Abstract
Objective
The National Family Health Survey‐4 in India provided the first nationally representative estimates of hysterectomy among women aged 15–49. This paper aims to examine the national and state‐level age‐specific prevalence of hysterectomy, individual and household level factors associated with the procedure, and state‐level indicators that may explain variation across states.
Design
Cross‐sectional, nationally representative household survey.
Setting
National Family Health Survey was conducted across all Indian states and union territories between 2015 and 2016.
Population
The survey covered 699 686 women between the ages of 15 and 49 years.
Methods
Descriptive analyses and multivariate logistic regression.
Main outcome measures
Women who reported ever having a hysterectomy and age at hysterectomy.
Results
Age‐specific prevalence of hysterectomy was 0.36% (0.33,0.39) among women aged 15‐29; 3.59% (3.45,3.74) among women aged 30‐39; and 9.20% (8.94,9.46) among women 40‐49 years. There was considerable variation in prevalence by state. Four states reported age‐specific prevalence similar to high‐income settings. Approximately two‐thirds of hysterectomies were conducted in private facilities, with similar patterns across age groups. At the national level, higher age and parity (at least two children); not having had formal schooling; rural residence (adjusted odds ratio [AOR] 1.36; 95% CI 1.27,1.45; P < 0.01) and higher wealth status were associated with higher odds of hysterectomy. Previously sterilised women had lower odds (AOR 0.64; 95% CI 0.61,0,68; P < 0.01) of reporting hysterectomy. Exploratory analyses suggest state‐level factors associated with prevalence of hysterectomy include caesarean section, female illiteracy, and women's employment.
Conclusions
Hysterectomy patterns among women aged 15–49 in India indicate the critical need to ensure treatment options for gynaecological morbidity and to address hysterectomy among young women in particular.
Funding
This study was part of the RASTA initiative of the Population Council's India country office under the Evidence Project supported by USAID.
Tweetable abstract
Hysterectomy patterns in India highlight the need for alternatives to treat gynaecological morbidity among younger women.
Keywords: epidemiology, gynaecology, hysterectomy, India, menstrual bleeding
Tweetable abstract
Hysterectomy patterns in India highlight the need for alternatives to treat gynaecological morbidity among younger women.
Introduction
Decades of research, predominantly in high‐income countries, have examined the prevalence, indications, and long‐term side effects of hysterectomy, or removal of the uterus. Between one‐quarter to one‐half of women in the USA, Germany, Western Australia, Ireland, and the UK will have undergone a hysterectomy by age 65–70 years.1, 2, 3, 4, 5 Although differences in measurement approaches, availability of data, and population age structures limit cross‐country comparisons, estimates suggest that incidence varies widely across countries. The age‐standardized incidence per 100 000 women ranges from 173/100 000 women in Denmark to 295/100 000 in Germany, and 510/100 000 in the USA.6, 7, 8, 9 Available data from high‐income settings indicate that hysterectomy is most commonly performed on women between 44 and 54 years.9, 10, 11 Evidence on the long‐term effects of hysterectomy suggests an increased risk of morbidity such as cardiovascular disease and osteoporosis, with higher risk among younger women and women who have undergone oophorectomy, the removal of one or both ovaries.12, 13, 14, 15, 16, 17
Population‐based data from low‐ and middle‐income countries, although limited, suggest lower prevalence compared with high‐income settings.18 A study in rural China estimated hysterectomy prevalence of 3.3% among women aged 25–69, at a mean age of 44 years.19 In India, community‐based cross‐sectional studies—conducted mostly in rural settings in different age and population groups—estimate a prevalence between 1.7 and 7.8%.20, 21, 22, 23, 24 However, a cohort study among rural, low‐income women in Gujarat suggested an incidence comparable to high‐income countries and a median age below 40 years.25 Further, qualitative and facility‐based research in several states has indicated the potential of medically unnecessary hysterectomy among low‐income women, driven by a complex set of factors such as lack of appropriate gynaecological care, menstrual taboos, attitudes towards the post‐reproductive uterus, provider or patient‐induced moral hazard, and inappropriate use of health insurance.26, 27, 28, 29
Accordingly, hysterectomy has emerged as an important issue in debates on health care and medical ethics in India. As health systems and epidemiological patterns vary widely across India,30 national and state‐representative prevalence estimates of hysterectomy are required. In 2013, Indian researchers, activists, and policymakers recommended the inclusion of hysterectomy in the National Family Health Survey (NFHS) in order to inform policy on women's health.29 This paper utilises findings of the NFHS to examine national and state‐level prevalence of hysterectomy in India, identify individual and household level factors associated with the procedure, and explore variation across states.
