Abstract
The use of neurotrophic factors as therapeutic agents for neurodegenerative diseases is considered as an approach aimed at restoring and maintaining neuronal function in the peripheral and central nervous system. Since the neuroprotective effect is depending on chronic delivery of the neurotrophic factors a sustained application, e.g., via cell‐based delivery is necessary. Human mesenchymal stem cells (hMSCs) were lentivirally modified to overexpress brain‐derived neurotrophic factor (BDNF) and to express fluorescent marker genes for easy visualization. Since genetically modified cells should be site‐specifically retained (e.g., by encapsulation) in the patients to avoid adverse effects the cells were additionally differentiated to chondrocytes to hypothetically improve their vitality and survival in a delivery matrix. Different polycations for lentiviral transduction were investigated for their efficiency. The success of differentiation was determined by analysis of chondrocyte marker genes and the neuroprotective effect of BDNF‐overexpressing cells was exemplarily investigated on neurons of the peripheral auditory system. The genetically modified hMSCs overexpressed BDNF from under 1 to 125 ng ml−1 day−1 depending on the donor and transfection method. Using protamine sulfate the transfection efficacy was superior compared to the use of polybrene. The BDNF secreted by the MSCs was significantly neuroprotective in comparison to the relevant controls even though the produced mean concentrations were lower than the effective concentrations for recombinant industrially produced proteins described in literature. The presented system of BDNF‐overexpressing hMSCs is neuroprotective and is therefore considered as a promising method for sustained delivery of proteins in therapeutically relevant amounts to degenerating neuronal structures.
Keywords: cell‐based therapy, chronic therapy, drug delivery, endogenous pharmacotherapy, neuroprotection, spiral ganglion neuron
Significance.
It is known that hMSCs have a high potential for neuroprotective therapy but up to now only few approaches found their way into clinical trials. We hypothesize that hMSC‐based drug delivery may benefit from developing appropriate encapsulation techniques and genetical modification of the hMSCs to overexpress specific neuroprotective factors. We developed hMSCs overexpressing BDNF and differentiated them to chondrocytes to hypothetically improve their vitality and survival in a delivery matrix. The BDNF‐overexpressing hMSCs protect neurons significantly better from degeneration than native MSCs. This finding has a high potential for future clinical application of hMSCs in human neuronal disorders therapy.
1. INTRODUCTION
Many neurons within the vertebrate nervous system require the presence of neurotrophic factors for development and differentiation (Oppenheim, 1991). Later, neurotrophin signaling plays an important role in maintenance and modulation of synaptic connections (Weissmiller & Wu, 2012).
The family of neurotrophic factors or neurotrophins consists of nerve growth factor (NGF), brain‐derived neurotrophic factor (BDNF), neurotrophin 3 (NT‐3), and neurotrophin 4 (NT‐4). In order to elicit a survival response, each binds to a tyrosine receptor kinase (Trk) and each of the neurotrophins can similarly respond through an apoptotic pathway initiated by binding to the 75 kD neurotrophin receptor (p75NTR). The spatial and temporal balance achieved between neuronal survival and death depends on the overall level of neurotrophin present and the type of receptors that are expressed (Chao, 2003).
In the central nervous system, BDNF is the predominant neurotrophin complemented by the substantial expression of TrkB (Weissmiller & Wu, 2012). Studies from heterozygous mice expressing reduced levels of BDNF reveal that this factor is essential for multiple functions throughout adulthood such as proper memory acquisition, memory retention, and long‐term potentiation (Korte et al., 1995). Several studies have shown the involvement of BDNF in the pathogenesis of neurodegenerative diseases and psychiatric disorders, like depression and schizophrenia (Balaratnasingam & Janca, 2012). Additionally, BDNF acts on cholinergic neurons, which are depleted in Alzheimer's disease (Benussi, Binetti, & Ghidoni, 2017) and on dopaminergic neurons of the substantia nigra, which are lost in Parkinson's disease (Bhardwaj & Deshmukh, 2018).
In the peripheral nervous system BDNF has shown neurotrophic actions on small fiber sensory neurons involved in sensory neuropathies (Geral, Angelova, & Lesieur, 2013) and on the peripheral auditory neurons, the spiral ganglion neurons (SGN) which are protected from degeneration by application of recombinant BDNF (Leake, Hradek, Hetherington, & Stakhovskaya, 2011; Schwieger, Warnecke, Lenarz, Esser, & Scheper, 2015; Wefstaedt, Scheper, Lenarz, & Stover, 2005).
