Abstract
Background
Radium‐223 dichloride (radium‐223) is approved for patients with castration‐resistant prostate cancer (CRPC), symptomatic bone metastases, and no visceral disease using a dosing regimen of 6 injections (55 kBq/kg intravenously; 1 injection every 4 weeks). Early results from international, open‐label, phase 1/2 study NCT01934790 showed that re‐treatment with radium‐223 was well tolerated with favorable effects on disease progression. Here we report safety and efficacy findings from 2‐year follow‐up of the radium‐223 re‐treatment study.
Methods
Patients with CRPC and bone metastases who completed 6 initial radium‐223 injections with no disease progression in bone and later progressed were eligible for radium‐223 re‐treatment (up to 6 additional radium‐223 injections), provided that hematologic parameters were adequate and chemotherapy had not been administered after the initial course of radium‐223. Concomitant cytotoxic agents were not allowed during re‐treatment but were allowed at the investigator's discretion during follow‐up; other concomitant agents for prostate cancer (including abiraterone acetate or enzalutamide) were allowed at investigator's discretion. The primary objective was safety. Exploratory objectives included time to radiographic bone progression, radiographic progression‐free survival (rPFS), time to total alkaline phosphatase (tALP), and prostate‐specific antigen (PSA) progression, overall survival (OS), time to first symptomatic skeletal event (SSE), and SSE‐free survival, all calculated from re‐treatment start. Evaluation of safety and exploratory efficacy objectives included active 2‐year follow‐up. Safety results from active follow‐up and updated efficacy are reported.
Results
Overall, 44 patients were re‐treated with radium‐223; 29 (66%) completed all 6 injections, and 34 (77%) entered 2‐year active follow‐up, during which no new safety concerns and no serious drug‐related adverse events were noted. rPFS events (progression or death) occurred in 19 (43%) of 44 patients; median rPFS was 9.9 months. Radiographic bone progression occurred in 5 (11%) of 44 patients. Median OS was 24.4 months. Median times to first SSE and SSE‐free survival were 16.7 and 12.8 months, respectively. Median time to tALP progression was not reached; median time to PSA progression was 2.2 months.
Conclusions
Re‐treatment with radium‐223 in this selected patient population was well tolerated, led to minimal hematologic toxicity, and provided continued disease control in bone at 2‐year follow‐up.
Keywords: alkaline phosphatase, prostate‐specific antigen, safety, SSEs, survival, symptomatic skeletal events
1. INTRODUCTION
Radium‐223 dichloride (radium‐223) is the first targeted alpha therapy approved for patients with metastatic castration‐resistant prostate cancer (mCRPC). In the pivotal phase 3 ALSYMPCA trial, radium‐223 plus best standard of care significantly prolonged overall survival (OS) (hazard ratio, 0.70; 95% confidence interval [CI] = 0.58‐0.83; P < .001) and delayed time to first symptomatic skeletal event (SSE) (hazard ratio, 0.66; 95% CI = 0.52‐0.83; P < .001) versus placebo plus best standard of care in patients with mCRPC.1, 2 Radium‐223‐treated patients also reported significant benefits in quality of life (QOL), with more patients experiencing meaningful QOL improvement (P = .020) and slower QOL decline over time (P = .006) compared with placebo.1, 3 Radium‐223 was well tolerated with a low incidence of myelosuppression.1, 4 Based on these results, radium‐223 was approved for the treatment of patients with castration‐resistant prostate cancer (CRPC), symptomatic bone metastases, and no known visceral disease using a dosing regimen of 6 injections (55 kBq/kg intravenously [IV]; 1 injection every 4 weeks).5, 6
In ALSYMPCA, radium‐223 treatment was associated with declines in total alkaline phosphatase (tALP) levels that correlated with increased OS.7 After completion of the 6‐injection regimen, tALP levels began to rise (median time to tALP increase: 7.4 months, radium‐223, vs 3.8 months, placebo), and despite initial success, disease progression in bone eventually occurred.1, 7 The favorable safety profile and low myelosuppression rates observed with radium‐223 suggested the possibility that patients who tolerate and benefit from initial radium‐223 treatment may also tolerate and further benefit from a rechallenge with up to 6 new radium‐223 injections.
