Abstract
Background
Patients undergoing prolonged pelvic surgery may develop compartment syndrome of one or both lower limbs in the absence of direct trauma or pre‐existing vascular disease (well leg compartment syndrome). This condition may have devastating consequences for postoperative recovery, including loss of life or limb, and irreversible disability.
Methods
These guidelines represent the collaboration of a multidisciplinary group of colorectal, vascular and orthopaedic surgeons, acting on behalf of their specialty associations in the UK and Ireland. A systematic analysis of the available peer‐reviewed literature was undertaken to provide an evidence base from which these guidelines were developed.
Results
These guidelines encompass the risk factors (both patient‐ and procedure‐related), diagnosis and management of the condition. Key recommendations for the adoption of perioperative strategies to facilitate prevention and effective treatment of well leg compartment syndrome are presented.
Conclusion
All surgeons who carry out abdominopelvic surgical procedures should be aware of well leg compartment syndrome, and instigate policies within their own institution to reduce the risk of this potentially life‐changing complication.

Key recommendations
All surgeons who undertake pelvic procedures on patients maintained in the Lloyd‐Davies/lithotomy positions should be aware of well leg compartment syndrome (WLCS).
The risk of WLCS must be noted specifically in the preoperative team brief and WHO time out. Strategies agreed to minimize the risk to each patient must be recorded before commencement of surgery.
Unless mandated by other patient safety considerations, the patient's legs should be kept at a level below the heart for the maximum duration possible during a procedure.
Where elevation of the legs is required to facilitate surgery, the maximum unbroken period of elevation should not exceed 4 h. The patient's legs should be kept at a lower level than the heart for a minimum of 15 min after each 4‐h interval. The duration of elevation and the time allowed for recovery should be monitored and documented in the patient's operation note/anaesthetic chart.
Intraoperative hypotension should be corrected where possible and intraoperative fluid therapy optimized to avoid both excessive fluid administration, or inadequate tissue perfusion.
Any patient who has undergone pelvic surgery in the lithotomy position, whether or not combined with Trendelenberg tilt, who complains of postoperative leg pain should be suspected of having WLCS.
The initial diagnosis of WLCS is entirely clinical, so assessment of the patient with suspected WLCS must be methodical, focused and thorough, and documented clearly and contemporaneously.
- Assessment must include:
- Accurate history – pain, paraesthesia, numbness, weakness, paralysis.
- Pain – onset, site, nature.
- Presence of paraesthesia or numbness.
- Inspection: swelling – unilateral/bilateral, oedema.
- Palpation: tension in compartments, palpable difference between sides; tenderness in each compartment.
- Passive stretch exacerbation of pain: dorsiflexion of toes, plantar flexion of toes, dorsiflexion/plantar flexion of ankle.
- Assessment of sensation, pulses and capillary refill.
If WLCS is suspected, immediate referral should be made to the orthopaedic or vascular surgery team, according to local protocols.
If clinical assessment confirms a definite or likely diagnosis of WLCS, this is a limb/life‐threatening surgical emergency requiring immediate decompression by open four‐compartment fasciotomy. Treatment should be under way within 1 h of diagnosis.
There should be a low threshold for reassessment in patients whose symptoms persist or deteriorate.
If initial assessment is equivocal, for example in a sedated/unconscious patient, in whom adequate clinical assessment cannot be undertaken, and there is a high index of suspicion, measurement of compartment pressures may be used to confirm or exclude compartment syndrome.
If compartment pressure measurement is undertaken, and the difference between diastolic pressure and compartment pressure is less than 30 mmHg in any compartment, an immediate (within 1 h), bilateral four‐compartment fasciotomy should be undertaken.
Reassessment of the compartments in theatre should be carried out between 48 and 72 h after decompression.
After fasciotomy, early discussion with, and involvement of, plastic and reconstructive surgeons is recommended for patients with significant tissue loss.
Introduction
Acute lower limb compartment syndrome may follow prolonged abdominopelvic surgery in the Lloyd‐Davies/lithotomy position and complicate the postoperative management of patients undergoing major pelvic surgery. It may result in life‐changing morbidity or even death. The purpose of this guideline is to clarify the nature of this condition and summarize the available evidence regarding pathogenesis, to ensure that appropriate arrangements can be made for prevention, early diagnosis and effective treatment.
Search strategy and review of the literature
A PubMed search was carried out using the following keywords: laparoscopic surgery, robotic surgery, lower limb, lower extremity, lithotomy, Lloyd Davies, compartment syndrome, well leg compartment syndrome. Other papers were identified using references from the original results, with exclusion of non‐English language papers. Owing to the rarity of the condition, there have been no RCTs or prospective case–control studies. The best level of evidence is presented by retrospective cohort studies (level III evidence), with the majority of published work taking the form of case series (level IV) with expert opinion (level V). Although this is a substantial limitation to the creation of this guideline, it must be accepted that more robust studies have not been undertaken. However, it is possible to produce key recommendations from the available evidence that may be adopted easily into clinical practice.
Definition of compartment syndrome
Compartment syndrome is defined as a condition in which fascial compartment pressure exceeds perfusion pressure, causing tissue ischaemia and necrosis1. Although commonest in the lower extremity, compartment syndrome has also been described in the hands, forearms, buttocks, thighs and abdomen. Well leg compartment syndrome (WLCS) is defined as an acute lower limb compartment syndrome that develops in the absence of trauma, and may occur without pre‐existing vascular disease. It is characteristically associated with prolonged operative positioning with the hips and knees flexed, especially in patients maintained with a head‐down tilt (Lloyd‐Davies/Trendelenburg position).
