of molecules in the pathway, demonstrating that phase separation not only produces changes in spatial organization, but also can alter biochemical activities. Second, the physical properties of phase separated LAT clusters resulted in the sorting of molecules through electrostatic attraction or repulsion; positively charged proteins concentrated in LAT clusters while negatively charged proteins were excluded, producing a distinct chemical environment from the surrounding membrane. Finally, in vitro phase separation of LAT and its binding partners was correlated with in vivo MAPK signaling in T cells, suggesting that clustering enhances MAPK activation.
A very recent study examined how composition regulates the interaction of LAT clusters with dynamic, actomyosin networks at the T cell plasma membrane (35). During activation of Jurkat T cells by α-TCR antibodies attached to a fluid lipid bilayer (mimicking an APC), LAT clusters form at the periphery of the cell-membrane interface and move radially toward its center through the actions of cortical actomyosin. Nck, and presumably its ligand, WASP, were found to dissociate from clusters approximately half-way through this trajectory. At this same position, the actin architecture changes from a peripheral branched network to a central network of actin arcs. In biochemical reconstitutions it was shown that Nck and the WASP homolog, N-WASP, contain basic regions that, at high density, act as clutches that couple LAT cluster movement to actomyosin movement. Thus, in cells, the dissociation of Nck/WASP likely weakens the association of LAT clusters with cortical actomyosin, and may be necessary to maintain radial movement through the two different actin networks. Consistent with this idea, clusters that constitutively contain a basic clutch were found to deviate from their normal trajectory from periphery to center of the cell-membrane interface. These observations show that cluster composition can be specifically tuned to control interactions with the local environment (in this case, cortical actomyosin), a principle that likely applies to many signaling pathways.
The TCR also forms clusters following engagement with an MHC-peptide complex on an APC (36). Super-resolution microscopy shows that the TCR localizes at LAT clusters (41), suggesting TCR clusters mostly associate with LAT clusters. Yet no concrete evidence exists for a direct, stable molecular connection between the TCR and LAT. However, a recent in vitro study showed that LAT can be semi-processively phosphorylated on membranes by TCR-bound ZAP-70 (69). As LAT is phosphorylated, Grb2 may be able to bind its phosphotyrosine residues and drive LLPS before LAT can laterally diffuse away from the TCR-ZAP-70 complex. In this way, semi-processive phosphorylation of LAT in the vicinity of activated TCRs could explain the observed co-localization of activated TCR clusters with LAT clusters, in the absence of direct binding of the two proteins. TCR clustering appears to be important for intracellular signaling, since stable ZAP-70 recruitment to the TCR requires TCR clustering; ZAP-70 transiently associates with a single TCR molecules but persistently localizes with TCR clusters (144). Thus, clustering of TCRs is potentially a mechanism that enables signal amplification by kinetic proofreading, a mechanism used by the T cell to discriminate between agonist and endogenous ligands to ensure that the T cell mounts a specific response to an APC (25, 100).
In addition to LAT and the TCR, many other immune receptors form clusters, some of which can co-localize. For example, during T cell activation, PD-1 and CD28 clusters are mostly co-localized, while PD-1 and TCR clusters are only partially co-localized (71). Co-localization of receptor clusters likely has functional consequences, since PD-1 clusters recruit the phosphatase Shp2, which preferentially dephosphorylates CD28 over the TCR (71). Thus, T cells can sort clusters to regulate downstream signaling. However, the mechanisms by which PD-1 and CD28 receptors form clusters and how the clusters co-localize are currently unknown. It is likely that other clusters of immune receptors on T cells, B cells, and other types of immune cells form with the same general principles described above for LAT and the TCR. Many of these receptors bind to multivalent adaptor proteins including Grb2, Gads, and Nck (17, 24, 152, 158). Further investigations are needed to understand the role of LLPS in clustering, interactions, and functions of immune cell receptors.
Cell adhesion receptors
In multicellular organisms, adhesion receptors allow cells to interact with and acquire information from their environments. Receptors mediate adhesion both to other cells (e.g. by IG domain containing proteins), and to the extracellular matrix (ECM) (by integrins). Cell adhesion molecules often form micron-sized clusters on the plasma membrane (23) with many cytoplasmic adaptor proteins, physically and functionally coupling to diverse downstream signaling pathways including MAPK, JNK, Rac, and the actin cytoskeleton (85). There is a growing body of evidence that many of these cell adhesion clusters may form through LLPS.
Nephrin and the glomerular filtration barrier
Nephrin is a transmembrane receptor that regulates cell-cell adhesion in podocyte cells of the kidney to form the glomerular filtration barrier (98). It is a member of a large family of cell adhesion molecules that participate in diverse processes including muscle formation, tissue patterning, and synaptogenesis (116). The extracellular domain of nephrin is composed of eight IgG-like motifs and a fibronectin (FN) type III domain (98). Nephrin extracellular domains on adjacent cells interact in homotypic fashion to form the slit diaphragm, a size-selective intercellular barrier that is the final element of the kidney filter (98).
Upon stimuli including crosslinking by antibodies and nearby integrin engagement the nephrin cytoplasmic domain can be phosphorylated by Src family kinases on up to 5 tyrosine residues (154, 155). Three of these tyrosine residues are consensus binding motifs for the SH2 domain of the adaptor protein Nck (pYDXV) (6, 14, 154). Recruitment of Nck to nephrin is required for local remodeling of the actin cytoskeleton and proper maintenance of the kidney filtration barrier (78). In addition to its SH2 domain, Nck has three SH3 domains, which bind the numerous (six to nine) PRMs of N-WASP, leading to actin assembly at sites of nephrin adhesion (14), which appears to be necessary for maintenance of the slit diaphragm (78). Nephrin phosphorylation increases following kidney injury (79, 108, 154), and mutation of the phosphorylation sites causes progressive kidney disease in mice (108), demonstrating the physiologic importance of these modifications.
The multivalency of these interactions suggests the potential for assembly and concomitant phase separation (Figure 4). Indeed, in both three-dimensional solution and when the phosphorylated intracellular tail of nephrin is attached to supported lipid bilayers, addition of Nck and N-WASP leads to LLPS, forming liquid droplets in solution and micrometer-scale clusters on membranes, respectively (6, 7, 91). Induced phosphorylation of transmembrane Nephrin in cells also leads to formation of micron-scale clusters at the plasma membrane, and larger, dense clusters at the cell periphery (82).
Figure 4.
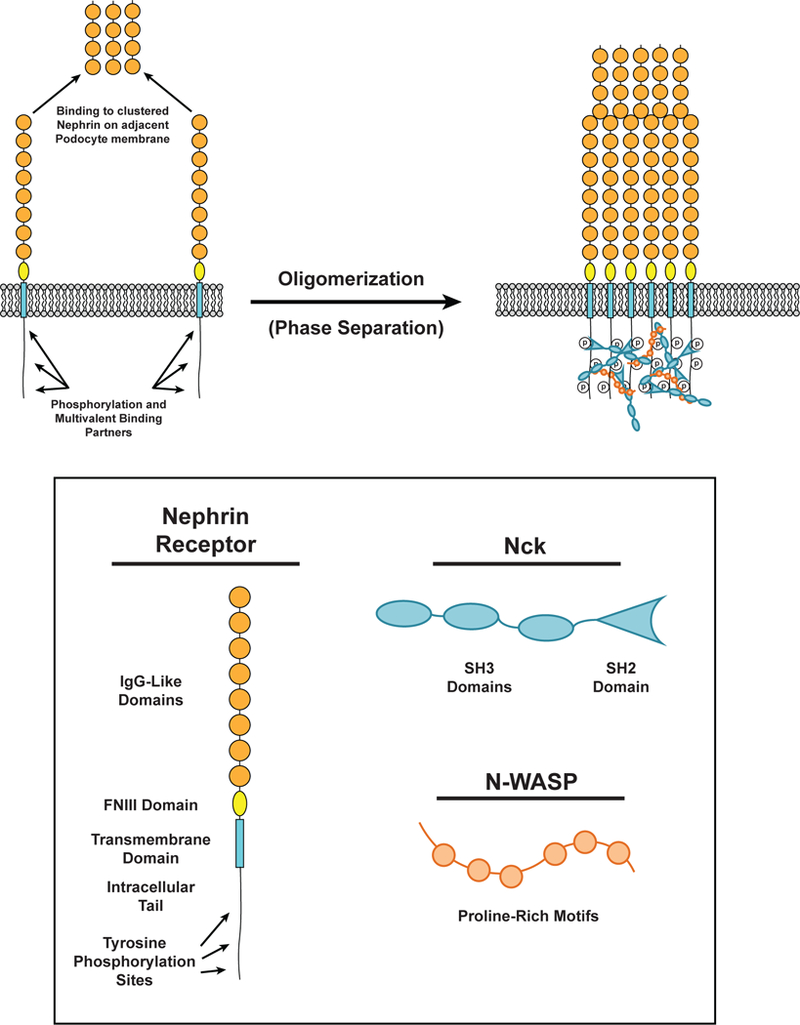
Phase separation in Nephrin in kidney podocytes. (LEFT) The distal-most IgG-like extracellular domain (Orange) of nephrin receptors can bind the distal-most IgG-like extracellular domain of nephrin receptors on the apposing podocyte membrane. The intracellular tail of nephrin is phosphorylated at three tyrosine residues. These phosphorylated residues can be bound by Nck (Cyan). (RIGHT) Phosphorylated tyrosine residues on nephrin can be bound by the SH2 domain of Nck. Nck SH3 domains bind to proline-rich motifs on N-WASP (Orange) to form a phase-separated cluster of nephrin, Nck, and N-WASP. Extracellular interactions between the distal-most IgG-like domains of nephrin also contribute to cluster formation.