The neurotrophic actions of BDNF have been established with diverse neuronal populations [for review see (Geral et al., 2013)]. Although there are strong rationales suggesting that increasing supply of neurotrophins to degenerating neurons may be a potent way to restore neuronal function in neurodegenerative conditions, at present, recombinant BDNF delivery in clinical trials has not been therapeutically successful (Bezdjian et al., 2016). Delivering neurotrophins into the brain (Weissmiller & Wu, 2012) or inner ear has proven to be a non‐trivial matter. Due to the presence of the blood‐brain barrier (BBB) or blood cochlear barrier (BCB) that make it almost impossible for large proteins and complex compounds to cross from the blood into the brain or inner ear CNS diseases and hearing disorders are difficult to treat. In addition, the cortical and subcortical circuits of the brain are interconnected resulting in crosstalk among multiple regions, so coming up with a treatment strategy that selectively targets affected neurons only, but not those unaffected ones, is a great challenge that has to be carefully considered (Weissmiller & Wu, 2012). The same is true for the inner ear. In case of unilateral treatment using neurotrophins a diffusion of the factor to the contralateral site or to the brain cannot be excluded due to the cochlear aqueduct and liquor exchange. To further compound these issues, neurotrophins are relatively large, polar molecules that cannot readily cross BBB and BCB and therefore must be administered directly to the target region. Next to this obstacle recombinant BDNF and the other neurotrophins need to be delivered frequently due to rapid protein breakdown (Poduslo & Curran, 1996) and have high manufacturing costs. Gene therapy using recombinant viral vectors such as adeno‐associated virus (AAV) or lentivirus vectors offers a way to produce therapeutic proteins continuously following a single administration with infection of target cells. However, different types of vector systems have different drawbacks which include toxicity and inflammation, infection of non‐relevant cell types or distribution into the body and risk of insertional mutagenesis into the genome associated with certain vector systems. To avoid these issues, alternative therapeutic strategies have been proposed including transplantation of cells releasing neurotrophins. The effectivity of such constructs is shown in in vivo models of various neuronal disorders. For example, transduction of bone marrow stromal cells to overexpress BDNF resulted in a significant increase in axonal growth at sites of spinal cord injury in rats (Lu, Jones, & Tuszynski, 2005). And BDNF‐overexpressing fibroblasts were neuroprotective in an animal model of sensory neural hearing loss (Warnecke et al., 2012).
For individualized therapies and for acceptance by the patient it may be beneficial not to use xenogenic or allogenic cells but autologous ones. Human mesenchymal stem cells (MSCs) are a promising cell source allowing autologous treatment. They have a therapeutic relevance which is based on one hand on their capacity of differentiating into cell types of the musculo‐skeletal system and on the contrary on secretion of soluble factors including neurotrophic factors such as NGF, BDNF and GDNF (Arnhold et al., 2006). MSC‐derived BDNF was already shown to have a protective effect on SGN (Wilkins et al., 2009).
To enhance the therapeutic effect achievable with MSCs we here developed a lentivirally based system for BDNF‐overexpression and secretion by human mesenchymal stem cells (hMSCs). The transplantation of BDNF‐overexpressing hMSCs should be linked to a delivery system avoiding cell migration and allowing nutrition and growth factor diffusion such as encapsulated cell devices (Konerding et al., 2017; Wahlberg et al., 2012) or hydrogel entrapment (Hütten et al., 2013). For this purpose, it may be beneficial to differentiate the hMSC into chondrocytes: firstly, differentiated cells are less carcinogenic or tumorigenic, and secondly, chondrocytes are able to withstand hypoxic conditions and low nutrient supply. The genetically modified and chondrognically differentiated hMSCs were evaluated for their BDNF production and neuroprotective potential on primary auditory neurons as well as for the expression of representative genes that are relevant for chondrogenic differentiation.
2. MATERIALS AND METHODS
An overview of the performed experiments and schematic presentation of lentiviral vector constructs and chondrogenic differentiation is presented in Figure 1.