Background
Incidence
Although no attempt has been made to collect risk‐adjusted data in large populations, clinically evident WLCS appears relatively uncommon. The incidence of the condition varies from as high as one in 500 patients undergoing cystectomy2, to one in 3500 (0·03 per cent) or six of 52 319 (0·01 per cent) undergoing abdominopelvic surgery in the lithotomy position3, 4. However, the lowest reported figures almost certainly represent an underestimate of the true incidence of the condition as many cases, especially those with less severe clinical features, are probably not reported. It seems likely, with the advent of laparoscopic or robotic surgery, resulting in more prolonged abdominopelvic operations undertaken in extreme Trendelenberg tilt and the decreased venous return associated with a pneumoperitoneum, that more patients will be put at risk of WLCS, although there have been no studies that have addressed this specifically.
Sequelae of well leg compartment syndrome
In a German survey5 of 21 patients with WLCS following gynaecological surgery (all carried out in the lithotomy position), six were left with permanent neurological disability. Expert review concluded that malpractice had occurred in over half of all of the cases, because preventative measures had not been undertaken clearly, and delays had occurred in diagnosis and/or treatment.
A comprehensive review6 of Danish cases of WLCS where claims for compensation had occurred found that 24 of 40 were successful. Only one patient made a full recovery; 13 patients had a sensory defect in the leg, 14 had pain and a sensory defect, and 12 had paresis, pain and sensory defects.
Pathophysiology
The leg is divided into four fascial compartments (Fig. 1), namely the anterior, lateral, deep posterior and superficial posterior compartments7. The anterior compartment is the most commonly affected by compartment syndrome as it is rigidly bound by the tibia, fibula, interosseous septum and deep fascia8. However, delayed diagnosis is associated with all four compartments being affected. WLCS results from tissue hypoperfusion, caused by a decrease in the perfusion pressure owing to elevation of the lower limb in patients in the lithotomy position. This is further exaggerated (where relevant) by head‐down (Trendelenburg) tilt. This reduces tissue oxygen availability, which causes cells in the affected regions to switch to anaerobic metabolism, generating lactate and other intermediary metabolites of incomplete fuel substrate oxidation. The ischaemia causes hypoxic disruption of capillary endothelium, allowing leakage of fluid and plasma proteins into the interstitial space. This leads to interstitial oedema and a rise in intracompartmental pressure9.
Figure 1.
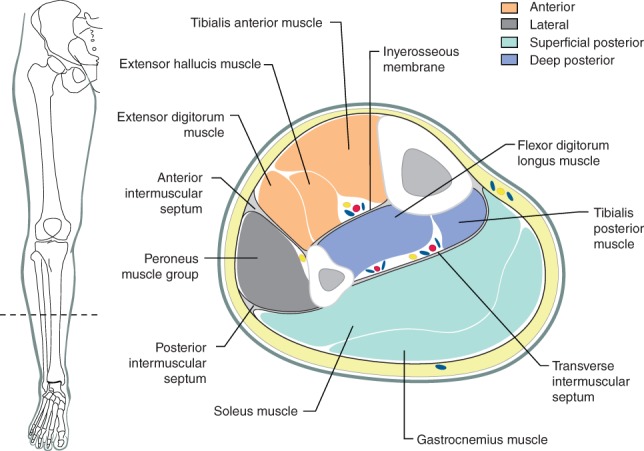
Lower limb compartments
The injury associated with tissue hypoperfusion itself may be reversible for up to 4–6 h. However, on levelling out the patient and lowering the limbs, thus restoring perfusion, a reperfusion injury may then occur. This results from the release of oxygen‐free radicals and other vasoactive mediators as tissues are reoxygenated, damaging the endothelium further. This causes a further increase in permeability to plasma proteins and aggravates interstitial oedema, resulting in a further, often abrupt rise in intracompartmental pressure10.
Normal compartment pressures in the lower extremity range from 0 to 8 mmHg. The first clinically significant effects of raised intracompartmental pressure probably begin to develop at pressures of 20–30 mmHg. The compliance of the fascial compartments of the leg is exceeded at pressures of 30–33 mmHg11. Pressures rise steeply thereafter, resulting in significant impairment of tissue perfusion, worsening ischaemia and, ultimately, tissue necrosis. If left untreated, cell lysis and release of intracellular proteins lead to myoglobinuria and renal damage, followed by multiple organ dysfunction syndrome12, 13.
Risk factors and prevention
Patient‐related
Age
Younger patients may be at greater risk of WLCS. Among those with tibial fractures, a threefold higher rate of acute lower limb compartment syndrome has been noted in patients under 35 years of age, compared with older patients14. This may reflect greater muscle bulk in the compartment and the presence of a stronger, thicker enclosing fascia, but it is unclear whether these alone are sufficient explanations for the apparent difference11. It is also unclear whether the same factors might predispose younger patients to WLCS after pelvic surgery, or whether they are specific to compartment syndrome complicating trauma. Although younger patients may need prolonged pelvic surgery, for example to treat inflammatory bowel disease, it seems likely that most patients undergoing surgery of this nature will tend to be older than the typical population of patients with a tibial fracture.
Obesity
Obesity may increase the risk of developing WLCS. In patients with a BMI above 25 kg/m2 a linear relationship has been reported between increasing BMI and a decrease in ankle BP in the elevated lithotomy position15. There is also a correlation between BMI and intramuscular pressure in the hemilithotomy position, which may explain this association16. There have been no studies analysing the impact of the individual weight of the lower limb on the risk of WLCS but, when positioning, care must be taken that excessive pressure on the popliteal fossa and posterior compartments is avoided in patients with a high BMI.
Vascular disease
As one might expect given the pathophysiology, patients with pre‐existing lower limb vascular disease may be at increased risk of further impairment of tissue perfusion when placed in the lithotomy position, especially with a head‐down tilt, and therefore be at greater risk of WLCS17. This impairment may result from the simple effects of gravity in a circulatory system already compromised by a degree of vascular stenosis, leading to a critical degree of ischaemia that might not otherwise have been apparent in a supine or erect position12.