An international open‐label phase 1/2 study (NCT01934790) was conducted to assess the safety of re‐treatment with up to 6 radium‐223 injections, administered at 4‐week intervals, to patients with mCRPC who previously received and tolerated a full course (6 injections) of radium‐223. Early results showed that re‐treating patients with up to 6 additional radium‐223 injections was well tolerated with favorable effects on disease progression.8 Among 44 patients, 1 (2%) had radiographic bone progression and 8 (18%) had radiographic soft tissue progression (3 in lymph nodes and 5 in viscera). Median radiographic progression‐free survival (rPFS) was 9.9 months, and median time to prostate‐specific antigen (PSA) progression was 2.2 months. Median time to tALP progression, median OS, time to first SSE, and SSE‐free survival were not reached. Overall, 3 (7%) of 44 patients experienced grade 3 hematologic treatment‐emergent adverse events (TEAEs) (2 anemia, 1 thrombocytopenia); there were no cases of grade 4 hematologic TEAEs.8 Here we present the extended safety and efficacy results from a 2‐year follow‐up of this study evaluating radium‐223 re‐treatment in patients with mCRPC.
2. MATERIALS AND METHODS
2.1. Patients
Eligibility criteria for the phase 1/2 radium‐223 re‐treatment study have been described in detail.8 Briefly, patients included men greater than or equal to 18 years of age with pathologically confirmed CRPC who had initially completed 6 injections of radium‐223, with no evidence of bone progression during treatment. Eligible patients had (a) radiologic progression (according to adapted Prostate Cancer Working Group 2 [PCWG2] criteria), (b) biochemical/clinical progression (defined as 2 subsequent values showing PSA increase ≥ 2 ng/mL), or (c) substantial worsening of pain due to bone metastases (based on investigator's determination) after initial treatment; Eastern Cooperative Oncology Group performance status (ECOG PS) less than or equal to 2; life expectancy greater than or equal to 6 months; and adequate hematologic, liver, and kidney function. Patients were excluded if they had visceral metastases greater than or equal to 1 cm in diameter measured less than or equal to 30 days from treatment start or radium‐223‐related serious or grade 3 or 4 adverse events (AEs) that did not resolve or led to treatment discontinuation. Patients must not have received chemotherapy after their initial course of radium‐223. Concomitant abiraterone or enzalutamide was permitted; concomitant chemotherapy was not permitted during the treatment period. Other agents, including denosumab and zoledronic acid, were permitted at the investigator's discretion. All patients provided written informed consent. During active follow‐up, systemic anticancer therapies were permitted at the investigator's discretion.
2.2. Study design and treatment
Figure 1 presents the design of this international, open‐label, phase 1/2 study. Patients received up to 6 additional doses of radium‐223 (55 kBq/kg IV), 1 injection every 4 weeks. The first injection could not be administered earlier than 30 days after the last radium‐223 dose of the initial treatment course. The planned active follow‐up period was up to 2 years after the last dose of radium‐223. No radium‐223 was administered during the follow‐up period.
Figure 1.
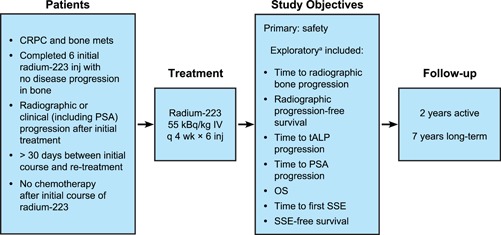
Radium‐223 re‐treatment study design. aExploratory endpoints were calculated from start of radium‐223 re‐treatment. CRPC, castration‐resistant prostate cancer; inj, injection; mets, metastases; OS, overall survival; PSA, prostate‐specific antigen; SSE, symptomatic skeletal event; tALP, total alkaline phosphatase [Color figure can be viewed at wileyonlinelibrary.com]
The study was approved by institutional review boards at each participating center, was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guidelines, and was registered at ClinicalTrials.gov, number NCT01934790.
2.3. Endpoints
The primary endpoint was safety. During the active follow‐up period, AEs and serious AEs were recorded if considered to be related to the study medication or if related to an SSE, and all occurrences of leukemia, myelodysplastic syndrome, aplastic anemia, myelofibrosis, primary bone cancer, or any other new primary malignancy were recorded.
