Abstract
Background
Long‐term prognosis of patients with chronic hepatitis C infection (HCV) remains incompletely characterized. We investigated the long‐term prognosis of liver disease in patients with chronic HCV infection who have not received antiviral therapy.
Methods
A total of 2304 patients with chronic HCV who were not received interferon‐based therapy were included.
Results
In the assessment of 1‐year disease state of liver transition probabilities, progression to chronic hepatitis occurred in 12% to 14% of patients across all age groups in male asymptomatic carriers. In male patients with chronic hepatitis, progression to cirrhosis was observed mostly in the 60 to 69 (7.6%) and ≥70 age groups (9.6%). In addition, in male patients with cirrhosis, HCC development occurred in approximately 5% of patients over the age of 40. In female asymptomatic carriers, progression to chronic hepatitis was observed in 6% to 14% of patients across all age groups. In female patients with chronic hepatitis, progression to cirrhosis was observed mostly in the 60 to 69 (8.7%) and ≥70 (7.4%) age groups. In addition, in female patients with cirrhosis, HCC development occurred in 0.9% to 3.3% of patients over the age of 50. Under assumptions of either chronic hepatitis or asymptomatic carrier state at age 40 as the starting condition for simulation over the following 40 years, the probability of HCC gradually increased with age and was higher in male patients.
Conclusions
There is a risk of cirrhosis or HCC development in HCV patients with not only chronic hepatitis but the asymptomatic carrier state as well.
Keywords: hepatitis C virus, interferon, Markov chain model, natural history, transition probability
Highlight
Yearly transition probabilities for each liver state (asymptomatic carrier, chronic hepatitis, cirrhosis, and hepatocellular carcinoma [HCC]) were calculated using a Markov chain model.
Poor liver disease outcomes such as the development of cirrhosis or HCC are possible not only in patients with chronic hepatitis but also in patients who are asymptomatic carriers at age ≥40 years.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- DAA
direct‐acting antiviral
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IFN
interferon
- PY
person‐year
- SVR
sustained virological response
1. INTRODUCTION
Approximately 180 million individuals worldwide are chronically infected with hepatitis C virus (HCV). It is a common cause of chronic liver disease and hepatocellular carcinoma (HCC) in Japan, the United States, and many European countries.1, 2 Chronic infection with HCV is a major cause of progressive liver damage and long‐term sequelae such as cirrhosis and HCC.
Interferon (IFN)‐based therapy has been performed to treat HCV patients with chronic hepatitis or decompensated cirrhosis. There have been many reports that IFN‐based therapy is useful for reducing serum alanine aminotransferase (ALT) levels, clearing HCV RNA, and improving the liver fibrosis in patients with chronic HCV infection.3, 4, 5, 6, 7 Elimination of HCV has also been reported to decrease the occurrence of HCC.6, 8 In other words, IFN‐based therapy evidently decreases the incidence of liver disease–related mortality and prolongs life expectancy.9, 10, 11 Direct‐acting antivirals (DAAs) have recently been developed to treat chronic HCV infection. They have resulted in higher rates of sustained virological response (SVR), shorter and simpler regimens, and minimal treatment‐related side effects. Many patients with chronic HCV infection, including asymptomatic carriers as well as patients with chronic hepatitis and decompensated cirrhosis, have recently received DAA therapy and achieved SVR in Japan.12, 13, 14 However, the long‐term prognosis of patients with chronic HCV infection, especially asymptomatic carriers, remains incompletely characterized. Therefore, clarifying the natural history of chronic HCV infection in asymptomatic carriers is important for estimating the benefits of DAA‐based therapy.
Markov chain models are useful for simulating the natural history of chronic diseases.15, 16 Disease states obtained from patients are individually determined and incorporated into a system of transitional probabilities from 1 state to another inside of a cycle (generally lasting 1 year).
In the present study, we simulated the long‐term prognosis of liver disease in chronic HCV patients who did not receive antiviral therapy using the Markov chain model.
2. MATERIALS AND METHODS
2.1. Patients
This study protocol was approved by the institutional review boards of Ogaki Municipal Hospital and Hiroshima University. It was in compliance with the Declaration of Helsinki. Written informed consent was obtained from all study patients for use of their laboratory data.
