ABSTRACT
Introduction: We previously reported our amyotrophic lateral sclerosis (ALS) video televisit experience. Here we report on video televisit versus in‐clinic costs, adjusting for perceived medical usefulness (MU). Methods: We take the patient‐perspective and a focused institutional‐perspective. Costs are adjusted for patient/caregiver and physician perceptions of visit MU. The base‐case reflects our outpatient ALS practice. Results: In the base‐case, from the patient perspective, in‐clinic visits cost $1,116 and video televisits cost $89 ($119 after MU‐adjustment). From the institutional perspective, clinic visits cost $799, and video televisits cost $354 ($472 after MU‐adjustment). Adjusted cost‐savings per televisit are $997 (patient) and $327 (institution). Sensitivity analyses on 5 variables accounted for uncertainty in base‐case assumptions. Conclusions: Video televisits provide marked adjusted cost‐savings for patients and institutions. Adjusted costs are sensitive to perceived MU of video televisits. Future research should explore the ability of video televisits to reduce healthcare resource usage. Muscle Nerve 60: 147–154, 2019
Keywords: ALS, cost analysis, multidisciplinary, TeleHealth, telemedicine, video televisits
Abbreviations
- AAN
American Academy of Neurology
- ALS
amyotrophic lateral sclerosis
- HIPAA
Health Insurance Portability and Accountability Act of 1996
- IT
information technology
- MGH
Massachusetts General Hospital
- MU
medical usefulness
- TelePALS
TeleHealth for people with ALS.
Over the course of the disease, people with amyotrophic lateral sclerosis (ALS) develop increasing difficulty with mobility and speech, leaving their caregivers with an ever‐increasing burden of care.1 Multidisciplinary ALS clinics were developed to bring specialists together and reduce the burden of care for people with ALS,2, 3, 4, 5, 6 but not all people with ALS can continue to attend a clinic7 and those who do find it increasingly taxing and costly to travel to clinic. Even early in the disease, when patients are still active, clinics are centrally located in mostly urban areas and visits are long and require substantial commitment from patients and loved ones.
Telemedicine is in use to provide care for several chronic conditions.8 A few groups have reported on the use of various forms of telemedicine to provide care to people with ALS,9, 10, 11, 12, 13, 14 and telemedicine is viewed favorably by both people with ALS and ALS health care providers.13, 15 We recently reported on our experience implementing a video televisit program to care for people with ALS seen at the Massachusetts General Hospital (MGH) multidisciplinary ALS clinic.16 Our adoption of video televisits in the ALS patient population was supported by a grant from the ALS Association, philanthropy, and the adoption of an internal hospital program to support physicians using telemedicine. More broadly, such resources are not available, resulting in substantial interest, but slow uptake of video televisits for people with ALS nationally despite a warm reception for televisits in ALS and a growing literature demonstrating the cost‐effectiveness of telemedicine for outpatient care.17
Our objective was to assess the cost of video televisits for people with ALS in their homes relative to multidisciplinary care provided in‐clinic. We incorporated both the patient perspective and a partial institutional perspective focused on the costs of operating a multidisciplinary clinic.
MATERIALS AND METHODS
For cost‐estimation and adjustment, we employ methods used in cost‐effectiveness analyses. Cost‐effectiveness is a method for comparing both the cost and effectiveness of a medical innovation to the existing standard of care. Because cost‐effectiveness analyses rely on a host of specific assumptions, results are presented as a base case (the cost‐effectiveness based on the most likely assumptions about cost and outcomes) and sensitivity analyses (cost‐effectiveness presented over a range of varied assumptions) that provide an understanding of how robust the cost‐effectiveness is over a range of assumptions for key variables.
Costs were assessed using available data and estimates, or assumptions based on our clinical observations when data was unavailable. Costs for each type of visit were adjusted for medical usefulness (MU), which was rated by the ALS providers and people with ALS for in‐clinic and televisits. To help guide our base‐case assumptions, we used data from the MGH Telehealth for People with ALS (TelePALS) program.16 In brief, visits are conducted by ALS physicians at MGH using a secure, encrypted Health Insurance Portability and Accountability Act (HIPAA)‐compliant videoconferencing software application (Vidyo, Inc., Hackensack, NJ) to connect directly with ALS patients in their homes using a patient's home computer, tablet, or smartphone (iOS or Android compatible). We performed a retrospective analysis of all cases collected from September 2014 to January 2016 to compare the costs, correcting for perceived MU of video compared with in‐clinic visits.
All people with ALS seen at least once in the MGH ALS Multidisciplinary Clinic, who had a broadband connection in their home and a home computer, tablet, or smartphone were eligible to participate in the MGH TelePALS program. We analyzed those visits occurring between September 2014 and January 2016, during which time we conducted video televisits with 97 people with ALS.
Cost: Visit Costs
Patient Perspective
From the patient perspective, estimates of the costs of attending an in‐clinic visit include the cost of travel for the patient and caregiver (if accompanied), lodging (if staying overnight), lost work for both the patient and caregiver, if employed, and visit copayment (see Base‐Case section and Tables 1 and 2 for description of patient‐perspective cost assumptions for the base case).