Intraoperative
Type of surgery
WLCS can occur in any type of abdominopelvic surgery where the patient is positioned with their hips and knees flexed, especially when a period of head‐down tilt is required. This includes colorectal, urological and gynaecological procedures5, 6. Indeed, WLCS has been reported in all the relevant surgical specialties, and after open, laparoscopic and robotic procedures. Laparoscopic and robotic procedures might expose patients to a greater risk of WLCS than open procedures, as a result of prolonged operating times, a greater degree of head‐down tilt, and possibly reduced venous drainage associated with pneumoperitoneum18.
Positioning
The position in which a patient is placed, and the duration for which it is maintained, are key factors in the development of WLCS. The most important consideration, even in a patient who would conventionally be regarded as having been positioned optimally for prolonged pelvic surgery, is the position of the lower limbs with reference to the heart. Although operative positioning of patients is often undertaken by other members of the theatre team, ensuring appropriate exposure to facilitate adequate surgery, avoidance of inappropriate or unsafe positioning, and the prevention of adverse consequences of even appropriate positioning, remain the ultimate responsibility of the operating surgeon.
Elevation of a patient's legs from the supine position has been said to result in a 2‐mmHg drop in mean arterial BP (MAP) in the calf for every vertical 2·5‐cm rise in height above the heart19. Therefore, a patient with a MAP of 90 mmHg, but whose lower limbs are raised so that the midpoint of the calf is elevated 50 cm (a common surgical position), would have a MAP of only 50 mmHg at this level. The addition of a head‐down Trendelenberg position been shown to result in a significant decrease in deep muscle mixed tissue oxygen saturation, owing to a combination of hydrostatic, blood and intra‐abdominal pressures, compounding the relative tissue underperfusion20. Some studies have suggested that a head‐down tilt of as little as 15° (as opposed to hip abduction and flexion) may be the dominant factor in impaired limb perfusion during prolonged pelvic dissection21. Even short periods of head‐down tilt, systemic hypotension or co‐existing peripheral vascular disease (or a combination of these) may therefore result in significant lower limb tissue ischaemia.
Ankle position may also affect leg compartment pressures, with full dorsiflexion increasing it in all four compartments, whereas knee position (full extension of the knee with full dorsiflexion of the ankle) affects only pressures in the superficial posterior and lateral compartments9, 22.
Great care must be taken at all times to protect the legs in order to avoid compression of the popliteal fossae and calves from support boots, other devices, or even the excessive use of material for lower limb wrapping. The upper part of the patients' calves should be clear of the support boots. Care should be taken to avoid dorsiflexion of the ankles, which should be maintained in a neutral or slightly plantar‐flexed position, and this should also be documented contemporaneously in the operative care chart.
There is no evidence to suggest that any one form of lower limb support is preferable for avoiding WLCS. There are no clinical outcome data, but anterior compartment pressures have been compared in Allen stirrups (a boot‐like device supporting the calf), posterior knee supports and ankle slings23. Both stirrups and knee supports resulted in significant increases in compartment pressures when the legs were elevated, although these pressures were reduced by pneumatic calf compression. Ankle slings did not increase compartment pressures significantly when the legs were elevated. Although it is unclear whether this might protect against WLCS, ankle slings are relatively unstable; they are acceptable for brief procedures (less than 30 min), but are usually inappropriate for more prolonged operations. Even though lower limb positioning may be extremely important, it should be noted that there are some case reports of WLCS developing in patients in the supine position24, 25, 26, 27.
For operations that are anticipated to be extremely long (for example complex reconstructive or exenterative pelvic surgery), it may be safer to start the procedure with the patient in the supine position, and to adopt the Lloyd‐Davies/lithotomy position only when the pelvic phase of the procedure commences. Alternatively, it may be appropriate to position the lower limbs in supports, but to ensure that they are kept level with the patient's heart for as long as possible, and to document this.
Where adequate pelvic exposure is needed for more than 4 h to complete a procedure, the time at which the lower limbs are elevated above the level of the patient's heart should be recorded routinely, and arrangements made to notify the surgeon as the 4‐h cut‐off approaches, so that preparations can be made to lower the legs at that time.
Duration of leg elevation and head‐down tilt
The minimum degree and duration of head‐down tilt required for safe and effective exposure of the operative field should be adopted at all times, and the tilt corrected at the earliest opportunity.
Given the pathophysiology described above, it seems reasonable to conclude that, in general, the likelihood of WLCS developing is time‐critical. The duration of compartment ischaemia is likely to be reflected in the extent of reperfusion injury when the legs are lowered. It is likely that subclinical degrees of muscle ischaemia and raised compartment pressures, which are asymptomatic (or at least minimally symptomatic), may occur more frequently, but go undetected, or at least unreported. The degree of compartment ischaemia in WLCS is probably the result of overall arterial perfusion and local perfusion abnormalities in the calf resulting from the degree of limb elevation and head‐down tilt. However, the extent (if any) of fluid overadministration, the presence of pre‐existing vascular insufficiency, and the duration for which the lower limbs are exposed to these factors are likely to also be important.
Lower limb compartment pressures have been measured in patients undergoing colorectal procedures in the lithotomy position. Compartment pressures increased immediately on putting the legs into the lithotomy position, but remained below 20 mmHg. They increased further with Trendelenburg tilt. Compartment pressures then continued to rise, reaching a mean of 30 mmHg at a mean of 5 h. After returning the patients to the supine position, all compartment pressures had returned to less than 10 mmHg within 15 min28.
The majority of accounts of WLCS in abdominopelvic surgery report that patients have been maintained for at least 4 h in the Lloyd‐Davies/lithotomy position. It should be noted that there are, however, several case reports in which WLCS developed within 4 h29. Of 125 case reports identified by Christoffersen and colleagues6, the median duration of surgery was 7·5 (range 2–12) h. These were a mix of colorectal, gynaecological, obstetric, plastic surgical and urological procedures.
