Abstract
In this post hoc analysis of the randomized controlled LixiLan‐O trial in insulin‐naive patients with type 2 diabetes mellitus (T2DM) not controlled with metformin, with or without a second oral antihyperglycaemic drug (OAD), the efficacy and safety of the fixed‐ratio combination, iGlarLixi (insulin glargine 100 U [iGlar] and lixisenatide [Lixi]), compared to its individual components was assessed in two patient subgroups: group 1) baseline HbA1c ≥9% (n = 134); group 2) inadequate control (HbA1c ≥7.0% and ≤9.0%) despite administration of two OADs at screening (n = 725). Treatment with iGlarLixi resulted in significantly greater reduction in least squares mean HbA1c compared to treatment with iGlar or Lixi alone in both subgroups (group 1: 2.9%, 2.5%, 1.7% and group 2: 1.5%, 1.2%, 0.7%, respectively). Target HbA1c less than 7% was achieved in more than 70% of patients using iGlarLixi in both subgroups, while mitigating the weight gain observed with use of iGlar alone. Rates of hypoglycaemic events were low overall. These results suggest that treatment with iGlarLixi achieves superior glycaemic control compared to treatment with iGlar or Lixi alone in T2DM patients with HbA1c ≥9% or in those inadequately controlled with two OADs.
Keywords: glycaemic control, iGlarLixi, insulin glargine 100 U, lixisenatide, type 2 diabetes mellitus
1. INTRODUCTION
The 2018 American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) consensus report on the management of hyperglycaemia in type 2 diabetes mellitus (T2DM) recommends that glycaemic targets should be individualized based on patient preferences and goals, and on the risk of adverse treatment effects, and that combination therapy may be considered in patients presenting with glycated haemoglobin (HbA1c) levels more than 1.5% above their target.1 In addition, for patients with HbA1c more than 2% above target or more than 10%, recommendations include combination therapy with both basal insulin and a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA), or a fixed‐ratio combination thereof, or a basal‐prandial combination.1 This consensus report is aligned with the diabetes management guidelines from the UK National Institute for Health and Care Excellence, which also recommend consideration of fixed‐ratio mixed insulin combinations (premixed insulins).2 For patients uncontrolled with two oral antihyperglycaemic drugs (OADs), both reports recommend treatment intensification with a third OAD, insulin initiation or a GLP‐1 RA.1, 2, 3
Despite current recommendations, treatment of diabetes worldwide remains suboptimal, with many patients failing to achieve targets, notwithstanding the approval of over 40 new treatment options worldwide since 2005.4, 5, 6, 7, 8 While the reasons for suboptimal glycaemic control are multiple, major contributing factors include non‐adherence to treatment, therapeutic inertia and resource limitations.1, 6, 8, 9 Adverse events, including hypoglycaemia and weight gain, may also affect patient adherence and healthcare professionals' confidence in therapy.1, 6, 8, 9 Moreover, employment of a stepwise approach to treatment intensification may prolong the time required to reach effective treatment(s)10 and possibly contributes to treatment non‐adherence. Treatment approaches that simplify therapy and accelerate the achievement of target HbA1c, such as early treatment with a fixed‐ratio combination of basal insulin and a GLP‐1 RA,1, 10, 11 could help address therapeutic inertia, improve outcomes and prevent complications.
The once‐daily, titratable, fixed‐ratio combination of basal insulin glargine 100 U (iGlar) and the GLP‐1 RA lixisenatide (Lixi), iGlarLixi, allows for a single daily injection that targets both fasting and postprandial glucose. The LixiLan‐O trial (NCT02058147) enrolled 1170 patients with T2DM who were inadequately controlled with metformin, with or without a second OAD, and found greater HbA1c reductions at Week 30 with iGlarLixi vs iGlar or Lixi alone, with no increased risk of hypoglycaemia vs iGlar.12 iGlarLixi also mitigated the weight gain observed with use of iGlar alone.
In this post hoc subgroup analysis of patients from the LixiLan‐O trial, we assessed whether intensification to iGlarLixi was efficacious in achieving glycaemic targets in patients with HbA1c at least 9% and those with inadequate glycaemic control (HbA1c ≥7.0% and ≤9.0%) with two OADs. These subgroups of patients were selected because they are predicted to be more difficult to treat, with a lower likelihood of reaching target HbA1c. These are also patients who, according to current guidelines, may require an injectable combination therapy to rapidly achieve glycaemic control or may represent a patient population for whom treatment with two OADs is insufficient.