Table 1.
Travel, lodging, and missed work assumptions by geography and visit type (base‐case).
| In‐clinic visit | |||||
|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintiles 3&4 | Quintile 5 | Televisit | |
| Mode of travel | Drive | Drive (75%) Fly (25%) | Drive (10%) Fly (90%) | Fly (100%) | None |
| Lodging | None | 1 night (75%) | 1 night (50%) 2 nights (50%) |
1 night (25%) 2 nights (75%) |
None |
| Travel time/missed work days | 1 day | 2 days | 2 days (50%) 3 days (50%) |
3 days (25%) 3 days (75%) |
3 h |
Table 2.
Cost assumptions for travel, lodging, and missed work (base‐case)
| Quintile 1 | Quintile 2 | Quintiles 3&4 | Quintile 5 | Televisit | |
|---|---|---|---|---|---|
| Driving | $50 | $150 | $250 | $400 | N/A |
| Flying | $150 | $225 | $300 | $300 | N/A |
| Hotel | $223 | N/A | |||
| Missed work (hourly) | $21* | $21* | |||
| Co‐pay | $25 | $25 | |||
Calculated from an annual salary of $44,510 (US Census Bureau mean national income for working adults in 2015).
Healthcare Institution Perspective
From the institutional perspective, costs for in‐clinic visits include provider time, multidisciplinary care personnel time and room costs. Cost items such as travel, lodging, and lost wages are not considered for this perspective, because these factors do not affect the institutional cost.
Costs for video televisits include provider time, video infrastructure costs, follow‐up time by multidisciplinary care team, and information technology (IT) personnel time (see Base‐Case section and Table 3 for description of institution‐perspective cost assumptions for the base‐case).
Table 3.
Patient characteristic assumptions for in‐clinic and televisits (base‐case).
| Patient characteristics (in‐clinic visits) | |
| % of patients who are ambulatory* | 54% |
| % of ambulatory patients who work | 30% |
| % of ambulatory patients who bring a caregiver to clinic | 75% |
| % of non‐ambulatory patients who work | 5% |
| % of non‐ambulatory patients who bring a caregiver to clinic | 100% |
| Patient characteristics (televisits) | |
| % of patients who are independent with televisit | 40% |
| % of independent televisit patients who work | 10% |
| % of independent televisit patients who have a caregiver at televisit | 90% |
| % of non‐independent televisit patients who work | 0% |
Calculated from MGH televisit cohort.
MU
In‐clinic and televisits are different modalities, and their costs cannot be directly compared without accounting for the relative effectiveness of each type of visit. The physical examination is limited to observation, and the multidisciplinary team may or may not be present. Thus, it is possible that either the provider or patient will view the visit as less useful than an in‐clinic visit. Yet if the cost is lower, then the value might still be high. In this analysis, we adjust visit costs using the perceived MU of each type of visit so that we can appropriately compare costs for each type of visit. Of note, visit preference is not the same as MU; preference is driven by a combination of cost, convenience, and perceived usefulness of a visit. Because preference may incorporate cost, it cannot be used to adjust the cost.
MU
To determine the MU of televisits, we analyzed results of a brief survey of patients and providers that we gave as a part of a quality improvement initiative for our TelePALS program. The survey was given to 3 patients and caregivers and 9 providers who have experienced both in‐clinic visits and video televisits. Survey participants were provided with 10 vignettes and asked how useful a televisit was, relative to an in‐clinic visit for each clinical scenario, including routine follow‐up visits in early and late stage patients, goals of care discussions, and assessment of physical complaints from people with ALS. Answers about MU were expressed relative to the MU of an in‐clinic visit and the modified Likert responses ranged from 0% (not at all medically useful) to 150% as useful as an in‐clinic visit. We used these answers to guide our base‐case assumptions about the MU of video televisits from both the patient and provider perspective.
Annual Costs
People with ALS are seen in‐clinic at our institution approximately every 3 months. We calculated the annual cost of in‐clinic visits from the patient and institutional perspectives and the MU‐adjusted costs of patients seen for a year (1) entirely by televisit, (2) two in‐clinic and two televisits, and (3) three in‐clinic and two televisits.
Base‐Case Assumptions
Patient‐Perspective Cost Assumptions
Mode of travel and duration of visit/stay
Based on time studies of our MGH multidisciplinary clinic, visits are assumed to last 2 h, including 30 min of physician time.18 Based on the MGH experience, video televisits are assumed to last 30 min.
Based on the geographic distribution of our previously published cohort of video televisit participants, we identified population quintiles by geography (people in the first quintile, for example, live within 64.3 miles of MGH) (Fig. 1). For each quintile, we then made informed assumptions about travel time and modality, and accommodations (when required), lost work time, and thus costs of travel to the MGH clinic (Table 1). Because patients and caregivers are assumed to lose work time only if they are employed, we made assumptions about rates of employment based on observations in our clinic. We also assumed that people attending a video televisit lost 3 h of work.