In both biochemical and cellular settings, nephrin clusters are consistent with phase separated structures. In vitro, for fixed density/concentration of p-nephrin/N-WASP, clustering occurs sharply above a threshold concentration of Nck. Decreasing the number of pTyr sites on nephrin or SH3 domains of Nck raises the threshold. Paralleling these behaviors, in cells the probability of observing nephrin clusters increases with expression level of Nck, in a manner dependent on SH3 valency (82). In both settings, clusters exhibit liquid-like dynamics including the ability to fuse, the rapid rearrangement of molecules within clusters, and the rapid exchange of molecules between clusters and the surrounding solution or membrane (6, 82).
Local assembly of the actin cytoskeleton at nephrin clusters is important for proper maintenance of the filtration barrier in podocytes (78, 98). In vitro reconstitution experiments suggest that LLPS of nephrin, Nck, and N-WASP may play an important role in stimulating this Arp2/3 complex-dependent actin polymerization (6). Recent quantitative analyses of this activity have revealed that actin assembles preferentially on clusters not only because N-WASP is concentrated there, but also because the specific activities (actin polymerization rate per molecule) of N-WASP and the Arp2/3 complex are higher there than in surrounding regions of the membrane (Case et al., submitted). This increased specific activity is due to increased membrane dwell time of N-WASP and the Arp2/3 complex, which results from the highly crosslinked nature of the phospho-Nephrin/Nck/N-WASP assembly (Case et al., submitted). A similar dependence of activity on membrane dwell time has also been observed for clusters formed by pLAT, Grb2, and full length Sos1, which activate Ras ((70); Huang et al., submitted). In both systems, activity is mediated by slow, multi-step, non-equilibrium processes, suggesting that the dwell time dependence is due to effects analogous to kinetic proofreading (67, 70, 100). Since many transmembrane receptors signal through analogous multi-step, non-equilibrium pathways and assemble into clusters through multivalent crosslinking, this enhancement of signaling by LLPS may be quite general. It remains an interesting question whether, as in classical kinetic proofreading during protein and DNA synthesis, the dwell time dependence in signaling systems could also be used to generate specificity for one downstream output over another.
Tight junctions
Epithelial monolayers are maintained by several types of cell-cell junctions including adherens junctions, desmosomes, and tight junctions (52). Tight junctions are the apical-most structure and are responsible for establishing the paracellular seal that creates a selectively permeable barrier (52). Tight junctions visualized with freeze fracture electron microscopy appear as rows of punctate membrane contacts (150). By light microscopy, tight junctions appear as a discrete band containing numerous transmembrane and multivalent cytoplasmic adaptor proteins, many of which rapidly exchange with the cytoplasmic pool (134). The visible appearance, rapid dynamics, and nature of the molecular components suggest that LLPS could play a role in forming tight junctions.
Tight junctions contain three classes of integral membrane proteins: Claudins, Tight Junction-Associated Marvel domain-containing Proteins (TAMPs), and Junctional Adhesion Molecules (JAMs) (150). Claudins are the main mediators of the paracellular seal, while JAMs, such as JAM-A, and TAMPs, such as Occludin, seem to have more redundant or overlapping functions in regulating permeability. Crosslinking and native PAGE shows that Claudin-2 forms homodimers, likely through interactions between transmembrane domains, while Claudin-4 and Occludin are monomers (151). The short C-terminal intracellular tails of Claudins and JAMs bind to PDZ domains in cytoplasmic ligands while the tail of Occludin binds to positively charged surfaces of its ligands (111).
Most of the cytoplasmic ligands of these receptors contain multiple modular interaction domains. ZO-1 and ZO-2 contain three N-terminal PDZ domains, a central SH3 domain, a region with homology to guanylate kinase, and a C-terminal actin-binding region (42). Deletion of ZO-1 and ZO-2 abolishes tight junction formation, suggesting these multivalent binding partners are essential for tight junction assembly (149). Relatedly, PSD-95, a postsynaptic density protein with similar domain structure to ZO-1 undergoes LLPS in vitro when mixed with SynGAP, a coiled-coiled trimer with PDZ binding motifs (171, 172). These studies provide the first evidence that multivalent interactions between PDZ domains and PDZ binding motifs are sufficient to drive LLPS in some contexts.
In addition to ZO-1 and ZO-2, there are many other multivalent binding partners at tight junctions including MUPP-1 (13 PDZ domains), MAGI-1 (two WW domains and six PDZ domains), PAT-J (ten PDZ domains), AF-6 (two Ras-binding domains, one PDZ domain, and an actin-binding domain), Cingulin (a homodimer with a globular head domain that mediates protein interactions), and Amotl1 (glutamine rich domain similar to those driving LLPS in other proteins (174), coiled-coiled domain and one PDZ binding motif) (133). We speculate that LLPS of these scaffolds with their transmembrane binding partners could promote tight junction formation. FRAP data suggest that while Claudins are stably localized at tight junctions, Occludins freely diffuse in the membrane and ZO-1 dynamically exchanges with the cytoplasmic pool (134). Such heterogeneity in dynamic behavior has been observed in three-dimensional biomolecular condensates (159) and may occur in membrane-associated clusters as well. Additionally, ZO-1 dynamics are actively regulated by the actin cytoskeleton. When myosin contractility is inhibited or the ZO-1 actin-binding domain is deleted, ZO-1 becomes much more stably associated with tight junctions (169). Analogous behavior has been observed in other contexts. For example, uncoupling N-WASP from the Arp2/3 complex decreases the rate of N-WASP turnover in signaling clusters generated by vaccinia virus (161), and abrogating ATP-hydrolysis by the RNA helicase Dhh1 greatly slows its exchange between P body condensates and the cytoplasm (21). The control of dynamics by active processes is another theme likely to span three-dimensional and membrane-associated clusters (5).
Integrins and focal adhesions
Integrins are transmembrane receptors that mediate cell adhesion to multivalent ECM ligands such as fibronectin and collagen (74). Integrins are obligate heterodimers composed of an α and a β subunit. Integrins have large extracellular domains that impart ligand specificity and small (20–50 amino acid) cytoplasmic tails that mediate intracellular signaling. Integrins form clusters, termed focal adhesions, with numerous cytoplasmic adaptor proteins, kinases, and actin binding proteins that connect cells with their surroundings. Focal adhesions serve as sites of force transmission between the intracellular actin cytoskeleton and the ECM to drive tissue morphogenesis, cell movement, and ECM remodeling. They act as signaling hubs to control the cell cycle, and cell differentiation and death. Thus, focal adhesions mediate an array of functions involving biochemical and physical interactions between the cell and its environment.
Integrins lack catalytic activity and do not directly bind actin. Rather, they signal by recruiting cytoplasmic proteins such as kindlin, talin, vinculin, FAK, paxillin, α-actinin, and p130Cas (23). Focal adhesions initially form as small (< 200 nm diameter) “nascent” clusters that disassemble within ~1 min at the cell edge. A subset of these clusters become stabilized and mature through force-dependent growth and compositional changes. FRAP experiments reveal that kindlin, paxillin, FAK, and α-actinin rapidly exchange with the cytoplasm, while talin, vinculin, and tensin are less dynamic when cells are plated on stiff substrates (139). Neighboring focal adhesions occasionally fuse (141, 146). The cellular properties and molecular construction of focal adhesions suggests that LLPS may contribute to their formation and function (Figure 5).
Figure 5.
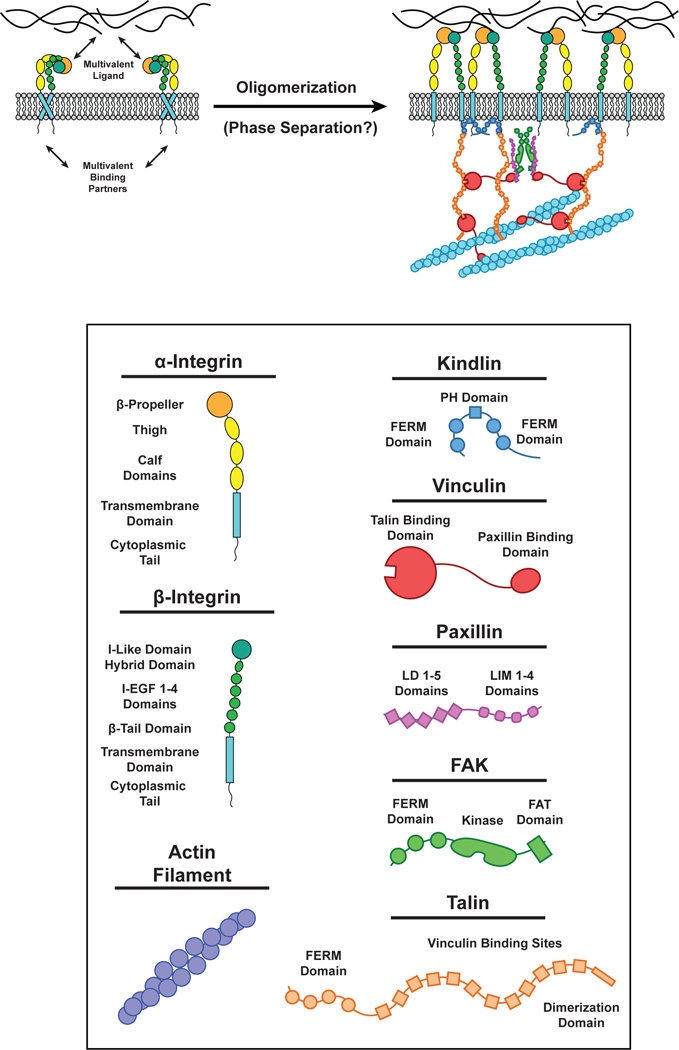
Multivalent interactions at Focal Adhesions. (LEFT) Integrin receptors in the inactive conformation. (RIGHT) Integrin receptors can be activated when the extracellular domain binds multivalent components of the extracellular matrix (ECM) and the intraceullar domain of β-integrin binds Kindlin (Blue) and/or Talin (Orange). Kindlin dimerizes by interactions in the FERM domain. Talin is composed of ten Vinculin (Red) binding sites and can bind actin filaments by a site in its C-terminus dimerization domain. Vinculin can bind Talin (via its N-terminal Talin binding domain) and Paxillin (Purple) LD motifs and actin filaments by a site on its C-terminus. Paxillin LD motifs can bind the Vinculin C-terminus and FAK FAT domains. FAK (Green) can dimerize by interactions between its FERM domains. Each of these unique interactions between multiple proteins results in the formation of a highly interconnected oligomeric protein network in focal adhesions.