Exploratory time‐to‐event endpoints included rPFS defined as the time from treatment start to radiographic progression or death by any cause, time to radiographic bone progression, time to tALP progression defined as greater than or equal to 25% increase above the nadir value to at least 1.5 × upper limit of normal, time to PSA progression defined as greater than or equal to 25% increase above nadir and an increase in absolute value greater than or equal to 2 ng/mL above nadir, OS, time to first SSE, and SSE‐free survival defined as time from treatment start to first SSE or death. SSEs included external beam radiotherapy (EBRT) to relieve skeletal pain, new symptomatic pathologic bone fractures, spinal cord compression, and tumor‐related orthopedic surgical intervention. Time‐to‐event exploratory efficacy endpoints were calculated from the day of first radium‐223 injection (day 1) to the end of the 2‐year follow‐up period.
2.4. Assessments
Assessments conducted during treatment were described in the initial report.8 Assessments during active follow‐up were vital signs, physical condition, complete blood counts, blood chemistries including tALP and PSA, ECOG PS, and SSE evaluations every 4 weeks for 12 weeks and then every 12 weeks until 2 years after the last dose of radium‐223 was received. Radiographic progression was evaluated by magnetic resonance imaging, or computed tomography of the abdomen and pelvis, chest x‐ray, and whole‐body technetium‐99 bone scans every 12 weeks until disease progression, start of a new anticancer treatment, or as needed to confirm suspected lesions. If progression was detected by bone scan, a confirmatory bone scan was required greater than or equal to 6 weeks later per PCWG2 criteria to confirm radiographic bone progression.
2.5. Statistics
The treatment period began with the first patient first visit (FPFV) on 22 December 2013. The final data cutoff was 11 June 2015, after all 44 patients had either completed treatment and the end‐of‐treatment visit (30 days after the last injection) or had discontinued treatment. Results obtained during the treatment period have been reported.8 The planned active follow‐up period was up to 2 years after the last dose of radium‐223; 34 patients entered follow‐up and were included in the 2‐year active follow‐up analysis.
The safety data reported here are from the active follow‐up period (interim data cutoff), 11 June 2015, to last patient last visit (LPLV) 12 April 2017 (n = 34). The exploratory efficacy data reported here are cumulative from FPFV to 12 April 2017 (N = 44). All efficacy analyses were considered exploratory and descriptive with no hypothesis testing. Sample size was based on clinical and practical considerations; no statistical assumptions were made. All analyses reported here used the safety population, defined as all patients who received at least 1 radium‐223 injection. Baseline value was defined as the last value observed before day 1. Time‐to‐event endpoints were summarized using Kaplan‐Meier estimates. A Kaplan‐Meier curve was generated for each time‐to‐event endpoint.
3. RESULTS
3.1. Patients
Radium‐223 re‐treatment was administered to 44 patients. Median time from end of initial radium‐223 treatment to re‐treatment was 6 months (range, 1.2‐17.1 months). Overall, 29 (66%) of 44 patients completed re‐treatment with all 6 radium‐223 injections, and 34 (77%) entered the 2‐year active follow‐up (Figure 2 ).
Figure 2.
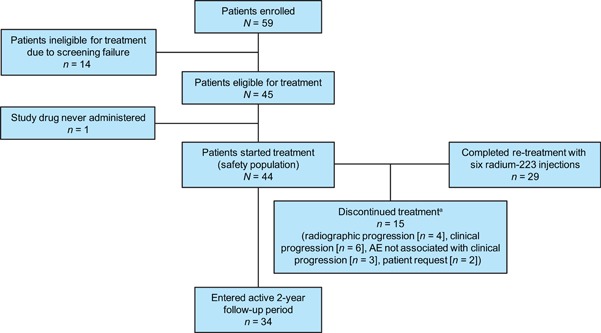
CONSORT diagram. aTypes of clinical progression leading to discontinuation were prostate‐specific antigen progression (n = 5) and Eastern Cooperative Oncology Group (ECOG) performance status change (n = 1). The reason for discontinuation due to patient request was inability to travel to the clinic (n = 2). AE, adverse event [Color figure can be viewed at wileyonlinelibrary.com]
Table 1 shows the demographic and baseline characteristics of the 44 patients in the safety data set. All patients had at least 2 prior hormonal regimens, 32 (73%) experienced disease progression after prior abiraterone or enzalutamide, and 20 (45%) had at least 1 prior chemotherapy regimen. During the 2‐year active follow‐up, 24 (71%) of 34 patients received at least 1 surgical therapeutic procedure, 25 (74%) patients received systemic anticancer therapy (ie, antineoplastic drugs and immunomodulating agents) (Table 2), 11 (32%) received EBRT, and 2 (6%) received bisphosphonates or denosumab. Of note, radium‐223 was given to 3 (9%) patients during active follow‐up, 2 of whom were enrolled in another clinical trial.
