Abstract
Abstract
In this study, an active antibacterial packaging film was developed by coating a polyethylene terephthalate/polyvinylidene chloride/retort casting polypropylene (PPR) plastic multilayer film with plantaricin BM‐1 and chitosan. The characteristics of the active packaging film and its antibacterial effect for chilled meat preservation were evaluated. Our results indicated that the barrier properties against oxygen were improved significantly and the tensile strength and the elongation at break were changed slightly. The active plantaricin film significantly (P < 0.05) decreased the viable counts of Listeria monocytogenes by 3.6 log10CFU/mL in liquid medium and approximately 1.4 log10CFU/g in meat stored at 4 °C for 8 days compared with the control. Moreover, the viable counts of aerobes and anaerobes in the meat packaged with the active plantaricin film were significantly (P < 0.05) decreased by approximately 0.6 log10CFU/g and 1.1 log10CFU/g when compared with that packaged with PPR film stored at 4 °C for 12 days. The total volatile base (TVB‐N) in the meat packaged with the active plantaricin film was significantly (P < 0.05) lower than that in the control during the entire storage period. Our results indicated that the active film could extend the meat shelf life by inhibiting the L. monocytogenes and the background spoilage bacteria in chilled meat stored at 4 °C. This outcome suggests that plastic multilayer film incorporating plantaricin BM‐1 can be potentially used for fresh meat packaging.
Practical Application
Fresh meat is highly perishable product. This study developed a plantaricin BM‐1 active plastic multilayer film that can inhibit the growth of microorganisms in chilled meat during storage at 4 °C.
Keywords: Listeria monocytogenes, meat preservation, plantaricin, packaging film
Introduction
Fresh meat is a highly perishable product because it is a rich nutrient matrix that offers an excellent environment favorable for spoilage microorganisms and foodborne pathogens (Aymerich, Picouet, & Monfort, 2008). L. monocytogenes is a foodborne pathogen that widely exists in different environments and foods such as raw milk, meat, fish, fruits, and vegetables. It occurs at different stages of the meat production process, even during the slicing or packaging of the product (Lara‐Lledó, Olaimat, & Holley, 2012). In addition, L. monocytogenes is a facultatively anaerobic, psychrotroph bacterium (Vázquez‐Boland et al., 2001) that grows at refrigerator temperatures, and it is tolerant to low pH and certain preservatives such as sodium chloride, which are inhibitory to other food pathogens (Alves, Martinez, Lavrador, & De Martinis, 2006). L. monocytogenes causes human listeriosis, although rare, has a mortality of 15% to 30% in affected individuals, including pregnant women, newborns, elderly, and immunocompromised people (Rodrigues, Sá, & Melo, 2017).
Antimicrobial and intelligent packaging may provide a promising method to satisfy consumer requirements for meat preservation, that is, high quality, easy‐to‐handle, safe, less chemically preserved, and inhibits the growth of spoilage organisms (Chen et al., 2012). To control the undesirable growth of microorganisms on the surface of the meat, coating of antimicrobial substances in packaging materials has been the most studied area (Zhou, Xu, & Liu, 2010). Bacteriocins are antimicrobial peptides/proteins produced by bacteria and have been incorporated into plastic films that could inhibit the growth of some foodborne pathogens and extend the shelf life of fresh meat (Woraprayote et al., 2013). The most studied bacteriocins in antilisterial active packaging are nisin (Neetoo, Ye, & Chen, 2007; Pattanayaiying, H‐Kittikun, & Cutter, 2015), pediocin PA‐1/AcH (Woraprayote et al., 2013), enterocin416K1 (Anacarso et al., 2011; Iseppi et al., 2008), and bacteriocin 32Y (Ercolini, Storia, Villani, & Mauriello, 2006). Of these bacteriocins, nisin is commercially available, and it has been used to decontaminate artificially contaminated pieces of raw pork (Murray & Richard, 1997). However, there are several limitations when employing nisin as an antimicrobial in meat due to its low solubility and interactions within phospholipids, emulsifiers, and other food components (Alves et al., 2006).
Soaking, blow processing, extrusion, pouch contact, and coating are common methods for incorporating bacteriocins within plastic films (Zhang et al., 2017). Coating the packaging material with an antimicrobial agent, effect of high temperature, and shearing on the antimicrobial agent during extrusion allow the antimicrobial agent to persist at a high concentration on the surface of the packaging material (Zhou et al., 2010). However, direct coating of bacteriocin on a plastic film has been limited due to its hydrophobic characteristics. (Woraprayote et al., 2013). To solve this problem, chitosan, an environmentally friendly material with intrinsic antimicrobial properties, was combined with a bacteriocin to enhance the adsorption of the bacteriocin and facilitate its coating in the film (Siripatrawan & Harte, 2010).
In this study, a plastic multilayer film with antibacterial activity that incorporates plantaricin BM‐1 and chitosan was developed to inhibit the growth of microorganisms and extend the shelf life of the chilled meat. We evaluated the film characteristics and inhibitory effects as a part of a model system for pork to facilitate the potential use of the antibacterial film for meat preservation.
