Abstract
BACKGROUND
Human alphaB crystallin (HspB5) contains the alpha crystallin core domain, a series of antiparallel beta-strands organized into the characteristic beta sandwich of small heat shock proteins (sHsp). The full 3-dimensional structure for alpha crystallin has not been determined and the mechanism for the biological activity remains elusive because sHsp participate in multiple interactions with a broad range of target proteins that favor self-assembly of polydisperse fibrils and complexes. We selected human alphaB crystallin to study interactive sequences because it is involved in many human condensation, amyloid, and aggregation diseases and it is very sensitive to the destabilization of unfolding proteins. Sophisticated methods are being used to analyze and complete the structure of alphaB crystallin with the expectation of understanding sHsp function. This review considers the identification interactive sites on the surface of the alphaB crystallin, which may be the key to understanding the multifunctional activity of human alphaB crystallin.
SCOPE OF REVIEW
This review summarizes the research on the identification of the bioactive interactive sequences responsible for the function of human alphaB crystallin, a sHsp with chaperone-like activity.
MAJOR CONCLUSIONS
The multifunctional activity of human alphaB crystallin results from the interactive peptide sequences exposed on the surface of the molecule. The multiple, non-covalent, interactive sequences can account for the selectivity and sensitivity of alphaB crystallin to the initiation of protein unfolding.
GENERAL SIGNIFICANCE
Human alphaB crystallin may be an important part of an endogenous protective mechanism in aging cells and tissues.
1. INTRODUCTION
The interactive sequences in the small heat shock protein (sHsp) human alphaB crystallin (sHspB5) were determined using a novel experimental method known as pin arrays[1]. AlphaB crystallin was selected for study of the interactive sites because it is highly-soluble, and is protective against protein condensation, aggregation and amyloid formation in diseases of aging. In contrast to alpha A crystallin (sHspB4) and most sHsp, alphaB crystallin contains no cysteines. The use of human alphaB crystallin in our studies avoided the complications of sulfhydryl chemistry in the characterization of the functional interactions with destabilized target proteins. The study of the protein-protein interactions responsible for functional activity are complicated enough without including disulfide formation. Since human alphaB crystallin interacts with a variety of target proteins, and the number of target proteins is so large, early studies often considered sHSP to be promiscuous in their binding and the protein-protein interactions to be non-specific. Continued experimentation demonstrated that alphaB crystallin interactions were selective and depended on weak non-covalent interactions at a number of sites throughout the alphaB crystallin sequence. When mapped to the surface of computer models of alphaB crystallin, the interactive sequences appeared to form a network of highly sensitive areas for protein-protein interactions[2].
Pin array experiments allowed a systematic assessment of interactions between sequential peptides in the protein sequence of human alphaB crystallin and target proteins (figure 1). Sequential peptides from human alphaB crystallin were immobilized on pins organized in an array that matched a 96 well plate. Each well contained a known concentration of a target protein. In each well, the interactions between the synthetic peptides on each pin and the target protein were measured using a simple colorimetric method where the intensity of the color corresponded with the relative strength of the interaction between the peptide sequence and the target protein. The target proteins that were studied included cytoskeletal proteins and filaments, crystallins, chaperone target proteins, and signaling molecules[3]. The negative controls, myoglobin and apomyoglobin, which are rich in alpha helix, had no significant interactions with alphaB crystallin sequences. While the interactive sequences were separated along the primary sequence of human alphaB crystallin, the binding sequences for each category of target protein were connected when mapped to a space filling model of alphaB crystallin (2).
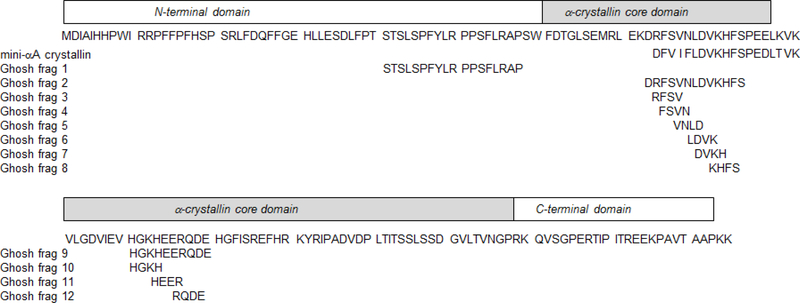
FIGURE 1: Interactive Sequences in AlphaB Crystallin Identified Using a Pin Array.
The full length sequence of human alphaB crystallin (top row) is compared with the 19 residue sequence of the mini-alpha A crystallin, MAC, (second row) discovered by Sharma. Pin array studies identified a number of interactive peptide sequences in human alphaB Crystallin listed as Ghosh frag 1–12. Ghosh frag 2 is the sequence in the C-terminal domain of alphaB crystallin that corresponds to MAC in alpha A crystallin. Ghosh frag 1 is an interactive peptide sequence in the N-terminal domain of alphaB crystallin. Ghosh frags 3–8 are four amino acid bioactive sequences based on the alphaB crystallin sequence corresponding to MAC. Ghosh frag 9 is a second bioactive sequence identified in the alpha crystallin core domain and Ghosh frags 10–12 are four amino acid bioactive sequences based on Ghosh frag 9. The functional activity of Ghosh peptide fragments are compared in figure 4.