There is evidence that multivalent interactions with extracellular ligands and intracellular adaptor proteins are both required for integrin clustering and focal adhesion formation (27). Soluble, monovalent ligands are unable to induce integrin cluster formation, while multivalent ligands or monovalent ligands added in tandem with multivalent antibodies promote integrin clustering (27, 102). Knocking out the cytoplasmic adaptors talin and kindlin inhibits integrin cluster formation, even in the presence of multivalent ligand (145). Thus, multivalent extracellular ligands are necessary, but not sufficient, for integrin cluster formation. Focal adhesions are enriched with a variety of intracellular multivalent proteins, many of which interact with each other (164). For example, talin and paxillin bind to different regions of vinculin, and each contains numerous vinculin binding sites (22). Talin also contains a single binding site for paxillin (170). These interactions provide a possible mechanism for vinculin to build higher-order talin/paxillin assemblies. Most of talin’s twelve vinculin binding sites are only are exposed when talin is stretched by force (32). Thus, the valency of talin can be increased by mechanical force, likely contributing to the known force-dependence of focal adhesion size (23). Paxillin, FAK, and p130Cas have also been observed to form complexes (64). FAK and paxillin have multiple binding sites for each other (124), the FAK C-terminus also contains multiple PRMs that bind the SH3 domain of p130Cas (59), and paxillin can bind the p130Cas C-terminus (173). p130Cas contains 15 tyrosine residues that, when phosphorylated, are binding sites for the SH2 domain of either Nck or Crk (11). As described above, these adaptors, in turn can bind to multivalent PRM proteins such as N-WASP, C3G, and EPS15, resulting in LLPS (125). These molecular interactions and the physical properties of focal adhesions suggest that LLPS may be a significant contributor to these cellular structures.
Super-resolution imaging indicates that focal adhesions do not behave as simple fluids, in that they are not isotropic in all dimensions. Rather they are spatially organized to form a stratified structure (80). Some proteins, such as paxillin and FAK, localize with integrins near the plasma membrane while others, such as actin, VASP and Zyxin, localize ~30 nm above the membrane. Talin, which has a long, rod-like structure, is oriented with its N-terminus co-localized with integrin and its C-terminus co-localized with actin. The length of talin regulates the distance between actin and the membrane and thus may control the thickness of focal adhesions perpendicular to the membrane (94). Thus, focal adhesions may behave more akin to liquid crystals, with disorder in two dimensions and order in the third (note, however, that because this order likely arises because of directionality imparted by the membrane rather than spontaneously, focal adhesions are not liquid crystals in a formal sense). Recent work has shown that in response to mechanical forces the extracellular domains of integrins also orient in a direction parallel to the membrane (142), suggesting that under force, focal adhesions may also become anisotropic in this direction as well. Super-resolution imaging of other membrane-associated clusters will be needed to determine whether anisotropic organization is generally observed.
Canonical Wnt signaling
Wnts are secreted proteins that bind to the extracellular domain of Frizzled (Fz) and LRP5/6 transmembrane receptors to regulate many aspects of development and adult homeostasis (137). In canonical Wnt signaling, binding of Wnt to Fz and LRP5/6 induces receptor heterodimerization and triggers clustering of the receptors into a macromolecular assembly that inhibits the degradation of β-catenin (29, 137). In the absence of Wnt, β-catenin is constantly targeted for proteasome-mediated degradation by the cytoplasmic β-catenin destruction complex. The Fz / LRP5/6 assembly on the membrane shares many molecular components with the destruction complex, including Axin and GSK3. While the exact mechanism by which Wnt signaling inhibits β-catenin degradation remains unclear, evidence suggests that activated Fz / LRP5/6 clusters either titrate components away from the destruction complex or directly inhibit the activity of the destruction complex (137). Following Wnt binding, Fz and LRP5/6 bind to multivalent cytoplasmic adaptors Disheveled (Dvl) and Axin as well as many other effectors, such as GSK3 and Cdk14, to form clusters (137).
When Dvl is overexpressed in tissue culture cells or is at high levels in cancer, liquid-like Dvl condensates are observed in the cytoplasm (127, 136). These condensates can undergo fusion and their components rapidly exchange with the cytoplasm (127). Both Dvl and Axin contain DIX domains, which interact in a weak, head to tail fashion that promotes assembly of either homotypic or heterotypic polymers (44, 126). Dvl also contains a DEP domain that mediates high affinity homodimerization, effectively crosslinking the DIX domain polymers into higher-order structures (51). The formation of cytoplasmic Dvl condensates and subsequent inhibition of β-catenin degradation requires both assembly of the Dvl and Axin DIX domains (44, 126) as well as crosslinking by the Dvl DEP domain (51). Thus, crosslinking of heterotypic Dvl-Axin polymers allows for higher-order oligomerization and subsequent LLPS (51).
Although Dvl forms cytoplasmic condensates when expressed at high levels, at endogenous levels in healthy cells the protein localizes predominantly to two-dimensional clusters containing Wnt, Fz, LRP, Axin, and GSK3 on the plasma membrane (57, 136). Membrane association is mediated by interactions of the DEP domain and C-terminus of Dvl with the intracellular domain of Fz, as well as potential interactions between Axin and GSK3 and the intracellular domain of LRP5/6 (29, 97, 101, 143). Thus, in normal canonical Wnt signaling, LLPS of Dvl and Axin polymers promotes formation of two-dimensional clusters on the plasma membrane. The mechanistic lessons learned from the three-dimensional condensates likely apply to the two-dimensional membrane system as well (Figure 6).
Figure 6.
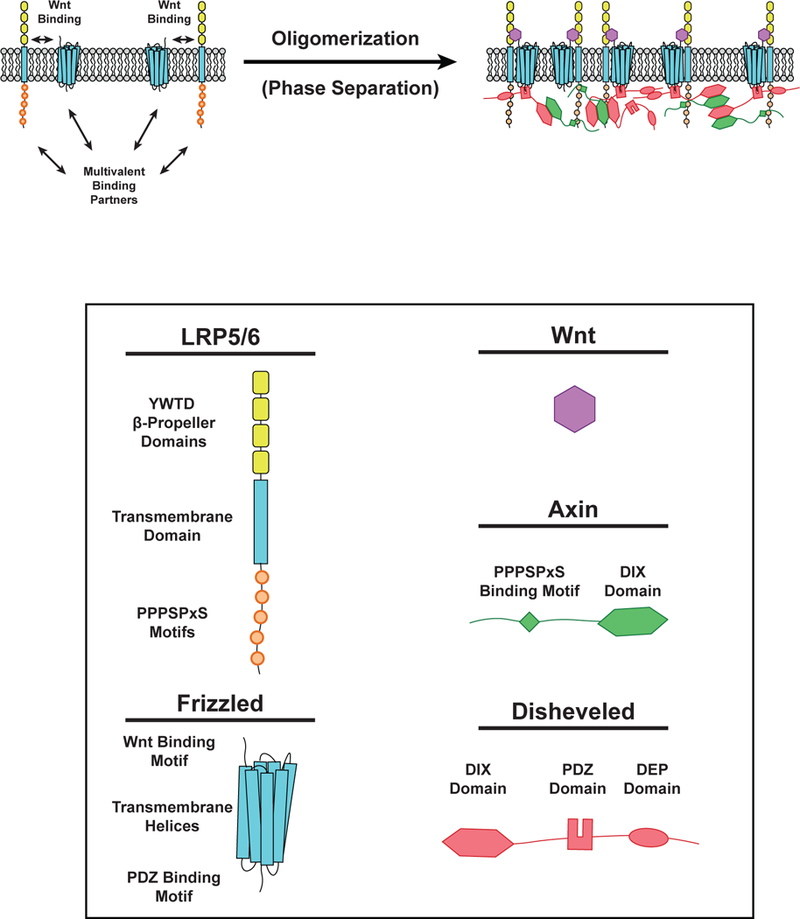
Phase separation in Wnt Signaling. (LEFT) Wnt (Purple) can bind to LRP5/6 (single-pass transmembrane receptor) and Frizzled (Fz) receptors (multipass transmembrane receptor) to initiate Wnt signaling. LRP5/6 can bind Axin (Green) while Fz can bind Disheveled (Dvl, Red). These interactions induce the formation of phase-separated clusters on the cell membrane. (RIGHT) Following Wnt binding, phosphorylation of PPPSPxS motifs on LRP5/6 enables binding of Axin via an undefined region of its C-terminus. Disheveled can bind the Fz receptor intracellular tail by its PDZ domain. Polymerization of DIX domains in both Axin and Dvl promote clustering of membrane-associated proteins at Fz and LRP5/6 receptors. Dvl also contains a C-terminal dimerization domain that enhances multivalent interactions within the Wnt signalosome.