Materials and Methods
Bacterial strains
Lactobacillus plantarum BM‐1 was isolated from a traditionally fermented Chinese meat product (Zhang et al., 2013) and inoculated in de Man–Rogosa–Sharpe broth (MRS; LuQiao Company, Beijing, China) at 2% (v/v) and incubated at 37 °C for 20 hr. L. monocytogenes CMCC 54003 was used as an indicator strain, and cultured in trypticase soy‐yeast extract broth (TSB‐YE; AoBoXing Company, Beijing, China) at 37 °C for 16 hr. All strains were stored at –80 °C in 15% (v/v) glycerol.
Bacteriocin preparation
L. plantarum BM‐1 was inoculated in MRS broth at 2% (v/v) and incubated at 37 °C for 20 hr. Plantaricin BM‐1 was purified using a two‐step procedure consisting of pH‐mediated cell adsorption–desorption and cation exchange (SP Sepharose Fast Flow column, GE Healthcare) as described (Zhang et al., 2013). The purified plantaricin BM‐1 was dialyzed and sterilized by filtration through a 0.22‐µm Millex GP filter (Millipore, Bedford, MA, USA). The active fractions were collected and freeze‐dried for further use (Freeze dryer FD‐1A‐80, Marin Christ), and the bacteriocin titer was determined by the agar well diffusion method (Zhang et al., 2013) and expressed in arbitrary units (AU) per milliliter (AU/mL). One AU was defined as the reciprocal of the highest twofold serial dilution in distilled water that showed a clear zone.
Preparation of the active plastic multilayer film
The coating solution was prepared using a method slightly modified from Ye, Neetoo, and Chen (2008). Chitosan (2 g) (Sinopharm Chemical Reagent Co., Ltd, Beijing, China) and hydroxypropyl methylcellulose (HPMC, 3 g; Alfa Aesar Chemical Co., LTD, Tianjin, China) were dissolved in 100 mL of 1% (w/v) acetic acid separately and stirred overnight at room temperature. The coating solution was prepared by mixing 13 parts of chitosan solution and two parts of HPMC solution. The bacteriocin coating solutions were prepared by mixing 2.6 g purified plantaricin BM‐1 into 30 mL of the coating solution. A polyethylene terephthalate/polyvinylidene chloride/retort casting polypropylene (PPR) plastic multilayer film was purchased from a local company (Tianjin Plastics Co., Ltd, Tianjin, China.) and taped to 32 × 55 cm2 glass plates. Then the open area of PPR film was coated with the coating solution using a MS‐ZN320A Laboratory blade coater (Xin Kerui Machinery Technology co., LTD, Xiamen, China). The coating thickness was controlled by adjusting the microcontroller to 100 µm. Finally, the chitosan coated films (PPR‐CH) containing 173 µg/cm2 of chitosan, and the bacteriocin coated films (PPR‐CH‐BM‐1) containing 173 µg/cm2 of chitosan and 867 µg/cm2 of bacteriocin. A PPR plastic multilayer film was used as the control. All films were air‐dried at room temperature overnight.
Film properties
The tensile strength (TS) and elongation at break (E%) of the plastic multilayer films were evaluated using a using a universal testing machine (C43.104, MTS Industrial Systems CO., LTD, Guangzhou, China). The initial grip separation and the cross‐head speed were set at 10 and 500 mm/min, respectively. The tensile strength was calculated by dividing the maximum stress by the cross‐sectional area of the specimen, and the elongation value was expressed in percent, with the ratio of the extended length at the break point of the initial length. Five specimens (10 × 10 mm2) cut from each film were tested.
The oxygen transmission rate (OTR) of the plastic multilayer film was tested using an oxygen permeability instrument (OX‐TRAN MODEL 2/21, Danbell Instrument CO., LTD, Beijing, China). The test was done at 100% oxygen at 24 °C and a relative humidity of 75%. Three repetitions were performed.
The water vapor transmission rate (WVTR) of the plastic multilayer films was measured at 23 °C and 65% relative humidity using a water vapor permeability meter (W303, Guangzhou Standard International Packaging Equipment Co., LTD, Guangzhou, China). Three independent measurements were performed.
Antimicrobial effect of active films against L. monocytogenes in broth
Each film, including PPR, PPR‐CH, and PPR‐CH‐BCM‐1, was sliced into 1‐cm diameter disks, added to 10 mL of Tryptic Soy Broth (TSB) liquid medium inoculated with 103 CFU/mL of L. monocytogenes CMCC 54003, and incubated at 37 °C. The optical density (OD540) of the cultures was determined every half‐hour. Colonies of L. monocytogenes CMCC 54003 were counted using plate counts every 3 hr. All experiments were repeated three times.
Antimicrobial effect of active films against L. monocytogenes in fresh pork
Fresh pork meat was purchased from a local supermarket and immediately cut into pieces of similar thickness weighing 25 ± 1 g. The sliced meat was soaked in 100 mL sterile water containing L. monocytogenes at an initial count of 1.0 × 104 CFU/mL for 5 min. After soaking, the samples were removed from the suspension and the residual moisture was drained. Then, samples were wrapped in 10 × 10 cm barrier pouches made from the PPR, PPR‐CH, and PPR‐CH‐BM‐1 multilayer films. All of the samples were stored at 4 °C for 8 days, and colonies of L. monocytogenes CMCC 54003 were counted every 2 days during the storage period by plating on PALCAM agar (Woraprayote et al., 2013).