Table 1.
Demographics and baseline characteristics
| Re‐treatment study | |
|---|---|
| Parameter | N = 44 |
| Age, median (range), y | 71 (52‐91) |
| ECOG PS, n (%) | |
| 0 | 14 (32) |
| 1 | 27 (61) |
| 2 a | 3 (7) |
| Extent of disease, bone metastases, n (%) | |
| <6 | 18 (41) |
| 6‐20 | 15 (34) |
| >20, not superscan | 6 (14) |
| Superscan | 5 (11) |
| Prior systemic anticancer treatment, n (%) | |
| Docetaxel | 20 (45) |
| Abiraterone | 27 (61) |
| Enzalutamide | 13 (30) |
| Prior bone‐targeted treatment, n (%) | |
| Bisphosphonates | 5 (11) |
| Denosumab | 22 (50) |
| Laboratory values, median (range) | |
| Hemoglobin, g/dL | 12 (9‐16) |
| Albumin, g/L | 39 (32‐44) |
| PSA, µg/L | 68 (<1‐2349) |
| LDH, U/L | 203 (115‐532) |
| tALP, U/L | 85 (29‐705) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; PSA, prostate‐specific antigen; tALP, total alkaline phosphatase.
0 patients had ECOG PS greater than 2.
Table 2.
Systemic anticancer therapies administered during active follow‐up
| Systemic anticancer therapy | Active follow‐up n = 34 |
|---|---|
| Patients with ≥1 systemic anticancer therapy, n (%) | 25 (74) |
| Anticancer therapy, n (%) a | |
| Cabazitaxel | 16 (47) |
| Enzalutamide | 16 (47) |
| Docetaxel | 11 (32) |
| Abiraterone acetate | 8 (24) |
| Immunostimulants | 4 (12) |
| Monoclonal antibodies | 4 (12) |
| Cyclophosphamide | 3 (9) |
| Radium‐223 dichloride | 3 (9) |
| Antineoplastic agents | 2 (6) |
| Cabozantinib | 2 (6) |
| Dexamethasone | 2 (6) |
| Carboplatin | 1 (3) |
| Cisplatin | 1 (3) |
| Diethylstilbestrol | 1 (3) |
| Estramustine phosphate sodium | 1 (3) |
| Etoposide | 1 (3) |
| Insulin | 1 (3) |
| Methylprednisolone | 1 (3) |
| Prednisolone | 1 (3) |
| Prednisone | 1 (3) |
| Samarium (Sm 153) lexidronam pentasodium | 1 (3) |
| Other antineoplastic agents | 1 (3) |
A patient may have taken more than 1 medication. If a patient had more than 1 medication in a category, the patient was counted once in that category. Medications were coded using the World Health Organization Drug Dictionary (WHO‐DD), version 2005/Q3.
3.2. Safety
No new safety concerns were observed during active follow‐up. AEs during active follow‐up and their status relative to the treatment period are shown in Table 3. Four (12%) of 34 patients had grade 3 AEs during active follow‐up: spinal cord compression, arthralgia, anemia, and muscular weakness; all other AEs were grade 1 or 2. One patient suffered a grade 2 fractured humerus during active follow‐up, in addition to 2 pathologic fractures that occurred during the treatment phase of the study; he had received abiraterone treatment before entering the study, but no concomitant abiraterone during the treatment period. Patients recovered from the instances of grade 2 fractured humerus and grade 3 spinal cord compression (1 patient each) that occurred during active follow‐up. The only treatment‐related AE was the grade 3 anemia in 1 (3%) of 34 patients; this event was the worsening of an existing AE, not a new event. No serious treatment‐related AEs were reported. At the end of active follow‐up, 2 (6%) patients had anemia (grade 1) and no patient had any grade neutropenia or thrombocytopenia; changes in hematologic laboratory values over time are shown in Figure 3.