As the factor common to published case reports (where this is stated) has generally been an operating time exceeding 4 h, it is recommended that care is taken to document the interval during which the legs are elevated above the heart, and to restrict this to a maximum unbroken period of 4 h5, 6, 30. Where technical difficulties or operative challenges prevent safe lowering of the patient's legs (for example, because of the need to control pelvic bleeding), the risk to the patient from lowering the legs may exceed the risk of WLCS. The reasons for exceeding the 4‐h period of elevation should, under those circumstances, be documented clearly when the relevant operation note is completed.
There are no clinical data to support the minimum time that should be allowed to elapse before the limbs can safely be elevated again, in order to minimize the risk of WLCS. Based on the change in compartment pressures noted above, it is recommended that the legs are returned to the same level as the heart for at least 15 min, before being elevated again. Lowering the legs appears to be safe and, in particular, there is no evidence to suggest that this results in an increase in the incidence of surgical‐site infection31.
Antiembolism stockings and/or intermittent pneumatic compression devices
Antiembolism/thromboembolism deterrent (TED) stockings generate a constant pressure of at least 25 mmHg32, 33, whereas intermittent pneumatic compression devices produce an intermittent pressure of approximately 40 mmHg for 12 s each minute34. As the risk of compartment syndrome increases at pressures above 30 mmHg, the application of antiembolism stockings and intermittent pneumatic compression devices could increase the risk of a patient developing compartment syndrome. It has been suggested that both modalities of thromboprophylaxis should not be used concurrently during extended surgery in the lithotomy or Lloyd‐Davies position, because of this risk35.
Current National Institute for Health and Care Excellence guidance36 requires only that patients should be offered either modality, and there may be specific contraindications to one or other form of thromboprophylaxis. However, the potential risk of causing (or at least increasing the risk of) WLCS seems unlikely to represent adequate justification for not using one or both forms of prophylaxis, properly undertaken, in high‐risk circumstances such as prolonged pelvic surgery. There are no randomized trials or even observational studies that have compared the risks of WLCS in patients with, and without TED stockings or intermittent pneumatic calf compression. The theoretical possibility that measures taken to reduce the risks of thromboembolism (taken alone or together) might increase the risk of WLCS has therefore to be evaluated based on the balance of the relative risks of these two potentially fatal postoperative complications.
In the absence of any form of thromboprophylaxis, the risk of an asymptomatic deep vein thrombosis (DVT) is up to 25 per cent and the risk of a fatal pulmonary embolism is 0·5 per cent following abdominopelvic surgery37. It therefore seems inappropriate to avoid carefully applied TED stockings and/or intermittent pneumatic calf compression devices, purely because of potential concerns about causing WLCS.
Fluid and BP management
Current enhanced recovery after surgery protocols suggest that traditional perioperative intravenous fluid therapy of 3·5–7 l of fluid on the day of surgery can lead to delays in return of normal gastrointestinal function, impairment in wound and anastomotic healing, and a decrease in tissue oxygenation38. A recent meta‐analysis39 showed that fewer patients suffered complications with a restrictive fluid protocol, and the risk of infection was lower. However, perioperative fluid restriction has not been shown to reduce postoperative hospital stay40. Although perioperative fluid restriction may improve patient outcomes, it may also, at least in theory, lead to impaired tissue perfusion, and excessive hypotension; it should therefore be avoided, especially while the legs are elevated, to minimize the risk of WLCS. No evidence has currently been identified to implicate the perioperative use of inotropes/vasoconstrictors in the aetiology of WLCS.
Other operative factors
Care must be taken to avoid vascular compromise in the lower limbs as a result of intra‐abdominal retractor placement. WLCS has been reported to have developed as consequence of compression of the external iliac vein by a disposable intra‐abdominal retractor41.
Preoperative planning and time out
Theatre teams undertaking expected prolonged (more than 2 h) pelvic surgery on patients in the Lloyd‐Davies/lithotomy position must recognize that they are potentially exposing their patient to a risk of WLCS. Although, in the authors' view, WLCS is insufficiently common to mandate routine discussion during consent, the needs and likely preferences of the patient should be considered so that, where appropriate (for example in an athlete or other active individual), a discussion satisfying the requirements of Montgomery v Lanarkshire Health Board 201542 can be undertaken and documented.
The nature of the proposed surgery and the fact that the patient is likely to be placed, at least for a period, in a position that puts them at risk of WLCS should be noted specifically during the team brief at the start of the theatre list and the WHO preoperative time out, to draw attention to the potential risk of this complication.
A plan should be agreed before operation, between the surgical, anaesthetic and theatre nursing teams, to minimize this risk (notably with regard to the duration of limb elevation and head‐down tilt). This plan and the resulting actions should be documented in the contemporaneous theatre records, notably the operation record. Some centres have already instituted these practices in every at‐risk procedure43.
Diagnosis
Intraoperative
Intraoperative monitoring of compartment pressures with needle transducers is technically possible, but unlikely to be helpful as it is not simply the duration of ischaemia, but also the subsequent reperfusion that leads to WLCS12. For example, in patients undergoing intra‐abdominal colorectal surgery, anterior compartment pressures can reach up to 70 mmHg for intervals while in the lithotomy position, without this resulting in WLCS once the legs are returned to a supine position28. Intraoperative compartment pressure monitoring therefore seems unlikely to be a useful basis on which to guide practice, and routine monitoring is therefore not currently recommended.
Postoperative
Leg pain after pelvic surgery is a red flag symptom and mandates immediate assessment. It should lead automatically to clinical suspicion of WLCS44. WLCS should be considered the most likely diagnosis in any patient complaining of otherwise unexplained unilateral or bilateral leg pain after pelvic surgery.