Nearly twenty years ago, a protective fragment of the sHSP, alpha A crystallin, Hsp4B, was identified in the Sharma laboratory using crosslinking, proteolysis, and sequencing[4]. The functional element was KFVIFLDVKHFSPEDLTVK which became known as a mini-alpha crystallin chaperone, MAC. A corresponding fragment, DRFSVNLDVKHFSPEELKVK, was identified in alphaB crystallin [5]. Chaperone-like activity was observed using a synthetic peptide in assays for the aggregation of oxidized gamma-crystallin or alcohol dehydrogenase (ADH)[6]. At the time, it was hypothesized that each category of target protein interacted with an independent site on alpha crystallin, which was the basis for the experiments using the pin arrays to determine the sequence of each binding site.
The results of the pin arrays were consistent with the Sharma results but the pin arrays determined that sequences as small as four amino acids in the core domain had bioactivity (figure 1). When the interactive sequences for different target proteins were mapped to the surface of alphaB crystallin, the surface exposed regions were interconnected. In addition, the side-chain interactions with the destabilized, unfolding target proteins were through weak noncovalent protein-protein interactions. Recent reports confirmed the interactive structure of the functional core domain of alphaB crystallin and the MAC interactive sites using X-ray diffraction and solution NMR [7, 8]. There is little doubt about the importance of the original findings by the Sharma laboratory for understanding the functional elements in alpha crystallins which continue to be confirmed using advanced technology (summarized in another review article in this issue).
STRUCTURE
The full length sequence of human alphaB crystallin is 175 amino acids and it contains the characteristic “alpha crystallin core domain” of approximately 85 amino acids in the C-terminal region [8, 9]. Similar to all sHsp there are C- and N- terminal extensions in human alphaB crystallin. The earliest successful X-ray diffraction studies of sHsp were conducted using crystals of sHsp16.5 from the archaeal Methanococcus jannaschii [10], and Triticum aestivum (wheat) sHsp16.9 [11]. In both molecules, the prominent structural motif was the alpha crystallin core domain consisting of anti-parallel beta strands in beta sheets that were organized in a beta-sandwich resembling an immunoglobulin fold. The results of X-ray diffraction confirmed previous circular dichroism studies and demonstrated that the lens crystallins were predominantly beta strands similar to all sHsp, even when the sequence identity was minimal [12, 13]. Dimerization was the basis for the self-assembly of larger oligomers involving the N- and C-terminal extensions, which are similar only in closely related sHsp [9, 14]. The C-terminal extension of human alphaB crystallin included an I-X-I motif which is conserved in most sHsp. Diffraction data for the full length human alphaB crystallin has not yet been obtained. Instead of forming crystals, concentrated solutions of human alpha crystallin favor polydisperse, globular oligomers and X-ray diffraction of human alphaB crystallin was possible only by removal of the N- and C-sequence extensions [9, 15, 16]. Computer modeling and energy minimization resulted in an alphaB structure containing the characteristic beta sandwich and immunoglobulin – like fold that was fit to sHsp 16.5 and sHsp 16.9 with an excellent Cα RMSD of= 2.06 angstroms [1]. In the absence of a detailed atomic structure of the full length human alphaB crystallin molecule, there continues to be uncertainty about the exact configuration of the N- and C- termini which are characteristically dynamic, often disordered d, and contribute to the multifunctional structure and activity of alphaB crystallin [9, 14]. A full length model for alphaB crystallin based on solid-state NMR, small angle X-ray scattering and electron microscopy confirmed the dynamic nature of the human alphaB crystallin in the formation of multimeric oligomers[17].
ALPHA CRYSTALLIN as a MODEL for PROTEIN AGGREGATION
Protein-protein interactions are fundamental to the self-assembly of the cytoskeleton, dimerization of surface receptors, and formation of amyloid aggregates in pathology, mechanisms known to be modulated by alphaB crystallin [2, 18–20]. Historically, the earliest studies of the protein-protein interactions involving alpha crystallin were in the formation of light scattering High Molecular Weight aggregates (HMW) in lens opacification (figures 2 & 3). An increase in light scattering and lens opacity correlated with a progressive increase in water-insoluble protein and cross-linked HMW aggregates [21, 22] where protein deamidation [22]and disulfide crosslinks between crystallins increased. Alpha crystallin was a major component of HMW aggregates which appeared to account for increased light scattering in cataract [21, 23, 24]. As a result there was an emphasis on covalent –S-S- interactions between alpha crystallin and other crystallins as the cause of light scattering resulting from protein destabilization, unfolding and aggregation in aging human lenses. These results were based on the analysis of mature cataracts. In fact, covalent disulfide bonds are unnecessary for the formation of HMW and may be important in the late stage of cataract formation. A few years later, the realization that mammalian alpha crystallin was related to sHsp in drosophila [25] inspired an experimental demonstration of the protective effects of alpha crystallin against the aggregation of unfolding proteins [26]. As a result of those experiments, the historic emphasis on alpha crystallin as a cause of cataract had to be reconsidered. Then a new hypothesis considered alpha crystallin to be protective against protein aggregation and cataract, rather than causative. The presence of alphaB crystallin in cells and tissues involved with disorders of aging, including cardiovascular and neurodegenerative disease, raised the possibility that alphaB crystallin might be protective against aggregation and fibrillation of amyloidogenic proteins in neurodegeneration, not just aggregation of lens crystallins in aging cataract. It needs to be noted that the protective activity of alphaB crystallin does not require –SH or –S-S- interactions.