Figure 1.
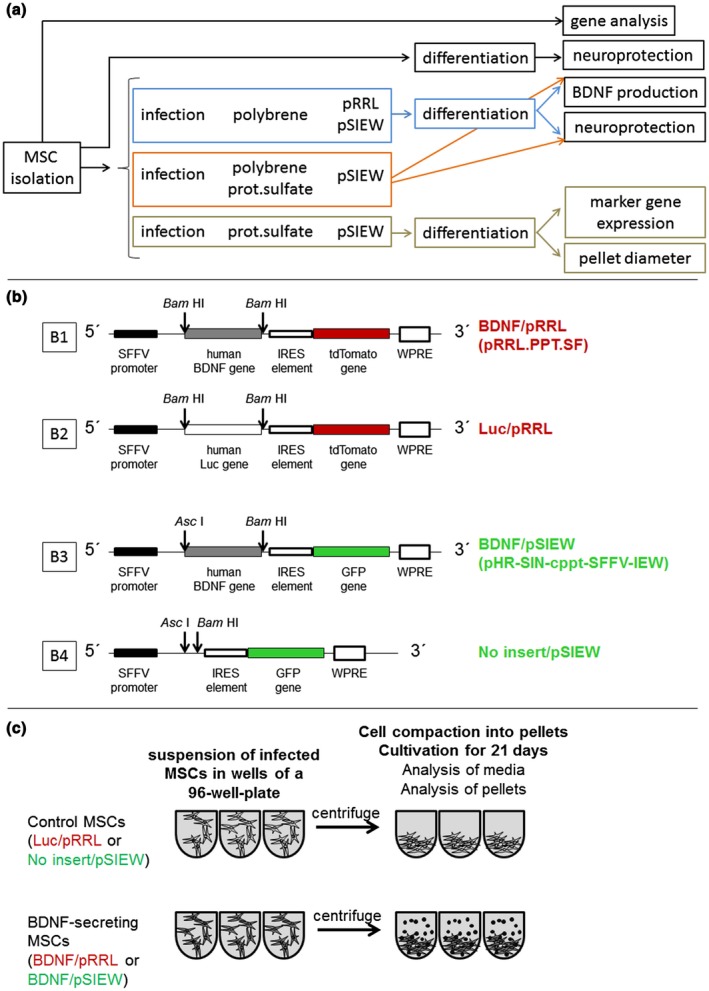
Work flow (a) and schematic presentation of lentiviral vector constructs (b1‐4) and chondrogenic differentiation of genetically modified hMSCs (c). SFFV: spleen focus forming virus promoter, IRES: internal ribosomal entry site, WPRE: Woodchuck Hepatitis Virus Posttranscriptional Response Element. Luc: Luciferase. Restriction sites used for cloning of the BDNF or luciferase transgenes, respectively, are indicated by arrows (b1‐4). The infected hMSCs were centrifuged and TNF‐β3 was added to induce differentiation or not (non‐induced controls) (c)
MSCs were isolated from three different donors (A, B, C) and the endogenous gene expression of selected genes was investigated (Figure 2) with the aim to predict the cells' potential for in vitro‐chondrogenic differentiation. Also, the effect of conditioned medium from chondrogenically differentiated MSCs on neuronal survival was measured (Figure 3).
Figure 2.
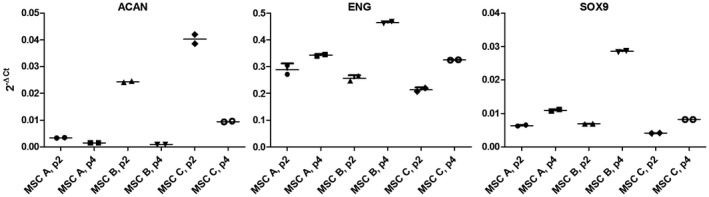
Gene expression in native hMSCs from three donors. Selected genes were analyzed in passage 2 (p.2) and 4 (p.4) during the expansion of human MSCs from donors A, B, and C by quantitative real‐time PCR. The level of gene expression is indicated by the 2−ΔCt value using the house‐keeping gene RPS29 as reference: ΔCt = Ctgene of interest—CtRPS29. ACAN: Aggrecan, END: Endoglin, SOX9: Sex Determing Region Y‐Box 9. Possibly high expression of ACAN in an early passage (here donor C, p.2) may predict poor in vitro chondrogenesis, while a low expression (here donor A) might indicate a higher potential for in vitro chondrogenesis
Figure 3.