Figure 1.
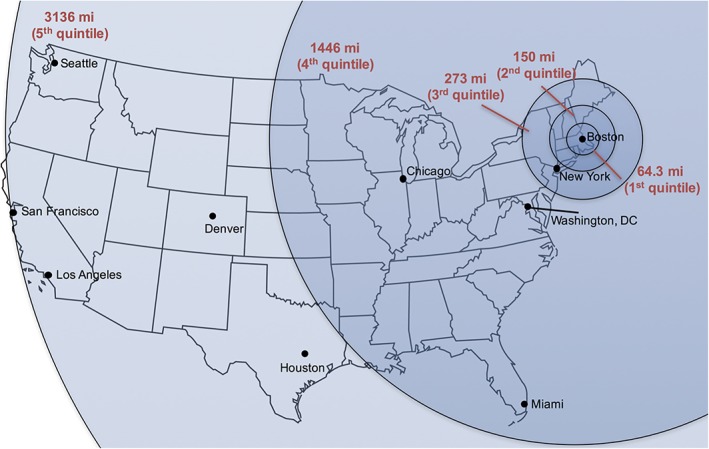
The map represents the distance between the MGH ALS clinic and the primary residence of ALS patients seen in TeleHealth (by quintile). mi: mile (reprint permission requested; status pending).
Patient and caregiver characteristics
Base‐case assumptions about patient and caregiver characteristics are based on clinic demographics and clinical observations at the MGH clinic (Table 3). For example, we assume that 54% of patients seen in clinic are ambulatory, that 75% of ambulatory patients are accompanied to in‐clinic visits by a caregiver, and that caregivers always accompany nonambulatory patients. We assume that 30% of people with ALS who are ambulatory are employed and 5% of people with ALS who are nonambulatory are employed. For the purposes of the base case, we assume that all caregivers are employed. We assume that 40% of patients can perform a televisit independently, and even so, a caregiver accompanies these patients to a televisit 90% of the time. By definition, a caregiver always accompanies nonindependent patients to televisits. Video televisit software is provided free to patients from the institution.
Institutional Cost Estimates
Institutional costs for in‐clinic visits include provider payments, the cost of stocking, staffing, cleaning, and maintaining 1 clinic room, and multidisciplinary personnel in clinic (Table 4).
Table 4.
Institutional cost estimates (base‐case).
| Justification | ||
|---|---|---|
| Costs for in‐clinic visit | ||
| Physician time | $119 | 2.49 RVUs at $49 each; level 4 follow‐up encounter (code 99214) |
| Room cost | $100 | Internal estimate |
| Multidisciplinary team | $580 | Per‐patient visit cost of multidisciplinary team (15) |
| Total | $799 | |
| Costs for televisit | ||
| Physician time | $119 | 2.49 RVUs at $49 each; level 4 follow‐up encounter (CPT code 99214) |
| Software license | $50 | Internal estimate |
| IT team | $22 | 30 min of time (annual salary $90,000) |
| Multidisciplinary team | ||
| Clinic coordinator (15 min) | $5 | 15 min of time (annual salary $40,000) |
| Practice manager | $50 | Annual salary $100,000 |
| Nurse follow‐up | $54 | 1.5 h post‐televisit follow‐up (annual salary $75,000) |
| Physical therapy follow‐up | $36 | 1 h post‐televisit follow‐up (annual salary $75,000) |
| Speech therapy follow‐up | $18 | 30 min post‐televisit follow‐up CPT RVUs annual salary $75,000) |
| Total | $354 | |
CPT, current procedural terminology; RVU, relative value unit.
Institutional costs for video televisits include provider payments, video televisit software and IT personnel. Multidisciplinary care team members are not routinely involved in the actual video televisit, yet there are often numerous follow‐up items that require asynchronous input from the multidisciplinary clinic personnel, so their time is also included (Table 4).
MU
In our survey, the average video televisit was rated 74% (range, 39–120%) as MU as an in‐clinic visit by providers and 78% (range, 50–115%) as medically useful by patients/caregivers. Thus, for our base‐case, both the patient and provider MU were set at 0.75 (i.e., 1 video televisit is three‐quarters as effective as an in‐person visit). For the base‐case to calculate adjusted costs of a televisit, the real costs are divided by 0.75.
Sensitivity Analyses
Patient‐perspective costs may vary dramatically depending upon variations in geography, employment status, and disease status. Institutional costs may vary based on personnel and space costs. And MU may differ depending upon the goals of a visit. We performed sensitivity analyses to explore the effect of these factors on our cost‐effectiveness analyses.
1) Travel Costs for Patients
For this sensitivity analysis, we varied the percent of people in each zone, from 100% in Zone 1 to 100% in Zone 5. This was expected to have no impact on the either the institutional perspective costs for in‐clinic visits or televisit costs for patients or institutions.
2) Effect of Ambulatory Status
For this sensitivity analysis, we varied the percent of people with ALS who are ambulatory from 0% to 100%. We assumed that nonambulatory patients are not independent for televisits. Because ambulatory status affects assumptions about patient employment and the percent of patients accompanied by caregivers to visits, this was expected to impact patient‐perspective costs for in‐clinic and televisits. It was not expected to impact the institutional perspective.