2. METHODS
2.1. LixiLan‐O study
The LixiLan‐O study design and main results have been published previously.12 Briefly, the LixiLan‐O study was a 30‐week, open‐label, randomized, multicentre, Phase 3 clinical trial, which enrolled insulin‐naive patients with T2DM, aged at least 18 years, with inadequate glycaemic control despite treatment for at least 3 months with metformin, with or without a second OAD. Inadequate glycaemic control was defined as HbA1c ≥7.5% and ≤10.0% for patients treated with metformin alone and as HbA1c ≥7.0% and ≤9.0% for those treated with metformin and a second OAD. Eligible patients entered a 4‐week run‐in phase during which all OADs except metformin were discontinued. In the current post hoc study the efficacy and safety of iGlarLixi, as compared to its individual components, was assessed in two patient subgroups: group 1) those with baseline, after run‐in, HbA1c ≥9%; group 2) those with inadequate control (HbA1c ≥7.0% and ≤9.0%) despite administration of two OADs at screening. The study (NCT02058147) was designed and monitored in accordance with Good Clinical Practice, the International Conference on Harmonisation and the Declaration of Helsinki. Institutional review boards or ethics committees at each study site approved the protocol. Each patient gave written informed consent. The manuscript was prepared to conform with the Consolidated Standards of Reporting Trials guidelines.
2.2. Interventions
At the end of a run‐in period, patients were randomized (2:2:1) to receive iGlarLixi, iGlar or Lixi. iGlarLixi was self‐administered once daily, during the hour (0‐60 minutes) before breakfast, using a SoloSTAR pen (Sanofi, Paris, France); doses ranged from 10 U/5 μg to 60 U/20 μg of iGlar/Lixi, respectively. iGlar was self‐administered once daily, at any time of the day but at approximately the same time every day, using a disposable prefilled Lantus SoloSTAR pen (Sanofi) (100 U/mL), with doses starting at 10 U and capped at 60 U. Lixi was self‐administered once daily, during the hour (0‐60 minutes) before breakfast, using disposable prefilled pens (Sanofi); the dose was 10 μg for 2 weeks and was up‐titrated to a 20 μg maintenance dose. The same dose adjustment algorithm was recommended for iGlar and iGlarLixi. After the first week, the dose was titrated once weekly, based on insulin glargine dose, until the patient reached a target fasting self‐monitored plasma glucose level of 80 to 100 mg/dL without hypoglycaemia episodes.
2.3. Post hoc analysis
Efficacy outcomes in the two subgroups included effect of treatment on HbA1c and body weight, and final iGlar and Lixi doses. Safety outcomes in these subgroup analyses included gastrointestinal treatment‐emergent adverse events (TEAEs). In addition, the proportion of patients with clinically important hypoglycaemia, accompanied by plasma glucose <54 mg/dL, was assessed. Severe symptomatic hypoglycaemia was defined as an episode requiring another person's active assistance to administer carbohydrate or glucagon, or to undertake other resuscitative actions.
2.4. Statistical analyses
Differences between treatments were determined using an analysis of covariance model with treatment groups, randomization strata of HbA1c at screening (<8%, ≥8%) and country as fixed effects, and using baseline value as a covariate, unless otherwise stated. Differences in proportion were analysed using the Cochran‐Mantel‐Haenszel method. Safety analysis was performed descriptively.
The analysis populations were the modified intent‐to‐treat (mITT) population, comprising all randomized patients for whom baseline and at least one post‐baseline assessments were available, and the safety population, comprising all randomized patients who received at least one dose of study drug.
3. RESULTS
3.1. Patient disposition and demographics
At the end of the run‐in period, 6% (71/1167) of the patients randomized to LixiLan‐O were using two OADs at screening and had HbA1c of at least 9% at baseline, after run‐in. These patients were therefore included in both subgroup analyses. The two subgroup analyses included 134 patients with baseline HbA1c of at least 9% (subgroup 1) and 725 patients receiving two OADs at screening (subgroup 2); mITT populations comprised 133 and 722 patients, respectively. Demographics and baseline characteristics were well balanced across treatment groups within each subgroup, and were generally similar to those of the overall study cohort (Table S1).