Immediate assessment requires taking an accurate history, recording the site, onset and character of the pain. Enquiry should be made regarding the presence of paraesthesia, numbness, weakness and paralysis. The sensory/motor effects of epidural analgesia or the potential masking effect of patient‐controlled analgesia should be taken into account, but should never be assumed to account for symptoms and signs. Although the use of analgesics has been implicated in the delayed diagnosis of compartment syndrome in patients with limb fracture, the same does not apply to WLCS.
Symptoms are typically bilateral, but are frequently asymmetrical, and may even be unilateral. Approximately half of patients complain of pain appearing almost immediately on waking from anaesthesia and over 99 per cent do so within 24 h of return to theatre recovery6. Notably, the pain is severe and unremitting, and patients may complain that the leg pain greatly exceeds that of the abdominal surgery.
Pain that is severe, unremitting and exacerbated by passive dorsiflexion of the toes and ankles is a cardinal symptom, together with the presence of tension and/or tenderness in the leg compartments. Active dorsiflexion of the ankle, or even of the toes, may be impossible owing to pain and also to impairment of peroneal nerve function.
In patients with unilateral symptoms, there may be a palpable difference between sides. Limb neurology and perfusion, including capillary refill and distal pulses, should be documented but they do not contribute to early diagnosis of the condition45, 46. It should be emphasized that evidence of vascular insufficiency or numbness is not required for diagnosis; indeed, these are features of a diagnosis made too late, and consequently of irreversible injury.
The British Orthopaedic Association Standards for Trauma (BOAST)45 state that ‘in high‐risk patients, regional anaesthesia should be avoided as it can mask the symptoms of compartment syndrome. In addition patient‐controlled analgesia with intravenous opiates can also mask the symptoms’. However, this is most relevant to the diagnosis of traumatic compartment syndrome (where the analgesia is aimed at treating pain in the injured limb in which the compartment syndrome might be diagnosed). It is not relevant to epidural analgesia (where the aim is to provide effective control of abdominal wound pain, usually without affecting sensation in the legs). Epidural anaesthesia should not be a barrier to the effective diagnosis of WLCS44, 47.
If initial clinical assessment is equivocal, a repeat clinical assessment in 30 min is mandatory, as WLCS is usually a rapidly progressive condition.
Exclusion of deep vein thrombosis
Although DVT is a possible explanation for postoperative leg pain when associated with a swollen, tense lower limb, WLCS and DVT may co‐exist; therefore, an attempt to diagnose DVT should not delay consideration and treatment of WLCS. The High Court in England found in 2013 that it was not reasonable to delay diagnosis and treatment of WLCS in order to exclude DVT and pulmonary embolism first, in a patient who had recently undergone abdominoperineal excision for rectal cancer48.
Special investigations
Diagnosis of WLCS is, in almost all cases, based on clinical assessment. Special investigations are not usually required for the diagnosis of WLCS in a conscious patient. They should not delay treatment, which, in almost all patients, requires consideration of urgent fasciotomy if neuromuscular function and, ultimately, the legs are to be preserved. They may be of value, however, in an obtunded/unconscious patient, in whom it may not be possible to diagnose WLCS without measurement of compartment pressures. The relevant technique and interpretation of results are outlined below.
Compartment pressure measurement
Compartment pressure measurement is not recommended for the routine diagnosis of WLCS. Clinical judgement is usually sufficient for the diagnosis in a conscious patient. However, in patients whose level of consciousness is impaired and the diagnosis of WLCS is difficult to establish, compartment pressure measurement (Fig. 2) may be valuable to support clinical judgement. Unless a raised pressure is identified in one compartment, measurement should be undertaken in all four compartments of both legs and done by the surgeon who will be responsible for performing fasciotomy. The measurement of compartment pressures may also be used to support/refute the diagnosis in a patient with equivocal findings complaining of limb pain, without other positive physical signs. Facilities for measurement are available in the standard anaesthetic room.
Figure 2.
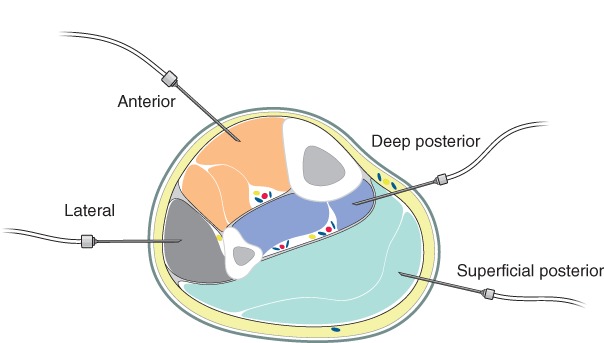
Compartment pressure measurement
There are differing opinions regarding whether compartment pressure alone, perfusion pressure (mean BP minus compartment pressure) or the difference between diastolic pressure and compartment pressure (so‐called delta P, Δp) is the most useful in the diagnosis of compartment syndrome. The Vascular Society of Great Britain and Ireland considers Δp to be the easiest and most reliable measurement for routine use. A Δp below 30 mmHg is indicative of compartment syndrome and mandates immediate bilateral four‐compartment fasciotomy. A Δp of less than 30 mmHg in a single compartment is a positive result for that leg. A negative result requires that all four compartments in the leg have a Δp of at least 30 mmHg.
Laboratory investigations
Abnormal laboratory investigations indicate that significant tissue damage has already occurred and should not be relied on to make the timely diagnosis of WLCS. Measurement of the serum concentration of the muscle enzyme creatine phosphokinase (CK) may provide an indication of severe muscle damage. A CK value of over 2000 units/l is uncommon after surgery and, if associated with symptoms, should be interpreted as indicating compartment syndrome49. A CK concentration of over 4000 units/l may be diagnostic when associated with a chloride level of greater than 104 mg/dl, and blood urea nitrogen level of less than 10 mg/dl50. However, these significant increases are usually a late feature of WLCS46. Similarly, urinary myoglobin may be present if rhabdomyolysis has occurred, but this too is an ominous and late development, frequently associated with a high risk of acute renal failure. Urgent referral to a renal physician is required under such circumstances.