Receptor Tyrosine Kinases
Many receptor tyrosine kinases (RTKs) form clusters in response to extracellular stimuli (99). Here, we focus on the most abundant class of RTKs, ephrin (Eph) receptors (77, 129) and suggest that principles governing cluster formation of Eph receptors can be applied to other RTKs (Figure 7).
Figure 7.
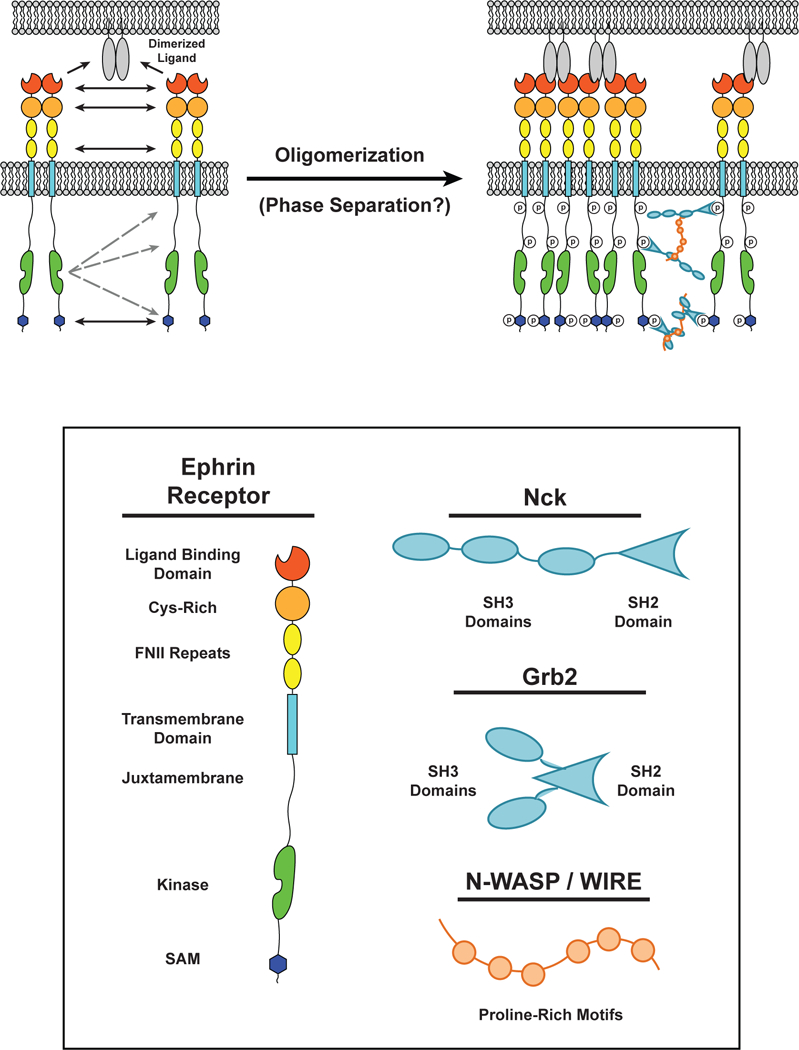
Multiple interactions drive dimerization and cluster formation of ephrin receptors. (LEFT) Interactions between two ephrin receptor EC ligand binding domains (LBDs), Cys-rich domains, and fibronectin-like (FN) domains can result in ephrin receptor dimerization in the absence of ligands. Dimerized ligands expressed on the surface of an apposing cell can seed cluster formation. (RIGHT) Upon binding to a ligand, the kinase domain of one ephrin receptor can transphosphorylate intracellular tyrosine residues on surrounding receptors. These residues can serve as docking sites for Src Homolgy (SH) SH2 / SH3 adaptor proteins, such as Grb2 and Nck, which can oligomerize through multivalent interactions with proteins containing Proline-Rich Motifs (PRMs), such as N-WASP. The intracellular Sterile Alpha Motifs (SAMs) also self-associate to promote receptor oligomerization.
Eph receptors regulate cell-cell recognition in the nervous, vascular, immune, and skeletal systems (87). Ephrin receptors bind to GPI-anchored ephrin A or transmembrane ephrin B on neighboring cells (77). Upon ligand binding, Eph receptors oligomerize into clusters that initiate and sustain downstream signal transduction (110). Both extracellular and intracellular multivalent interactions promote assembly of Eph receptors (Figure 7).
Eph receptors contain four extracellular (EC) domains (the ligand binding domain (LBD), a cysteine-rich domain, and two fibronectin (FN) repeats), a single-pass transmembrane helix, and up to four intracellular domains (the juxtamembrane region, a tyrosine kinase domain, a sterile alpha motif (SAM), and a PTB-domain binding motif (PBM)) (77). Crystal structures have been determined for complexes of the full EC domains bound to ephrin ligands (63, 129). These revealed interactions between the LBD and ephrin previously observed in complexes of the isolated domains (62). However, extensive additional contacts were observed in the crystal lattice between neighboring receptors and/or ligands. These involved conserved surfaces of the receptor LBD, cysteine-rich domain, and FN domains. These contacts generate a complex network of both parallel and antiparallel receptor arrays. As organized on the cell surface, these interactions would occur both in trans (between cells) and in cis (on a given cell membrane). Mutation of either the trans or cis interfaces prevented the formation of Eph receptor oligomers in cells, leading to a model wherein EC domain interactions between receptors on the same cellular membrane could ‘seed’ Eph receptor oligomer formation and macroscopic assembly (Figure 7).
Intracellular regions of the Eph receptors likely also contribute to assembly and potentially LLPS. Upon extracellular ligand binding, the intracellular domains are phosphorylated on several tyrosine residues by the kinase domain to create docking sites for the multivalent SH2 / SH3 adaptor proteins Grb2, CrkII, and Nck (103, 109). These, in turn bind to PRMs within the proteins WIRE and N-WASP (103). As described above for nephrin, such interactions have a strong propensity to drive LLPS (6). In addition, the SAM of Eph receptors may dimerize or oligomerize, as observed for other SAMs (10), further contributing to receptor assembly (Figure 7). These domains and interactions raise the possibility that Eph receptor clusters are phase-separated compartments on cell membranes. In support of this, a recent study used computational modeling and quantitative microscopy to demonstrate that Eph receptor clusters initially form through addition of individual Eph receptors. However, these smaller clusters then merge to form larger clusters (113), a behavior that is consistent with LLPS (6). Further investigation is needed to determine whether Eph receptor clusters are indeed formed through phase separation and how LLPS may regulate functional consequences of Eph receptor cluster formation in human health and disease.
Conclusions and Perspectives
Cell biological studies have demonstrated that many receptors assemble into large clusters upon activation, and in some cases this higher-order assembly has been shown to be important for downstream signaling. Several themes emerge from consideration of many such systems. First, clustering is often mediated by multivalent interactions capable of generating networks consistent with ideas from classical polymer chemistry. Second, these multivalent interactions occur in both the extracellular and intracellular regions of the receptors, often through heterotypic interactions and sometimes also through homotypic interactions. Third, a frequently observed molecular mechanism for clustering involves a multiply tyrosine-phosphorylated disordered protein (either a receptor cytoplasmic tail or a proximal adaptor protein), which interacts directly with proteins containing an SH2 domain and multiple SH3 domains, which in turn bind proteins with large proline rich regions containing multiple SH3 binding motifs. For some receptor-ligand combinations, these interactions have been shown to promote macroscopic phase separation in vitro, with correlated behavior in cells. For others we have argued that a role for phase separation is likely based on the behavior of the cellular clusters and the features of the molecules that form them.
Two interesting topics that have not yet been extensively explored in this area are the relationship between protein phase separation and lipid phase separation, and the regulation and functional importance of receptor cluster size. We discuss these below in the context of future directions for studies of the biophysics, biochemistry, and cell biology of receptor signaling.
Phase separation of lipids in the plasma membrane
Like many macromolecules, lipids have the ability to phase separate under certain conditions (60). The mechanisms that regulate the self-organization of lipids have been intensely studied using cellular and in vitro experimental approaches and computational modeling (20, 66, 90, 93). In vitro experimentation using planar supported lipid bilayers (SLBs) and giant unilamellar vesicles (GUVs) has revealed that lipid mixtures containing combinations of sterols, saturated, and unstaturated lipids will spontaneously undergo liquid-liquid phase separation (LLPS) to form macroscopic phase-separated domains (65). Sterols and saturated lipids partition into liquid ordered (LO) domains while unsaturated lipids partition into liquid disordered (LD) domains (33, 162). Similar phenomena have been observed using giant plasma membrane vesicles (GPMVs) that are blebbed from cells, and thus are composed of representative cellular lipid and membrane-associated protein mixtures (12, 89, 153). Thus, the plasma membrane of cells contains components that are capable of demixing into separate lipid domains (131). However, long-lived, micron-scale domains like those observed in SLBs, GUVs, GPMVs, have not been generally observed in the plasma membranes of mammalian cells (although they have been observed in some specialized membranes, including the yeast vacuole and erythrocyte plasma membrane) (88, 119, 148, 156). Rather, super-resolution light microscopy and environmentally sensitive membrane dyes have revealed the presence of short-lived, nanoscopic (< 100 nm) domains in the plasma membrane of mammalian cells (40, 115), suggesting that active processes prevent spontaneous formation of large membrane domains. In fact, several studies have demonstrated that the cortical actin cytoskeleton plays an active role in modulating membrane organization of lipids and proteins (55, 56, 117). These data indicate that ATP-dependent actomyosin contraction, rather than passive lipid phase separation, may drive lipid clustering and define the size and dynamics of lipid domains on the plasma membrane.