3.2. Efficacy outcomes
In line with the overall cohort, statistically significantly greater improvements in HbA1c at Week 30 were achieved with treatment with iGlarLixi as compared to treatment with iGlar or Lixi alone in both subgroups (Figure 1).
Figure 1.
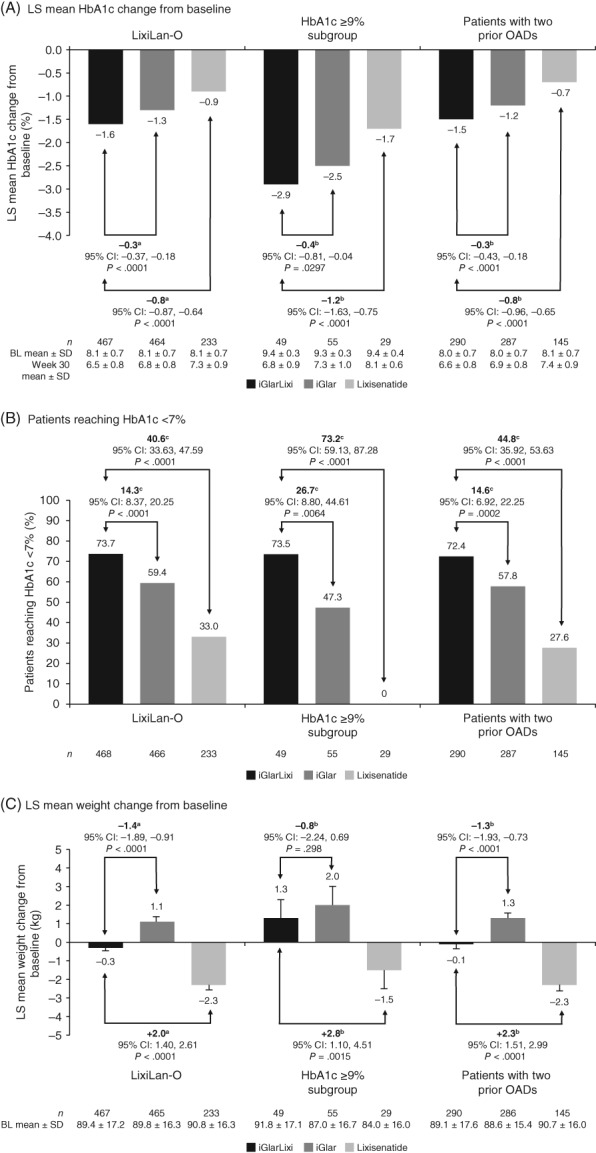
HbA1c and body weight outcomes for patients with T2DM from the overall LixiLan‐O study population,12 for patients with baseline HbA1c ≥9% and for patients with two OADs according to randomization strata at screening (mITT population). Error bars indicate SE. ANCOVA, analysis of covariance; BL, baseline; CI, confidence interval; HbA1c, glycated haemoglobin; iGlar, insulin glargine 100 U; iGlarLixi, insulin glargine and lixisenatide; Lixi, lixisenatide; LOCF, last observation carried forward; LS, least squares; mITT, modified intent‐to‐treat; MMRM, mixed‐effect model with repeated measures; OAD, oral antihyperglycaemic drug; SD, standard deviation; SE, standard error; T2DM, type 2 diabetes mellitus. aOverall LixiLan‐O data based on MMRM analysis. bLS mean difference for iGlarLixi vs iGlar or lixisenatide alone, ANCOVA; LOCF was used to handle missing data. cDifferences in proportion of patients achieving HbA1c <7% were analysed based on weighted average differences between treatment groups from each strata using a Cochran‐Mantel‐Haenszel method
3.2.1. Patients with HbA1c of at least 9% at baseline
Treatment with iGlarLixi, iGlar and Lixi reduced least squares (LS) mean HbA1c by 2.9%, 2.5% and 1.7%, respectively (P = .0297 for iGlarLixi vs iGlar; P < .0001 for iGlarLixi vs Lixi); final mean HbA1c at Week 30 was 6.8%, 7.3% and 8.1%, respectively (Figure 1A). Furthermore, 73.5% of patients achieved HbA1c levels below 7% by Week 30 with iGlarLixi vs 47.3% of patients with iGlar and no patients with Lixi (Figure 1B). Patients using iGlarLixi tended to gain less weight as compared to those using iGlar (LS mean weight gain, 1.3 kg vs 2.0 kg; P = .3) (Figure 1C).