MRI
T2‐weighted MRI has been suggested to be useful in demonstrating and localizing muscle oedema51. However, the findings are not specific and a scan should not be allowed to delay treatment. MRI should certainly not replace clinical judgement.
Near‐infrared spectrometry
Near‐infrared spectrometry (NIRS) is a non‐invasive technique that can identify muscle deoxygenation and perfusion, and may be of value in the assessment of acute and chronic compartment syndromes. Tissue oxygen saturation was reduced significantly when affected legs were compared with unaffected legs in patients with acute traumatic compartment syndrome52 and the reduction in tissue oxygen saturation appears to persist, even after fasciotomy53. However, Bariteau and colleagues54 aborted a trial of NIRS prematurely after false‐negative results were observed in patients with acute compartment syndrome. This might, however, have been because tissue ischaemia had not yet developed at that time point55. All studies of NIRS to date have been undertaken in acute compartment syndrome following trauma, and not WLCS. There is insufficient evidence to recommend NIRS for the diagnosis of WLCS and, in particular, to assume that a satisfactory NIRS result excludes WLCS.
Management
The management of WLCS is no different from the treatment of compartment syndrome secondary to trauma. The greater the level of intracompartmental pressure, the more rapidly ischaemic tissue injury occurs. In addition, a less significantly raised intracompartmental pressure maintained for longer may cause equally severe damage56. BOAST45 recommend two‐incision, four‐compartment decompression. When suspected, WLCS should be considered a surgical emergency and treatment should be commenced within 1 h of diagnosis.
Once compartment syndrome is suspected, an immediate referral should be made to colleagues in either orthopaedic or vascular surgery, depending on local protocols. There is usually no place for conservative treatment for suspected WLCS, and arrangements should be made for immediate transfer to theatre and fasciotomy. A delay in fasciotomy of more than 12 h increases the risk of permanent sequelae. Full recovery is more likely if fasciotomy is undertaken within 6 h of diagnosis57.
In patients with a reduced level of consciousness in whom WLCS is suspected and there are therefore no reliable physical signs, bilateral measurement of compartment pressure in all four compartments may be appropriate. Concurrent measurement of diastolic BP is required to calculate Δp. A Δp of less than 30 mmHg measured in any compartment mandates immediate bilateral four‐compartment fasciotomy. As WLCS develops rapidly and 99 per cent develops within 24 h of return to recovery, it follows that it is safer to undertake bilateral four‐compartment fasciotomies in patients with a reduced level of consciousness. There should be a low threshold for re‐evaluation in borderline cases.
After fasciotomy, return to theatre for examination of the affected limb(s) under anaesthesia, assessment and, where appropriate, debridement of affected tissues should be arranged routinely 48–72 h later. Subsequent debridement and wound management includes delayed primary closure, skin grafting and healing by secondary intention. The wound management will depend on surgeon experience, preference and local arrangements. Early liaison with plastic and reconstructive surgeons for advice and clinical input is recommended for challenging wounds.
The knowledge, skills and procedures required to assess and manage compartment syndrome, and therefore WLCS, are prescribed by the Trauma and Orthopaedic Surgery58 and Vascular Surgery59 curricula.

Acknowledgements
These guidelines have been formally endorsed by the councils, on behalf of the membership of the following organizations to represent the applicable and relevant professional standards for the matters concerned, on behalf of the Association of Coloproctology of Great Britain and Ireland, the Vascular Society of Great Britain and Ireland, the British Orthopaedic Association, the British Association of Urological Surgeons and the Association of Surgeons of Great Britain and Ireland.
Disclosure: The authors declare no conflict of interest.
The copyright line for this article was changed on 15 July 2019 after original online publication.
References
- 1. McQueen MM. Acute Compartment Syndrome. Fractures in Adults (7th edn). Lippincott Williams & Wilkins: Philadelphia, 2010. [Google Scholar]
- 2. Simms MS, Terry TR. Well leg compartment syndrome after pelvic and perineal surgery in the lithotomy position. Postgrad Med J 2005; 81: 534–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halliwill JR, Hewitt SA, Joyner MJ, Warner MA. Effect of various lithotomy positions on lower‐extremity blood pressure. Anesthesiology 1998; 89: 1373–1376. [DOI] [PubMed] [Google Scholar]
- 4. Warner ME, LaMaster LM, Thoeming AK, Marienau ME, Warner MA. Compartment syndrome in surgical patients. Anesthesiology 2001; 94: 705–708. [DOI] [PubMed] [Google Scholar]
- 5. Bauer EC, Koch N, Erichsen CJ, Juettner T, Rein D, Janni W et al Survey of compartment syndrome of the lower extremity after gynecological operations. Langenbecks Arch Surg 2014; 399: 343–348. [DOI] [PubMed] [Google Scholar]
- 6. Christoffersen JK, Hove LD, Mikkelsen KL, Krogsgaard MR. Well leg compartment syndrome after abdominal surgery. World J Surg 2017; 41: 433–438. [DOI] [PubMed] [Google Scholar]
- 7. von Keudell AG, Weaver MJ, Appleton PT, Bae DS, Dyer GSM, Heng M et al Diagnosis and treatment of acute extremity compartment syndrome. Lancet 2015; 386: 1299–1310. [DOI] [PubMed] [Google Scholar]