Antimicrobial effect of plantaricin BM‐1‐activated films on fresh pork meat
Fresh pork meat was cut into sections of 25 ± 1 g and stored in the open for 3 hr, then the sliced meat was wrapped in barrier pouches described as above. The wrapped samples were sealed using a vacuum‐packaging machine (PR4257, The Magic Seal Company, Guangdong, China). All samples were stored at 4 °C for 12 days, and the total bacterial count, pH, and TVB‐N of the meat were determined every 2 days. All experiments were repeated three times.
Total aerobe and anaerobe counts of the meat were determined by colony counting method using Plate Count Agar (PCA) (LuQiao Company, Beijing, China) according to the China National Standard (GB 4789.2‐2010). Briefly, a sample of 25 ± 1 g of sliced meat was added to 225 mL sterilized physiological saline solution (0.85% NaCl) and homogenized for 1 min. Tenfold serial dilutions were made in sterilized physiological saline solution, then a 1 mL sample of the suspension was added to the plate count agar, and the plates were incubated at 37 °C for 48 hr under aerobic and anaerobic conditions, respectively. Colony forming units (CFU/g) were counted to determine the total aerobic and anaerobic counts.
The pH of the pork meat was determined according to the China National Standard (GB/T 9695.5‐2008). Briefly, meat sample (10 ± 0.1 g) was homogenized with distilled water (100 mL, pH 7.0) for 30 s, then the pH of sample suspension was measured using a PHS‐3B digital pH meter (Ray Magnetic Instruments, Shanghai, China).
A semi‐automatic Kjeldahl apparatus (KDN‐08A, Hongji Instrument Co. Ltd, Shanghai, China) was used to determine the TVB‐N using the semi‐micro Kjeldahl method according to the China National Standard GB (5009.44‐2016). Briefly, meat sample (10 ± 0.1 g) was homogenized with distilled water (100 mL, pH 7.0) for 30 min. After filtering, the extract was treated with a magnesia solution (10 g/L) and subjected to steam distillation. The volatile base components were absorbed by a boric acid receiver and determined by titration.
Statistical analyses
Experiments were performed at least in triplicate. Data value was presented as the mean ± SD. Significant differences of antimicrobial effect and mechanical properties between three films were identified by a general linear model analysis of variance (ANOVA) using the IBM SPSS Statistics software (version 17.0), and Tukey's test was used for comparison of mean values at a significant level of 0.05.
Results
Mechanical properties of active films
According to our results, the TS of the three films were 27.9 to 33.7 MPa, and the E% of the three films fell in a range of 110.61% to 130.88%. There were no significant differences of the TS and E% among the three films (P > 0.05; Table 1). Moreover, the OTR of the PPR‐CH‐BM‐1 plastic multilayer film was 19.79 ± 0.75 mL/m2·day, which is significantly higher (P < 0.05) than the PPR‐CH film (16.09 ± 0.90 mL/m2·day) and significantly lower (P < 0.05) than the PPR film (22.56 ± 0.44 mL/m2·day) (P < 0.05). The WVTR of the PPR‐CH‐BM‐1 was greatly higher (P < 0.05) than that of the PPR plastic multilayer film, and there was no significant difference of PPR‐CH film in the WVTR with PPR‐CH‐BM‐1 and PPR film (P > 0.05).
Table 1.
Mechanical properties of active plastic multilayer films
| Films | TS (MPa) | E% (%) | OTR (mL/m·day) | WVTR (g/m2·day) |
|---|---|---|---|---|
| PPR | 29.01 ± 5.99a | 128.33 ± 32.67a | 22.56 ± 0.44a | 2.03 ± 0.48b |
| PPR‐CH | 27.98 ± 5.40a | 130.88 ± 39.96a | 16.09 ± 0.90c | 2.65 ± 0.11ab |
| PPR‐CH‐BM‐1 | 33.68 ± 4.28a | 110.61 ± 26.09a | 19.79 ± 0.75b | 2.77 ± 0.25a |
a,b,cMeans with different small letters in the same column represent a significant difference at α = 0.05 level.
Antimicrobial activity of the active films against L. monocytogenes in a TSB culture
Three different films were individually added into a liquid TSB liquid culture inoculated with L. monocytogenes at an initial count of 3.22 log10CFU/mL. The optical density (OD) of the L. monocytogenes in the PPR film treatment group increased gradually and reached the highest level of 1.6 at 10 hr and then decreased gradually to 0.9 at the end of the culture period at 24 hr. In contrast, the optical density of L. monocytogenes in the PPR‐CH‐BM‐1 treatment group increased slowly during the entire experimental duration and reached the highest level of 0.6 at 24 hr (Figure 1A). Moreover, the viable counts of L. monocytogenes in the PPR and PPR‐CH groups increased gradually and reached the logarithmic phase at 8.22 log10CFU/mL and 8.35 log10CFU/mL, respectively, at 10 hr and then decreased to 7.25 log10CFU/mL and 7.35 log10CFU/mL at the end of the culture period. In the PPR‐CH‐BM‐1 film treatment, the viable counts of L. monocytogenes decreased from an initial 3.22 log10CFU/ml to 1.85 log10CFU/ml at 6 hr, this value was significantly (P < 0.05) lower than the values of 3.6 and 3.3 log10CFU/ml in the PPR and PPR‐CH treatment groups and then increased gradually to 3.85 log10CFU/ml by the end of the experiment (Figure 1B). During the entire experimental duration, there were no significant differences (P > 0.05) in the OD or viable counts of L. monocytogenes between the PPR and PPR‐CH film treatments. Our results suggested that the PPR‐CH‐BM‐1 film significantly inhibited the growth of L. monocytogenes in a TSB culture.