In addition to interactions with extracellular ligands and intracellular adaptor proteins, transmembrane receptors can also interact with lipids in the plasma membrane. Thus, lipid phase separation and protein phase separation should be coupled—the phase separation of membrane lipids should influence the phase separation of transmembrane receptors, and vice-versa. Indeed, self-organization of lipids within membranes is able to modulate protein localization within lipid domains (95, 117). Lorent and colleagues (95) report that both the surface area and length of the transmembrane domain and the palmitoylation state of membrane-associated proteins contribute to the targeting of proteins to either LO or LD regions of membranes. Using a combination of computational modeling, in vitro experimentation with cell-derived GPMVs, and live-cell experiments, these authors predict and describe how proteins that are palmitoylated and have long, narrow transmembrane domains are more likely to associate with LO domains that are tightly packed with saturated lipids, sterols, and glycosylated lipids. Concentrating like-proteins to specific lipids should prime receptors for phase separation prior to receiving any extracellular signal, essentially decreasing the degree of protein-mediated crosslinking needed to induce LLPS. Similarly, phase separation of transmembrane proteins through protein interactions should locally favor the formation of a lipid domain. In both aspects, the coupling of protein and lipid phase separation will enhance formation of membrane compartments with distinct physical properties and chemical compositions from the surroundings. Moreover, since both lipids and proteins can be linked to the actin cytoskeleton, actomyosin contraction could further modulate, in some cases even dominate, the formation and dynamics of these compartments.
With recent technological advances in in vitro biochemical reconstitutions and in vivo microscopic imaging, we can now begin to experimentally address how the propensities of both lipids and proteins to phase separate, and the ability of each to interact with the actin cytoskeleton, can act together to organize the plasma membrane. Such work will be essential for understanding the physical mechanisms of transmembrane signal transduction.
Receptor cluster size
In vitro, membrane-associated protein LLPS produces clusters that have decaying size distributions, consistent with thermodynamic control (6). The distribution at any given time is dependent on a number of factors, including the rates of nucleation, association and dissociation of molecules, and cluster fusion and lateral mobility (8). However, many integral membrane proteins have been observed to form clusters in cells with peaked distributions (i.e. with a preferred size; e.g. (26, 121, 146)). This could be explained by so-called equilibrium cluster phases, which produce clusters of discrete sizes through a combination of short range attraction and long range repulsion (128, 138). Alternatively, active processes may control size distributions. The actin cytoskeleton and proteins adhered to it can form barriers that restrain molecular movement, which could modulate cluster sizes (43, 49, 50). In vitro, actomyosin dynamics can alter cluster size distributions and drive motion of clusters (35, 82), behaviors that could also be relevant in cells. For GPI anchored proteins, and likely some transmembrane receptors as well, actomyosin contraction provides an active mechanism, distinct from phase separation, to produce clusters (56, 84, 117), which would also produce different size distributions. Finally, a recent theoretical model suggests that competing enzymes (e.g. a kinase and a phosphatase), if they favor and disfavor phase separation, respectively, could give peaked distributions of cluster sizes, whose means are defined by the relative rates of the two enzymes (166). Active processes are an important area of investigation of biomolecular condensates in general, and should also be examined in the context of membrane systems.
How might cluster size impact the biochemical and cellular activities of clusters? Molecules in the center of a cluster are in a different chemical environment than those at the periphery (as manifest, for example, in macroscopic surface tension). This could impart different activities to molecules in the center than at the periphery (note that the size scale of “center” and “periphery” will depend on the specific system, and perhaps on the particular activity being measured). In small clusters, the average specific activity of the cluster as a whole will thus change as size increases, since the fraction of central molecules increases with size. But for larger clusters the fraction of central molecules becomes asymptotic with size. In this regime, the average specific activity of the cluster will not change appreciably with size for reactions without diffusible intermediates. However, for reactions with diffusible intermediates, size is still likely to be important in this larger range. Examples include signaling systems where intermediates dissociate from the membrane and then rebind before diffusing away (76, 112). In such cases, a larger cluster will increase the probability that the intermediate will encounter other cluster molecules before diffusing away, thus increasing specific activity. Finally, larger clusters could transmit greater amounts of force than smaller clusters. This could provide a physical justification for the increasing size of focal adhesions in the presence of increasing forces (23, 120), and may be relevant for other systems, such as LAT clusters in T cells and B cells, which respond to and transmit cytoskeletal forces as well (35, 68). Further analyses of the size distributions of clusters and the relationship between size and activity will be necessary to explore these various possibilities.
In conclusion, recent investigations demonstrate that LLPS could be a general mechanism by which cell membrane receptors assemble into clusters. While LLPS has only been demonstrated for a small number of signaling systems, many cell surface receptors share molecular attributes that could promote phase separation, such as binding to multivalent extracellular and intracellular ligands. Additionally, many receptors have cellular behaviors consistent with phase separation, such as forming dynamic, nanometer- to micron-scale structures. Future investigations of transmembrane signaling should thus consider phase separation as a potential mechanism underlying the formation, regulation and function of clusters of cell-surface receptors.
References
- 1.Ahmad N, Gabius H-J, André S, Kaltner H, Sabesan S, et al. 2004. Galectin-3 Precipitates as a Pentamer with Synthetic Multivalent Carbohydrates and Forms Heterogeneous Cross-linked Complexes. J. Biol. Chem. 279(12):10841–47 [DOI] [PubMed] [Google Scholar]
- 2.Asherie N, Pande J, Lomakin A, Ogun O, Hanson SRA, et al. 1998. Oligomerization and phase separation in globular protein solutions. Biophys. Chem. 75(3):213–27 [DOI] [PubMed] [Google Scholar]
- 3.Atanasova M, Whitty A. 2012. Understanding cytokine and growth factor receptor activation mechanisms. Crit. Rev. Biochem. Mol. Biol. 47(6):502–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balagopalan L, Kortum RL, Coussens NP, Barr VA, Samelson LE. 2015. The Linker for Activation of T Cells (LAT) Signaling Hub: From Signaling Complexes to Microclusters. J. Biol. Chem. 290(44):26422–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banani SF, Lee HO, Hyman AA, Rosen MK. 2017. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18(5):285–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banjade S, Rosen MK. 2014. Phase transitions of multivalent proteins can promote clustering of membrane receptors. Elife. 3:e04123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banjade S, Wu Q, Mittal A, Peeples WB, Pappu RV, Rosen MK. 2015. Conserved interdomain linker promotes phase separation of the multivalent adaptor protein Nck. Proc. Natl. Acad. Sci. U. S. A. 112(47):E6426–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barabasi AL, Stanley HE. 1995. Fractal concepts in surface growth. Cambridge Univ. Press [Google Scholar]
- 9.Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. 2005. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat. Immunol. 6(1):80–89 [DOI] [PubMed] [Google Scholar]
- 10.Baron MK, Boeckers TM, Vaida B, Faham S, Gingery M, et al. 2006. An Architectural Framework That May Lie at the Core of the Postsynaptic Density. Science. 311(5760):531 LP-535 [DOI] [PubMed] [Google Scholar]
- 11.Barrett A, Pellet-Many C, Zachary IC, Evans IM, Frankel P. 2013. p130Cas: a key signalling node in health and disease. Cell. Signal. 25(4):766–77 [DOI] [PubMed] [Google Scholar]
- 12.Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, et al. 2007. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl. Acad. Sci. U. S. A. 104(9):3165–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bienz M 2014. Signalosome assembly by domains undergoing dynamic head-to-tail polymerization. Trends Biochem. Sci. 39(10):487–95 [DOI] [PubMed] [Google Scholar]
- 14.Blasutig IM, New LA, Thanabalasuriar A, Dayarathna TK, Goudreault M, et al. 2008. Phosphorylated YDXV Motifs and Nck SH2/SH3 Adaptors Act Cooperatively To Induce Actin Reorganization. Mol. Cell. Biol. 28(6):2035–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boscher C, Zheng YZ, Lakshminarayan R, Johannes L, Dennis JW, et al. 2012. Galectin-3 Protein Regulates Mobility of N-cadherin and GM1 Ganglioside at Cell-Cell Junctions of Mammary Carcinoma Cells. J. Biol. Chem. 287(39):32940–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewer CF, Miceli MC, Baum LG. 2002. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 12(5):616–23 [DOI] [PubMed] [Google Scholar]
- 17.Brooks SR, Kirkham PM, Freeberg L, Carter RH. 2004. Binding of Cytoplasmic Proteins to the CD19 Intracellular Domain Is High Affinity, Competitive, and Multimeric. J. Immunol. 172(12):7556 LP-7564 [DOI] [PubMed] [Google Scholar]