- 8. Mubarak SJ, Hargens AR. Acute compartment syndromes. Surg Clin North Am 1983; 63: 539–565. [DOI] [PubMed] [Google Scholar]
- 9. Beraldo S, Dodds SR. Lower limb acute compartment syndrome after colorectal surgery in prolonged lithotomy position. Dis Colon Rectum 2006; 49: 1772–1780. [DOI] [PubMed] [Google Scholar]
- 10. Grace PA. Ischaemia–reperfusion injury. Br J Surg 1994; 81: 637–647. [DOI] [PubMed] [Google Scholar]
- 11. Shadgan B, Menon M, Sanders D, Berry G, Martin C Jr, Duffy P et al Current thinking about acute compartment syndrome of the lower extremity. Can J Surg 2010; 53: 329–334. [PMC free article] [PubMed] [Google Scholar]
- 12. Martin JT. Compartment syndromes: concepts and perspectives for the anesthesiologist. Anesth Analg 1992; 75: 275–283. [DOI] [PubMed] [Google Scholar]
- 13. Burzstein S, Carlson GL. Crush injury In Scientific Foundations of Trauma, Cooper GJ, Dudley HAF, Gann DS, Little RA, Maynard RL. (eds). CRC Press: Boca Raton, 1997; 285–299. [Google Scholar]
- 14. McQueen MM, Gaston P, Court‐Brown CM. Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br 2000; 82: 200–203. [PubMed] [Google Scholar]
- 15. Peters P, Baker SR, Leopold PW, Taub NA, Burnand KG. Compartment syndrome following prolonged pelvic surgery. Br J Surg 1994; 81: 1128–1131. [DOI] [PubMed] [Google Scholar]
- 16. Tan V, Pepe MD, Glaser DL, Seldes RM, Heppenstall RB, Esterhai JL Jr. Well‐leg compartment pressures during hemilithotomy position for fracture fixation. J Orthop Trauma 2000; 14: 157–161. [DOI] [PubMed] [Google Scholar]
- 17. Canterbury TD, Wheeler WE, Scott‐Conner CE. Effects of the lithotomy position on arterial blood flow in the lower extremities. W V Med J 1992; 88: 100–101. [PubMed] [Google Scholar]
- 18. Rao MM, Jayne D. Lower limb compartment syndrome following laparoscopic colorectal surgery: a review. Colorectal Dis 2011; 13: 494–499. [DOI] [PubMed] [Google Scholar]
- 19. Enderby GE. Postural ischaemia and blood‐pressure. Lancet 1954; 266: 185–187. [DOI] [PubMed] [Google Scholar]
- 20. Tong C, Olympio M, Bodin S, Edwards A, Hemal A. Leg muscle hypoxia and RALUS In Annual Meeting of the ASA, San Diego, California, USA, 2010; A194. [Google Scholar]
- 21. Horgan AF, Geddes S, Finlay IG. Lloyd‐Davies position with Trendelenburg – a disaster waiting to happen? Dis Colon Rectum 1999; 42: 916–919. [DOI] [PubMed] [Google Scholar]
- 22. Gershuni DH, Yaru NC, Hargens AR, Lieber RL, O'Hara RC, Akeson WH. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am 1984; 66: 1415–1420. [PubMed] [Google Scholar]
- 23. Pfeffer SD, Halliwill JR, Warner MA. Effects of lithotomy position and external compression on lower leg muscle compartment pressure. Anesthesiology 2001; 95: 632–636. [DOI] [PubMed] [Google Scholar]
- 24. O'Shea E, Power K. Well leg compartment syndrome following prolonged surgery in the supine position. Can J Anaesth 2008; 55: 794–795. [DOI] [PubMed] [Google Scholar]
- 25. Kavouni A, Ion L. Bilateral well‐leg compartment syndrome after supine position surgery. Ann Plast Surg 2000; 44: 462–463. [PubMed] [Google Scholar]
- 26. Beadnell SW, Saunderson JR, Sorenson DC. Compartment syndrome following oral and maxillofacial surgery. J Oral Maxillofac Surg 1988; 46: 232–234. [DOI] [PubMed] [Google Scholar]
- 27. O'Connor D, Breslin D, Barry M. Well‐leg compartment syndrome following supine position surgery. Anaesth Intensive Care 2010; 38: 595. [PubMed] [Google Scholar]
- 28. Chase J, Harford F, Pinzur MS, Zussman M. Intraoperative lower extremity compartment pressures in lithotomy‐positioned patients. Dis Colon Rectum 2000; 43: 678–680. [DOI] [PubMed] [Google Scholar]
- 29. Stornelli N, Wydra FB, Mitchell JJ, Stahel PF, Fabbri S. The dangers of lithotomy positioning in the operating room: case report of bilateral lower extremity compartment syndrome after a 90‐minutes surgical procedure. Patient Saf Surg 2016; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pridgeon S, Bishop CV, Adshead J. Lower limb compartment syndrome as a complication of robot‐assisted radical prostatectomy: the UK experience. BJU Int 2013; 112: 485–488. [DOI] [PubMed] [Google Scholar]
- 31. Balen P. Nerve damage due to positioning during surgery. Clin Risk 2016; 22: 69–75. [Google Scholar]
- 32. Murthy G, Ballard RE, Breit GA, Watenpaugh DE, Hargens AR. Intramuscular pressures beneath elastic and inelastic leggings. Ann Vasc Surg 1994; 8: 543–548. [DOI] [PubMed] [Google Scholar]
- 33. Veraart JC, Pronk G, Neumann HA. Pressure differences of elastic compression stockings at the ankle region. Dermatol Surg 1997; 23: 935–939. [DOI] [PubMed] [Google Scholar]
- 34. Gilbart MK, Oglivie‐Harris DJ, Broadhurst C, Clarfield M. Anterior tibial compartment pressures during intermittent sequential pneumatic compression therapy. Am J Sports Med 1995; 23: 769–772. [DOI] [PubMed] [Google Scholar]
- 35. Lachmann EA, Rook JL, Tunkel R, Nagler W. Complications associated with intermittent pneumatic compression. Arch Phys Med Rehabil 1992; 73: 482–485. [PubMed] [Google Scholar]
- 36. National Institute for Health and Care Excellence (NICE). Venous Thromboembolism in over 16s: Reducing the Risk of Hospital‐acquired Deep Vein Thrombosis or Pulmonary Embolism. NICE Guideline [NG89]; 2018. https://www.nice.org.uk/guidance/ng89/evidence [accessed 1 November 2018]. [PubMed]
- 37. Scottish Intercollegiate Guidelines Network (SIGN) . Prevention and Management of Venous Thromboembolism SIGN publication no. 122; 2010. http://www.sign.ac.uk [accessed 1 November 2018].