3) Effect of Patient Employment Status
For this sensitivity analysis, we varied the percent of patients who are employed from 5% to 80% of ambulatory patients and half as many nonambulatory patients.
4) Effect of Clinic Personnel and IT Personnel Costs
For this sensitivity analysis, we varied the costs of multidisciplinary personnel from $0 (nonmultidisciplinary visit) to 150% of base‐case (base‐case is $580 per in‐clinic visit and $163 per video televisit). Eliminating the multidisciplinary team from an in‐clinic visit was also assumed to reduce the MU, so the MU of in‐clinic and televisits was assumed to be equal when multidisciplinary team costs were eliminated.
Low‐volume televisit programs may be costlier to institutions because IT staff may be underused. With fewer telemedicine patients, the per‐patient cost of IT staff will increase. So, we also varied the cost of IT support for televisits up to 500% of base‐case.
5) Effect of Perceived MU
For sensitivity analyses, we anticipated that patient and physician ratings of MU would diverge, so our model can incorporate different ratings for patient and physician perspectives on MU, and although patient/caregiver and provider ratings of MU diverged very little in our survey, for our sensitivity analysis, we varied MU values for patients and providers based on the minimum and maximum ratings we found for each group. Televisit MU for patients/caregivers ranged from a minimum of 50% (half as useful as an in‐clinic visit) to a maximum of 120% (20% more useful than an in‐clinic visit). Televisit MU for providers ranged from 40% to 115%.
RESULTS
Base‐Case
The patient‐perspective base‐case costs are $1,116 per in‐clinic visit and $119 per televisit, after correcting for MU (Table 5, Row 1), an MU‐adjusted savings of 89%.
Table 5.
Base case and sensitivity analyses: patient perspective costs*
| Patient costs per in‐clinic visit | MU adjusted patient costs per televisit | Annual patient cost (4 in‐clinic visits) | Annual MU‐adjusted patient cost (4 televisits) | Annual MU‐adjusted patient cost (2 in‐clinic/2 televisits) | Annual MU‐adjusted patient cost (3 in‐clinic/2 televisits) | Annual cost saving (‐) or burden (+) of all televisits vs. all In‐clinic | Annual cost saving (‐) or burden (+) of 2 clinic/2 televisits vs. all in‐clinic | Annual cost saving (‐) or burden (+) of 3 clinic/2 televisits vs. all in‐clinic | |
|---|---|---|---|---|---|---|---|---|---|
| Base‐case | $1,116 | $119 | $4,464 | $476 | $2,470 | $3,586 | ‐89% | ‐45% | ‐20% |
| All patients live in zone 1 | $278 | $119 | $1,111 | $476 | $794 | $1,071 | ‐57% | ‐29% | ‐3.6% |
| All patients live in zone 2 | $794 | $3,176 | $1,826 | $2,620 | ‐85% | ‐43% | ‐18% | ||
| All patients live in zones 3 and 4 | $1,454 | $5,815 | $3,145 | $4,599 | ‐92% | ‐46% | ‐21% | ||
| All patients live in zone 5 | $1,601 | $6,403 | $3,440 | $5,040 | ‐93% | ‐46% | ‐21% | ||
| All patients ambulatory | $1,137 | $119 | $4,547 | $476 | $2,511 | $3,648 | ‐90% | ‐45% | ‐20% |
| No patients ambulatory | $1,092 | $119 | $4,367 | $476 | $2,421 | $3,513 | ‐89% | ‐45% | ‐20% |
| All patients employed | $1,409 | $196 | $5,636 | $784 | $3,210 | $4,619 | ‐86% | ‐43% | ‐18% |
| No patients employed | $1,050 | $116 | $4,198 | $462 | $2,330 | $3,380 | ‐89% | ‐44% | ‐19% |
| 80% of ambulatory patients and 40% of non‐ambulatory employed | $1,271 | $143 | $5,084 | $572 | $2,828 | $4,099 | ‐89% | ‐44% | ‐19% |
| No visit preference | $1,116 | $74 | $4,464 | $297 | $2,827 | $4,166 | ‐94% | ‐47% | ‐22% |
| 5x in‐clinic preference | $1,116 | $178 | $4,464 | $714 | $2,589 | $3,705 | ‐84% | ‐42% | ‐17% |
Cells with light shading show base‐case scenario costs.
From an institutional perspective, the base‐case cost of an in‐clinic visit is $799, and it is $472 for an MU‐adjusted televisit (Table 6, row 1), an MU‐adjusted savings of 41%.
Table 6.