- 18.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, et al. 2002. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 158(7):1263–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunnell SC, Singer AL, Hong DI, Jacque BH, Jordan MS, et al. 2006. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol Cell Biol. 26(19):7155–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carquin M, D’Auria L, Pollet H, Bongarzone ER, Tyteca D. 2016. Recent progress on lipid lateral heterogeneity in plasma membranes: From rafts to submicrometric domains. Prog. Lipid Res. 62:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll JS, Munchel SE, Weis K. 2011. The DExD/H box ATPase Dhh1 functions in translational repression, mRNA decay, and processing body dynamics. J. Cell Biol. 194(4):527–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Case LB, Baird MA, Shtengel G, Campbell SL, Hess HF, et al. 2015. Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat. Cell Biol. 17(7):880–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Case LB, Waterman CM. 2015. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 17(8):955–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castello A, Gaya M, Tucholski J, Oellerich T, Lu K-H, et al. 2013. Nck-mediated recruitment of BCAP to the BCR regulates the PI(3)K-Akt pathway in B cells. Nat. Immunol. 14:966. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborty AK, Weiss A. 2014. Insights into the initiation of TCR signaling. Nat. Immunol. 15:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamma I, Letellier M, Butler C, Tessier B, Lim K-H, et al. 2016. Mapping the dynamics and nanoscale organization of synaptic adhesion proteins using monomeric streptavidin. Nat. Commun. 7:10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cluzel C, Saltel F, Lussi J, Paulhe F, Imhof BA, Wehrle-Haller B. 2005. The mechanisms and dynamics of (alpha)v(beta)3 integrin clustering in living cells. J Cell Biol. 171(2):383–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen RJ, Benedek GB. 1982. Equilibrium and kinetic theory of polymerization and the sol-gel transition. J. Phys. Chem. 86(19):3696–3714 [Google Scholar]
- 29.Cong F, Schweizer L, Varmus H. 2004. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 131(20):5103–15 [DOI] [PubMed] [Google Scholar]
- 30.Dam TK, Oscarson S, Roy R, Das SK, Page D, et al. 2005. Thermodynamic, Kinetic, and Electron Microscopy Studies of Concanavalin A and Dioclea grandiflora Lectin Cross-linked with Synthetic Divalent Carbohydrates. J. Biol. Chem. 280(10):8640–46 [DOI] [PubMed] [Google Scholar]
- 31.DeFranco AL. 1992. Tyrosine phosphorylation and the mechanism of signal transduction by the B‐lymphocyte antigen receptor. Eur. J. Biochem. 210(2):381–88 [DOI] [PubMed] [Google Scholar]
- 32.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. 2009. Stretching single talin rod molecules activates vinculin binding. Science. 323(5914):638–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, et al. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80(3):1417–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dill KA, Bromberg S. 2003. Molecular driving forces : statistical thermodynamics in chemistry and biology. Garland Science; 666 pp. [Google Scholar]
- 35.Ditlev JA, Vega AR, Köster DV, Su X, Lakoduk A, et al. 2018. A Composition-Dependent Molecular Clutch Between T Cell Signaling Clusters and Actin. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douglass AD, Vale RD. 2005. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 121(6):937–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dustin ML, Choudhuri K. 2016. Signaling and Polarized Communication Across the T Cell Immunological Synapse. Annu. Rev. Cell Dev. Biol. 32(1):303–25 [DOI] [PubMed] [Google Scholar]
- 38.Dustin ML, Groves JT. 2012. Receptor Signaling Clusters in the Immune Synapse. Annu. Rev. Biophys. 41(1):543–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Earl LA, Bi S, Baum LG. 2011. Galectin multimerization and lattice formation are regulated by linker region structure. Glycobiology. 21(1):6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, et al. 2009. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 457(7233):1159–62 [DOI] [PubMed] [Google Scholar]
- 41.Eilon S, Valarie B, SL E. 2012. Super-resolution characterization of TCR-dependent signaling clusters. Immunol. Rev. 251(1):21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fanning AS, Anderson JM. 2009. Zonula occludens-1 and −2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci. 1165(1):113–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feric M, Brangwynne CP. 2013. A nuclear F-actin scaffold stabilizes ribonucleoprotein droplets against gravity in large cells. Nat. Cell Biol. 15(10):1253–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiedler M, Mendoza-Topaz C, Rutherford TJ, Mieszczanek J, Bienz M. 2011. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating β-catenin. Proc. Natl. Acad. Sci. U. S. A. 108(5):1937–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flory PJ. 1942. Thermodynamics of High Polymer Solutions. J. Chem. Phys. 10(1):51–61 [Google Scholar]
- 46.Flory PJ. 1953. Principles of Polymer Chemistry. Ithaca, NY: Cornell University Press [Google Scholar]
- 47.Flory PJ, Krigbaum WR. 1951. Thermodynamics of High Polymer Solutions. Annu. Rev. Phys. Chem. 2(1):383–402 [Google Scholar]
- 48.Freeman Rosenzweig ES, Xu B, Kuhn Cuellar L, Martinez-Sanchez A, Schaffer M, et al. 2017. The Eukaryotic CO2-Concentrating Organelle Is Liquid-like and Exhibits Dynamic Reorganization. Cell. 171(1):148–162.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman SA, Vega A, Riedl M, Collins RF, Ostrowski PP, et al. 2018. Transmembrane Pickets Connect Cyto- and Pericellular Skeletons Forming Barriers to Receptor Engagement. Cell. 172(1):305–317.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujiwara TK, Iwasawa K, Kalay Z, Tsunoyama TA, Watanabe Y, et al. 2016. Confined diffusion of transmembrane proteins and lipids induced by the same actin meshwork lining the plasma membrane. Mol. Biol. Cell. 27(7):1101–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gammons MV, Renko M, Johnson CM, Rutherford TJ, Bienz M. 2016. Wnt Signalosome Assembly by DEP Domain Swapping of Dishevelled. Mol. Cell. 64(1):92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia MA, Nelson WJ, Chavez N. 2018. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 10(4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Germain RN. 1997. T-cell signaling: the importance of receptor clustering. Curr. Biol. 7(10):R640–4 [DOI] [PubMed] [Google Scholar]
- 54.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. 2002. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 124(50):14922–33 [DOI] [PubMed] [Google Scholar]
- 55.Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, et al. 2008. Nanoclusters of GPI-Anchored Proteins Are Formed by Cortical Actin-Driven Activity. Cell. 135(6):1085–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gowrishankar K, Ghosh S, Saha S, C. R, Mayor S, Rao M. 2012. Active Remodeling of Cortical Actin Regulates Spatiotemporal Organization of Cell Surface Molecules. Cell. 149(6):1353–67 [DOI] [PubMed] [Google Scholar]
- 57.Hagemann AIH, Kurz J, Kauffeld S, Chen Q, Reeves PM, et al. 2014. In vivo analysis of formation and endocytosis of the Wnt/β-catenin signaling complex in zebrafish embryos. J. Cell Sci. 127(Pt 18):3970–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harmon TS, Holehouse AS, Rosen MK, Pappu RV. 2017. Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. Elife. 6:e30294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. 1996. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J. Biol. Chem. 271(23):13649–55 [DOI] [PubMed] [Google Scholar]
- 60.Heberle FA, Feigenson GW. 2011. Phase separation in lipid membranes. Cold Spring Harb. Perspect. Biol. 3(4):a004630–a004630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heldin CH. 1995. Dimerization of cell surface receptors in signal transduction. Cell. 80(2):213–23 [DOI] [PubMed] [Google Scholar]
- 62.Himanen J-P, Saha N, Nikolov DB. 2007. Cell–cell signaling via Eph receptors and ephrins. Curr. Opin. Cell Biol. 19(5):534–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, et al. 2010. Architecture of Eph receptor clusters. Proc. Natl. Acad. Sci. 107(24):10860 LP-10865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffmann J-E, Fermin Y, Stricker RLO, Ickstadt K, Zamir E. 2014. Symmetric exchange of multi-protein building blocks between stationary focal adhesions and the cytosol. Elife. 3:e02257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Honerkamp-Smith AR, Cicuta P, Collins MD, Veatch SL, den Nijs M, et al. 2008. Line Tensions, Correlation Lengths, and Critical Exponents in Lipid Membranes Near Critical Points. Biophys. J. 95(1):236–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Honerkamp-Smith AR, Veatch SL, Keller SL. 2009. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta - Biomembr. 1788(1):53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hopfield JJ. 1974. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc. Natl. Acad. Sci. U. S. A. 71(10):4135–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Houtman JCD, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, et al. 2006. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol. 13(9):798–805 [DOI] [PubMed] [Google Scholar]
- 69.Huang WYC, Ditlev JA, Chiang H-K, Rosen MK, Groves JT. 2017. Allosteric Modulation of Grb2 Recruitment to the Intrinsically Disordered Scaffold Protein, LAT, by Remote Site Phosphorylation. J. Am. Chem. Soc. 139(49):18009–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang WYC, Yan Q, Lin W-C, Chung JK, Hansen SD, et al. 2016. Phosphotyrosine-mediated LAT assembly on membranes drives kinetic bifurcation in recruitment dynamics of the Ras activator SOS. Proc. Natl. Acad. Sci. 113(29):8218–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, et al. 2017. T cell costimulatory receptor CD28 is a primary target for PD-1–mediated inhibition. Science. 355(6332):1428 LP-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huse M 2017. Mechanical forces in the immune system. Nat. Rev. Immunol. 17:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyman AA, Weber CA, Jülicher F. 2014. Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 30(1):39–58 [DOI] [PubMed] [Google Scholar]