Figure 1.
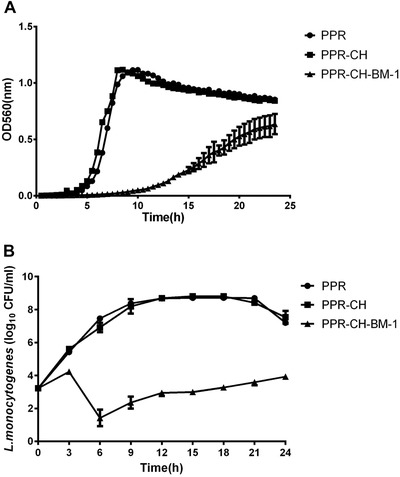
Antimicrobial effect of the active plastic multilayer films against L. monocytogenes CMCC54003 in a liquid TSB culture medium. (A) Refers to the optical density of L. monocytogenes CMCC54003, (B) Refers to the viable cell count of L. monocytogenes CMCC54003.
Antimicrobial activity of the active films against L. monocytogenes in chilled meat
This experiment evaluated the effect of the active films on the growth of L. monocytogenes in chilled meat. The results showed that the bacterial counts of L. monocytogenes increased gradually from initial 2.91 log10CFU/g to 4.24 and 4.07 log10CFU/g in fresh pork meat packaged with the PPR and PPR‐CH films, and there was a significant difference (P < 0.05) of L. monocytogenes counts between the PPR and PPR‐CH films on day 2, day 6, and day 8 (Figure 2). However, the bacterial count of L. monocytogenes in the PPR‐CH‐BM‐1 treatment group decreased from 2.91 to 2.24 log10CFU/g after 4 days of storage and was still maintained at a significantly lower level (P < 0.05). At the end of storage, it increased gradually to 2.67 log10CFU/g, which was an approximate decrease of 1.4 log10CFU/g from the values of the PPR and PPR‐CH groups. Our results indicated that the PPR‐CH‐BM‐1 film could significantly inhibit the growth of L. monocytogenes in fresh pork meat stored at 4 °C.
Figure 2.
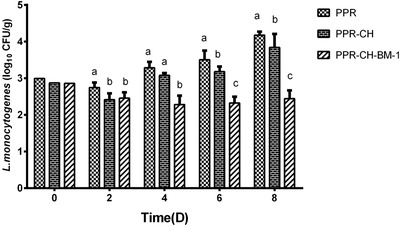
Effect of the active plastic multilayer film on the growth of L. monocytogenes CMCC54003 on fresh meat stored at 4 °C. The viable cell counts of L. monocytogenes CMCC54003 were analyzed by plate counting every 2 days. a, b, cMeans with different small letters in the same condition represent a significant difference at P < 0.05.
Antimicrobial activity of the active films on fresh meat
Fresh meat samples were exposed to the environment for a certain time, and the initial counts of aerobic and anaerobic bacteria were 3.6 and 3.22 log10CFU/g, respectively. As shown in Figure 3A, the total aerobic counts of the PPR and PPR‐CH film groups increased gradually from an initial count of 3.6 log10CFU/g to 6.18 log10CFU/g and 6.2 log10CFU/g at the end of storage, which were above the China National Standard for meat quality of 6.0 log10CFU/g. Moreover, although the total aerobic counts in the PPR and PPR‐CH groups increased gradually with a similar trend, the total aerobic counts in PPR‐CH group were lower (P < 0.05) than that in PPR group from 4 to 9 days. In PPR‐CH‐BM‐1 treatment group, the total aerobic counts were stably maintained at the initial level during the first 6 days of storage, and then increased gradually to 5.62 log10CFU/g at the end of storage, which was significantly (P < 0.05) lower than the approximate 0.6 and 0.58 log10CFU/g in the PPR and PPR‐CH groups, respectively.
Figure 3.
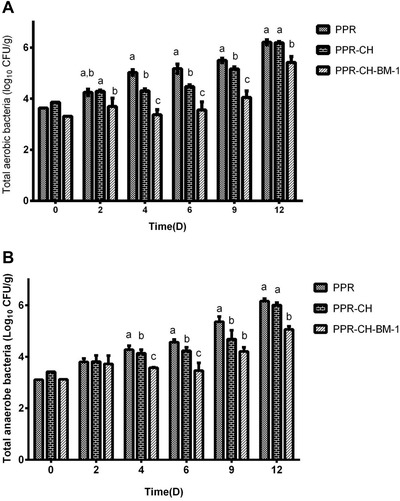
Effect of the active plastic multilayer film on the counts of total aerobic bacteria (A) and total anaerobic bacteria (B) on packaged chilled meats stored at 4 °C. a, b, cMeans with different small letters in the same condition represent a significant difference at P < 0.05.
Moreover, the total anaerobe counts in the PPR and PPR‐CH film groups increased gradually from an initial count of 3.22 log10CFU/g to 6.16 log10CFU/g and 6.0 log10CFU/g at the end of storage. From 4 to 9 days of storage, the total anaerobic counts of the meat treated with PPR‐CH remained lower (P < 0.05) than that in the PPR film group. Furthermore, the total anaerobic counts of the PPR‐CH‐BM‐1 group increased slowly and reached the highest level of 5.06 log10CFU/g at the end of storage, which were significantly (P < 0.05) lower than that in the PPR and PPR‐CH groups. All of these results suggested that the plantaricin BM‐1 active film could inhibit the growth of both aerobic and anaerobic bacteria in the meat.