Table 3.
Adverse events during active follow‐up
| Patients with adverse events, n (%) | Active follow‐up n = 34 | Adverse event status a |
|---|---|---|
| Anemia | 3 (9) | Ongoing b |
| Arthralgia | 2 (6) | 1 ongoing; 1 new |
| Bone pain | 2 (6) | 1 ongoing; 1 new |
| Humerus fracture | 1 (3) | New |
| Muscular weakness | 1 (3) | New |
| Musculoskeletal pain | 1 (3) | New c |
| Osteonecrosis of jaw | 1 (3) | Ongoing |
| Paresthesia | 1 (3) | Ongoing |
| Spinal cord compression | 1 (3) | New |
| Spinal pain | 2 (6) | 1 ongoing; 1 new |
Adverse event status is given relative to the treatment period: “ongoing” indicates events that began during the treatment period and continued in the active follow‐up; “new” indicates events that were new during active follow‐up.
All worsening of an existing event that started during the treatment period.
Patient had other events of musculoskeletal pain during the treatment period but at different body locations.
Figure 3.
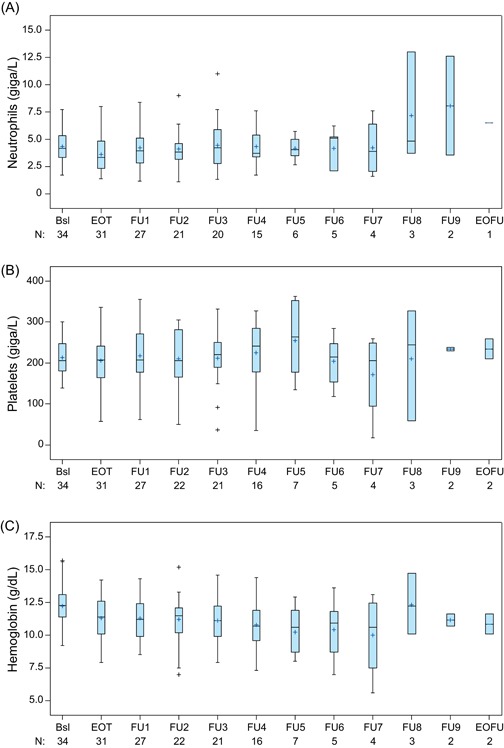
Neutrophil (A), platelet (B), and hemoglobin (C) values over time, from baseline to end of active follow‐up. Note: Whiskers represent maximum and minimum values within limits of 1.5 quartile ranges above and below the upper and lower quartiles, respectively. Bsl, baseline; EOFU, end of active follow‐up; EOT, end of treatment; FU, active follow‐up; +, outlier value [Color figure can be viewed at wileyonlinelibrary.com]
No new primary malignancies were reported during the active follow‐up period. Death occurred in 28 (64%) of the 44 patients in the safety data set, including 23 (52%) during active follow‐up. The most common cause of death was progressive disease in 25 (89%) of 28 patients; other deaths were related to pulmonary fibrosis, adverse events not related to disease progression, and an unknown cause.
3.3. Exploratory efficacy
All patients in the safety data set (N = 44) were included in the exploratory efficacy analysis. Radiographic bone progression occurred in 5 (11%) patients. The median time to radiographic bone progression was not estimated because 39 (89%) patients were censored (Figure 4A). The maximum follow‐up time for radiographic bone progression was 16.3 months. Median rPFS was 9.9 months (Figure 4B), and 19 (43%) patients had an rPFS event (radiographic progression or death), 6 of which occurred during the active follow‐up period, including 2 in bone, 1 non‐bone event, and 3 deaths. Censoring affected 25 (57%) patients. The maximum follow‐up time for rPFS was 17.7 months. Median OS was 24.4 months (Figure 4C), and 28 of 44 (64%) patients died. The median follow‐up for OS was 31.6 months. OS was estimated at 78% at 12 months and 50% at 24 months.