A total of 8954 consecutive patients who tested positive for HCV antibodies were treated at our institution, between October 1994 and September 2014. Of these, 2743 (32 469 person‐year [PY] units) met the following inclusion criteria: (1) detectable levels of HCV RNA for longer than 6 months; (2) negative of hepatitis B virus infection; (3) negative of human immunodeficiency virus co‐infection; (4) no other causes of chronic liver disease such as alcohol consumption >80 g/day, hepatotoxic drugs, autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, and Wilson's disease; (5) HCC surveillance performed during the follow‐up period; (6) follow‐up duration greater than 3 years from the first visit; (7) no incidence of HCC for at least 1 year after the first visit; and (8) complete clinical data. Of these 2743 patients, 1011 patients received IFN‐based therapy and the remaining 1732 patients did not receive IFN‐based therapy during the follow‐up period. Of the 1011 patients who received IFN‐based therapy, 439 patients started this therapy at the start of follow‐up. The remaining 572 patients received IFN‐based therapy later during the follow‐up period; their clinical data before the start of IFN‐based therapy was used for analysis. Consequently, 2304 patients (1732 patients with no IFN‐based therapy during the follow‐up period and 572 patients with IFN‐based therapy during the follow‐up period) were included in the study (Figure 1), resulting in 20 140 PY units.
Figure 1.
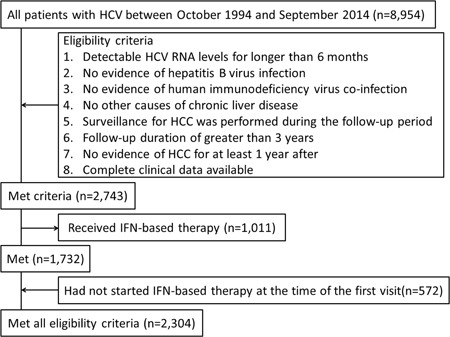
Flowchart of the patient selection process. HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN, interferon
Yearly liver disease states were acquired by surveillance of HCC program data of the study patients. The day of the initial visit was decided as the start of follow‐up. The last of follow‐up in patients who did not receive IFN‐based therapy was defined as the day of the final visit for patients who remained alive or HCC‐free, as the day of HCC diagnosis for patients who developed HCC, or as the day of death for patients who perished in the course of the follow‐up period. Regarding patients who received IFN‐based therapy during follow‐up, the last of follow‐up was decided as the day when IFN‐based therapy began.
Since there was little data of liver pathology for confirming cirrhosis, we assessed the fibrosis‐4 (FIB‐4) index as a scoring system of hepatic fibrosis. It was previously studied to have useful in predicting fibrosis in patients with chronic HCV infection.17, 18 This index is calculated as aspartate aminotransferase (AST) [IU/L] × age [years]/platelet count [109/L] × ALT [IU/L]1/2.
2.1.1. HCV RNA and genotype were confirmed using PCR
At our institution, the decision to offer IFN‐based therapy to patients with chronic HCV infection was determined based on the clinical guidelines.19, 20, 21 The 1732 patients who did not receive IFN‐based therapy for chronic HCV infection had no indications for IFN‐based therapy or declined treatment. If a patient with an indication for IFN‐based therapy declined after being provided with sufficient information about the treatment, they received hepatoprotective medications (eg, ursodeoxycholic acid) as an alternative therapy.
2.2. HCC surveillance
HCC surveillance was conducted every 3 to 6 months. It included ultrasonography and blood tests, including measurement of the tumor marker α‐fetoprotein according to the Clinical Practice Guidelines for Hepatocellular Carcinoma in Japan.22 If a nodular lesion was detected by ultrasonography or a tumor marker was found to be elevated, additional imaging studies (computed tomography, magnetic resonance imaging, or both) were performed. The diagnosis of HCC was based on imaging characteristics specified by the guidelines of the American Association for the Study of Liver Diseases.23, 24
2.3. Disease states and diagnosis of liver
In the present study, we decided disease states of liver as asymptomatic carrier, chronic hepatitis, cirrhosis, and HCC. Disease states were defined according to the following criteria25, 26:
2.4. Markov chain model analysis
The form (Figure 2) and hypothesis of the Markov chain model in the present study are in the following manner. The Markov model composed of four disease states (asymptomatic carrier, chronic hepatitis, cirrhosis, and HCC) that were collectively exhaustive and mutually exclusive.
-
1.
Transitions in the four disease states of liver appeared at 1‐year intervals.
-
2.
The disease state of liver transitions in the next year relied only on the disease state of this year.
-
3.
HCC was assumed to be an absorbing state, defined as having no later transitions to other liver disease states.