The pH of the meat packaged with the three films during storage was also measured. The initial pH of the meat was approximately 5.6. The pH of all samples was slightly decreased at the beginning of storage, and then it started to increase gradually. During the whole storage, there were no significant differences (P > 0.05) of pH among the three groups (Figure 4).
Figure 4.
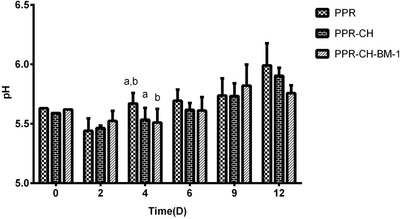
Effect of the active plastic multilayer film on the pH of meat stored at 4 °C. a, b, cMeans with different small letters in the same condition represent a significant difference at P < 0.05.
The TVB‐N is one of the most important indicators of meat spoilage. According to Figure 5, the TVB‐N of all samples increased gradually during the entire storage period. The TVB‐N of the meats in the PPR and PPR‐CH groups increased gradually to 10.0 and 10.9 mg/100 g at the end of storage, respectively. There was no significant difference of TVB‐N between the PPR and PPR‐CH groups during the entire storage period. Moreover, the TVB‐N in the PPR‐CH‐BM‐1 group increased slowly and was still lower than 9.0 mg/100 g even at the end of the storage period, which was significantly (P < 0.05) lower than that of the PPR and PPR‐CH films. It is clear that all TVB‐N values were lower than 15 mg/100 g that indicates the spoilage of fresh meat.
Figure 5.
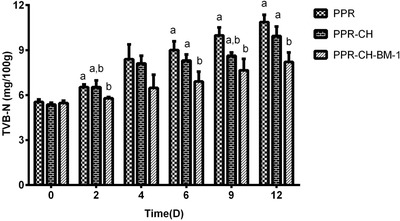
Effect of the active plastic multilayer film on the TVB‐N of meat stored at 4 °C. a, b, cMeans with different small letters in the same condition represent significant difference at P < 0.05.
Discussion
In this study, we first investigated the characteristics of the PPR, PPR‐CH, and PPR‐CH‐BM‐1 films, including TS, E%, OTR, and WVTR. TS represents the film's resistance to elongation, and E% is related to its stretching capacity (Sánchezgonzález, Quintero Saavedra, & Chiralt, 2013). According to our results, the TS of the PPR‐CH‐BM‐1 film was slightly higher than that of the PPR‐CH and PPR multilayer films, but there were no significant differences in TS and E% among these films, indicating that the chitosan and plantaricin BM‐1 did not affect the TS and E% of the active films. A similar previous result indicated that nisin incorporated in a pectin/polylactic acid (pectin/PLA) film did not affect the TS, flexibility and toughness of the pectin/PLA film (Jin, Liu, Zhang, & Kevin, 2010). However, Wu, Teng, Liu, Tian, and Wang (2018) reported that anchoring nisin onto the oxidized cellulose membrane increased the tensile strengths of the active film.
The OTR showed that the chitosan plastic film (with or without plantaricin BM‐1) also had a significantly (P < 0.05) lower OTR compared with the control plastic film due to the presence of chitosan. It is possible that chitosan has a membranous structure and a good gas permselectivity for CO2 and O2. So, it was always combined with other antimicrobial applications in active packaging (Yuan, Chen, & Li, 2016). Additionally, Abdollahi, Rezaei, and Farzi (2012) found that chitosan combined with other antimicrobials influences the OTR, causing the oxygen transmittance rating to increase. In this study, the OTR of the PPR‐CH‐BM‐1 film was significantly higher than that of the PPR‐CH film but significantly lower than that of the PPR film (P < 0.05), which may be due to the uneven film surface caused by other antimicrobial substances such as plantaricin BM‐1, and it increased the oxygen transmittance rate. The WVTR of the PPR‐CH‐BM‐1 film was higher than that of the PPR film group, but it was still lower than for other common plastic films. The chitosan and the plantaricin BM‐1 are all absorbent materials, so it will influence the film of WVTR. The film characteristics experiments demonstrated that the PPR‐CH‐BM‐1 film is strong, flexible, tough, and a good oxygen and water vapor barrier suitable for food packaging.