3.2.2. Patients with two OADs at screening
Treatment with iGlarLixi, iGlar and Lixi reduced LS mean HbA1c by 1.5%, 1.2% and 0.7%, respectively (P < .0001 for both iGlarLixi vs iGlar and iGlarLixi vs Lixi), from a mean baseline value of 8.0%, 8.0% and 8.1%, respectively; final mean HbA1c at Week 30 was 6.6%, 6.9% and 7.4%, respectively (Figure 1A). Moreover, 72.4% of patients achieved HbA1c levels below 7% by Week 30 with iGlarLixi vs 57.8% of patients with iGlar and 27.6% of patients with Lixi (Figure 1B). Treatment with iGlarLixi resulted in significantly less weight gain than treatment with iGlar (LS mean weight change, −0.1 vs +1.3 kg; P < .0001) (Figure 1C).
3.3. Final iGlar and Lixi doses
In the overall study population and in both subgroups, final doses of iGlar were similar in the iGlarLixi and iGlar treatment groups (40‐45 U) (Table S2). For iGlarLixi, final mean doses of the Lixi component were similar (16‐17 μg) in the overall study cohort and in both subgroups (Table S2).
3.4. Safety outcomes
Consistent with the entire LixiLan‐O study population, the rates of gastrointestinal TEAEs in the iGlarLixi arm were lower as compared to the Lixi arm, and higher as compared to the iGlar arm in both subgroups. The rates of gastrointestinal TEAEs leading to discontinuation were low in both subgroups (Table 1).
Table 1.
Clinically important hypoglycaemia outcomes and gastrointestinal disorders (safety population)
|
All patients
(N = 1169) |
Patients with baseline HbA1c ≥9%
(n = 134) |
Patients with two OADs according to randomization strata at screening
(n = 724) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
iGlarLixi
(n = 469) |
iGlar
(n = 467) |
Lixi
(n = 233) |
iGlarLixi
(n = 50) |
iGlar
(n = 55) |
Lixi
(n = 29) |
iGlarLixi
(n = 291) |
iGlar
(n = 288) |
Lixi
(n = 145) |
|
| Clinically important hypoglycaemia a | |||||||||
| Patients with events, n (%) | 38 (8.1) | 32 (6.9) | 4 (1.7) | 6 (12.0) | 1 (1.8) | 1 (3.4) | 24 (8.2) | 24 (8.3) | 3 (2.1) |
| Events per patient per year, n | 0.24 | 0.14 | 0.06 | 0.43 | 0.07 | 0.13 | 0.23 | 0.17 | 0.07 |
| Gastrointestinal disorders | |||||||||
| Gastrointestinal disorders, overall | 102 (21.7) | 59 (12.6) | 86 (36.9) | 14 (28.0) | 8 (14.5) | 14 (48.3) | 64 (22.0) | 36 (12.5) | 54 (37.2) |
| Nausea | 45 (9.6) | 17 (3.6) | 56 (24.0) | 6 (12.0) | 4 (7.3) | 10 (34.5) | 31 (10.7) | 12 (4.2) | 37 (25.5) |
| Discontinuation because of nausea | 2 (0.4) | 0 | 6 (2.6) | 1 (2.0) | 0 | 0 | 1 (0.3) | 0 | 5 (3.4) |
| Vomiting | 15 (3.2) | 7 (1.5) | 15 (6.4) | 0 | 0 | 1 (3.4) | 13 (4.5) | 5 (1.7) | 7 (4.8) |
| Discontinuation because of vomiting | 2 (0.4) | 0 | 4 (1.7) | 0 | 0 | 0 | 2 (0.7) | 0 | 3 (2.1) |
| Diarrhoea | 42 (9.0) | 20 (4.3) | 21 (9.0) | 8 (16.0) | 1 (1.8) | 3 (10.3) | 26 (8.9) | 13 (4.5) | 12 (8.3) |
| Discontinuation because of diarrhoea | 1 (0.2) | 0 | 2 (0.9) | 0 | 0 | 1 (3.4) | 0 | 0 | 1 (0.7) |
Note: Patient‐years of exposure was calculated as time from the first to the last injection of study drug plus 1 day. Number of events per patient‐year was calculated as number of events divided by total patient‐years of exposure.
Abbreviations: HbA1c, glycated haemoglobin; iGlar, insulin glargine 100 U; iGlarLixi, insulin glargine and lixisenatide; Lixi, lixisenatide; OAD, oral antihyperglycaemic drug.