-
4.
1‐year transition probability estimates were stratified by age and sex.
Figure 2.
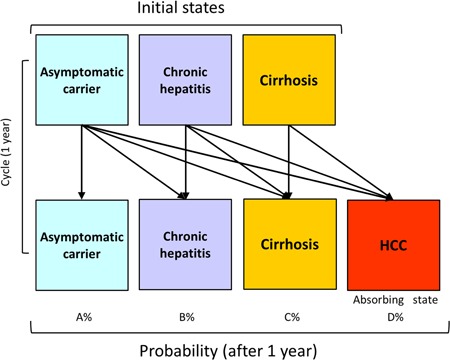
Structure of the Markov chain model. The transition between HCV‐related liver disease states based on the Markov model is shown. HCC is the absorbing state from which no transitions to other states can occur. HCC, hepatocellular carcinoma; HCV, hepatitis C virus
Annual transition probabilities for each state of liver were analyzed according to sex and 10‐year age groups. Next, the initial starting conditions were specified as asymptomatic carrier and chronic hepatitis at age 40. The % probabilities for transition crosswise liver states for the following 40 years were simulated according to yearly transition probability processions in the study patients, stratified by sex.
2.5. Statistical methods
Quantitative data are expressed as median values (interquartile range). Data analysis was carried out by JMP (version 9, SAS Institute, Cary, NC).
3. RESULTS
3.1. Characteristics of patients
Table 1 shows the baseline characteristics of the study patients. The patients comprised of 1174 (51.0%) males and 1130 (49.0%) females with a median (interquartile range) age of 61.0 (52.0‐68.0) years. There were 548 (23.8%) asymptomatic carriers, 932 (40.5%) patients with chronic hepatitis, and 824 (35.8%) patients with cirrhosis at the start of follow‐up (Table 2). Table S1 also shows the number of liver disease states at the start of follow‐up among study patients stratified by sex and 10‐year age group. During follow‐up, 473 patients developed HCC. The median follow‐up was 10.7 (6.7‐15.0) years.
Table 1.
Patient characteristics (N = 2304)
| Age, y a | 61.0 (52.0‐68.0) |
| Sex, male/female | 1174/1130 |
| AST, IU/L a | 42 (28‐70) |
| ALT, IU/L a | 45 (27‐80) |
| Albumin, g/dL a | 4.1 (3.8‐4.3) |
| Total bilirubin, mg/dL a | 0.6 (0.4‐0.8) |
| Prothrombin time, % a | 96.0 (85.0‐107.0) |
| Platelet count, × 104/mm3 a | 16.6 (12.5‐20.9) |
| α‐fetoprotein, ng/mL a | 3.9 (2.2‐8.0) |
| FIB‐4 index a | 2.4 (1.5‐4.1) |
| HCV genotype, 1/2/unknown | 1197/631/476 |
| HCV RNA, log10 IU/mL a | 5.6 (4.5‐6.1) |
| Follow‐up duration, y a | 10.7 (6.7‐15.0) |
| Development of HCC | 473 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Values are expressed as medians (interquartile range).
Table 2.
Age‐ and sex‐specific 1‐y liver disease state transition probability matrices
| 1‐y transition probability | ||||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| Asymptomatic carrier | Chronic hepatitis | Cirrhosis | HCC | Asymptomatic carrier | Chronic hepatitis | Cirrhosis | HCC | |
| Initial state | ||||||||
| Age: 30‐39 y | ||||||||
| Asymptomatic carrier | 81.3% | 12.5% | 6.2% | 0.0% | 94.0% | 6.0% | 0.0% | 0.0% |
| Chronic hepatitis | 97.8% | 2.2% | 0.0% | 99.4% | 0.6% | 0.0% | ||
| Cirrhosis | 100.0% | 0.0% | 100.0% | 0.0% | ||||
| Age: 40‐49 y | ||||||||
| Asymptomatic carrier | 87.2% | 12.8% | 0.0% | 0.0% | 85.0% | 13.7% | 1.3% | 0.0% |
| Chronic hepatitis | 95.6% | 4.4% | 0.0% | 98.3% | 1.7% | 0.0% | ||
| Cirrhosis | 95.3% | 4.7% | 100.0% | 0.0% | ||||
| Age: 50‐59 y | ||||||||
| Asymptomatic carrier | 84.3% | 13.6% | 2.1% | 0.0% | 90.1% | 8.9% | 1.0% | 0.0% |
| Chronic hepatitis | 94.9% | 4.6% | 0.5% | 94.9% | 5.0% | 0.1% | ||
| Cirrhosis | 95.8% | 4.2% | 99.1% | 0.9% | ||||
| Age: 60‐69 y | ||||||||
| Asymptomatic carrier | 84.7% | 11.3% | 4.0% | 0.0% | 88.7% | 7.6% | 3.7% | 0.0% |
| Chronic hepatitis | 91.7% | 7.6% | 0.7% | 91.1% | 8.7% | 0.2% | ||
| Cirrhosis | 95.1% | 4.9% | 97.3% | 2.7% | ||||
| Age: ≥70 y | ||||||||
| Asymptomatic carrier | 83.0% | 9.0% | 7.7% | 0.3% | 89.4% | 7.0% | 3.4% | 0.2% |
| Chronic hepatitis | 88.4% | 9.6% | 2.0% | 92.4% | 7.4% | 0.2% | ||
| Cirrhosis | 95.2% | 4.8% | 96.7% | 3.3% | ||||
Abbreviation: HCC, hepatocellular carcinoma.