Figure 4.
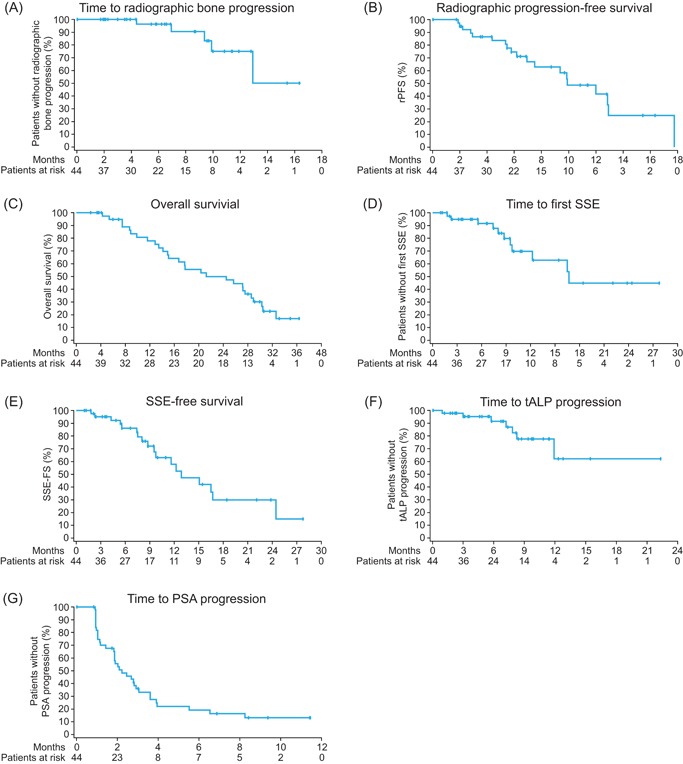
Kaplan‐Meier analysis of time to radiographic bone progression (A), radiographic progression‐free survival (B), overall survival (C), time to first symptomatic skeletal event (SSE) (D), SSE‐free survival (E), time to total alkaline phosphatase (tALP) progression (F), and time to prostate‐specific antigen (PSA) progression (G) [Color figure can be viewed at wileyonlinelibrary.com]
The median time to first SSE was 16.7 months (Figure 4D); 14 of 44 (32%) patients experienced at least 1 SSE, although 3 patients were censored due to new cancer treatment, leaving 11 for inclusion in the Kaplan‐Meier analysis. First SSEs were EBRT for bone pain in 10 (23%) patients, pathologic bone fracture in 3 (7%), and spinal cord compression in 1 (2%). Median SSE‐free survival was 12.8 months (Figure 4E), and 18 (41%) patients had an SSE‐free survival event, which included 7 deaths. Maximum follow‐up time for first SSE or SSE‐free survival was 27.8 months.
Median time to tALP progression was not reached by the end of the 2‐year active follow‐up (Figure 4F) because of 37 (84%) patients being censored; 7 (16%) patients had tALP progression. Maximum follow‐up time for tALP progression was 22.3 months. Median time to PSA progression was 2.2 months (Figure 4G); 35 of 44 (80%) patients had PSA progression, and 9 (21%) were censored. Maximum follow‐up time for PSA progression was 11.4 months.
4. DISCUSSION
Re‐treatment with radium‐223 was well tolerated, led to minimal hematologic toxicity, and provided continued disease control in bone in this group of selected patients. This 2‐year active follow‐up analysis provides a more comprehensive assessment of the safety of radium‐223 re‐treatment. The only treatment‐related AE reported during active follow‐up was 1 case of grade 3 anemia reported as the worsening of an existing event. Three additional grade 3 AEs were reported: spinal cord compression, arthralgia, and muscular weakness, each in 1 patient and none related to treatment. The spinal cord compression resolved. One patient suffered a grade 2 fractured humerus that resolved. No serious treatment‐related AEs were reported. Most of the AEs and serious AEs reported in the study resolved before the active follow‐up period began; the exception was anemia, which appeared to worsen during follow‐up in 3 (9%) patients. The exploratory efficacy findings suggest a potential benefit from re‐treatment, and further evaluation in a larger prospective study is warranted.