Clinically important hypoglycaemia: symptoms typical of hypoglycaemia accompanied by plasma glucose <54 mg/dL.
Rates of clinically important hypoglycaemia were similar in the iGlarLixi and iGlar arms in the overall population and in the subgroup using two OADs (Table 1), but were numerically higher in the iGlarLixi arm vs the iGlar arm in the subgroup with HbA1c of at least 9%. As the number of patients who experienced hypoglycaemia events was low, no meaningful statistical testing could be performed. One patient in the iGlar arm of the subgroup using two OADs experienced severe symptomatic hypoglycaemia.
4. DISCUSSION
In these post hoc analyses of insulin‐naive patients with T2DM who were using metformin, with HbA1c of at least 9% or who were inadequately controlled with two OADs at screening, treatment with iGlarLixi resulted in a greater reduction in HbA1c as compared to treatment with iGlar or Lixi alone. In both subgroups, over 70% of patients treated with iGlarLixi achieved HbA1c less than 7%. Consequently, the fixed‐ratio combination of iGlar and Lixi, delivered as a single daily injection in the iGlarLixi group, with its complementary mechanism of action targeting both fasting and postprandial hyperglycaemia, is a viable treatment option for patients with T2DM who have HbA1c levels of at least 9% or who have failed to achieve glycaemic control with two OADs.
There are some limitations to this post hoc analysis. The original trial was not designed or powered to detect differences between treatments within these two subgroups. Additionally, the LixiLan‐O study did not apply forced titration, but allowed the investigator to make clinical judgements concerning dosing while avoiding hypoglycaemic episodes. Despite achievement of a similar fasting plasma glucose at the end of the study in both groups,12 with a similar unit of insulin glargine (Table S2) and a similar titration algorithm, a greater proportion of patients treated with iGlarLixi achieved the HbA1c target as compared with those treated with iGlar. In both arms, there was a proportion of patients who did not reach HbA1c less than 7%, and they may have benefitted from further up‐titration. Finally, sample sizes, particularly for the subgroup with HbA1c of at least 9%, were rather small. Patient populations with HbA1c of at least 9% are often not well represented in randomized clinical trials, and data focusing on this group are limited. The findings presented here would benefit from validation in a prospective, randomized trial in a larger patient cohort or in a real‐world setting.
The results of these subgroup analyses, within the context of the limitations of a post hoc analysis, are in line with the recent ADA/EASD consensus statement1 and the NICE guidelines2 that recommend initiation of a combination of basal insulin and a GLP‐1 RA in patients with HbA1c more than 2% above target or greater than 10% overall. The achievement of HbA1c less than 7% by more than 70% of patients via a single therapeutic intervention may facilitate treatment intensification in this difficult‐to‐treat patient group.
CONFLICT OF INTEREST
M. J. D. serves on Advisory Panels for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi‐Aventis and Servier; serves as a consultant to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novo Nordisk and Sanofi‐Aventis; has received research support (grants in support of investigator trials) from Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk and Sanofi‐Aventis; and is a member of speakers bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novo Nordisk, Sanofi‐Aventis and Takeda.
D. R‐J. serves on Advisory Panels for, is a Board member of and consultant to, and has received research support from AstraZeneca, Eli Lilly, Novo Nordisk and Sanofi; and is a member of speakers bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Sanofi and Takeda.
T. M. B. serves on Advisory Panels for AstraZeneca, Boehringer Ingelheim, Napp Pharmaceuticals, Novo Nordisk and Sanofi; and has received research support from AstraZeneca, Bayer and Shire.
F. J. L‐G. serves on Advisory Panels for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk and Sanofi; is a Board member of AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk and Sanofi; and is a member of speakers bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novo Nordisk and Sanofi.
G. R. G. serves on Advisory Panels for AbbVie, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Pfizer and Sanofi; and is a member of speakers bureaus for Amgen, AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Eli Lilly, LifeScan, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi, Servier and Takeda.
D. Z. has nothing to disclose.
M. B. is an employee of and stock/shareholder in Sanofi; is Honorary Associate Professor at the University of Swansea; and is Non‐Executive Director of Ashford and St Peter's Hospital NHS Foundation Trust.