3.2. 1‐Year disease state of liver transition probability processions
The 1‐year disease state of liver transition probabilities in the four disease states of liver were analyzed according to 20 140 PY units. Table 2 shows the 1‐year disease state of liver transition probability processions in the study patients according to sex and 10‐year age group.
In male asymptomatic carriers, progression to chronic hepatitis was observed in 12% to 14% over all age groups. In male patients with chronic hepatitis, progression to cirrhosis was observed mostly in the 60 to 69 (7.6%) and ≥70 age groups (9.6%). In male patients with cirrhosis, HCC development was observed in the 40 to 49 (4.4%), 50 to 59 (4.2%), 60 to 69 (4.9%), and ≥70 age groups (4.8%).
In female asymptomatic carriers, progression to chronic hepatitis was observed in 6% to 14% over all age groups. In female patients with chronic hepatitis, progression to cirrhosis was observed mostly in the 60 to 69 (8.7%) and ≥70 age groups (7.4%). In female patients with cirrhosis, HCC development was observed in the 50 to 59 (0.9%), 60 to 69 (2.7%), and ≥70 age groups (3.3%).
3.3. Simulation of the % probabilities for transition crosswise states of liver in asymptomatic carriers at age 40 by the Markov chain model
The asymptomatic carrier state at age 40 was the primary starting state for simulation over following 40 years. In both male (Figure 3a) and female (Figure 3b) patients, the probability of developing chronic hepatitis gradually increased with age until the 50 seconds. The probability of developing cirrhosis gradually increased with age until 70 seconds. The probability of developing HCC increased gradually with age. The probability of HCC was higher in male vs female patients in the same age group. In male patients, the probability of HCC was higher than the probability of cirrhosis by age 80. Conversely, in female patients, the probability of HCC was lower than the probability of cirrhosis by age 80.
Figure 3.
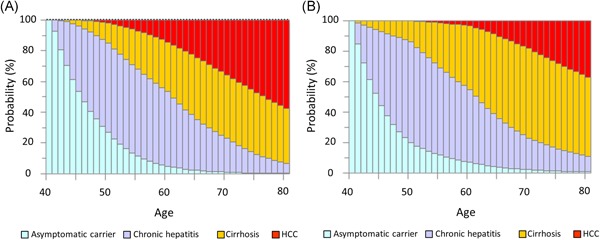
Natural history of liver disease over 40 years in hypothetical cohorts of asymptomatic carriers at age 40. A, Males: At age 50, asymptomatic carrier status accounted for 25.4% of patients, chronic hepatitis for 58.4%, cirrhosis for 14.4%, and HCC for 2.2%. At age 60, asymptomatic carrier status accounted for 4.6% of patients, chronic hepatitis for 48.0%, cirrhosis for 32.5%, and HCC for 14.9%. At age 70, asymptomatic carrier status accounted for 0.9% of patients, chronic hepatitis for 21.9%, cirrhosis for 41.1%, and HCC for 36.1%. At age 80, asymptomatic carrier status accounted for 0.1% of patients, chronic hepatitis for 6.6%, cirrhosis for 35.3%, and HCC for 58.0%. B, Females: At age 50, asymptomatic carrier status accounted for 19.7% of patients, chronic hepatitis for 66.5%, cirrhosis for 13.8%, and HCC for 0.0%. At age 60, asymptomatic carrier status accounted for 7.0% of patients, chronic hepatitis for 47.9%, cirrhosis for 42.0%, and HCC for 3.1%. At age 70, asymptomatic carrier status accounted for 2.1% of patients, chronic hepatitis for 20.9%, cirrhosis for 58.8%, and HCC for 18.2%. At age 80, asymptomatic carrier status accounted for 0.7% of patients, chronic hepatitis for 10.1%, cirrhosis for 52.2%, and HCC for 37.0%. HCC, hepatocellular carcinoma
3.4. Simulation of the % probabilities for transition crosswise states of liver in patients with chronic hepatitis at age 40 by the Markov chain model
The chronic hepatitis state at age 40 was the primary starting state for simulation over following 40 years. In both male (Figure 4a) and female (Figure 4b) patients, the probability of developing cirrhosis gradually increased with age until the 70 seconds. In male patients, the probability of developing HCC gradually increased with age. In female patients, the probability of HCC gradually increased with age starting in the 50 seconds. In male patients, the probability of HCC was higher than the probability of cirrhosis by age 70. Conversely, in female patients, the probability of HCC was lower than the probability of cirrhosis across all age groups.