Methods
This analysis draws from the fourth round of the NHFS (NFHS‐4) conducted in all 29 states and 7 union territories of India in 2015–2016. The total population of India was 1.2 billion in 2011, of which 26% (312 million) were aged 15–49 years.31 NFHS, a nationally representative sample survey, covered 723 875 eligible women aged 15–49 in 572 000 households. Interviews were conducted with 699 686 women, a 97% response rate. The NFHS‐4 employed a stratified two‐stage sampling process. In the first stage, primary sampling units (PSUs) were selected with population proportion to size sampling. PSUs were villages in rural areas and Census Enumeration Blocks in urban areas. In the second stage, in every selected rural and urban cluster, households were randomly selected by systematic sampling.32, 33 The hysterectomy module, asked of all women, included questions on whether a woman had ever undergone an operation to remove the uterus, how many years ago it was performed, and where it was conducted (public/private hospital) and for what medical indication. Regarding standards for reporting patient and public involvement in research (GRIPP2‐SF), we note that this is a secondary analysis of publicly available data collected under the direction of the Ministry of Health and Family Welfare of the Government of India; we did not directly engage with patients in either the development of the research or analysis. We presented a draft of findings at a 2018 consultation with community‐based organisations, government representatives, physician bodies, and researchers for input, and will continue to use the findings for future advocacy.34
This paper presents findings from three analyses:
Prevalence estimates by age and across states, along with place of procedure and indication. As the survey only covers women aged 15–49, we do not report overall prevalence but instead report age‐specific prevalence in three age groups in descriptive analyses. A dichotomous variable for hysterectomy status was generated. There were 32 cases with the reported age at hysterectomy as less than 13 years, the average age of menarche in India. Sensitivity analyses indicated that inclusion of these cases did not affect estimates; we retained them in analyses while noting there may be limitations in the accuracy of age reporting. Data regarding place of hysterectomy were collected according to type of facility visited by the respondent, categorised as public, private or others. Public included government/municipal hospital, primary health center, community health center, subcenter, government mobile clinic, camp or other public facilities. Private included private hospital/clinic, mobile clinics and other private facilities. Other facilities included non‐government organisations, trust hospitals and other healthcare facilities.
Multivariate logistic regression to identify predictors of hysterectomy nationally and four states with the highest prevalence. We examined crude odds ratios across a range of background characteristics. The adjusted model included variables associated with hysterectomy in crude odds ratios (P ≤ 0.05) and variables reported in the published literature on hysterectomy in India. Adjusted Wald tests were used to examine P‐values for variables with more than two categories. We controlled for age (15–29; 30–39, 40–49 years), parity (0–1, 2, 3+), residence (urban/rural), religion (Hindu, Muslim, and others); caste (Scheduled Tribe and Scheduled Caste and others), household wealth index (a composite score given in the NFHS based on household assets categorised into three categories: poor, middle, and rich), years of schooling/education (none, 1–5 years, 5–10 years, and more than 10 years), and sterilisation status (yes/no). Analysis of predictors at the national level included a fixed effect term to adjust for state‐level variation. Pregnancy complications were not included, as information was limited to the last birth of the respondent. Individual‐level models did not include women's employment status, freedom of mobility or decision‐making, as these data were only available from a sub‐sample of respondents. We used state‐level prevalence among women aged 15–49 to identify the four highest prevalence states in order to explore whether predictors vary across states.
An exploratory linear regression analysis to identify state‐level socio‐economic and health system factors associated with hysterectomy prevalence at the state level. Variables were included to explore potential sources of variation across states, based on the literature25, 35 and availability of data in the NFHS. These included economic development indicated by literacy and wealth index, utilisation of healthcare facilities by women indicated by proportion of last births delivered in institutions, women's employment status in the last 12 months, freedom of mobility to go to the market, health facility or outside the village, role in decision‐making for at least one of: health care, large household purchases, and daily needs, as well as state‐level indicators of reproductive healthcare interventions, specifically the proportion of caesarean sections and sterilisation levels.
All three analyses were conducted using the svy function in STATA Version 13 to account for survey design and sampling weights. Results are presented as weighted estimates and 95% confidence intervals.
Results
The prevalence of hysterectomy across age groups was estimated to be: 0.36% (0.33,0.39) among women 15‐29; 3.59% (3.45,3.74) among women 30‐39; and 9.20% (8.94,9.46) among women 40‐49 years (Table 1). Median age at hysterectomy was 24 years among women 15‐29 years at the the time of the survey; 30 years among women 30‐39 years at the time of the survey; and 37 years among women 40‐49 years at the time of the survey. Age distribution at the time of procedure is presented in Figure 1A. Private facilities were utilised in approximately two‐thirds of cases, with similar patterns across age groups: 65.7% (15–29 years), 71.3% (30–39 years), and 66.6% (40–49 years). Excessive menstrual bleeding or pain was self‐reported as an indication for hysterectomy by over half of women aged 15–49, followed by fibroids (14.2–20.7%) and uterine disorder (rupture) (13.3–14.9%) (Table 1). Cancer, uterine prolapse and severe postpartum haemorrhage were reported by less than 10% of women in this age group. The majority of women (88%) reported only one primary indication; the remainder reported multiple reasons.
Table 1.