Patients in the re‐treatment study were a very select group and included patients who already had benefited from prior radium‐223 therapy. Most patients in the re‐treatment study had received and failed prior treatment, including docetaxel (45%), abiraterone (61%), and enzalutamide (30%); however, the survival was better than might be expected. Whether this was due to patient selection or radium‐223 treatment cannot be ascertained using this study design.
While direct comparisons between the re‐treatment study and ALSYMPCA are not possible, it is noteworthy that efficacy assessments after re‐treatment closely paralleled those from initial radium‐223 treatment in ALSYMPCA.1 Median OS was 24.4 months in re‐treatment compared with 14.9 months in ALSYMPCA; median time to first SSE was 16.7 months with re‐treatment versus 15.6 months in ALSYMPCA; and median SSE‐free survival was 12.8 months with re‐treatment compared with 9.0 months with radium‐223 in ALSYMPCA. It must be emphasized that patients in the re‐treatment study were a very select group, not only because they had successfully received a full course of 6 radium‐223 injections without progression or safety concerns, but also because they had not received chemotherapy after their initial course of radium‐223 and their disease had not progressed outside the bone, which improved their prognosis even though they had received multiple lines of treatment. These patients therefore represent a population very different from patients enrolled in ALSYMPCA. Additionally, chemotherapy, radiotherapy, and/or immunotherapy administered during follow‐up may have affected the study results, although most of the efficacy endpoints had been reached before the follow‐up period. These results are promising and provide a rationale for further study to more precisely define benefits of re‐treatment with radium‐223.
Limitations of the study focus on the design (nonrandomized), and the fact that patients were selected for non‐bone progression during their first course of radium‐223 and for their continued good performance status and good hematologic parameters at baseline before the repeat course of radium‐223. Outcomes for a control population with similar parameters are not available. DNA repair alterations were not assessed in this study in a systematic manner, but such studies could be informative with regard to understanding those individuals having more favorable responses to radium‐223. Patients with DNA‐repair defects have tumors that may be more susceptible to DNA‐damaging agents, such as radium‐223 or platinum‐based chemotherapy.9, 10, 11, 12
5. CONCLUSIONS
Selected patients with CRPC and bone metastases who tolerated and benefited from an initial radium‐223 treatment regimen, and whose disease subsequently progressed, appear to benefit from re‐treatment with radium‐223 at the same dose and schedule. After 2‐year follow‐up, re‐treatment with radium‐223 showed minimal hematologic toxicity and sustained benefit in terms of OS and low rates of bone progression; no cumulative or unexpected toxicity was reported. A larger prospective study is needed to further evaluate the efficacy and safety of re‐treatment with radium‐223; however, based on these results, radium‐223 re‐treatment for patients who would have been eligible for this trial is feasible.
CONFLICTS OF INTEREST
The authors would like to declare the following conflicts of interest: Sartor reports personal fees from AAA, Astellas, Blue Earth Diagnostics, Inc, EMD Serono, Hinova, Myovant, and Pfizer; grants and personal fees from AstraZeneca, Bayer, Constellation, Dendreon, Endocyte, Johnson & Johnson, Progenics, and Sanofi; and grants from Innocrin, Invitae, Merck, Roche, and SOTIO, during the conduct of the study. Heinrich reports grants and nonfinancial support from Bayer, during the conduct of the study; personal fees from Astellas, Eisai, Ipsen, and Janssen‐Cilag; grants and personal fees from AstraZeneca, Bayer, Roche, Merck Sharp & Dohme, and Bristol‐Myers Squibb, outside the submitted work. Mariados reports serving on a speakers bureau for Bayer. Méndez Vidal reports personal fees from Bayer Healthcare during the conduct of the study, and from Janssen‐Cilag, Sanofi Aventis, Astellas, Medivation, Roche, Novartis, and Pfizer, outside the submitted work. Peer reports grants and personal fees from Sanofi and personal fees from Bayer, Pfizer, Janssen, Astellas, Teva, Novartis, Bristol‐Myers Squibb, Merck Sharp & Dohme, and Roche, outside the submitted work. Procopio reports grants and personal fees from Bayer and Jannsen, and has been an advisory board member for Bayer, Janssen, and AstraZeneca, outside the submitted work. Rosenbaum reports travel fees to ASCO from Bayer, outside the submitted work. Trigo reports personal fees from AstraZeneca, Bayer, Merck Sharp & Dohme, Eisai, and Takeda, and grants and personal fees from Bristol‐Myers Squibb, outside the submitted work. Trandafir, Wagner, and Li are employees at Bayer HealthCare. The other authors declare no potential conflicts of interest.