Figure 4.
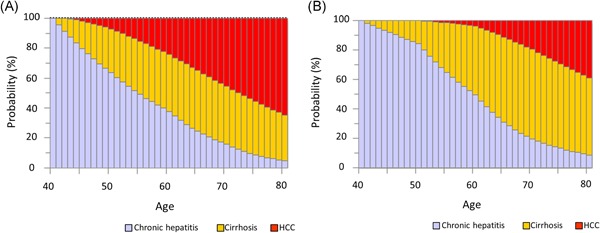
Natural history of liver disease over 40 years in hypothetical cohorts of patients with chronic hepatitis at age 40. A, Males: At age 50, chronic hepatitis accounted for 63.7% of patients, cirrhosis for 29.0%, and HCC for 7.3%. At age 60, chronic hepatitis accounted for 37.7% of patients, cirrhosis for 38.0%, and HCC for 24.3%. At age 70, chronic hepatitis accounted for 15.9% of patients, cirrhosis for 38.6%, and HCC for 45.5%. At age 80, chronic hepatitis accounted for 4.6% of patients, cirrhosis for 30.7%, and HCC for 64.7%. B, Females: At age 50, chronic hepatitis accounted for 84.6% of patients, cirrhosis for 15.4%, and HCC for 0.0%. At age 60, chronic hepatitis accounted for 49.9% of patients, cirrhosis for 46.5%, and HCC for 3.6%. At age 70, chronic hepatitis accounted for 19.7% of patients, cirrhosis for 60.8%, and HCC for 19.5%. At age 80, chronic hepatitis accounted for 9.0% of patients, cirrhosis for 52.4%, and HCC for 38.6%. HCC, hepatocellular carcinoma
4. DISCUSSION
The present study, which included a great number of patients with chronic hepatitis C (20 140 PY units) who did not receive IFN‐based antiviral therapy during the follow‐up period, clarified the long‐term natural history of liver‐related disease in this cohort. In the assessment of 1‐year liver disease state transition probability matrices, progression to chronic hepatitis from the asymptomatic carrier state was observed in 12% to 14% of male patients and 6% to 14% of female patients with chronic hepatitis C across all age groups. In male patients with cirrhosis, the transition probability of developing HCC over the age of 40 was approximately 5%. Conversely, in female patients with cirrhosis, the transition probability of developing HCC was approximately 3% over the age of 60. Thus, there is a risk of HCC development in all males over the age of 40 and older female HCV patients with cirrhosis. The results also suggest that there is a risk of progression to chronic hepatitis from the asymptomatic carrier state in both male and female patients with chronic HCV infection, even at a relatively young age. Based on the Markov chain simulation in HCV patients with the asymptomatic carrier or the chronic hepatitis state at age 40, transition to the state of HCC was further found in male patients. Therefore, chronic HCV infection is a risk factor for liver disease progression during long‐term follow‐up even in asymptomatic carriers.