- 38. Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009; 144: 961–969. [DOI] [PubMed] [Google Scholar]
- 39. Schol PB, Terink IM, Lancé MD, Scheepers HC. Liberal or restrictive fluid management during elective surgery: a systematic review and meta‐analysis. J Clin Anesth 2016; 35: 26–39. [DOI] [PubMed] [Google Scholar]
- 40. MacKay G, Fearon K, McConnachie A, Serpell MG, Molloy RG, O'Dwyer PJ. Randomized clinical trial of the effect of postoperative intravenous fluid restriction on recovery after elective colorectal surgery. Br J Surg 2006; 93: 1469–1474. [DOI] [PubMed] [Google Scholar]
- 41. Kikuchi T, Maeda H. Two cases of compartment syndrome of the lower extremities during surgery for gynecological malignancies. J Anesth 2016; 30: 481–485. [DOI] [PubMed] [Google Scholar]
- 42. Montgomery v Lanarkshire Health Board [2015] SC 11 [2015] 1 AC 1430.
- 43. Rosevear HM, Lightfoot AJ, Zahs M, Waxman SW, Winfield HN. Lessons learned from a case of calf compartment syndrome after robot‐assisted laparoscopic prostatectomy. J Endourol 2010; 24: 1597–1601. [DOI] [PubMed] [Google Scholar]
- 44. Mar GJ, Barrington MJ, McGuirk BR. Acute compartment syndrome of the lower limb and the effect of postoperative analgesia on diagnosis. Br J Anaesth 2009; 102: 3–11. [DOI] [PubMed] [Google Scholar]
- 45. British Orthopaedic Association Standards for Trauma (BOAST) . BOAST 10 – Diagnosis and Management of Compartment Syndrome of the Limbs; 2015. https://www.boa.ac.uk/resources/boasts/boast‐10‐pdf.html [accessed 1 November 2018].
- 46. Köstler W, Strohm PC, Südkamp NP. Acute compartment syndrome of the limb. Injury 2004; 35: 1221–1227. [DOI] [PubMed] [Google Scholar]
- 47. Montgomery CJ, Ready LB. Epidural opioid analgesia does not obscure diagnosis of compartment syndrome resulting from prolonged lithotomy position. Anesthesiology 1991; 75: 541–543. [DOI] [PubMed] [Google Scholar]
- 48. Orwell v Salford Royal NHS Foundation Trust , England and Wales High Court (Queen's Bench Division), EWHC 3245 (QB) Sess. (28 October 2013).
- 49. Lampert R, Weih EH, Breucking E, Kirchhoff S, Lazica B, Lang K. [Postoperative bilateral compartment syndrome resulting from prolonged urological surgery in lithotomy position. Serum creatine kinase activity (CK) as a warning signal in sedated, artificially respirated patients.] Anaesthesist 1995; 44: 43–47. [DOI] [PubMed] [Google Scholar]
- 50. Valdez C, Schroeder E, Amdur R, Pascual J, Sarani B. Serum creatine kinase levels are associated with extremity compartment syndrome. J Trauma Acute Care Surg 2013; 74: 441–447. [DOI] [PubMed] [Google Scholar]
- 51. Ikeya E, Taguchi J, Ohta K, Miyazaki Y, Hashimoto O, Yagi K et al Compartment syndrome of bilateral lower extremities following laparoscopic surgery of rectal cancer in lithotomy position: report of a case. Surg Today 2006; 36: 1122–1125. [DOI] [PubMed] [Google Scholar]
- 52. Shuler MS, Reisman WM, Kinsey TL, Whitesides TE Jr, Hammerberg EM, Davila MG et al Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am 2010; 92: 863–870. [DOI] [PubMed] [Google Scholar]
- 53. Giannotti G, Cohn SM, Brown M, Varela JE, McKenney MG, Wiseberg JA. Utility of near‐infrared spectroscopy in the diagnosis of lower extremity compartment syndrome. J Trauma 2000; 48: 396–401. [DOI] [PubMed] [Google Scholar]
- 54. Bariteau JT, Beutel BG, Kamal R, Hayda R, Born C. The use of near‐infrared spectrometry for the diagnosis of lower‐extremity compartment syndrome. Orthopedics 2011; 34: 178. [DOI] [PubMed] [Google Scholar]
- 55. Boezeman RP, Moll FL, Ünlü Ç, de Vries JP. Systematic review of clinical applications of monitoring muscle tissue oxygenation with near‐infrared spectroscopy in vascular disease. Microvasc Res 2016; 104: 11–22. [DOI] [PubMed] [Google Scholar]
- 56. Boesgaard‐Kjer DH, Boesgaard‐Kjer D, Kjer JJ. Well‐leg compartment syndrome after gynecological laparoscopic surgery. Acta Obstet Gynecol Scand 2013; 92: 598–600. [DOI] [PubMed] [Google Scholar]
- 57. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br 2003; 85: 625–632. [PubMed] [Google Scholar]
- 58. Intercollegiate Surgical Curriculum Programme . Trauma and Orthopaedic Surgery; 2017. https://www.iscp.ac.uk/curriculum/surgical/specialty_year_syllabus.aspx?enc=+e2oZ1qwvgMteu/azG9FTVLt8CJ4IHS1keA07oN0qYc= [accessed 1 November 2018].
- 59. Intercollegiate Surgical Curriculum Programme . Vascular Surgery; 2016. https://www.iscp.ac.uk/curriculum/surgical/specialty_year_syllabus.aspx?enc=faEFWbLB8NTxNjoRSS+HBQ== [accessed 1 November 2018].