Age‐specific prevalence of hysterectomy, place of procedure, and self‐reported indications for hysterectomy, India, 2015–2016
| 15–29 years (n = 1292) | 30–39 years (n = 6740) | 40–49 years (n = 14 021) | |
|---|---|---|---|
| Prevalence of hysterectomy | 0.36 (0.33, 0.39) | 3.59 (3.45, 3.74) | 9.20 (8.94, 9.46) |
| Median age at hysterectomy | 24 | 30 | 37 |
| Place hysterectomy performed | |||
| Public facilities | 34.29 (30.61, 38.18) | 28.67 (26.94, 30.46) | 33.94 (32.62, 35.29) |
| Private and other facilities | 65.71 (61.82, 69.39) | 71.33 (69.54, 73.06) | 66.06 (64.71, 67.38) |
| Self‐reported indications for hysterectomy a | |||
| Excessive menstrual bleeding/pain | 51.20 (47.18, 55.20) | 54.78 (52.95, 56.60) | 55.99 (54.63, 57.34) |
| Fibroids/cysts | 14.22 (11.64, 17.27) | 17.86 (16.51, 19.31) | 20.73 (19.64, 21.86) |
| Uterine disorder (rupture) | 13.29 (10.82, 16.22) | 14.91 (13.67, 16.25) | 13.41 (12.5, 14.37) |
| Cancer | 6.19 (4.46, 8.52) | 5.93 (5.19, 6.76) | 5.31 (4.79, 5.90) |
| Uterine prolapse | 7.87 (6.13, 10.05) | 8.88 (7.87, 9.82) | 7.33 (6.70, 8.01) |
| Severe postpartum haemorrhage | 5.02 (3.60, 6.96) | 3.79 (3.12, 4.60) | 3.15 (2.77, 3.59) |
| Other | 12.8 (10.44, 15.60) | 7.63 (6.66, 8.73) | 6.86 (6.19, 7.60) |
Multiple response question. n, Number of cases of hysterectomy in each age group.
Figure 1.
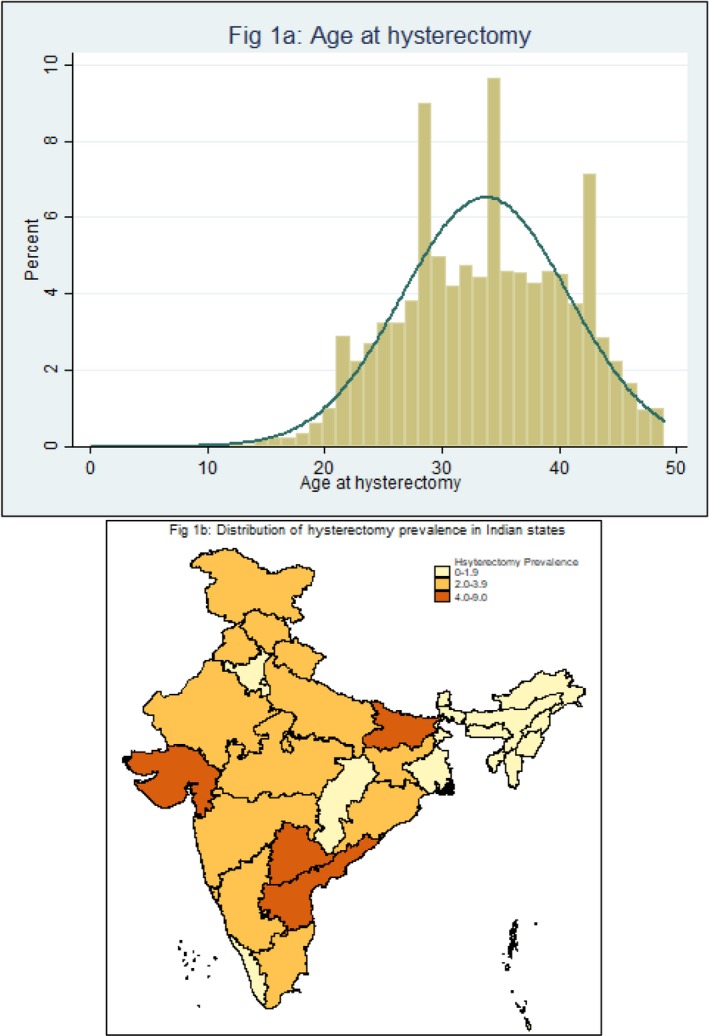
(A,B) Distribution of age at hysterectomy and prevalence of hysterectomy among women aged 15–49 in Indian states, 2015–2016.
Prevalence estimates varied considerably across all states of India (Figure 1B). Prevalence among women aged 15–49 was below 2% in all north‐east Indian states, Punjab, Chhattisgarh, West Bengal, and Kerala (Table S1). Prevalence estimates were highest in four states, where the proportion of hysterectomy cases among women aged 40–49 years was: Andhra Pradesh (22.4%), Bihar (14.5%), Gujarat (12.6%), and Telangana (20.1%). Almost one‐half (46.1%; 95% CI 44.8–47.5) of women who reported having undergone hysterectomy had already been previously sterilised, as reported for contraception use (Table 2). Prevalence of caesarean section and the proportion of women with hysterectomy who had been previously sterilised varied widely across the four higher prevalence states (Table 2).
Table 2.
State‐wise estimates of age‐specific prevalence and other variables for selected four states, India, 2015–2016
| State | N | Age‐specific prevalence of hysterectomy | Among women aged 15–49 | |||
|---|---|---|---|---|---|---|
| 15–29 | 30–39 | 40–49 | % births delivered by caesarean section | % sterilised of women who had hysterectomy | ||
| Gujarat | 22 932 | 0.2 | 4.2 | 12.6 | 18.4 | 46.6 |
| Bihar | 45 812 | 1.0 | 8.2 | 14.5 | 6.2 | 21.6 |
| Andhra Pradesh | 10 428 | 1.1 | 9.6 | 22.4 | 40.1 | 85.6 |
| Telangana | 7567 | 1.1 | 9.5 | 20.1 | 57.7 | 59.4 |
| India | 699 686 | 0.4 | 3.6 | 9.2 | 17.2 | 46.2 |
N, total weighted sample size.