Base case and sensitivity analyses: institutional perspective costs.*
| Institutional cost per in‐clinic visit | MU‐adjusted institutional cost per televisit | Annual institutional cost (4 in‐clinic visits) | Annual MU‐adjusted institutional cost (4 televisits) | Annual MU‐adjusted institutional cost (2 in‐clinic/2 televisits) | Annual MU‐adjusted institutional cost (3 in‐clinic/2 televisits) | Annual cost saving (‐) or burden (+) of all televisits vs. all in‐clinic | Annual cost saving (‐) or burden (+) of 2 clinic/2 televisits vs. All in‐clinic | Annual cost saving (‐) or burden (+) of 3 clinic/2 televisits vs. All in‐clinic | |
|---|---|---|---|---|---|---|---|---|---|
| Base‐case | $799 | $472 | $3,196 | $1,886 | $2,541 | $3,340 | ‐41% | ‐20% | 5% |
| No multidisciplinary costs and no visit preference | $219 | $191 | $876 | $763 | $820 | $1,039 | ‐13% | ‐6% | 19% |
| 150% Multidisciplinary costs | $1,089 | $580 | $4,356 | $2,321 | $3,339 | $4,428 | ‐47% | ‐23% | 2% |
| 150% Multidisciplinary costs +500% IT costs | $1,089 | $696 | $4,356 | $2,782 | $3,569 | $4,658 | ‐36% | ‐18% | 7% |
| No visit preference | $799 | $308 | $3,676 | $1,230 | $2,453 | $3,372 | ‐67% | ‐33% | ‐8% |
| 5x in‐clinic preference | $799 | $884 | $3,196 | $3,537 | $3,367 | $4,166 | 11% | 5% | 30% |
Cells with light shading show base‐case scenario costs. Cells with dark shading indicate scenarios under which televisits are NOT cost‐savings relative to in‐clinic visits.
Base‐Case Annual Costs
Assuming all visits in a year were televisits, after MU adjustment, patients would save 89% (Table 5, row 1) for a total annual savings of $3,988 compared with the annual cost of all in‐clinic visits. Institutions would save 41% (Table 6, row 1) for a total annual savings of $1,310 compared with the annual cost of all in‐clinic visits.
In reality, patients and providers may decide on a mix of in‐clinic and televisits throughout the year. A mix of 2 in‐clinic and 2 televisits leads to an annual MU‐adjusted cost‐savings to the patients of $1994 (45%) and to institutions of $655 (20%). Under some circumstances, patients and providers may increase the frequency of care using televisits. A mix of 2 televisits and 3 in‐clinic visits per year (increase of 1 visit per year) leads to an adjusted cost‐savings to patients of $878 (20%) and an adjusted cost burden to institutions of +$144 (+5%).
Using our current assumptions, if televisits and in‐clinic visits were reimbursed equally, the cost to payers would be affected only by the number of visits per year; thus only the final scenario (3 in‐clinic, 2 televisits) would increase payer expense.
Sensitivity Analyses
1) Geographic Patient Mix and Travel Costs
Assuming that all patients live in Zone 1 (Fig. 1) reduces the patient‐perspective cost of an in‐clinic visit by 75% to $278. Under this assumption, televisits provide only a 57% MU‐adjusted savings to patients compared with televisit. If all patients reside in Zone 5, the patient‐perspective cost of an in‐clinic visit increases to $1601, leading to a MU‐adjusted televisit savings of 93%. Annual MU‐adjusted cost savings are tabulated in Table 5.
Patient geography has no effect on institutional‐perspective costs.
2) Effect of Ambulatory Status
Changing base‐case assumptions about patient ambulatory status has little impact on MU‐adjusted costs (Table 5). The most extreme assumptions (all ambulatory or all nonambulatory) only alter in‐clinic costs by ‐$21 to +$24, respectively. This small change owes to the interplay between employment status, ambulatory status, and caregiver accompaniment.
There is no effect on institutional‐perspective costs.
3) Effect of Employment Status
Changing assumptions about the percent of patients employed substantially changes patient‐perspective visit costs, but because it affects both in‐clinic and televisits, there is little effect on the MU‐adjusted cost‐savings of televisits (Table 5). The most extreme assumptions (all patients employed, and no patients employed) meet with small changes in patient‐perspective, MU‐adjusted cost‐savings of televisits the difference ranges only from 86% to 89% relative to in‐clinic visits.
There is no effect on institutional‐perspective costs.
4) Effect of Clinic Personnel Costs
We explored cost impacts assuming that there was no multidisciplinary team for either televisits or in‐clinic visits (Table 6). Under this circumstance, the MU of televisits and in‐clinic visits would be closer to the same, so we also assumed equivalent MU. Under these assumptions, the institution‐perspective adjusted cost of both in‐clinic and televisits decrease. Although televisits remain cost saving compared with in‐clinic visits, the cost‐savings are only 13%; less robust than in the base‐case.
On the other hand, if the multidisciplinary team personnel costs are increased to 150% of the base‐case for each visit type, then the institution‐perspective adjusted cost savings of a televisit increases to 47%. Increasing IT salary to 500% above our base‐case then blunts the institution‐perspective MU‐adjusted cost‐savings of a televisit to 36% compared with an in‐clinic visit.
From the institution perspective, incorporating televisits remains MU‐adjusted cost‐saving annually under all circumstances except when comparing all in‐clinic visits to a combination of in‐clinic and televisits that effectively increases the number of annual visits from 4 to 5 (including 3 in‐clinic and 2 televisits).