ACKNOWLEDGMENTS
We thank the patients who volunteered to participate in this study; their family members and caregivers; the study center staff members who cared for the patients; the sponsor staff involved in data collection and analyses; and Richard McCabe, PhD (SciStrategy Communications) for medical writing assistance in the development of the manuscript. Research and manuscript support was provided by Bayer.
Sartor O, Heinrich D, Mariados N, et al. Re‐treatment with radium‐223: 2‐year follow‐up from an international, open‐label, phase 1/2 study in patients with castration‐resistant prostate cancer and bone metastases. The Prostate. 2019;79:1683‐1691. 10.1002/pros.23893
References
REFERENCES
- 1. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium‐223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213‐223. [DOI] [PubMed] [Google Scholar]
- 2. Sartor O, Coleman R, Nilsson S, et al. Effect of radium‐223 dichloride on symptomatic skeletal events in patients with castration‐resistant prostate cancer and bone metastases: results from a phase 3, double‐blind, randomised trial. Lancet Oncol. 2014;15(7):738‐746. [DOI] [PubMed] [Google Scholar]
- 3. Nilsson S, Cislo P, Sartor O, et al. Patient‐reported quality‐of‐life analysis of radium‐223 dichloride from the phase III ALSYMPCA study. Ann Oncol. 2016;27(5):868‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vogelzang NJ, Coleman RE, Michalski JM, et al. Hematologic safety of radium‐223 dichloride: baseline prognostic factors associated with myelosuppression in the ALSYMPCA trial. Clin Genitourin Cancer. 2017;15(1):42‐52 e48. [DOI] [PubMed] [Google Scholar]
- 5.Xofigo (radium Ra 223 dichloride) injection, for intravenous use [package insert]. Wayne NJ: Bayer HealthCare Pharmaceuticals Inc; May 2013. http://hcp.xofigo‐us.com/index.php. Accessed 17 October 2018.
- 6. Zimmerman BE, Bergeron DE, Cessna JT, Fitzgerald R, Pibida L. Revision of the NIST standard for (223)Ra: new measurements and review of 2008 data. J Res Natl Inst Stand Technol. 2015;120:37‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sartor O, Coleman RE, Nilsson S, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate‐specific antigen dynamics in the phase 3 ALSYMPCA trial with radium‐223. Ann Oncol. 2017;28(5):1090‐1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sartor O, Heinrich D, Mariados N, et al. Re‐treatment with radium‐223: first experience from an international, open‐label, phase I/II study in patients with castration‐resistant prostate cancer and bone metastases. Ann Oncol. 2017;28(10):2464‐2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Isaacsson Velho P, Qazi F, Hassan S, et al. Efficacy of radium‐223 in bone‐metastatic castration‐resistant prostate cancer with and without homologous repair gene defects. Eur Urol. 2019;76(2):170‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steinberger AE, Cotogno P, Ledet EM, Lewis B, Sartor O. Exceptional duration of radium‐223 in prostate cancer with a BRCA2 mutation. Clin Genitourin Cancer. 2017;15(1):e69‐e71. [DOI] [PubMed] [Google Scholar]
- 11. Ramos JD, Mostaghel EA, Pritchard CC, Yu EY. DNA repair pathway alterations in metastatic castration‐resistant prostate cancer responders to radium‐223. Clin Genitourin Cancer. 2018;16(2):106‐110. [DOI] [PubMed] [Google Scholar]
- 12. Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic inactivation of BRCA2 in platinum‐sensitive metastatic castration‐resistant prostate cancer. Eur Urol. 2016;69(6):992‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]