Predictors of hysterectomy
Prevalence of hysterectomy varied by demographic and socio‐economic characteristics, with inconsistent relations across states. Unadjusted odds ratios indicate that older age, higher parity, and women's schooling were consistently associated with history of hysterectomy, nationally and in each of the four states (Table S2). Crude ratios also indicated that illiteracy, rural residence, and higher household wealth were associated with higher odds of hysterectomy at the national level and in the four selected states. Women who were identified as scheduled caste/scheduled tribe groups in two states (Bihar and Andhra Pradesh) had lower odds of hysterectomy, whereas odds were higher in Telangana. Crude odds ratios indicated higher odds among women who had been previously sterilised, nationally and across the four states.
At the national level, adjusted analyses indicate that higher age and parity (at least two children) were associated with higher odds of hysterectomy (Table 3). Evidence indicated that women with at least 5–10 years of education (adjusted odds ratio [AOR] 0.74, 95% CI 0.69–0.79; P < 0.01) had lower odds of reporting hysterectomy compared with women without formal schooling, with lower odds among women with more than 10 years as well. There was evidence that sterilisation was associated with lower odds of hysterectomy (AOR 0.64, 95% CI 0.61–0.68; P < 0.01). Rural residence was associated with higher odds (AOR 1.36, 95% CI 1.27–1.45; P < 0.01), along with higher wealth status (AOR for highest household wealth group = 1.76, 95% CI 1.65–1.88; P < 0.01).
Table 3.
Adjusted odds ratios with 95% confidence interval by background characteristics, 2015–2016
| n | India | Gujarat | Bihar | Andhra Pradesh | Telangana | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aOR (CI) | P‐value | aOR (CI) | P‐value | aOR (CI) | P‐value | aOR (CI) | P‐value | aOR (CI) | P‐value | ||
| Age of women | |||||||||||
| 15–29 years | 1292 | 1 | 1 | 1 | 1 | 1 | |||||
| 30–39 years | 6740 | 6.61 (5.96, 7.33) | 13.46 (7.92, 22.88) | 4.04 (3.27, 4.99) | 5.45 (3.77, 7.87) | 5.30 (3.77, 7.45) | |||||
| 40–49 years | 14 021 | 16.9 (15.19, 18.80) | <0.01 | 41.02 (24.27, 69.32) | <0.01 | 7.23 (5.82, 8.98) | <0.01 | 14.35 (10.12, 20.34) | <0.01 | 11.14 (7.53, 16.46) | <0.01 |
| No. of children | |||||||||||
| 0–1 | 2015 | 1 | 1 | 1 | 1 | 1 | |||||
| 2 | 7317 | 2.48 (2.25, 2.73) | 2.28 (1.57, 3.30) | 5.63 (3.95, 8.03) | 1.66 (1.15, 2.39) | 3.35 (2.27, 4.95) | |||||
| 3+ | 12 721 | 2.43 (2.21, 2.68) | <0.01 | 2.35 (1.59, 3.47) | <0.01 | 7.37 (5.08, 10.69) | <0.01 | 1.49 (1.02, 2.17) | 0.02 | 2.96 (1.93, 4.52) | <0.01 |
| Education | |||||||||||
| Uneducated | 11 039 | 1 | 1 | 1 | 1 | 1 | |||||
| 1‐5 years | 3675 | 1.01 (0.95, 1.08) | 0.91 (0.69, 1.20) | 1.12 (0.96, 1.32) | 1.08 (0.87, 1.35) | 1.22 (0.90, 1.65) | |||||
| 5‐10 years | 5586 | 0.74 (0.69, 0.79) | <0.01 | 0.67 (0.52, 0.87) | <0.01 | 0.86 (0.73, 1.01) | <0.01 | 0.8 (0.64, 0.99) | <0.01 | 0.39 (0.28, 0.54) | |
| 10+ years | 1753 | 0.45 (0.40, 0.49) | 0.51 (0.36, 0.72) | 0.54 (0.41, 0.70) | 0.27 (0.17, 0.42) | 0.23 (0.13, 0.39) | <0.01 | ||||
| Place of residence | |||||||||||
| Urban | 6594 | ||||||||||
| Rural | 15 459 | 1.36 (1.27, 1.45) | <0.01 | 1.26 (1.01, 1.57) | 0.05 | 1.08 (0.90, 1.29) | 0.39 | 1.31 (1.08, 1.59) | 0.01 | 1.97 (1.50, 2.60) | <0.01 |
| Caste | |||||||||||
| Others | 16 516 | ||||||||||
| SC/ST | 5537 | 0.85 (0.81, 0.90) | <0.01 | 0.88 (0.71, 1.09) | 0.23 | 0.84 (0.73, 0.98) | 0.03 | 0.93 (0.74, 1.17) | 0.55 | 1.16 (0.91, 1.47) | 0.23 |
| Religion | |||||||||||
| Hindu | 18 891 | ||||||||||
| Non‐Hindu | 3162 | 0.74 (0.69, 0.80) | <0.01 | 0.91 (0.65, 1.28) | 0.60 | 0.71 (0.60, 0.83) | <0.01 | 0.88 (0.68, 1.13) | 0.31 | 0.70 (0.50, 0.98) | 0.04 |
| Household wealth | |||||||||||
| Poor | 7233 | 1 | 1 | 1 | 1 | 1 | |||||
| Medium | 5177 | 1.45 (1.37, 1.54) | 1.17 (0.94, 1.46) | 1.46 (1.27, 1.68) | 1.35 (1.07, 1.71) | 1.25 (0.98, 1.59) | |||||
| Rich | 9643 | 1.76 (1.65, 1.88) | <0.01 | 1.63 (1.24, 2.15) | <0.01 | 1.58 (1.31, 1.92) | <0.01 | 1.53 (1.19, 1.96) | <0.01 | 2.04 (1.57, 2.65) | <0.01 |
| Sterilised | |||||||||||
| No | 11 881 | 1 | 1 | 1 | 1 | 1 | |||||
| Yes | 10 172 | 0.64 (0.61, 0.68) | <0.01 | 0.77 (0.63, 0.95) | 0.01 | 0.58 (0.51, 0.67) | <0.01 | 1.45 (1.11, 1.91) | 0.01 | 0.58 (0.46, 0.72) | <0.01 |
n, number of cases of hysterectomy in each sub‐group.