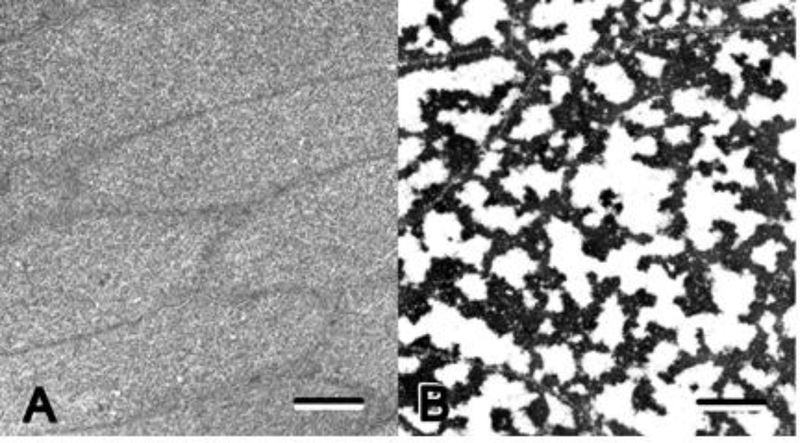
FIGURE 2: Cellular Opacification (cataract) in Mouse is an Excellent Model for the Study of Protein Aggregation and Amyloid Formation in vivo.
Transmission electron micrographs of transparent (left) and opaque (right) lens cells illustrate differences between cytoplasmic structure in the normal transparent lens and a dense cataract. In the transparent cells, soluble, concentrated proteins are distributed uniformly throughout the cells and fluctuations in the index of refraction are small relative to the wavelength of visible light (390–700nm). The distribution of the cytoplasmic proteins is uniform and homogeneous similar to window glass. In opaque cells, the cytoplasmic proteins self-assemble into condensed, light scattering aggregates separated by spaces filled with cytoplasmic fluid. The fluctuations in the index of refraction are large relative to the wavelength of visible light similar to frosted glass. In this example the condensed protein appears to have a filamentous structure connecting the cell membranes, but various morphologies are observed in opacities, and they can be very subtle [47–50]. A lens containing opaque cells like those in figure 2B would appear completely opaque as in the cataract in figure 3. It is well known, but not widely recognized, that only 3 percent of the cytoplasmic proteins need to self- assemble into HMW for complete cellular opacity. Regulation of the interactions between cytoplasmic proteins is a function of alphaB crystallin that is thought to assist in stabilization of transparent cytoplasm both during the differentiation of transparency and during aging when post-translational modification and proteolysis can favor aggregation and disordered cytoplasmic structure. (bar = one micron)
FIGURE 3: In Vivo Transparency and Opacity in Cells of a Mouse Lens Cataract.
In a transparent lens, protection against self-assembly of HMW aggregates can be evaluated easily in vivo. In a photograph of a normal live mouse eye at approximately 45 days of age, the lens is transparent (3A). Lens cell transparency allows the observation of changes in light scattering from individual cells using quantitative spectroscopic methods. Large HMW aggregates are responsible for the opacity (3B) observed in the lenses of numerous animal models for cataract, and dynamic light scattering can be used to measure the progressive transition from soluble protein monomers to multimeric light scattering complexes at the earliest stages of opacification. The protective activity of modulators of the self-assembly of proteins can be evaluated in vivo using these methods. A number of small molecular weight compounds and biomolecules have protective effects when administered during the early stages of aggregation in vivo [51, 52]. Pantethine (MW = 555 Daltons) is among the most effective molecules that protect against protein aggregation and opacity (3C) [53, 54]. Pantethine can enhance the natural protective action of alphaB crystallin.
Pin arrays were then used to evaluate the potential interactive sequences in human alphaB crystallin involved in recognition and inhibition of protein fibrillation of the amyloid forming proteins, Abeta1–42, alpha-synuclein, transthyretin, and beta2-microglobulin [27]. In an extensive series of experiments, the pin arrays identified five sequences in human alphaB crystallin that modulated fibrillation measured by the ThioflavinT fluorescence assays, often used to measure self-assembly of amyloid fibrils. Enhanced Thioflavin T fluorescence at 486 nm can be used to quantify the self-assembly of amyloid fibrils. The peptides derived from human alphaB crystallin were inhibitors of amyloid assembly. Unexpectedly, systematic truncation of these bioactive peptides of alphaB crystallin resulted in peptide fragments that were effective in assays of fibril formation. While longer sequences could simulate the effects of the full length protein, these studies determined that sequences as short as four amino acids (molecular weight <500) were bioactive and modulated fibrillation in the ThioflavinT assays (figure 4)[27].
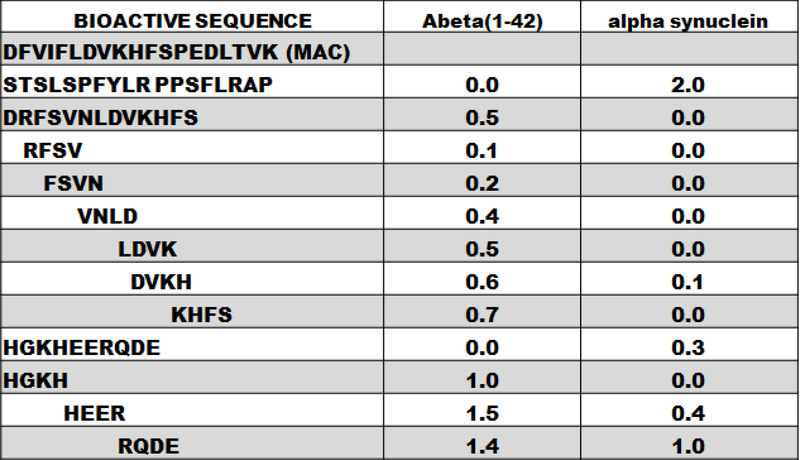
FIGURE 4: The Interactive Sequences in Human AlphaB Crystallin Enhanced or Inhibited the Self-Assembly of Amyloid Proteins.