Our investigation of the antilisterial effect of the active films indicated that the PPR‐CH plastic multilayer film had no obvious antilisterial effect, but the PPR‐CH‐BM‐1 active film significantly inhibited the growth of L. monocytogenes in liquid TSB medium (Figure 1A and 1B). A previous study demonstrated that plantaricin BM‐1 can inhibit some Gram‐positive pathogens, including L. monocytogenes and S. aureus (Zhang et al., 2017), and coating LDPE, DHDPE, and PE plastic films with it can prolong the exponential growth phase and cause a drastic decrease of L. monocytogenes (Zhang et al., 2013). Jin et al. (2010) found that pectin and nisin in a PLA film significantly decreased the bacterial counts. Compared with the control, it inhibited the growth rate and the final cell density of L. monocytogenes. In addition, some researchers also used measured inhibition zones of L. monocytogenes caused by active films on solid medium and demonstrated that plastic films with bacteriocins incorporated had a significant effect on L. monocytogenes. For example, lactocin 705 and lactocin AL705 incorporated into a multilayer film can inhibit L. innocua 7 for 6 weeks at 30, 10, and 5 °C (Blanco Massani, Morando, Vignolo, & Eisenberg, 2012), and the Lactobacillus curvatus 32Y incorporated into a polythene film by various methods inhibited the growth of L. monocytogenes V7. The coated film displayed clear and stable antilisterial activity (Mauriello, Ercolini, La Storia, Casaburi, & Villani, 2004). A similar finding that the contact method for incorporating bacteriocin in PE‐based films was more effective, homogeneous, and used less active solution than other methods has been reported (Massani, Fernandez, Ariosti, Eisenberg, & Vignolo, 2008). Therefore, in this study, we adopted the coating method to produce a PPR‐CH‐BM‐1 active film, and demonstrated that it strongly inhibited L. monocytogenes.
Similar results were obtained for chilled meat packaged with the three films. Although the PPR‐CH film had a slight antilisteral effect, the PPR‐CH‐BM‐1 active film effect was more significant. It clearly reduced the viable counts of L. monocytogenes and kept them at a lower level during the storage period (Figure 2). These results are in agreement with previous reports that lactocin incorporated in active synthetic films effectively reduced L. innocua 7 in wieners by approximately 2.5 log cycles at day 45 compared with synthetic films (Blanco et al., 2014). Bacteriocin 32Y coated films were used to wrap hamburger and pork steaks and reduced the initial counts of L. monocytogenesby nearly 1.0 log during the first 24 hr of storage (Mauriello et al., 2004). Enterocin 416K1 was coated on LLDPE films and decreased L. monocytogenes in frankfurter samples during the first 24 hr, and the effect even maintained up to the seventh day and then decreased (Iseppi et al., 2008). All of these studies demonstrated that bacteriocins incorporated within plastic films had good potential to control the risk of meat contamination by L. monocytogenes.
We determined the total aerobic and anaerobic counts to further investigate the antimicrobial activity of the active films for chilled meat. Refrigerated PPR‐CH multilayer film has slight antimicrobial activity, but the PPR‐CH‐BM‐1 film has excellent antimicrobial activity as indicated by both the total aerobic and anaerobic counts (Figure 3A and 3B). On the one hand, previous research showed that chitosan had antibacterial activity. The positive charges of chitosan interact with negatively charged residues of macromolecules on the microbial cell surface, causing membrane leakage (Siripatrawan & Harte, 2010). However, the antibacterial spectrum of chitosan is very narrow, so it is always combined with other antibacterial materials to enhance the antibacterial effect and extended the shelf life of a product (Ojagh, Rezaei, Razavi, & Hosseini, 2010). Ye et al. (2008) found that a nisin–chitosan plastic multilayer film can control the total aerobes in smoked salmon to less than 2.5 log CFU/cm2 and anaerobes to less than 1.9 log CFU/cm2 after 8 weeks of storage at 4 °C. On the other hand, bacteriocin has been widely used as an antibacterial agent in antimicrobial packaging to extend the shelf life of food. For example, nisin incorporated in a plastic film can inactivate a portion of the Salmonella typhimurium population of inoculated chicken skin and control the mesophilic and psychrotrophic counts of treated drumsticks (Natrajan & Sheldon, 2000). Additionally, previous reports indicated that plantaricin BM‐1 can significantly delay the growth of background spoilage bacteria in cooked ham (Zhou et al., 2010). In this study, we found that the antimicrobial activity of the PPR‐CH‐BM‐1 film was strong, but it became gradually weaker with time. This phenomenon may be associated with the transfer of the plantaricin BM‐1 molecules from the film to the meat, which may reduce the concentration of plantaricin BM‐1 on the surface of the film. A similar result was reported by Nguyen, Gidley, and Dykes (2008). Thus, it is important to control the release of the agent to maintain the concentration of active compound during storage (Han, 2005). Our previous study demonstrated that plantaricin BM‐1‐incorporated in a PVDC film has an excellent adsorption and release rate (Zhang et al., 2017). So, a PPR‐CH‐BM‐1 film that used the PVDC film as an internal material not only extended the antibacterial spectrum but also had the potential for adsorption and release in food packaging.
Conclusions
The pH and TVB‐N are the most important indicators that determine the deterioration of cold fresh meat (Dong et al., 2016; Hao, Yang, Yang, & Ma, 2013). With time, the pH of the meat showed a tendency to increase gradually (Figure 4) and had significant differences with the total aerobic and anaerobe counts, which may have been caused by small molecules such as amino acids and alkaline groups from the proteins and enzymes (Kumar & Tanwar, 2011). However, all samples showed a small decrease on the second day. Hao et al. (2013) hypothesized that the lactic acid bacteria played a decisive role in the pH decrease. The pH change of the PPR‐CH‐BM‐1 film was small, possibly because plantaricin BM‐1 from a traditionally fermented Chinese meat product can inhibit the growth of lactic acid bacteria (Zhou et al., 2010). Similar results that nisin incorporated in a PLLA/PVA/PCL multilayer film resulted in a smaller pH change have been reported (Zhang et al., 2017). TVB‐N caused by microbial spoilage affected proteins and enzymes that were produced by the alkaline substances such as amines, which leads to meat deterioration (Dong et al., 2016). The TVB‐N increased gradually in all of the meat samples with storage time, but the PPR‐CH‐BM‐1 films had significantly lower values than the PPR and PPR‐CH films (Figure 5). This result was similar to the total aerobic and anaerobic count results. Because the TVB‐N is related to the spoilage by bacterial decomposition of the meat muscle, higher values of the total aerobic and anaerobic counts should correspond with a higher TVB‐N during storage (Hao et al., 2013). Our results suggested that the active film incorporated with plantaricin BM‐1 and chitosan has good potential for active packaging of chilled meat to inhibit the growth of L. monocytogenes and background spoilage bacteria to prolong the shelf life of fresh meat.