These correlations varied by state in our comparison of the four high prevalence states of Andhra Pradesh, Bihar, Gujarat, and Telangana (Table 3). For example, sterilised women had higher odds of hysterectomy in Andhra Pradesh (AOR 1.45, 95% CI 1.11–1.91; P < 0.05), whereas the association was reversed in the other three states.
State‐level variation
Our exploratory analysis of variation across states, using state‐level variables, indicated evidence for a positive linear relation between three state‐level factors and the prevalence of hysterectomy (Table 4). With a 1% increase in the proportion of caesarean section deliveries (reported for last birth), there was evidence of a 0.07 increase in prevalence of hysterectomy (coefficient 0.07, 95% CI 0.02–0.12). Similarly, a 1% increase in the proportion of illiterate women (coefficient 0.10, 95% CI 0.06–0.15) and the proportion of working women in the state (coefficient 0.05, 95% CI 0.01–0.10) was associated with a 0.10 and 0.05 increase in the prevalence of hysterectomy, respectively. There was no evidence that health insurance coverage at the time of the survey, demographic factors such as poverty, urbanisation and caste, and other collected indicators on women's agency were associated with the prevalence of hysterectomy at the state level.
Table 4.
Linear regression model to assess the association between hysterectomy prevalence and state‐level variables, India, 2015–2016
| State‐level variable | Coefficient | P‐value | 95% CI | |
|---|---|---|---|---|
| % sterilised | 0.015 | 0.486 | −0.030 | 0.061 |
| % c‐sections | 0.069 | 0.014 | 0.015 | 0.122 |
| % health insurance | 0.003 | 0.834 | −0.025 | 0.031 |
| % illiterate | 0.097 | 0.001 | 0.042 | 0.152 |
| % Hindu religion | 0.000 | 0.982 | −0.027 | 0.027 |
| % SC/ST caste | −0.005 | 0.720 | −0.036 | 0.026 |
| % poor | −0.020 | 0.268 | −0.057 | 0.017 |
| % urban | −0.015 | 0.266 | −0.042 | 0.012 |
| % worked in last 12 months | 0.052 | 0.032 | 0.005 | 0.099 |
| % with freedom of mobility | 0.005 | 0.734 | −0.024 | 0.033 |
| % role in decision‐making | −0.008 | 0.748 | −0.061 | 0.044 |
| Constant | −1.053 | 0.699 | −6.601 | 4.495 |
Discussion
Main findings
This analysis examined hysterectomy among women aged 15–49 in India, with a focus on four high‐prevalence states. Prevalence was highest among women aged 40–49 at the time of the survey (9.20%), who underwent the procedure at a median age of 37. Over two‐thirds of hysterectomies were conducted in the private sector. The leading self‐reported medical indication was excessive menstrual bleeding/pain. At the national level, higher age, parity of at least two children, less than 5 years of education, higher wealth, and no history of sterilisation were associated with higher odds of hysterectomy.
Prevalence and evidence for correlates varied by state. Prevalence was highest in Andhra Pradesh, Telangana, Gujarat, and Bihar and lower in states such as Punjab, Chhattisgarh, West Bengal, and Kerala, as well as all eight north‐eastern states. In addition to individual characteristics, our exploratory analysis suggested that higher prevalence of caesarean section, female illiteracy, and female labour force participation at the state level were associated with a higher prevalence of hysterectomy among women aged 15–49.