There is no effect on patient‐perspective costs.
5) Effect of MU Ratings
When we increase the MU of a televisit to 120% for patients/caregivers and 115% for providers from our base‐case assumption of 75%, the patient‐perspective adjusted cost of a televisit decreases by 38% to $74 (Tables 5 and 6). The institutional adjusted cost of a televisit decreases by 35% to $308.
Assuming a strong bias for in‐clinic visits (televisit MU rating of 50% for patients/caregivers and 40% for providers), the MU‐adjusted patient perspective cost of televisits increases by 50% to $178, but remains an 84% MU‐adjusted cost‐savings compared with in‐clinic visits. The MU‐adjusted institutional cost of televisits increases by 87% to 884, making the adjusted cost higher than in‐clinic costs by 11%.
DISCUSSION
These results demonstrate substantial adjusted cost‐savings from televisits for both patients/caregivers and institutions under almost all assumptions about costs, patient population, geography, and a range of ratings of perceived MU for televisits.
Cost‐adjusted analyses can provide a framework for comparing interventions that differ both in cost and some measure of effectiveness. In this case, we used “perceived MU” of televisits (relative to in‐clinic visits) as the outcome measure of interest.
Both patients and providers rated MU lower for video televisits than for in‐clinic visits. Multidisciplinary clinic care for people with ALS is guided by the American Academy of Neurology (AAN) Guidelines.19, 20 Video televisits are designed to follow the AAN guidelines, yet the lower MU rating for video televisits compared with in‐clinic visits suggests that providers and people with ALS might believe that televisits cannot completely fulfill the AAN guidelines for ALS care as strictly as in‐clinic visits. Still, this reduction in perceived MU is modest and cost‐effectiveness is robust.
The analyses demonstrate televisit MU‐adjusted cost‐savings in our base‐case even though we made several assumptions that favor in‐clinic visits. First, we have not incorporated convenience directly into the cost adjustment. Incorporating convenience would certainly favor televisits. Second, we assume each televisit generates hefty follow‐up for the multidisciplinary team. We have likely over‐estimated the work time of the multidisciplinary team doing visit follow‐up. Third, we assume that caregivers are more frequently present for video televisits than in‐person clinic visits, thus increasing the costs of these visits. These were conservative assumptions that we made to add credibility to our analysis.
Patient Perspective Adjusted Costs
Our sensitivity analyses demonstrate the robust cost‐savings of televisits from the patient perspective across a wide variety of assumptions about employment, ambulatory status, distance from the ALS clinic, and perceived MU.
Our base‐case patient perspective MU rating was based on responses from just 3 people with ALS. Clearly, this is not a sampling meant to have any statistical power. Instead, it is meant to provide the basis for a reasonable assumption about MU. The sensitivity analysis tests the robustness of this assumption, and varying patient perspective MU ratings did not substantially impact the cost‐effectiveness of televisits relative to in‐clinic visits.
Likewise, varying ambulatory status had little effect on adjusted cost‐savings. ALS is progressively disabling and travel to visits becomes increasingly costly; it seemed possible that televisits would be more cost‐saving for people with advanced disease. Our sensitivity analysis did not confirm this hypothesis. Instead, a higher percentage of patients were employed earlier in the disease, so the increase in lost wages for in‐clinic visits at earlier stages balanced out the increased costs of travel later in the disease. Our perception is that patients prefer televisits later in the disease because of the decreased burden, and again, we have not included this in our current model to focus our analysis on true costs. Yet the importance of reducing burden later in the disease cannot be overlooked.
Of interest, varying employment status had an overall modest effect on the MU‐adjusted cost‐savings of televisits, because it impacted lost wages for both televisits and in‐clinic visits.
Geography impacted the adjusted cost‐savings of televisits substantially, yet televisits remained adjusted cost‐saving for patients even under extreme assumptions. MGH is a tertiary referral center; thus, our patient population is geographically dispersed. Yet, assuming that all patients live within 60 miles of the hospital, patients still saw a 36% cost‐savings using televisits.
In fact, across our analyses, no sensitivity analysis showed increased MU‐adjusted patient‐perspective costs for televisits relative to in‐clinic visits.
Annual cost‐savings from the patient perspective were 89% in the base‐case, and they remain cost‐saving whether televisits supplement or replace in‐clinic visits over the course of a year under all assumptions. Importantly, this includes annual costs for 3 in‐clinic and 2 televisits, a 25% increase in physician/patient contact over the year compared with traditional schemas of 4 in‐clinic visits annually.
Institutional Perspective Adjusted Costs
Institutional‐perspective MU‐adjusted costs also favor televisits with an adjusted cost‐saving of 41% per televisit in our base‐case. This leads to an annual adjusted cost‐savings if televisits are used to replace in‐clinic visits, and a very modest 5% adjusted cost‐burden if they supplement in‐clinic visits (3 in‐clinic and 2 televisits compared with 4 in‐clinic visits annually).