- 74.Hynes RO. 2002. IntegrinsBidirectional, Allosteric Signaling Machines. Cell. 110(6):673–87 [DOI] [PubMed] [Google Scholar]
- 75.Iversen L, Tu H-LL, Lin W-CC, Christensen SM, Abel SM, et al. 2014. Molecular kinetics. Ras activation by SOS: allosteric regulation by altered fluctuation dynamics. Science. 345(6192):50–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jadwin JA, Oh D, Curran TG, Ogiue-Ikeda M, Jia L, et al. 2016. Time-resolved multimodal analysis of Src Homology 2 (SH2) domain binding in signaling by receptor tyrosine kinases. Elife. 5:e11835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janes PW, Nievergall E, Lackmann M. 2012. Concepts and consequences of Eph receptor clustering. Semin. Cell Dev. Biol. 23(1):43–50 [DOI] [PubMed] [Google Scholar]
- 78.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, et al. 2006. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 440(7085):818–23 [DOI] [PubMed] [Google Scholar]
- 79.Jones N, New LA, Fortino MA, Eremina V, Ruston J, et al. 2009. Nck Proteins Maintain the Adult Glomerular Filtration Barrier. J. Am. Soc. Nephrol. 20(7):1533–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, et al. 2010. Nanoscale architecture of integrin-based cell adhesions. Nature. 468(7323):580–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kiessling LL, Gestwicki JE, Strong LE. 2000. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr. Opin. Chem. Biol. 4(6):696–703 [DOI] [PubMed] [Google Scholar]
- 82.Kim S, Kalappurakki JM, Mayor S, Rosen MK. Multivalent interactions generated membrane receptor clusters that move through actomyosin contraction. Prep. [Google Scholar]
- 83.Kortum RL, Balagopalan L, Alexander CP, Garcia J, Pinski JM, et al. 2013. The Ability of Sos1 to Oligomerize the Adaptor Protein LAT Is Separable from Its Guanine Nucleotide Exchange Activity in Vivo. Sci Signal. 6(301):ra99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Köster DV, Mayor S. 2016. Cortical actin and the plasma membrane: inextricably intertwined. Curr. Opin. Cell Biol. 38:81–89 [DOI] [PubMed] [Google Scholar]
- 85.Krauss RS. 2010. Regulation of promyogenic signal transduction by cell–cell contact and adhesion. Exp. Cell Res. 316(18):3042–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumari S, Depoil D, Martinelli R, Judokusumo E, Carmona G, et al. 2015. Actin foci facilitate activation of the phospholipase C-γ in primary T lymphocytes via the WASP pathway. Elife. 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lackmann M, Boyd AW. 2008. Eph, a Protein Family Coming of Age: More Confusion, Insight, or Complexity? Sci. Signal. 1(15):re2 LP-re2 [DOI] [PubMed] [Google Scholar]
- 88.Leonard C, Conrard L, Guthmann M, Pollet H, Carquin M, et al. 2017. Contribution of plasma membrane lipid domains to red blood cell (re)shaping. Sci. Rep. 7(1):4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levental I, Grzybek M, Simons K. 2011. Raft domains of variable properties and compositions in plasma membrane vesicles. Proc. Natl. Acad. Sci. 108(28):11411–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levental I, Veatch SL. 2016. The Continuing Mystery of Lipid Rafts. J. Mol. Biol. 428(24):4749–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li P, Banjade S, Cheng H-C, Kim S, Chen B, et al. 2012. Phase transitions in the assembly of multivalent signalling proteins. Nature. 483(7389):336–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin Y-H, Qiu D-C, Chang W-H, Yeh Y-Q, Jeng U-S, et al. 2017. The intrinsically disordered N-terminal domain of galectin-3 dynamically mediates multisite self-association of the protein through fuzzy interactions. J. Biol. Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lingwood D, Simons K. 2010. Lipid rafts as a membrane-organizing principle. Science. 327(5961):46–50 [DOI] [PubMed] [Google Scholar]
- 94.Liu J, Wang Y, Goh WI, Goh H, Baird MA, et al. 2015. Talin determines the nanoscale architecture of focal adhesions. Proc. Natl. Acad. Sci. U. S. A. 112(35):E4864–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lorent JH, Diaz-Rohrer B, Lin X, Spring K, Gorfe AA, et al. 2017. Structural determinants and functional consequences of protein affinity for membrane rafts. Nat. Commun. 8(1):1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mammen M, Choi S, Whitesides GM. 1998. Polyvalent Interactions in Biological Systems : Implications for Design and Use of Multivalent Ligands and Inhibitors **. Angew. Chem. Int. Ed. Engl. 37:2754–94 [DOI] [PubMed] [Google Scholar]
- 97.Mao J, Wang J, Liu B, Pan W, Farr GH, et al. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell. 7(4):801–9 [DOI] [PubMed] [Google Scholar]
- 98.Martin CE, Jones N. 2018. Nephrin Signaling in the Podocyte: An Updated View of Signal Regulation at the Slit Diaphragm and Beyond. Front. Endocrinol. (Lausanne). 9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayer BJ, Yu J. 2018. Protein Clusters in Phosphotyrosine Signal Transduction. J. Mol. Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McKeithan TW. 1995. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl. Acad. Sci. U. S. A. 92(11):5042–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mi K, Dolan PJ, Johnson GVW. 2006. The Low Density Lipoprotein Receptor-related Protein 6 Interacts with Glycogen Synthase Kinase 3 and Attenuates Activity. J. Biol. Chem. 281(8):4787–94 [DOI] [PubMed] [Google Scholar]
- 102.Miyamoto S, Akiyama SK, Yamada KM. 1995. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 267(5199):883–85 [DOI] [PubMed] [Google Scholar]
- 103.Mohamed AM, Boudreau JR, Yu FP, Liu J, Chin-Sang ID. 2012. The Caenorhabditis elegans Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance. PLoS Genet. 8(2):e1002513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mulyasasmita W, Lee JS, Heilshorn SC. 2011. Molecular-Level Engineering of Protein Physical Hydrogels for Predictive Sol–Gel Phase Behavior. Biomacromolecules. 12(10):3406–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muschol M, Rosenberger F. 1998. Liquid–liquid phase separation in supersaturated lysozyme solutions and associated precipitate formation/crystallization. J. Chem. Phys. 107(6):1953 [Google Scholar]
- 106.Nabi IR, Shankar J, Dennis JW. 2015. The galectin lattice at a glance. J. Cell Sci. 128(13):2213–19 [DOI] [PubMed] [Google Scholar]
- 107.Nag A, Monine M, Perelson AS, Goldstein B. 2012. Modeling and Simulation of Aggregation of Membrane Protein LAT with Molecular Variability in the Number of Binding Sites for Cytosolic Grb2-SOS1-Grb2. PLoS One. 7(3):e28758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.New LA, Martin CE, Scott RP, Platt MJ, Keyvani Chahi A, et al. 2016. Nephrin Tyrosine Phosphorylation Is Required to Stabilize and Restore Podocyte Foot Process Architecture. J. Am. Soc. Nephrol. 27(8):2422–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nievergall E, Lackmann M, Janes PW. 2012. Eph-dependent cell-cell adhesion and segregation in development and cancer. Cell. Mol. Life Sci. 69(11):1813–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nikolov DB, Xu K, Himanen JP. 2013. Eph/ephrin recognition and the role of Eph/ephrin clusters in signaling initiation. Biochim. Biophys. Acta - Proteins Proteomics. 1834(10):2160–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nomme J, Antanasijevic A, Caffrey M, Van Itallie CM, Anderson JM, et al. 2015. Structural Basis of a Key Factor Regulating the Affinity between the Zonula Occludens First PDZ Domain and Claudins. J. Biol. Chem. 290(27):16595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oh D, Ogiue-Ikeda M, Jadwin JA, Machida K, Mayer BJ, Yu J. 2012. Fast rebinding increases dwell time of Src homology 2 (SH2)-containing proteins near the plasma membrane. Proc Natl Acad Sci U S A. 109(35):14024–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ojosnegros S, Cutrale F, Rodríguez D, Otterstrom JJ, Chiu CL, et al. 2017. Eph-ephrin signaling modulated by polymerization and condensation of receptors. Proc. Natl. Acad. Sci. 114(50):13188 LP-13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Opdenakker G, Rudd PM, Wormald M, Dwek RA, Van Damme J. 1995. Cells regulate the activities of cytokines by glycosylation. FASEB J. 9(5):453–57 [DOI] [PubMed] [Google Scholar]
- 115.Owen DM, Williamson DJ, Magenau A, Gaus K. 2012. Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat. Commun. 3(1):1256. [DOI] [PubMed] [Google Scholar]
- 116.Özkan E, Chia PH, Wang RR, Goriatcheva N, Borek D, et al. 2014. Extracellular Architecture of the SYG-1/SYG-2 Adhesion Complex Instructs Synaptogenesis. Cell. 156(3):482–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raghupathy R, Anilkumar AA, Polley A, Singh PP, Yadav M, et al. 2015. Transbilayer Lipid Interactions Mediate Nanoclustering of Lipid-Anchored Proteins. Cell. 161(3):581–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raut AS, Kalonia DS. 2016. Pharmaceutical Perspective on Opalescence and Liquid–Liquid Phase Separation in Protein Solutions. Mol. Pharm. 13(5):1431–44 [DOI] [PubMed] [Google Scholar]
- 119.Rayermann SP, Rayermann GE, Cornell CE, Merz AJ, Keller SL. 2017. Hallmarks of Reversible Separation of Living, Unperturbed Cell Membranes into Two Liquid Phases. Biophys. J. 113(11):2425–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roca-Cusachs P, Iskratsch T, Sheetz MP. 2012. Finding the weakest link – exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 125(13):3025 LP-3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rogacki MK, Ottavia G, J. TS, Tianyi L, Sunetra B, et al. 2018. Dynamic lateral organization of opioid receptors (kappa, muwt and muN40D) in the plasma membrane at the nanoscale level. Traffic. 0(0): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Samelson LE. 2002. Signal Transduction Mediated by the T Cell Antigen Receptor: The Role of Adapter Proteins. Annu. Rev. Immunol. 20(1):371–94 [DOI] [PubMed] [Google Scholar]
- 123.San Miguel M, Grant Gunton. 1985. Phase separation in two-dimensional binary fluids. Phys. Rev. A, Gen. Phys. 31(2):1001–5 [DOI] [PubMed] [Google Scholar]