Strengths and limitations
The primary strengths of this analysis were the use of previously unavailable nationally representative data and state‐specific analyses within India. These data will provide baseline estimates to examine hysterectomy prevalence over time and across states, among women aged 15–49. Our understanding of the epidemiology of hysterectomy in India, however, is limited by the use of cross‐sectional data limited to women aged 15–49. National data in India inclusive of women over 49 would provide prevalence estimates comparable with other settings, the total population of women at risk of long‐term side effects of hysterectomy, and insight into whether correlates of hysterectomy vary by age. NFHS data also did not include health insurance status at the time of hysterectomy or mode of payment, precluding an analysis of the role of health financing. Our analysis of state‐level factors was limited by lack of available relevant data on health system performance, such as availability of gynaecological services. While recall error related to a major surgery is unlikely, women's understanding of the question may have affected estimates. Similarly, women's self‐reported age and medical indications for hysterectomy are subject to reporting and/or recall error. Categories of self‐reported reasons for hysterectomy, as currently reported in the NFHS, do not clearly differentiate obstetric from non‐obstetric cases. Lastly, the use of cross‐sectional data limit conclusions on causality related to risk factors.
Interpretation in light of other evidence
While the national prevalence estimate was low compared with other countries, prevalence of hysterectomy among women aged 40–49 years in four states (Andhra Pradesh, Bihar, Gujarat, and Telangana) was similar to age‐specific estimates in the USA and UK.1, 4 The median age at hysterectomy in India, as estimated in specific age groups (37 years among women 40–49 years) was considerably lower than that reported in high‐income settings.36 Although NFHS did not collect data on oophorectomy, evidence suggests it commonly accompanies hysterectomy: bilateral removal of the ovaries was conducted in 37% of hysterectomies in a major teaching hospital in New Delhi and among 59% of women who reported hysterectomy in rural Andhra Pradesh.37, 38 Even without oophorectomy, hysterectomy has been associated with earlier onset of menopause.39 Accordingly, women in India who underwent hysterectomy in their late thirties are at risk of menopause considerably earlier than the estimated global median age at natural menopause, 51 years.36
The majority of hysterectomy cases were for benign conditions, similar to patterns in high‐income settings.6 Over one‐half of women who underwent hysterectomy reported excessive menstrual bleeding as a medical indication. Standard treatment guidelines, including those in India, advise that heavy menstrual bleeding first be treated hormonally or with conservative surgery.40, 41 However, higher prevalence of hysterectomy in rural women is consistent with qualitative research that reports limited non‐surgical options for bleeding compared with urban women due to lack of treatment options and opportunity costs associated with pursuing non‐surgical treatment–suggesting that hysterectomy may be offered as primary treatment in some rural settings.26, 27
Evidence on the association of hysterectomy with age, less education, and rural residence is consistent with previous community‐based research in India.24, 25, 35 The correlation with higher household wealth emerges as a new finding compared with previous research, which has primarily focused on low‐income women in India. Further, the intersection of demographic, economic, and social factors that render some women more vulnerable remains less understood. Sterilisation presents a puzzling pattern. The NFHS reports that 75% of current contraceptive use among women was sterilisation, at a median age of 25.7.33 With the exception of Andhra Pradesh, adjusted analyses indicated that odds of hysterectomy were lower among women who had been sterilised—suggesting different risk factors for the two procedures or, potentially, use of hysterectomy as a replacement for sterilisation.
Variations in predictors across the four high‐prevalence states suggest that health systems factors and social context influence the risk of hysterectomy.25, 35 For example, women workers may experience pressure to remove the uterus, either to treat gynaecological ailments permanently or to preserve labour productivity, as suggested by qualitative research and recent news reports.26, 27, 42 Although the NFHS did not collect data on household insurance coverage at the time of hysterectomy, state‐level variation suggests a potential role of health financing.43 Further, the association with caesarean section at the state level suggests hysterectomy prevalence may be higher where medical intervention and/or gynaecological services are more common. Studies of within‐country variation are limited; one analysis has suggested that the density of gynaecologists influenced variation in hysterectomy across regions in the USA.44
Conclusion
Our analysis of NFHS data on hysterectomy underscores critical issues for women's health in India. The median age at the procedure and prevalence in specific states are of primary concern. Evidence on the long‐term effects of hysterectomy is predominantly from high‐income settings, where the median age is higher. Women in India who undergo hysterectomy at a younger age have a longer risk of exposure to lower estrogen levels and, potentially, elevated risk of side effects. Our findings confirm that treatment for gynaecological morbidity such as excessive bleeding must constitute a significant priority, in order to reduce the use of hysterectomy among women in their thirties. Moreover, the use of non‐surgical or conservative alternatives must be promoted further as treatment for gynaecological morbidity.
Although NFHS data provide an important start to strengthening the evidence base, wider population‐based research among all women above 15 years is required to estimate overall population prevalence of gynaecological morbidity and the use of hysterectomy, and to understand women's treatment history and long‐term side effects of hysterectomy in the Indian context. State‐level, comparative research is required to provide insight into health system factors that influence the use of hysterectomy, along with evaluations of strategies such as medical audits and quality monitoring of both hospitals and health financing schemes to reduce medically unnecessary procedures.
India's health system currently caters to a limited range of health services for women, largely related to pregnancy, delivery, family planning, and postpartum care.45 The recently launched programme to promote Comprehensive Primary Health Care signifies a positive trend in addressing chronic diseases, but much wider investment is required. In particular, primary care providers will need training in managing gynaecological morbidity. As momentum builds for a women's health agenda in India, evidence on hysterectomy highlights the critical need for a continuum of care for gynaecological services for women through the life cycle.45
Disclosure of interests
All authors declare they have no conflict of interest. Completed disclosure of interests forms are available to view online as supporting information.