The relative activity for each bioactive peptide identified in human alphaB crystallin was assessed using a Thioflavin T fluorescence assay for fibrillation of amyloid proteins. All experiments were conducted using 1:1 molar ratio of peptide to amyloid target protein. Target proteins were the amyloid protein Amyloid beta 1–42 (Abeta) associated with Alzheimer’s disease (column 2) or alpha synuclein (column 3) associated with Parkinson’s disease. In the Thioflavin T assay, relative fluorescence was measured and “1.0” is normal fibrillation in the absence of bioactive peptide, and “0.0” is complete protection against fibrillation observed with full size alphaB crystallin. Peptides STSLSPFYLRPPSFLRAP and HGKHEERQDE were the most effective inhibitors of Abeta fibril formation. While HGKH had no effect, HEER and RQDE enhanced fibril formation of Abeta. The protective activity of the peptides was different with alpha synuclein where STSLSPFYLRPPSFLRAP enhanced fibril formation and Abeta, where there was complete inhibition of fibril formation. In contrast, the sequence DRFSVNLDVKHFS and the four residue peptides, RFSV, FSVN, VNLD, LDVK, KHFS and HGKH were effective inhibitors of alpha synuclein fibrillation and less effective with Abeta. The studies determined that human alphaB crystallin contains a number of inhibitory peptide sequences that can modulate amyloid formation in vitro. This is an excellent example of how a peptide sequence that is an effective inhibitor of fibril formation of one amyloid protein can be ineffective against fibril formation of another amyloid protein.
Once we identified the sequences for the sites where the bioactive peptides interacted with the amyloid proteins, we used computer docking programs to model the interactions. The modeling showed that interactions occurred at the sequences in transthyretin or beta2-microglobulin known to form amyloid. While X-ray diffraction structures of the amyloidogenic sequences were unavailable for Abeta1–42 and alpha-synuclein, there are similarities in the primary sequences responsible for amyloid formation. These sequences were identified as interactive sites for the bioactive peptides derived from alphaB crystallin using molecular modeling. These findings are supported by recent structural studies that determined the capability of the alphaB crystallin core domain to prevent amyloid fibrillation and associated toxicity [7]. Recent NMR analysis of the interface between the mini-alpha crystallin derived from the beta 3 –beta 4 strands in the core of alpha A crystallin, identified residues responsible for binding between the target protein human gamma D crystallin (HGD) and an interactive site on the mini-alpha crystallin[8]. Apparent Kd values in the range of only 200–700 micromolar characterized weak reversible binding which preceded unfolding of the target HGD protein and formation of a stable, irreversible complex where much stronger Kd values were needed. While the affinities were not determined, the structural studies characterized weak interactive sites involved in dimer formation in the core alphaB crystallin domain [7]. These results suggested that surface exposed side chains of the interactive sequences can be influenced by subtle cellular changes in pH, ionic strength or osmolarity under physiological conditions to modify their binding to target proteins. This is an area that needs further study.
FUNCTION of the ALPHA CRYSTALLIN SEQUENCES
After the Sharma laboratory determined that a 19-residue peptide fragment in human alpha A crystallin had functional activity, it was only a matter of using new experimental methods to characterize the activity of component peptides and their involvement in the functional mechanism. Pin array studies of alphaB crystallin identified as many as seven interactive sequences in human alphaB crystallin involved in the interactions between human alphaB crystallin and target proteins. Five of the sequences were involved in interactions between human alphaB crystallin and amyloid proteins. On the basis of the pin array results, synthetic fragments as small as four amino acids in length were used in quantitative studies of bioactivity against fibrillation of amyloidogenic proteins (figures 1 and 4). When the surface exposed side chains of the bioactive peptides were mapped to 3-dimensional computer models of alphaB crystallin, the overlap of the bioactive surfaces appeared as a network. Exactly how a network of interactive sequences contributes to the functional mechanism of human alphaB crystallin remains to be determined. In theory, a network may be more sensitive to one energetic state than another, emphasizing the importance of measuring exact affinities for binding structurally diverse, dynamic sites in destabilized proteins. The pin array results combined with surface mapping generated new knowledge for the basis of protein -protein interactions and selective, rather than specific, interactions between alphaB crystallin and its targets. The possibility that alphaB crystallin need not bind directly to a functional target but instead binds indirectly to regulatory sites where modification can occur by phosphorylation, deamidation or proteolysis, needs to be considered. The results summarized in this article suggest that no individual amino acid sequence is required for protective activity and that the bioactivity of any sequence varies with the target protein. Whether or not a protective peptide adapts to any secondary structure or the activity depends on interactions with hydrophobic patches was not determined. Clearly, identification of the earliest initiating events of self-assembly will benefit our understanding of the alphaB crystallin mechanism.