Author Contributions
Hongxing Zhang and Hui Liu conceived and designed the experiments. Wenge Yang and Yuanhong Xie performed the experiments, collected test data, and drafted the manuscript. Wenge Yang and Junhua Jin analyzed the data.
Acknowledgments
The research was supported by the Research project of Beijing Municipal Education Commission (KM201810020016).
References
- Abdollahi, M. , Rezaei, M. , & Farzi, G. (2012). Improvement of active chitosan film properties with rosemary essential oil for food packaging. International Journal of Food Science and Technology, 47, 847–853. [Google Scholar]
- Alves, V. F. , Martinez, R. C. R. , Lavrador, M. A. S. , & De Martinis, E. C. P. (2006). Antilisterial activity of lactic acid bacteria inoculated on cooked ham. Meat Science, 74, 623–627. [DOI] [PubMed] [Google Scholar]
- Anacarso, I. , Niederhäusern, S. D. , Iseppi, R. , Sabia, C. , Bondi, M. , & Messi, P. (2011). Anti‐listerial activity of chitosan and Enterocin 416K1 in artificially contaminated RTE products. Food Control, 22, 2076–2080. [Google Scholar]
- Aymerich, T. , Picouet, P. A. , & Monfort, J. M. (2008). Decontamination technologies for meat products. Meat Science, 78, 114–129. [DOI] [PubMed] [Google Scholar]
- Blanco Massani, M. , Morando, P. J. , Vignolo, G. M. , & Eisenberg, P. (2012). Characterization of a multilayer film activated with Lactobacillus curvatus CRL705 bacteriocins. Journal of the Science of Food and Agriculture, 92, 1318–1323. [DOI] [PubMed] [Google Scholar]
- Blanco, M. M. , Molina, V. , Sanchez, M. , Renaud, V. , Eisenberg, P. , & Vignolo, G. (2014). Active polymers containing Lactobacillus curvatus CRL705 bacteriocins: Effectiveness assessment in Wieners. International Journal of Food Microbiology, 178, 7–12. [DOI] [PubMed] [Google Scholar]
- Chen, J. H. , Ren, Y. , Seow, J. , Liu, T. , Bang, W. S. , & Yuk, H. G. (2012). Intervention technologies for ensuring microbiological safety of meat: Current and future trends. Comprehensive Reviews in Food Science and Food Safety, 11, 119–132. [Google Scholar]
- Dong, T. , Zhang, Y. Q. , Qi, X. J. , Liang, M. , Song, S. X. , Liu, L. L. , & Shuang, Q. (2016). Evaluation of the effects of prepared antibacterial multilayer film on the quality and shelf‐life stability of chilled meat. Journal of Food Processing and Preservation, 41, 1–10. [Google Scholar]
- Ercolini, D. , Storia, A. L. , Villani, F. , & Mauriello, G. (2006). Effect of a bacteriocin‐activated polythene film on Listeria monocytogenes as evaluated by viable staining and epifluorescence microscopy. Journal of Applied Microbiology, 100, 765–772. [DOI] [PubMed] [Google Scholar]
- Han, J. H. (Ed.). (2005). Antimicrobial packaging systems Innovations in food packaging (pp. 80–107). New York, NY, Academic Press. [Google Scholar]
- Hao, J. M. , Yang, W. P. , Yang, H. , & Ma, L. Z. (2013). The Application of a compound natural preservative solution to chilled beef and mutton under vacuum packaging during refrigerated storage. Food Science and Technology Research, 19(4), 591–599. [Google Scholar]
- Iseppi, R. , Pilati, F. , Marini, M. , Toselli, M. , Niederhäusern, S. D. , Guerrieri, E. , … Bondi, M. (2008). Anti‐listerial activity of a polymeric film coated with hybrid coatings doped with Enterocin 416K1 for use as bioactive food packaging. International Journal of Food Microbiology, 123, 281–287. [DOI] [PubMed] [Google Scholar]
- Jin, T. , Liu, L. S. , Zhang, H. , & Kevin, H. (2010). Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against Listeria monocytogenes . International Journal of Food Microbiology, 44, 322–329. [Google Scholar]
- Kumar, D. , & Tanwar, V. K. (2011). Effects of incorporation of ground mustard on quality attributes of chicken nuggets. Journal of Food Science and Technology, 48, 759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara‐Lledó, M. , Olaimat, A. , & Holley, R. A. (2012). Inhibition of Listeria monocytogenes on bologna sausages by an antimicrobial film containing mustard extract or sinigrin. International Journal of Food Microbiology, 156, 25–31. [DOI] [PubMed] [Google Scholar]
- Massani, M. B. , Fernandez, M. R. , Ariosti, A. , Eisenberg, P. , & Vignolo, G. (2008). Development and characterization of an active polyethylene film containing Lactobacillus curvatus CRL705 bacteriocins. Food Additives and Contaminants, 25, 1424–1430. [DOI] [PubMed] [Google Scholar]