- 124.Scheswohl DM, Harrell JR, Rajfur Z, Gao G, Campbell SL, Schaller MD. 2008. Multiple paxillin binding sites regulate FAK function. J. Mol. Signal. 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schumacher C, Knudsen BS, Ohuchi T, Di Fiore PP, Glassman RH, Hanafusa H. 1995. The SH3 domain of Crk binds specifically to a conserved proline-rich motif in Eps15 and Eps15R. J. Biol. Chem. 270(25):15341–47 [DOI] [PubMed] [Google Scholar]
- 126.Schwarz-Romond T, Fiedler M, Shibata N, Butler PJG, Kikuchi A, et al. 2007. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 14(6):484–92 [DOI] [PubMed] [Google Scholar]
- 127.Schwarz-Romond T, Metcalfe C, Bienz M. 2007. Dynamic recruitment of axin by Dishevelled protein assemblies. J. Cell Sci. 120(Pt 14):2402–12 [DOI] [PubMed] [Google Scholar]
- 128.Sciortino F, Mossa S, Zaccarelli E, Tartaglia P. 2004. Equilibrium Cluster Phases and Low-Density Arrested Disordered States: The Role of Short-Range Attraction and Long-Range Repulsion. Phys. Rev. Lett. 93(5):55701. [DOI] [PubMed] [Google Scholar]
- 129. Seiradake E, Harlos K, Sutton G, Aricescu AR, Jones EY. 2010. An extracellular steric seeding mechanism for Eph-ephrin signaling platform assembly. Nat. Struct. &Amp; Mol. Biol. 17:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Semenov AN, Rubinstein M. 1998. Thermoreversible Gelation in Solutions of Associative Polymers. 1. Statics. Macromolecules. 31(4):1373–85 [Google Scholar]
- 131.Sezgin E, Kaiser H-J, Baumgart T, Schwille P, Simons K, Levental I. 2012. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 7(6):1042–51 [DOI] [PubMed] [Google Scholar]
- 132.Sezgin E, Levental I, Mayor S, Eggeling C. 2017. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18:361–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. 2011. Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annu. Rev. Physiol. 73(1):283–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen L, Weber CR, Turner JR. 2008. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 181(4):683–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shin Y, Brangwynne CP. 2017. Liquid phase condensation in cell physiology and disease. Science. 357(6357):eaaf4382 [DOI] [PubMed] [Google Scholar]
- 136.Smalley MJ, Signoret N, Robertson D, Tilley A, Hann A, et al. 2005. Dishevelled (Dvl-2) activates canonical Wnt signalling in the absence of cytoplasmic puncta. J. Cell Sci. 118(Pt 22):5279–89 [DOI] [PubMed] [Google Scholar]
- 137.Steinhart Z, Angers S. 2018. Wnt signaling in development and tissue homeostasis. Development. 145(11):dev146589 [DOI] [PubMed] [Google Scholar]
- 138.Stradner A, Sedgwick H, Cardinaux F, Poon WCK, Egelhaaf SU, Schurtenberger P. 2004. Equilibrium cluster formation in concentrated protein solutions and colloids. Nature. 432:492. [DOI] [PubMed] [Google Scholar]
- 139.Stutchbury B, Atherton P, Tsang R, Wang D-Y, Ballestrem C. 2017. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J. Cell Sci. 130(9):1612–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Su X, Ditlev JA, Hui E, Xing W, Banjade S, et al. 2016. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science. 352(6285):595–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Swaminathan V, Fischer RS, Waterman CM. 2016. The FAK–Arp2/3 interaction promotes leading edge advance and haptosensing by coupling nascent adhesions to lamellipodia actin. Mol. Biol. Cell. 27(7):1085–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Swaminathan V, Kalappurakkal JM, Mehta SB, Nordenfelt P, Moore TI, et al. 2017. Actin retrograde flow actively aligns and orients ligand-engaged integrins in focal adhesions. Proc. Natl. Acad. Sci. U. S. A. 114(40):10648–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tauriello DVF, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, et al. 2012. Wnt/β-catenin signaling requires interaction of the Dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc. Natl. Acad. Sci. U. S. A. 109(14):E812–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taylor MJ, Husain K, Gartner ZJ, Mayor S, Vale RD. 2017. A DNA-Based T Cell Receptor Reveals a Role for Receptor Clustering in Ligand Discrimination. Cell. 169(1):108–119.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Theodosiou M, Widmaier M, Böttcher RT, Rognoni E, Veelders M, et al. 2016. Kindlin-2 cooperates with talin to activate integrins and induces cell spreading by directly binding paxillin. Elife. 5:e10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thievessen I, Thompson PM, Berlemont S, Plevock KM, Plotnikov SV, et al. 2013. Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth. J. Cell Biol. 202(1):163–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tolar P, Spillane KM. 2014. Chapter Three - Force Generation in B-Cell Synapses: Mechanisms Coupling B-Cell Receptor Binding to Antigen Internalization and Affinity Discrimination In Cell Biology of the B Cell Receptor, ed HLBT-A in I Ploegh. 123:69–100. Academic Press; [DOI] [PubMed] [Google Scholar]
- 148.Toulmay A, Prinz WA. 2013. Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. J. Cell Biol. 202(1):35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, et al. 2006. ZO-1 and ZO-2 Independently Determine Where Claudins Are Polymerized in Tight-Junction Strand Formation. Cell. 126(4):741–54 [DOI] [PubMed] [Google Scholar]
- 150.Van Itallie CM, Anderson JM. 2014. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 36:157–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Van Itallie CM, Mitic LL, Anderson JM. 2011. Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. J. Biol. Chem. 286(5):3442–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vanshylla K, Bartsch C, Hitzing C, Krümpelmann L, Wienands J, Engels N. 2018. Grb2 and GRAP connect the B cell antigen receptor to Erk MAP kinase activation in human B cells. Sci. Rep. 8(1):4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Veatch SL, Cicuta P, Sengupta P, Honerkamp-Smith A, Holowka D, Baird B. 2008. Critical Fluctuations in Plasma Membrane Vesicles. ACS Chem. Biol. 3(5):287–93 [DOI] [PubMed] [Google Scholar]
- 154.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. 2006. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J. Clin. Invest. 116(5):1346–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Verma R, Venkatareddy M, Kalinowski A, Patel SR, Garg P. 2016. Integrin Ligation Results in Nephrin Tyrosine Phosphorylation In Vitro. PLoS One. 11(2):e0148906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang C-W, Miao Y-H, Chang Y-S. 2014. A sterol-enriched vacuolar microdomain mediates stationary phase lipophagy in budding yeast. J. Cell Biol. 206(3):357–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y, Annunziata O. 2008. Liquid-liquid phase transition of protein aqueous solutions isothermally induced by protein cross-linking. Langmuir. 24(6):2799–2807 [DOI] [PubMed] [Google Scholar]
- 158.Watanabe Y, Nakayama T, Nagakubo D, Hieshima K, Jin Z, et al. 2006. Dopamine selectively induces migration and homing of naive CD8+ T cells via dopamine receptor D3. J. Immunol. 176(2):848–56 [DOI] [PubMed] [Google Scholar]
- 159.Weidtkamp-Peters S, Lenser T, Negorev D, Gerstner N, Hofmann TG, et al. 2008. Dynamics of component exchange at PML nuclear bodies. J. Cell Sci. 121(16):2731 LP-2743 [DOI] [PubMed] [Google Scholar]
- 160.Weiss A 1991. Molecular and Genetic Insights into T Cell Antigen Receptor Structure and Function. Annu. Rev. Genet. 25(1):487–510 [DOI] [PubMed] [Google Scholar]
- 161.Weisswange I, Newsome TP, Schleich S, Way M. 2009. The rate of N-WASP exchange limits the extent of ARP2/3-complex-dependent actin-based motility. Nature. 458(7234):87–91 [DOI] [PubMed] [Google Scholar]
- 162.Wesołowska O, Michalak K, Maniewska J, Hendrich AB. 2009. Giant unilamellar vesicles - a perfect tool to visualize phase separation and lipid rafts in model systems. Acta Biochim. Pol. 56(1):33–39 [PubMed] [Google Scholar]
- 163.Wong Po Foo CTS, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC. 2009. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc. Natl. Acad. Sci. U. S. A. 106(52):22067–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu C 2007. Focal adhesion: a focal point in current cell biology and molecular medicine. Cell Adh. Migr. 1(1):13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu H 2013. Higher-Order Assemblies in a New Paradigm of Signal Transduction. Cell. 153(2):287–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wurtz JD, Lee CF. 2018. Chemical-Reaction-Controlled Phase Separated Drops: Formation, Size Selection, and Coarsening. Phys. Rev. Lett. 120(7):78102. [DOI] [PubMed] [Google Scholar]
- 167.Yang T, Baryshnikova OK, Mao H, Holden MA, Cremer PS. 2003. Investigations of bivalent antibody binding on fluid-supported phospholipid membranes: the effect of hapten density. J. Am. Chem. Soc. 125(16):4779–84 [DOI] [PubMed] [Google Scholar]
- 168.Yokoyama WM, Shevach EM. 1989. T cell activation via cell-surface antigens other than the CD3/T cell receptor complex. Year Immunol. 4:110–46 [PubMed] [Google Scholar]
- 169.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, et al. 2010. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc. Natl. Acad. Sci. 107(18):8237–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zacharchenko T, Qian X, Goult BT, Critchley DR, Lowy DR, et al. 2016. LD Motif Recognition by Talin: Structure of the Talin- DLC1 Complex LD Motif Recognition by Talin: Structure of the Talin-DLC1 Complex. Struct. Des. 24:1130–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zeng M, Chen X, Guan D, Xu J, Wu H, et al. 2018. Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell. 174(5):1172–1187.e16 [DOI] [PubMed] [Google Scholar]
- 172.Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, Zhang M. 2016. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell. 166(5):1163–1175.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang C, Miller DJ, Guibao CD, Donato DM, Hanks SK, Zheng JJ. 2017. Structural and functional insights into the interaction between the Cas family scaffolding protein p130Cas and the focal adhesion-associated protein paxillin. J. Biol. Chem. 292(44):18281–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang H, Elbaum-Garfinkle S, Langdon EM, Taylor N, Occhipinti P, et al. 2015. RNA Controls PolyQ Protein Phase Transitions. Mol. Cell. 60(2):220–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 92(1):83–92 [DOI] [PubMed] [Google Scholar]