These results raised new hypotheses about the mechanism(s) for the protective action of human alphaB crystallin and its selective binding with diverse target proteins. Exactly how the alphaB crystallin regulated the interactions with target proteins remains to be determined, but preliminary results are consistent with a mechanism where dynamic interactive surfaces on alphaB crystallin can recognize multiple interactive domains exposed on the surface of diverse targets from crystallins to cytoskeletal elements to amyloid proteins. The interactive domains for each target protein are not separated on the surface of alphaB crystallin. Instead the interactive domains for different categories of target proteins overlap. It should be noted that the interactive sequences on alphaB crystallin are used in normal dynamic self assembly of alpha crystallins which do not require disulfide bonds [1,2]. There remains much to discover about the biological importance of the interactive sequences in normal cell function and in protection against modified abnormal proteins. Quantification of the nature of the interactions, the affinities between interactive sites, and the effects of 3D surface topology on alphaB crystallin function, are needed to understand the mechanism of alphaB crystallin activity in cells.
Taken together the results using synthetic peptides (figure 4) based on interactive sequences in human alphaB crystallin and identified using pin arrays (figure 1), were consistent with the hypothesis that human alphaB crystallin, the archetype for all sHsp, is multifunctional because it has multiple surface-exposed, interactive sequences. While not specific for a single category of proteins, most targets for alphaB crystallin appeared to be self-assembling proteins, both normal and abnormal. AlphaB crystallin has target protein preferences. For example, lens crystallins appeared to be the favored targets for human alphaB crystallin. While the alpha crystallins self-assemble, there were also preferential interactions with the beta/gamma crystallins present at high concentrations in lens cells throughout the lifetime of an animal. Numerous studies support the hypothesis that alphaB crystallin protects against endogenous protein destabilization leading to the slow progressive unfolding and self-assembly of HMW aggregates during aging and cataract formation. The inhibition of aggregation by many of the peptides corresponded to the inhibition of aggregation by the full length protein. In lens, crystallins are the highest concentration of the cytoplasmic proteins by a factor of nearly ten. After crystallins, the predominate proteins in lens cells are those of the cytoskeleton including fibers, filaments and microtubules. The cytoskeleton is important in cellular restructuring during the proliferation, migration and elongation of lens cells to form a highly symmetric, refractive, transparent optical element in the eye (figures 2&3) [28, 29]. Within each cell, the cytoskeleton provides a scaffold for establishing short range transparent order in the concentrated crystallins [30]. It is well established that alphaB crystallin (and other sHsp) can interact with and regulate self-assembly of cytoskeletal proteins in a variety differentiating cells throughout the body and in cell culture [18, 20, 31, 32].
Having determined that several small sequences on the surface of alphaB crystallin have the ability to interact with amyloid forming proteins, we hypothesized that common physical properties were responsible for modification of potential interactions with unfolding target proteins. Peptide modifiers of protein-protein interactions needed to be studied in individual cells or tissues in model organisms where fundamental interactions found in the earliest phylogenies can be evaluated. The same protein-protein interactions and interactive surfaces on molecules in human cells were probably important in the simplest unicellular organisms even where there was little sequence identity. While the proteins and the sequences may have evolved, it can be expected that fundamental properties of the subtle interactions between proteins remain unchanged.
RECOGNITION of UNSTABLE TARGET PROTEINS
Given the diversity of the interactive sequences on the surface of human alphaB crystallin and the variety of self-assembling target proteins including crystallins, cytoskeletal proteins, and amyloid proteins, a series of experiments were conducted to evaluate the sensitivity of human alphaB crystallin to the initiation of protein self-assembly [33]. Hemoglobin S (HbS), sickle cell hemoglobin, was selected as the experimental model because the filament assembly of HbS results from a glu-6-val mutation in normal HbA, and it may be the most thoroughly studied self-assembly pathology in biology. Numerous structural and biophysical reports on all aspects of the HbS assembly have been published [34, 35]. Interactions were not expected between human alphaB crystallin and HbS or HbA in vivo or in vitro. Using a thermal unfolding assay, the experiments determined that alphaB crystallin bound 6% more HbS than native HbA at room temperature and 25% more at 37°C [33]. It was of special interest that alphaB crystallin distinguished native HbA from HbS under near physiological conditions. Continued destabilization with time or increasing temperature increased the interaction between alphaB crystallin and both HbS or HbA though the interactions with HbS were stronger than HbA. AlphaB crystallin appeared to be more sensitive than ultra violet circular dichroism (UVCD) to the earliest stages of protein unfolding in HbS. In terms of surface interactions, the alphaB crystallin recognized the difference between HbA and HbS while the difference in hydrophobic surface area was less than one percent. The molecular basis for the recognition of destabilized proteins is an area that needs much more attention. Continued study of the interactive domains in HbA and HbS for alphaB crystallin during initiation of unfolding is expected to provide new insight into mechanisms of alphaB crystallin function.
ENDOGENOUS PROTECTIVE PEPTIDES
Separate studies of alpha crystallin purified from different layers of lenses found that the oldest cells in the core of a lens contained modified alpha crystallins and crystallin fragments that may be more than 20% of the protein [36, 37]. While it is expected that the increase in fragments could cause aggregation and increased light scattering, the protective effects of fragments of alpha crystallin may maintain transparency. Mechanistically it is understandable that molecular aging of the proteins can result in aggregation in the absence of intact alphaB crystallin or protective fragments. An accumulation of crystallins altered by post-translational modification or mutated by genetic factors favors the disruption of transparent short range order by the formation of HMW aggregates. However, endogenous production of protective peptides could help protect against self-assembly of light scattering aggregates with aging [37].