- Mauriello, G. , Ercolini, D. , La Storia, A. , Casaburi, A. , & Villani, F. (2004). Development of polythene films for food packaging activated with an antilisterial bacteriocin from Lactobacillus curvatus 32Y. Journal of Applied Microbiology, 97, 314–322. [DOI] [PubMed] [Google Scholar]
- Murray, M. , & Richard, J. A. (1997). Comparative study of the antilisterial activity of Nisin A and Pediocin AcH in fresh ground pork stored aerobically at 5°C. Journal of Food Protection, 60, 1534–1540. [DOI] [PubMed] [Google Scholar]
- Natrajan, N. , & Sheldon, B. W. (2000). Efficacy of nisin‐coated polymer films to inactivate Salmonella Typhimurium on fresh broiler skin. Journal of Food Protection, 63, 1189–1196. [DOI] [PubMed] [Google Scholar]
- Neetoo, H. , Ye, M. , & Chen, H. Q. (2007). Effectiveness and stability of plastic films coated with nisin for inhibition of Listeria monocytogenes . Journal of Food Protection, 70(5), 1267–1271. [DOI] [PubMed] [Google Scholar]
- Nguyen, V. T. , Gidley, M. J. , & Dykes, G. A. (2008). Potential of a nisin‐containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiology, 25, 471–478. [DOI] [PubMed] [Google Scholar]
- Ojagh, S. M. , Rezaei, M. , Razavi, S. H. , & Hosseini, S. M. H. (2010). Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chemisty, 120, 193–198. [Google Scholar]
- Pattanayaiying, R. , H‐Kittikun, A. , & Cutter, C. N. (2015). Incorporation of nisin Z and lauric arginate into pullulan films to inhibit foodborne pathogens associated with fresh and ready‐to‐eat muscle foods. International Journal of Food Microbiology, 207, 77–82. [DOI] [PubMed] [Google Scholar]
- Rodrigues, C. S. , Sá, C. V. G. C.D. , & Melo, C. B. D. (2017). An overview of Listeria monocytogenes contamination in ready to eat meat, dairy and fishery foods. Ciência Rural, 47(2), 1–8. [Google Scholar]
- Sánchezgonzález, L. , Quintero Saavedra, J. I. , Chiralt, A , & (2013). Physical properties and antilisterial activity of bioactive edible films containing Lactobacillus plantarum . Food Hydrocolloid, 33, 92–98. [Google Scholar]
- Siripatrawan, U. , & Harte, B. R. (2010). Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocolloid, 24, 770–775. [Google Scholar]
- Vázquez‐Boland, J. A. , Kuhn, M. , Berche, P. , Chakraborty, T. , Domínguez‐Bernal, G. , Goebel, W. , & Kreft, J. (2001). Listeria pathogenesis and molecular virulence determinants. Clinical Microbiology Reviews 14, 584–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woraprayote, W. , Kingcha, Y. , Amonphanpokin, P. , Kruenate, J. , Zendo, T. , Sonomoto, K. , & Visessanguan, W. (2013). Anti‐listeria activity of poly(lactic acid)/sawdust particle biocomposite film impregnated with pediocin PA‐1/AcH and its use in raw sliced pork. International Journal of Food Microbiology, 167, 229–235. [DOI] [PubMed] [Google Scholar]
- Wu, H. , Teng, C. , Liu, B. , Tian, H. , & Wang, J. (2018). Characterization and long term antimicrobial activity of the nisin anchored cellulose films. International Journal of Biological Macromolecules, 113, 487–493. [DOI] [PubMed] [Google Scholar]
- Ye, M. , Neetoo, H. , & Chen, H. Q. (2008). Effectiveness of chitosan‐coated plastic films incorporating antimicrobials in inhibition of Listeria monocytogenes on cold‐smoked salmon. International Journal of Food Microbiology, 127, 235–240. [DOI] [PubMed] [Google Scholar]
- Yuan, G. , Chen, X. , & Li, D. (2016). Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Research International, 89, 117–128. [DOI] [PubMed] [Google Scholar]
- Zhang, H. X. , Liu, L. , Hao, Y. L. , Zhong, S. Q. , Liu, H. , Han, T. , & Xie, Y. H. (2013). Isolation and partial characterization of a bacteriocin produced by Lactobacillus plantarum BM‐1 isolated from a traditionally fermented Chinese meat product. Microbiol Immunol, 57, 746–755. [DOI] [PubMed] [Google Scholar]
- Zhang, M. , Gao, X. Z. , Zhang, H. X. , Liu, H. , Jin, J. H. , Yang, W. G. , & Xie, Y. H. (2017). Development and anti‐listerial activity of PE‐based biological preservative films incorporating plantaricin BM‐1. FEMS Microbiology Letters, 364(7), 283–288. [DOI] [PubMed] [Google Scholar]
- Zhou, G. H. , Xu, X. L. , & Liu, Y. (2010). Preservation technologies for fresh meat‐a review. Meat Science, 86, 119–128. [DOI] [PubMed] [Google Scholar]