Contribution to authorship
The authors jointly conceptualised the study. SD and AS developed an analysis plan, and AS conducted data analysis. SD, DN, and RV provided input into analyses and reviewed findings. SD led the writing of the manuscript with AS, with contributions to individual sections from DN and RV. All authors approved the final manuscript.
Details of ethics approval
The study conducted a secondary analysis of publicly available data with no identifiable information on survey respondents. The data can be downloaded from www.DHSprogram.com.
Funding
This study was supported by the RASTA initiative of the Population Council's India country office under the Evidence Project. The Evidence Project is made possible by the generous support of the American people through the U.S. Agency for International Development (USAID) under the terms of cooperative agreement no. AID‐OAA‐A‐13‐00087. The contents of this manuscript are the sole responsibility of the Evidence Project and Population Council and do not necessarily reflect the views of USAID or the United States Government.
Supporting information
Table S1. Age‐specific prevalence of hysterectomy and place of hysterectomy performed in Indian states, 2015‐2016.
Table S2. Prevalence of hysterectomy and unadjusted odds ratios (95% CI) by background characteristics, India, 2015‐2016.
Acknowledgements
The authors acknowledge the insightful comments of researchers, policymakers, and activists on a draft of this paper presented at a national consultation on hysterectomy in New Delhi in September 2018.
Desai S, Shuka A, Nambiar D, Ved R. Patterns of hysterectomy in India: a national and state‐level analysis of the Fourth National Family Health Survey (2015–2016). BJOG 2019; 126 (S4): 72–80
The copyright line for this article was changed on 30 August 2019 after original online publication.
References
- 1. Rositch AF, Nowak RG, Gravitt PE. Increased age and race‐specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer 2014;120:2032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ong S, Codd M, Coughlan M, O'Herlihy C. Prevalence of hysterectomy in Ireland. Int J Gynaecol Obstet 2000;69:243–7. [DOI] [PubMed] [Google Scholar]
- 3. Spilsbury K, Semmens J, Hammond I, Bolck A. Persistent high rates of hysterectomy in Western Australia: a population‐based study of 83 000 procedures over 23 years. BJOG 2006;113:804–9. [DOI] [PubMed] [Google Scholar]
- 4. Redburn J, Murphy M. Hysterectomy prevalence and adjusted cervical and uterine cancer rates in England and Wales. BJOG 2001;108:388–95. [DOI] [PubMed] [Google Scholar]
- 5. Stang A, Merrill RM, Kuss O. Prevalence‐corrected hysterectomy rates by age and indication in Germany 2005–2006. Arch Gynecol Obstet 2012;286:1193–200. [DOI] [PubMed] [Google Scholar]
- 6. Hammer A, Rositch AF, Kahlert J, Gravitt PE, Blaakaer J, Søgaard M. Global epidemiology of hysterectomy: possible impact on gynecological cancer rates. Am J Obstet Gynecol 2015;213:23–9. [DOI] [PubMed] [Google Scholar]
- 7. Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol 2008;198:34.e1–7. [DOI] [PubMed] [Google Scholar]
- 8. Stang A, Merrill RM, Kuss O. Hysterectomy in Germany: a DRG‐based nationwide analysis, 2005–2006. Dtsch Arztebl Int 2011;108:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lykke R, Blaakær J, Ottesen B, Gimbel H. Hysterectomy in Denmark 1977–2011: changes in rate, indications, and hospitalization. Eur J Obstet Gynecol Reprod Biol 2013;171:333–8. [DOI] [PubMed] [Google Scholar]
- 10. Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol 2007;110:1091–5. [DOI] [PubMed] [Google Scholar]
- 11. Hill EL, Graham ML, Shelley JM. Hysterectomy trends in Australia between 2000/01 and 2004/05. Aust N Z J Obstet Gynaecol 2010;50:153–8. [DOI] [PubMed] [Google Scholar]
- 12. Botkin MM‐W. The Association Between Osteoporosis and Early Menopause Following Hysterectomy. 2016. https://scholarworks.waldenu.edu/dissertations/2278/. Accessed 25 Jan 2019.