Chronic infection with HCV is the leading cause of end‐stage liver disease, HCC, and liver‐related death. Clinical outcomes after HCV infection are highly variable. HCV infection is infrequently diagnosed during the acute phase because most persons have either no symptoms or only mild symptoms. Asymptomatic HCV infection becomes chronic in most cases, and people are unaware of being infected until end‐stage liver disease occurs. Several longitudinal studies27, 28, 29, 30, 31, 32, 33, 34 were conducted to evaluate the clinical outcomes related to HCV infection. In these studies, rates of cirrhosis have ranged from 17% to 55%, rates of HCC have ranged from 1% to 23%, and rates of liver‐related death have ranged from 1% to 23% over an estimated infection period of 20 to 30 years.27, 35, 36 However, these studies mainly enrolled specific populations such as patients with post‐transfusion hepatitis,28 blood donors,30 drug abusers,31 and women vaccinated with contaminated immunoglobulins.27, 34 Therefore, the natural history of the chronic HCV infection remains incompletely characterized. Although our study was retrospective in nature, we clarified the natural history of chronic HCV infection in patients with chronic hepatitis as well as asymptomatic carriers at the start of follow‐up. The advantage of our study compared with previous reports was that we estimated the long‐term prognosis of liver disease in patients with persistent HCV infection over 40 years. Intervention in the form of DAA therapy to eradicate HCV is necessary even in asymptomatic carriers in view of the long‐term natural history of persistent HCV infection.
Recent years, various laboratory scoring systems of fibrosis have been developed in patients with chronic liver disease.17, 18, 37, 38, 39, 40 Vallet‐Pichard et al18 reported that the FIB‐4 index is accordant with liver fibrosis according to pathological assessment of liver biopsy specimens in patients with chronic HCV infection. They reported that the FIB‐4 index enabled the correct identification of chronic HCV patients with severe fibrosis (F3‐F4) and cirrhosis with an area under the receiver operating characteristic curve of 0.85 (95% confidence interval, 0.82‐0.89) and 0.91 (95% confidence interval, 0.86‐0.93), respectively.18 Additionally, previous studies have reported that older age, male sex, lower albumin levels and platelet count, and elevated ALT, AST, and α‐fetoprotein levels are significant risk factors for cirrhosis and HCC.41, 42, 43, 44, 45, 46 The FIB‐4 index is assessed using age, AST, ALT, and platelet count. In other words, the FIB‐4 index is considered to be a more complex scoring system for evaluating the incidence of liver‐related events in patients with chronic HCV infection.
The Markov model assesses data measured repeatedly with general outcomes. These types of styles explain how a transition of patient between a course of states of disease over long‐term periods, which is likeable in the explanation of processes of disease that certainly include progression across stages of advancing severity.47 In the present study, we analyzed the Markov chain model to estimate the 1‐year disease state of liver transition probabilities in four disease states of liver (ie, asymptomatic carrier, chronic hepatitis, cirrhosis, and HCC) in patients with the asymptomatic carrier state, chronic hepatitis, or cirrhosis who did not receive IFN‐based therapy. Particularly, we analyzed the % probabilities for transition crosswise liver states on the long‐term periods in asymptomatic carriers and chronic hepatitis patients at age 40.
The principal limitations of the present research involve its hospital‐based subjects and retrospective study. Therefore, further prospective studies with community‐based subjects are warranted. Another limitation was that the FIB‐4 index, originally an index of liver fibrosis, can overestimate the progression of liver fibrosis because it includes age, a strongly time‐dependent factor. In addition, liver cirrhosis was determined as FIB‐4 index or more 3.25 in this study. Consequently, it was considered that more or less patients with significant liver fibrosis stage were determined as liver cirrhosis. Further research by imaging elastography or pathological data for the confirmation of liver cirrhosis are warranted. Further, in this study, the asymptomatic carrier was defined as patients with ALT less than 35 IU/L. Therefore, it was possible that there were some patients with chronic hepatitis even in patients who were defined as asymptomatic carrier.
In conclusion, according to the Markov chain model, poor liver disease outcomes such as the progression of liver cirrhosis or HCC are possible not only in patients with chronic hepatitis but also in patients who are asymptomatic carriers at age ≥ 40 years. Further studies are warranted to confirm these findings in other populations.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Concept and study design: JT and TT. Data acquisition: All authors. Analyses of the data: MO, TA, and JT. Statistics: MO, TA, and JT. Supervision: TK. Preparing manuscript: TT. Review and approval: All authors. All authors approved the final version of the manuscript.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was supported by Health and Labour Sciences Research Grants (Research on Hepatitis) from the Ministry of Health, Labour and Welfare of Japan.