C. D‐B. is an employee of and stock/shareholder in Sanofi.
R. J. M. serves on Advisory Panels for Eli Lilly, Novo Nordisk, Sanofi; and is a member of speakers bureaus for Eli Lilly and Sanofi.
AUTHOR CONTRIBUTIONS
C. D‐B. and M. B. were involved in the conception and design of the study. T. M. B., M. J. D., R. J. M. and D. R‐J. were involved in the conduct of the study and in data acquisition. All authors were involved in data analysis and interpretation. All authors were involved in critically revising the manuscript, have provided final approval and take full accountability for the work.
DATA SHARING
Qualified researchers may request access to patient‐level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Patient‐level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details concerning Sanofi's data sharing criteria, eligible studies and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.
Supporting information
Table S1. Patient demographics and baseline characteristics (randomized population).
Table S2. iGlar and Lixi doses at Week 30 (mITT population).
ACKNOWLEDGMENTS
The authors thank the study participants, trial staff and investigators for their participation. The authors also thank Minzhi Liu and Yao Huang at BDM Consulting Inc. for performing statistical analyses. M. J. D. wishes to thank the National Institute of Health Research Leicester Biomedical Research Centre for supporting the research.
Coordination of the development of this manuscript and assistance with revision was provided by Helena Andersson, PhD, at Sanofi. Professional medical writing and editorial assistance, which was funded by Sanofi, was provided by Christina Holleywood, PhD and Catriona McKay, PhD at Caudex.
The subgroup analyses data have been presented previously at the 77th Scientific Sessions of the American Diabetes Association, San Diego, California, June 9‐13, 2017 (Davies M, et al. Diabetes. 2017;66(suppl. 1A):LB36 [Abstract 137‐LB]; Russell‐Jones D, et al. Diabetes. 2017;66(suppl. 1A):LB35 [Abstract 134‐LB]).
Davies MJ, Russell‐Jones D, Barber TM, et al. Glycaemic benefit of iGlarLixi in insulin‐naive type 2 diabetes patients with high HbA1c or those with inadequate glycaemic control on two oral antihyperglycaemic drugs in the LixiLan‐O randomized trial. Diabetes Obes Metab. 2019;21:1967–1972. 10.1111/dom.13791
Funding information The LixiLan‐O trial (NCT02058147) and this post hoc analysis were sponsored by Sanofi.
REFERENCES
- 1. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NICE . Type 2 diabetes in adults: management. NICE guideline (NG28; 2 December 2015). https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-1837338615493. Accessed March 26, 2019.
- 3. International Diabetes Federation Guideline Development Group . Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104:1‐52. [DOI] [PubMed] [Google Scholar]
- 4. Chan JC, Gagliardino JJ, Baik SH, et al.; IDMPS Investigators. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care. 2009;32:227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baptista DR, Thieme RD, Reis WC, Pontarolo R, Correr CJ. Proportion of Brazilian diabetes patients that achieve treatment goals: implications for better quality of care. Diabetol Metab Syndr. 2015;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jabbar A, Abdallah K, Hassoun A, et al. Patterns and trends in insulin initiation and intensification among patients with type 2 diabetes mellitus in the Middle East and North Africa region. Diabetes Res Clin Pract. 2019;149:18‐26. [DOI] [PubMed] [Google Scholar]
- 9. Moreira RO, Cobas R, Lopes Assis Coelho RC. Combination of basal insulin and GLP‐1 receptor agonist: is this the end of basal insulin alone in the treatment of type 2 diabetes? Diabetol Metab Syndr. 2018;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frias J, Puig Domingo M, Meneghini L, et al. More patients reach glycaemic control with a fixed‐ratio combination of insulin glargine and lixisenatide (iGlarLixi) than with basal insulin at 12 weeks of treatment: a post hoc time‐to‐control analysis of LixiLan‐O and LixiLan‐L. Diabetes Obes Metab. 2018;20:2314‐2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenstock J, Handelsman Y, Vidal J, et al. Propensity‐score‐matched comparative analyses of simultaneously administered fixed‐ratio insulin glargine 100 U and lixisenatide (iGlarLixi) vs sequential administration of insulin glargine and lixisenatide in uncontrolled type 2 diabetes. Diabetes Obes Metab. 2018;20:2821‐2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan‐O randomized trial. Diabetes Care. 2016;39:2026‐2035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient demographics and baseline characteristics (randomized population).
Table S2. iGlar and Lixi doses at Week 30 (mITT population).