In fact, from the institutional perspective, televisits demonstrate MU‐adjusted cost‐savings compared with in‐clinic visits under all sensitivity analyses except when the provider rating of MU falls below 50%. And in fact, from the institutional perspective, the adjusted cost‐savings or cost‐burden of televisits is exquisitely sensitive to the provider MU rating. When providers perceive a high MU, then there is substantial cost‐savings. When the providers view a low MU, televisits become a cost‐burden. In other words, it is critical that providers have a strong say in the development of video televisit programs and are thoughtful about the circumstances under which video televisits are implemented.
FUTURE DIRECTIONS
Based on these analyses, we are of the opinion that institutions, physicians, and patients should be aligned in advocating for payers to reimburse televisits for people with ALS. Our present analysis did not formally include the payor‐perspective, in part because cost and medical‐usefulness assumptions are most complex for the payor. Having said that, if televisits and in‐clinic visits were reimbursed the same, then providers and patients would be free to match the use of video televisits to scenarios under which video visits are most useful. Our brief quality improvement survey suggests that both providers and patients can make informed assessments of the MU of in‐clinic and video televisits after having experienced both visit types. Further study with a formal survey of a broad group of providers, patients, and caregivers would help bolster our understanding of patient, caregiver, and provider views of video televisits.
If the visit types were reimbursed equally, any potential for increased cost to payors (insurance) would come primarily from increased usage (more frequent visits). Markedly increased usage is likely a small risk, given that patient, caregiver, and physician time is limited. Furthermore, more frequent routine visits could lead to fewer expensive medical interactions, such as hospitalizations.
A full healthcare usage study would provide much‐needed insights into healthcare usage and the impact of video televisits and other forms of telemedicine in ALS. In fact, our “institutional perspective” analysis focuses exclusively on the costs of administering clinic. Arguably more important for payors is the effect of video televisits on healthcare usage, in particular, expensive resources such as hospitalization rates. To support such an analysis, we need to better understand hospitalization patterns in people with ALS and the gather data about the potential impact of televisits on hospitalization rates.
The MU of televisits may vary based on ALS disease state, and we did not model this complexity. In addition, as we previously noted, we did not include a “burden tax” for patients traveling to in‐clinic appointments. Future research could better define patient perception of MU of televisits in the early, middle, and advanced stages of the disease, with a focus palliative care for people with ALS rendered by means of video televisit.
Acknowledgment
We thank Dr. Merit Cudkowicz, Chair of Neurology at the Massachusetts General Hospital, for her support of the TeleNeurology Program. We also thank the ALS Association and Muscular Dystrophy Association for supporting innovation in ALS clinical care and research. Individual Author Contributions: Sabrina Paganoni: study concept/design, analysis/interpretation of data, drafting/revising the manuscript. Marc Van De Rijn: analysis/interpretation of data, drafting/revising the manuscript. Kristin Drake: analysis/interpretation of data, drafting/revising the manuscript. Katherine Burke: analysis/interpretation of data, drafting/revising the manuscript. Michael Doyle: analysis/interpretation of data, drafting/revising the manuscript. Amy Swartz Ellrodt: study concept/design, drafting/revising the manuscript. Katherine Nicholson: study concept/design, drafting/revising the manuscript. Nazem Atassi: study concept/design, drafting/revising the manuscript. Fabiola De Marchi: study concept/design, drafting/revising the manuscript. Suma Babu: study concept/design, drafting/revising the manuscript. Juan Estrada: study concept/design, drafting/revising the manuscript. Lee H. Schwamm: study concept/design, analysis/interpretation of data, drafting/revising the manuscript. James D. Berry: study concept/design, analysis/interpretation of data, drafting/revising the manuscript.
Ethical Publication Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Funding: This study was funded in part by the ALS Association.
Conflicts of Interest: Sabrina Paganoni has received funding from the NIH, Target ALS, the ALS Association, ALS Finding a Cure, Amylyx, the Salah Foundation, and the Spastic Paraplegia Foundation. Marc Van De Rijn has no relevant disclosures. Kristin Drake has no relevant disclosures. Katherine Burke has no relevant disclosures. Michael Doyle has no relevant disclosures. Amy Swartz Ellrodt has no relevant disclosures. Katherine Nicholson has received research funding from Biogen, Brainstorm Cell Therapeutics, ALS Finding a Cure and AAN/ALSA. Nazem Atassi has consulted for MT Pharma and Biogen, and has received research support from Biogen, Denali Therapeutics, Genentech, ALS Association, ALS One, Muscular Dystrophy Association, and NIH. Fabiola De Marchi has no relevant disclosures. Suma Babu has received research funding from Biogen, Brainstorm Cell Therapeutics, American Association of Neuromuscular and Electrodiagnostic Medicine and Muscular Dystrophy Association. Juan Estrada has no relevant disclosures. Lee H. Schwamm is Director of the MGH Center for TeleHealth and has received research support from the NIH, PCORI, and HRSA. James D. Berry has consulted for MT Pharma and Denali Therapeutics and has received research support from Voyager Therapeutics, GSK, Cytokinetics, Brainstorm Cell Therapeutics, ALS One, ALS Association, Muscular Dystrophy Association, and NIH.