Tada T, Toyoda H, Yasuda S, et al. Natural history of liver‐related disease in patients with chronic hepatitis C virus infection: an analysis using a Markov chain model. J Med Virol. 2019;91:1837‐1844. 10.1002/jmv.25533
References
REFERENCES
- 1. Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705‐714. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global alert and response (GAR): hepatitis C. 2011 (January 26). http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index4.html. Accessed September 1, 2017.
- 3. Marcellin P, Boyer N, Gervais A, et al. Long‐term histologic improvement and loss of detectable intrahepatic HCV RNA in patients with chronic hepatitis C and sustained response to interferon‐alpha therapy. Ann Intern Med. 1997;127:875‐881. [DOI] [PubMed] [Google Scholar]
- 4. Reichard O, Glaumann H, Frydén A, Norkrans G, Wejstål R, Weiland O. Long‐term follow‐up of chronic hepatitis C patients with sustained virological response to alpha‐interferon. J Hepatol. 1999;30:783‐787. [DOI] [PubMed] [Google Scholar]
- 5. Poynard T, Moussalli J, Ratziu V, Regimbeau C, Opolon P. Effect of interferon therapy on the natural history of hepatitis C virus‐related cirrhosis and hepatocellular carcinoma. Clin Liver Dis. 1999;3:869‐881. [DOI] [PubMed] [Google Scholar]
- 6. Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174‐181. [DOI] [PubMed] [Google Scholar]
- 7. Ikeda K, Saitoh S, Arase Y, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long‐term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124‐1130. [DOI] [PubMed] [Google Scholar]
- 8. Hung CH, Lee CM, Lu SN, et al. Long‐term effect of interferon alpha‐2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus‐related cirrhosis. J Viral Hepat. 2006;13:409‐414. [DOI] [PubMed] [Google Scholar]
- 9. Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687‐1695. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida H, Arakawa Y, Sata M, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology. 2002;123:483‐491. [DOI] [PubMed] [Google Scholar]
- 11. Kasahara A, Tanaka H, Okanoue T, et al. Interferon treatment improves survival in chronic hepatitis C patients showing biochemical as well as virological responses by preventing liver‐related death. J Viral Hepat. 2004;11:148‐156. [DOI] [PubMed] [Google Scholar]
- 12. Toyoda H, Atsukawa M, Takaguchi K, et al. Real‐world virological efficacy and safety of elbasvir and grazoprevir in patients with chronic hepatitis C virus genotype 1 infection in Japan. J Gastroenterol 2018. [Epub ahead of print]. [DOI] [PubMed]
- 13. xAtsukawa M, Tsubota A, Toyoda H, et al. Efficacy and safety of ombitasvir/paritaprevir/ritonavir and ribavirin for chronic hepatitis patients infected with genotype 2a in Japan. Hepatol Res 2018. [Epub ahead of print]. [DOI] [PubMed]
- 14. Toyoda H, Kumada T, Tada T, et al. Efficacy and tolerability of an IFN‐free regimen with DCV/ASV for elderly patients infected with HCV genotype 1B. J Hepatol. 2017;66:521‐527. [DOI] [PubMed] [Google Scholar]
- 15. Beck JR. Markov models of natural history. J Clin Epidemiol. 1988;41:619‐621. [DOI] [PubMed] [Google Scholar]
- 16. Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322‐338. [DOI] [PubMed] [Google Scholar]
- 17. Sterling RK, Lissen E, Clumeck N, et al. APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 18. Vallet‐Pichard A, Mallet V, Nalpas B, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32‐36. [DOI] [PubMed] [Google Scholar]
- 19. AASLD/IDSA HCV Guidance Panel . Hepatitis C guidance: AASLD‐IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932‐954. [DOI] [PubMed] [Google Scholar]
- 20. Pawlotsky JM, Negro F, Aghemo A, et al. European Association for Study of Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461‐511. [DOI] [PubMed] [Google Scholar]
- 21. Omata M, Kanda T, Wei L, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016;10:702‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Japan Society of Hepatology . Surveillance algorithm and diagnostic algorithm for hepatocellular carcinoma: clinical practice guidelines for hepatocellular carcinoma. Hepatology Res. 2010;40(suppl s1):6‐7. [DOI] [PubMed] [Google Scholar]
- 23. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208‐1236. [DOI] [PubMed] [Google Scholar]