From a phylogenetic perspective, alphaB crystallin homologs are endogenous to nearly all cells throughout the plant and animal kingdoms. As a predominant class of proteins in the lens, alpha crystallin was readily characterized in early biochemical studies. But alphaB crystallin is constitutively expressed in a diverse number of cells as well, indicating the potential importance of alphaB crystallin in a variety of molecular systems where self-assembly is important for differentiation, migration and protection against cell stress. A number of sHsp share common functions as well as a common structure with alphaB crystallin. The similarities in protective sequences can account for the shared functions. While the similar functional sequences are important, the differences in the surface exposure of interactive sequences appear to account for the diversity in the selectivity of the sHsp. The phenotype for the first functional sHsp may have appeared early in phylogeny. Systematic analysis of the surface exposure of the interactive domains across phyla could provide new insights into the fundamental basis for the protective mechanism against amyloid formation and aggregation diseases and reveal novel therapeutic targets for regulation of the self-assembly of amyloid proteins.
In mammals and zebra fish, the lens is an excellent model system for studies of alphaB crystallin function because the lens is a transparent optical element that transmits and focuses light [29]. Transparency and refraction result from the establishment of short range order (Fig 2 & 3) in very high cellular concentrations of cytoplasmic proteins known as alpha and beta/gamma crystallin [38–40]. The cytoskeleton participates in the establishment of short range order[30] possibly through production of a scaffold involving interactions between cytoskeletal elements and alphaB crystallin, as expression of beta/gamma crystallins increases during cell differentiation [41–43]. Careful and subtle regulation of the interactions between the beta/gamma crystallins and the cytoskeleton can result in a transparent structure without aggregation [44–47] during the elongation and migration of lens fibers during differentiation into symmetric layers of varying refractive power.
SUMMARY
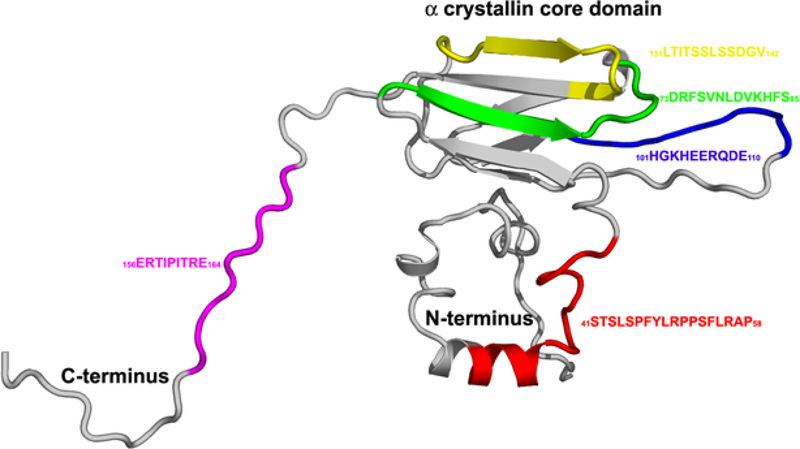
In humans, alphaB crystallin, sHspB5, is a multifunctional protein containing the alpha crystallin core domain, the fundamental beta-sandwich found in most small heat shock proteins, sHsp. The beta-strand structure is represented in sHsp across all phyla in both the plant and animal kingdoms, supporting the hypothesis that sHsp are critical for the response of cells to cell stress including cell differentiation and cell aging. Mechanisms for the self-assembly of protein complexes are the basis for cytoskeletal assembly that is necessary for normal cell function and for the pathological amyloid aggregates that are common in cataract, sickle cell disease, and neurodegeneration. Our hypothesis is that the self-assembly mechanisms critical for filament, fibril, and tubule formation in normal cells share common dynamics with the formation of amyloid and aggregate complexes in multifactorial aging diseases. In human alphaB crystallin, there is no single binding site for target proteins. Instead, multiple interactive sequences that map to the exposed surface of the molecule, regulate the function of αB crystallin in diverse biological self-assembly systems under normal or abnormal conditions (figure 5).
FIGURE 5: Model for the interactive sequences in a ribbon model of the small heat shock protein, human alphaB crystallin.
Possibly the most surprising result of the study of the interactive sequences in human alphaB crystallin was the identification of multiple interactive sequences. The sequences form a diverse network of potential protective sites for selective interaction with several categories of target proteins. The biological, biophysical and pharmacological advantages of combining a number of multifunctional interactive sites on a single intracellular protective molecule, instead of numerous soluble cytoplasmic peptides, as small as four amino acids, each with protective activity remains to be determined.
BBA HIGHLIGHTS.
The interactive sequences in human alphaB crystallin are reviewed.
Multifunctional interactive sequences were identified
The interactive sequences as small as four amino acids have protective activity independent of the intact alphaB crystallin.