REFERENCES
- 1. Bruletti G, Comini L, Scalvini S, Morini R, Luisa A, Paneroni M, et al. A two‐year longitudinal study on strain and needs in caregivers of advanced ALS patients. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:187–195. [DOI] [PubMed] [Google Scholar]
- 2. Paganoni S, Karam C, Joyce N, Bedlack R, Carter GT. Comprehensive rehabilitative care across the spectrum of amyotrophic lateral sclerosis. NeuroRehabilitation 2015;37:53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Traynor BJ, Alexander M, Corr B, Frost E, Hardiman O. Effect of a multidisciplinary amyotrophic lateral sclerosis (ALS) clinic on ALS survival: a population based study, 1996‐2000. J Neurol Neurosurg Psychiatry 2003;74:1258–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rooney J, Byrne S, Heverin M, Tobin K, Dick A, Donaghy C, et al. A multidisciplinary clinic approach improves survival in ALS: a comparative study of ALS in Ireland and Northern Ireland. J Neurol Neurosurg Psychiatry 2015;86:496–501. [DOI] [PubMed] [Google Scholar]
- 5. Chio A, Bottacchi E, Buffa C, Mutani R, Mora G, PARALS. Positive effects of tertiary centres for amyotrophic lateral sclerosis on outcome and use of hospital facilities. J Neurol Neurosurg Psychiatry 2006;77:948–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van den Berg JP, Kalmijn S, Lindeman E, Veldink JH, de Visser M, Van der Graaff MM, et al. Multidisciplinary ALS care improves quality of life in patients with ALS. Neurology 2005;65:1264–1267. [DOI] [PubMed] [Google Scholar]
- 7. Stephens HE, Young J, Felgoise SH, Simmons Z. A qualitative study of multidisciplinary ALS clinic use in the United States. Amyotroph Lateral Scler Frontotemporal Degener 2015;17:55–61. [DOI] [PubMed] [Google Scholar]
- 8. Daschle T, Dorsey ER. The return of the house call. Ann Intern Med 2015;162:587–588. [DOI] [PubMed] [Google Scholar]
- 9. Hobson EV, Baird WO, Cooper CL, Mawson S, Shaw PJ, McDermott CJ. Using technology to improve access to specialist care in amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler Frontotemporal Degener 2016;17:313–324. [DOI] [PubMed] [Google Scholar]
- 10. McClellan F, Washington M, Ruff R, Selkirk SM. Early and innovative symptomatic care to improve quality of life of ALS patients at Cleveland VA ALS Center. J Rehabil Res Dev 2013;50:vii–xvi. [DOI] [PubMed] [Google Scholar]
- 11. Nijeweme‐d'Hollosy WO, Janssen EP, Huis in 't Veld RM, Spoelstra J, Vollenbroek‐Hutten MM, Hermens HJ. Tele‐treatment of patients with amyotrophic lateral sclerosis (ALS). J Telemed Telecare 2006;12(Suppl 1):31–34. [DOI] [PubMed] [Google Scholar]
- 12. Selkirk SM, Washington MO, McClellan F, Flynn B, Seton JM, Strozewski R. Delivering tertiary centre specialty care to ALS patients via telemedicine: a retrospective cohort analysis. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:324–332. [DOI] [PubMed] [Google Scholar]
- 13. Pulley MT, Brittain R, Hodges W, Frazier C, Miller L, Matyjasik‐Liggett M, et al. Multidisciplinary ALS telemedicine care: the store and forward method. Muscle Nerve 2019;59:34–39. [DOI] [PubMed] [Google Scholar]
- 14. Paganoni S, Simmons Z. Telemedicine to innovate ALS multidisciplinary care: the time has come. Muscle Nerve 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Geronimo A, Wright C, Morris A, Walsh S, Snyder B, Simmons Z. Incorporation of TeleHealth into a multidisciplinary ALS clinic: feasibility and acceptability. Amyotroph Lateral Scler Frontotemporal Degener 2017;18:555–561. [DOI] [PubMed] [Google Scholar]
- 16. Van De Rijn M, Paganoni S, Levine‐Weinberg M, Campbell K, Swartz Ellrodt A, Estrada J, et al. Experience with telemedicine in a multi‐disciplinary ALS clinic. Amyotroph Lateral Scler Frontotemporal Degener 2018;19:143–148. [DOI] [PubMed] [Google Scholar]
- 17. Dullet NW, Geraghty EM, Kaufman T, Kissee JL, King J, Dharmar M, et al. Impact of a university‐based outpatient telemedicine program on time savings, travel costs, and environmental pollutants. Value Health 2017;20:542–546. [DOI] [PubMed] [Google Scholar]
- 18. Paganoni S, Nicholson K, Leigh F, Swoboda K, Chad D, Drake K, et al. Developing multidisciplinary clinics for neuromuscular care and research. Muscle Nerve 2017;56:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73:1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73:1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]