- 24. Bruix J, Sherman M. Management ofhepatocellular carcinoma: an update. Hepatology. 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Omata M, Kanda T, Wei L, et al. APASL consensus statements and recommendations for hepatitis C prevention, epidemiology, and laboratory testing. Hepatol Int. 2016;10:681‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asahina Y, Izumi N, Hiromitsu K, et al. JSH guidelines for the management of hepatitis C virus infection: A 2016 update for genotype 1 and 2. Hepatol Res. 2016;46:129‐165. [DOI] [PubMed] [Google Scholar]
- 27. Kenny‐Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti‐D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228‐1233. [DOI] [PubMed] [Google Scholar]
- 28. Seeff LB, Hollinger FB, Alter HJ, et al. Long‐term mortality and morbidity of transfusion‐associated non‐A, non‐B, and type C hepatitis: A National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455‐463. [DOI] [PubMed] [Google Scholar]
- 29. Suruki R, Hayashi K, Kusumoto K, et al. Alanine aminotransferase level as a predictor of hepatitis C virus‐associated hepatocellular carcinoma incidence in a community‐based population in Japan. Int J Cancer. 2006;119:192‐195. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka H, Tsukuma H, Yamano H, Oshima A, Shibata H. Prospective study on the risk of hepatocellular carcinoma among hepatitis C virus‐positive blood donors focusing on demographic factors, alanine aminotransferase level at donation and interaction with hepatitis B virus. Int J Cancer. 2004;112:1075‐1780. [DOI] [PubMed] [Google Scholar]
- 31. Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450‐456. [DOI] [PubMed] [Google Scholar]
- 32. Tong MJ, el‐Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion‐associated hepatitis C. N Engl J Med. 1995;332:1463‐1466. [DOI] [PubMed] [Google Scholar]
- 33. Uto H, Stuver SO, Hayashi K, et al. Increased rate of death related to presence of viremia among hepatitis C virus antibody‐positive subjects in a community‐based cohort study. Hepatology. 2009;50:393‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wiese M, Grüngreiff K, Güthoff W, Lafrenz M, Oesen U, Porst H. East German Hepatitis C Study Group. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany‐a 25‐year multicenter study. J Hepatol. 2005;43:590‐598. [DOI] [PubMed] [Google Scholar]
- 35. Yano M, Kumada H, Kage M, et al. The long‐term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334‐1340. [DOI] [PubMed] [Google Scholar]
- 36. Kobayashi M, Tanaka E, Sodeyama T, Urushihara A, Matsumoto A, Kiyosawa K. The natural course of chronic hepatitis C: a comparison between patients with genotypes 1 and 2 hepatitis C viruses. Hepatology. 1996;23:695‐699. [DOI] [PubMed] [Google Scholar]
- 37. Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734‐739. [DOI] [PubMed] [Google Scholar]
- 38. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518‐526. [DOI] [PubMed] [Google Scholar]
- 39. Forns X, Ampurdanès S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986‐992. [DOI] [PubMed] [Google Scholar]
- 40. Imbert‐Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, MULTIVIRC Group . Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069‐1075. [DOI] [PubMed] [Google Scholar]
- 41. Ono E, Shiratori Y, Okudaira T, et al. Platelet count reflects stage of chronic hepatitis C. Hepatol Res. 1999;15:192‐200. [Google Scholar]
- 42. Matsumura H, Moriyama M, Goto I, Tanaka N, Okubo H, Arakawa Y. Natural course of progression of liver fibrosis in Japanese patients with chronic liver disease type C—a study of 527 patients at one establishment. J Viral Hepat. 2000;7:268‐275. [DOI] [PubMed] [Google Scholar]
- 43. Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C‐related advanced liver disease. Gastroenterology. 2009;136:138‐148. HALT‐C Trial Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kurosaki M, Matsunaga K, Hirayama I, et al. The presence of steatosis and elevation of alanine aminotransferase levels are associated with fibrosis progression in chronic hepatitis C with non‐response to interferon therapy. J Hepatol. 2008;48:736‐742. [DOI] [PubMed] [Google Scholar]
- 45. Kumada T, Toyoda H, Kiriyama S, et al. Predictive value of tumor markers for hepatocarcinogenesis in patients with hepatitis C virus. J Gastroenterol. 2011;46:536‐544. [DOI] [PubMed] [Google Scholar]
- 46. Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143‐154. [DOI] [PubMed] [Google Scholar]
- 47. Jackson C. Multi‐state modelling with R: the msm package. Cambridge, UK: MRC Biostatistics Unit; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