ACKNOWLEGEMENTS
Funding from grant # EY04542 from N.E.I. is gratefully acknowledged. Technical assistance from J.M. Clark is appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ghosh JG, Clark JI, Insights into the domains required for dimerization and assembly of human alphaB crystallin, Protein science : a publication of the Protein Society, 14 (2005) 684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ghosh JG, Shenoy AK Jr., Clark JI, Interactions between important regulatory proteins and human alphaB crystallin, Biochemistry, 46 (2007) 6308–6317. [DOI] [PubMed] [Google Scholar]
- [3].Clark JI, Self-assembly of protein aggregates in ageing disorders: the lens and cataract model, Philos. Trans. R. Soc. Lond. B Biol. Sci, 368 (2013) 20120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sharma KK, Kumar RS, Kumar GS, Quinn PT, Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin, The Journal of biological chemistry, 275 (2000) 3767–3771. [DOI] [PubMed] [Google Scholar]
- [5].Bhattacharyya J, Padmanabha Udupa EG, Wang J, Sharma KK, Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity, Biochemistry, 45 (2006) 3069–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kumar RS, Sharma KK, Chaperone-like activity of a synthetic peptide toward oxidized gamma-crystallin, The journal of peptide research : official journal of the American Peptide Society, 56 (2000) 157–164. [DOI] [PubMed] [Google Scholar]
- [7].Hochberg GK, Ecroyd H, Liu C, Cox D, Cascio D, Sawaya MR, Collier MP, Stroud J, Carver JA, Baldwin AJ, Robinson CV, Eisenberg DS, Benesch JL, Laganowsky A, The structured core domain of alphaB-crystallin can prevent amyloid fibrillation and associated toxicity, Proc. Natl. Acad. Sci. U. S. A, 111 (2014) E1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Banerjee PR, Pande A, Shekhtman A, Pande J, Molecular mechanism of the chaperone function of mini-alpha-crystallin, a 19-residue peptide of human alpha-crystallin, Biochemistry, 54 (2015) 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Slingsby C, Wistow GJ, Clark AR, Evolution of crystallins for a role in the vertebrate eye lens, Protein science : a publication of the Protein Society, 22 (2013) 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim KK, Kim R, Kim SH, Crystal structure of a small heat-shock protein, Nature, 394 (1998) 595–599. [DOI] [PubMed] [Google Scholar]
- [11].van Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E, Crystal structure and assembly of a eukaryotic small heat shock protein, Nature structural biology, 8 (2001) 1025–1030. [DOI] [PubMed] [Google Scholar]
- [12].Li LK, Spector A, The optical rotatory dispersion and circular dichroism of calf lens alpha-crystallin, The Journal of biological chemistry, 242 (1967) 3234–3236. [PubMed] [Google Scholar]
- [13].Horwitz J, Some properties of the low molecular weight alpha-crystallin from normal human lens: comparison with bovine lens, Experimental eye research, 23 (1976) 471–481. [DOI] [PubMed] [Google Scholar]
- [14].Basha E, O’Neill H, Vierling E, Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions, Trends in biochemical sciences, 37 (2012) 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bagneris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C, Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20, Journal of molecular biology, 392 (2009) 1242–1252. [DOI] [PubMed] [Google Scholar]
- [16].Laganowsky A, Benesch JL, Landau M, Ding L, Sawaya MR, Cascio D, Huang Q, Robinson CV, Horwitz J, Eisenberg D, Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function, Protein science : a publication of the Protein Society, 19 (2010) 1031–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jehle S, Vollmar BS, Bardiaux B, Dove KK, Rajagopal P, Gonen T, Oschkinat H, Klevit RE, N-terminal domain of alphaB-crystallin provides a conformational switch for multimerization and structural heterogeneity, Proc. Natl. Acad. Sci. U. S. A, 108 (2011) 6409–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ghosh JG, Houck SA, Clark JI, Interactive domains in the molecular chaperone human alphaB crystallin modulate microtubule assembly and disassembly, PLoS One, 2 (2007) e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arai H, Atomi Y, Chaperone activity of alphaB-crystallin suppresses tubulin aggregation through complex formation, Cell structure and function, 22 (1997) 539–544. [DOI] [PubMed] [Google Scholar]
- [20].Xi JH, Bai F, McGaha R, Andley UP, Alpha-crystallin expression affects microtubule assembly and prevents their aggregation, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 20 (2006) 846–857. [DOI] [PubMed] [Google Scholar]
- [21].Harding J, Cataract: Bochemistry, Epidemiology and Pharmacology, Chapman and Hall, London, 1991. [Google Scholar]
- [22].Lampi KJ, Wilmarth PA, Murray MR, David LL, Lens beta-crystallins: the role of deamidation and related modifications in aging and cataract, Progress in biophysics and molecular biology, 115 (2014) 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jedziniak JA, Kinoshita JH, Yates EM, Hocker LO, Benedek GB, On the presence and mechanism of formation of heavy molecular weight aggregates in human normal and cataractous lenses, Experimental eye research, 15 (1973) 185–192. [DOI] [PubMed] [Google Scholar]
- [24].Jedziniak JA, Kinoshita JH, Yates EM, Benedek GB, The concentration and localization of heavy molecular weight aggregates in aging normal and cataractous human lenses, Experimental eye research, 20 (1975) 367–369. [DOI] [PubMed] [Google Scholar]