- 13. Svejme O, Ahlborg H, Nilsson JÅ, Karlsson M. Early menopause and risk of osteoporosis, fracture and mortality: a 34‐year prospective observational study in 390 women. BJOG 2012;119:810–6. [DOI] [PubMed] [Google Scholar]
- 14. Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neurodegener Dis 2012;10:175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population‐based cohort study. Eur Heart J 2011;32:745–50. [DOI] [PubMed] [Google Scholar]
- 16. Yeh JS, Cheng HM, Hsu PF, Sung SH, Liu WL, Fang HL, et al. Hysterectomy in young women associates with higher risk of stroke: a nationwide cohort study. Int J Cardiol 2013;168:2616–21. [DOI] [PubMed] [Google Scholar]
- 17. Fletcher HM, Bennett F, Simms‐Stewart D, Reid M, Williams NP, Wharfe GH, et al. Cardiovascular disease risk factors in menopausal Jamaican black women after hysterectomy and bilateral oophorectomy: an observational study. West Indian Med J 2010;59:625–32. [PubMed] [Google Scholar]
- 18. Desai S. The effect of health education on women's treatment‐seeking behaviour: Findings from a cluster randomised trial and an in‐depth investigation of hysterectomy in Gujarat. India: London School of Hygiene & Tropical Medicine; 2015. [Google Scholar]
- 19. Liu F, Pan Y, Liang Y, Zhang C, Deng Q, Li X, et al. The epidemiological profile of hysterectomy in rural Chinese women: a population‐based study. BMJ Open 2017;7:e015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhasin SK, Roy R, Agrawal S, Sharma R. An epidemiological study of major surgical procedures in an urban population of East Delhi. Indian J Surg 2011;73:131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarna A, Friedland BA, Srikrishnan AK, Katzen LL, Tun W, Abbott SA, et al. Sexually transmitted infections and reproductive health morbidity in a cohort of female sex workers screened for a microbicide feasibility study in Nellore, India. Glob J Health Sci 2013;5:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desai S, Sinha T, Mahal A. Prevalence of hysterectomy among rural and urban women with and without health insurance in Gujarat, India. Reprod Health Matters 2011;19:42–51. [DOI] [PubMed] [Google Scholar]
- 23. Patel V, Tanksale V, Sahasrabhojanee M, Gupte S, Nevrekar P. The burden and determinants of dysmenorrhoea: a population‐based survey of 2262 women in Goa, India. BJOG 2006;113:453–63. [DOI] [PubMed] [Google Scholar]
- 24. Singh A, Arora AK. Why hysterectomy rates are lower in India. Indian J Community Med 2008;33:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Desai S, Campbell OM, Sinha T, Mahal A, Cousens S. Incidence and determinants of hysterectomy in a low‐income setting in Gujarat, India. Health Policy Plan 2016;32:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Desai S. Pragmatic prevention, permanent solution: women's experiences with hysterectomy in rural India. Soc Sci Med 2016;151:11–8. [DOI] [PubMed] [Google Scholar]
- 27. Sardeshpande N. Why do young women accept hysterectomy? Findings from a study in Maharashtra, India. Int J Innov Appl Stud 2014;8:579. [Google Scholar]
- 28. Kameswari S, Vinjamuri P. Medical ethics: a case study of hysterectomy in Andhra Pradesh. Hyderabad: National Institute of Nutrition; 2009. [Google Scholar]
- 29. Prayas . Understanding the reason for rising number of hysterectomies in India: national consultation. Jaipur: Prayas; 2013. Report available at http://www.prayaschittor.org/pdf/Hysterectomy-report.pdf. Accessed 25 Jan 2019.
- 30. Dandona L, Dandona R, Kumar GA, Shukla D, Paul VK, Balakrishnan K, et al. Nations within a nation: variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. Lancet 2017;390:2437–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Census . Registrar General of India, Ministry of Home Affairs, Government of India. India; 2011.
- 32. Ram F. NFHS‐4: an Introduction. Demogr Ind 2014;43:1–8. [Google Scholar]
- 33. ICF, IIPS . National Family Health Survey (NFHS 4). Mumbai: International Institute for Population Sciences; 2018. [Google Scholar]
- 34. Prayas . New Evidence on Hysterectomy in India: Directions for Research, Advocacy and Programs. New Delhi: Prayas, Population Council and UNFPA 2019.
- 35. Prusty RK, Choithani C, Gupta SD. Predictors of hysterectomy among married women 15–49 years in India. Reprod Health 2018;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–74. [DOI] [PubMed] [Google Scholar]
- 37. Kameswari S. Case Study on Unindicated Hysterectomies in Andhra Pradesh. National Workshop on Rising Hysterectomies in India. New Delhi: August 2013.
- 38. Bhatla N. Hysterectomy: Clinical Indications and Patterns. Presentation made at Consultation on New Evidence on Hysterectomy, New Delhi 2018.
- 39. Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG 2005;112:956–62. [DOI] [PubMed] [Google Scholar]
- 40. NICE . Heavy menstrual bleeding: assessment and management; 2018. [PubMed]
- 41. Government of India . Clinical Pathways for Management of Key Indications of Hysterectomy; 2014.
- 42. Why many women in Maharashtra's Beed district have no wombs. The Hindu Business Line 2019; April 11, 2019.
- 43. Grover S, Palacios R. The first two years of RSBY in Delhi In: Palacios R, Das J, Sun C, eds. India's Health Insurance Scheme for the Poor: Evidence from the Early Experience of the Rashtriya Swasthya Bima Yojana. New Delhi: Centre for Policy Research; 2011. pp 153–88. [Google Scholar]
- 44. Geller SE, Burns LR, Brailer DJ. The impact of nonclinical factors on practice variations: the case of hysterectomies. Health Serv Res 1996;30:729. [PMC free article] [PubMed] [Google Scholar]
- 45. Ministry of Health and Family Welfare . The India Strategy for Women's, Children's and Adolescents’ Health (2018‐2030). New Delhi; 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Age‐specific prevalence of hysterectomy and place of hysterectomy performed in Indian states, 2015‐2016.
Table S2. Prevalence of hysterectomy and unadjusted odds ratios (95% CI) by background characteristics, India, 2015‐2016.