- [25].Ingolia TD, Craig EA, Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin, Proc. Natl. Acad. Sci. U. S. A, 79 (1982) 2360–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Horwitz J, Alpha-crystallin can function as a molecular chaperone, Proc. Natl. Acad. Sci. U. S. A, 89 (1992) 10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ghosh JG, Houck SA, Clark JI, Interactive sequences in the molecular chaperone, human alphaB crystallin modulate the fibrillation of amyloidogenic proteins, The international journal of biochemistry & cell biology, 40 (2008) 954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Greiling TM, Clark JI, New insights into the mechanism of lens development using zebra fish, International review of cell and molecular biology, 296 (2012) 1–61. [DOI] [PubMed] [Google Scholar]
- [29].Greiling TM, Clark JI, The transparent lens and cornea in the mouse and zebra fish eye, Seminars in cell & developmental biology, 19 (2008) 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Clark JI, Matsushima H, David LL, Clark JM, Lens cytoskeleton and transparency: a model, Eye, 13 ( Pt 3b) (1999) 417–424. [DOI] [PubMed] [Google Scholar]
- [31].Nicholl ID, Quinlan RA, Chaperone activity of alpha-crystallins modulates intermediate filament assembly, The EMBO journal, 13 (1994) 945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].FitzGerald PG, Graham D, Ultrastructural localization of alpha A-crystallin to the bovine lens fiber cell cytoskeleton, Current eye research, 10 (1991) 417–436. [DOI] [PubMed] [Google Scholar]
- [33].Clark TJ, Houck SA, Clark JI, Hemoglobin interactions with alphaB crystallin: a direct test of sensitivity to protein instability, PLoS One, 7 (2012) e40486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sickle Cell Disease, Raven Press, Ltd, New York, 1994. [Google Scholar]
- [35].Eaton WA, Hofrichter J, Sickle cell hemoglobin polymerization, Advances in protein chemistry, 40 (1990) 63–279. [DOI] [PubMed] [Google Scholar]
- [36].Anderson DM, Floyd KA, Barnes S, Clark JM, Clark JI, McHaourab H, Schey KL, A method to prevent protein delocalization in imaging mass spectrometry of non-adherent tissues: application to small vertebrate lens imaging, Analytical and bioanalytical chemistry, 407 (2015) 2311–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sharma KK, Santhoshkumar P, Lens aging: effects of crystallins, Biochimica et biophysica acta, 1790 (2009) 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Delaye M, Tardieu A, Short-range order of crystallin proteins accounts for eye lens transparency, Nature, 302 (1983) 415–417. [DOI] [PubMed] [Google Scholar]
- [39].Clark JI, Order and disorder in the transparent media of the eye, Experimental eye research, 78 (2004) 427–432. [DOI] [PubMed] [Google Scholar]
- [40].Bassnett S, Shi Y, Vrensen GF, Biological glass: structural determinants of eye lens transparency, Philos. Trans. R. Soc. Lond. B Biol. Sci, 366 (2011) 1250–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG, Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin, Investigative ophthalmology & visual science, 44 (2003) 5252–5258. [DOI] [PubMed] [Google Scholar]
- [42].FitzGerald PG, Lens intermediate filaments, Experimental eye research, 88 (2009) 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gokhin DS, Nowak RB, Kim NE, Arnett EE, Chen AC, Sah RL, Clark JI, Fowler VM, Tmod1 and CP49 synergize to control the fiber cell geometry, transparency, and mechanical stiffness of the mouse lens, PLoS One, 7 (2012) e48734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang Y, Lomakin A, McManus JJ, Ogun O, Benedek GB, Phase behavior of mixtures of human lens proteins Gamma D and Beta B1, Proc. Natl. Acad. Sci. U. S. A, 107 (2010) 13282–13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Banerjee PR, Pande A, Patrosz J, Thurston GM, Pande J, Cataract-associated mutant E107A of human gammaD-crystallin shows increased attraction to alpha-crystallin and enhanced light scattering, Proc. Natl. Acad. Sci. U. S. A, 108 (2011) 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP, Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse, Laboratory investigation; a journal of technical methods and pathology, 84 (2004) 679–692. [DOI] [PubMed] [Google Scholar]
- [47].Costello MJ, Burette A, Weber M, Metlapally S, Gilliland KO, Fowler WC, Mohamed A, Johnsen S, Electron tomography of fiber cell cytoplasm and dense cores of multilamellar bodies from human age-related nuclear cataracts, Experimental eye research, 101 (2012) 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Norose K, Lo WK, Clark JI, Sage EH, Howe CC, Lenses of SPARC-null mice exhibit an abnormal cell surface-basement membrane interface, Experimental eye research, 71 (2000) 295–307. [DOI] [PubMed] [Google Scholar]
- [49].Vaezy S, Clark JI, Characterization of the cellular microstructure of ocular lens using 2D power law analysis, Annals of biomedical engineering, 23 (1995) 482–490. [DOI] [PubMed] [Google Scholar]
- [50].Vaezy S, Clark JI, Clark JM, Quantitative analysis of the lens cell microstructure in selenite cataract using a two-dimensional Fourier analysis, Experimental eye research, 60 (1995) 245–255. [DOI] [PubMed] [Google Scholar]
- [51].Clark JI, Huang QL, Modulation of the chaperone-like activity of bovine alpha-crystallin, Proc. Natl. Acad. Sci. U. S. A, 93 (1996) 15185–15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Clark JI, Livesey JC, Steele JE, Delay or inhibition of rat lens opacification using pantethine and WR-77913, Experimental eye research, 62 (1996) 75–84. [DOI] [PubMed] [Google Scholar]
- [53].Randazzo J, Zhang P, Makita J, Blessing K, Kador PF, Orally active multi-functional antioxidants delay cataract formation in streptozotocin (type 1) diabetic and gamma-irradiated rats, PLoS One, 6 (2011) e18980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nahomi RB, Wang B, Raghavan CT, Voss O, Doseff AI, Santhoshkumar P, Nagaraj RH, Chaperone peptides of alpha-crystallin inhibit epithelial cell apoptosis, protein insolubilization, and opacification in experimental cataracts, The Journal of biological chemistry, 288 (2013) 13022–13035. [DOI] [PMC free article] [PubMed] [Google Scholar]