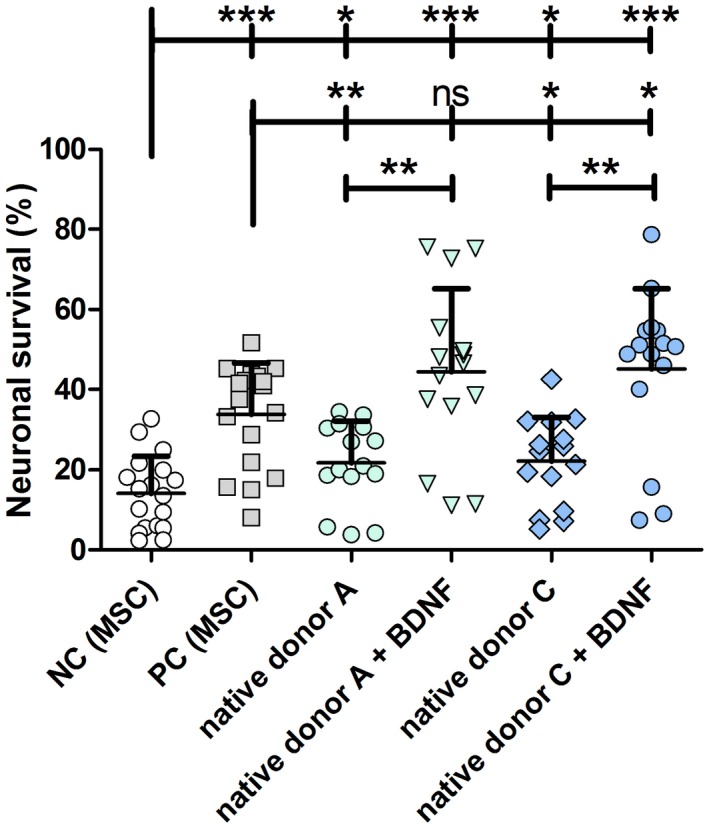
Neuronal survival rates of SGN treated with supernatant of chondrogenically differentiated native hMSCs. The conditioned supernatants of native MSCs collected during chondrogenesis from donors A and C were added to dissociated SGN. The conditioned medium of native hMSCs from both donors did have a neuroprotective effect compared to negative control (NC, MSC growth medium, including 5% FCS) but this effect was lower compared to the positive control (PC, MSC growth medium with 50 ng/ml recombinant BDNF). An additional supply of exogenous BDNF to the hMSC‐conditioned supernatant significantly increased the neuronal survival. This effect was even significantly higher in comparison to the PC for donor C, albeit not for donor A. *p < 0.05; **p < 0.01; ***p < 0.001; ns = not significant
MSCs were subsequently infected to co‐overexpress fluorescent marker proteins (pSIEW = green; pRRL = red) and BDNF. The rationale for use of a fluorescent marker protein during lentiviral modification is to obtain a permanent signal allowing to directly judge the efficacy of the transduction. It also allows following implantation of the cells in animal models in subsequent studies including detection of the cells in histological analysis. The effect of lentiviral infection with two different lentiviral systems (pRRL, pSIEW) and subsequent chondrogenic differentiation on BDNF production (Figure 4) and neuronal survival (Figure 5) was compared using conditioned media sampled during the course of differentiation without finding notable differences between both vector systems. Therefore, due to the higher green than red autofluorescence within fixated tissue, subsequent experiments were performed with the pRRL‐system (red fluorescence) only. BDNF secretion was optimized by comparing the effect of different polycations, polybrene, or protamine sulfate, on fluorescence intensity (Figure 6), BDNF production (Figure 7), and neuronal survival (Figure 8) in the pRRL vector system. Finally, chondrogenic differentiation was compared in the presence or absence of BDNF‐secretion using two different MSC populations and the expressions of several marker genes relevant for chondrogenic differentiation were analyzed (Figure 9) as well as the diameter of MSC‐pellets grown during the differentiation process (Figure 10).
Figure 4.
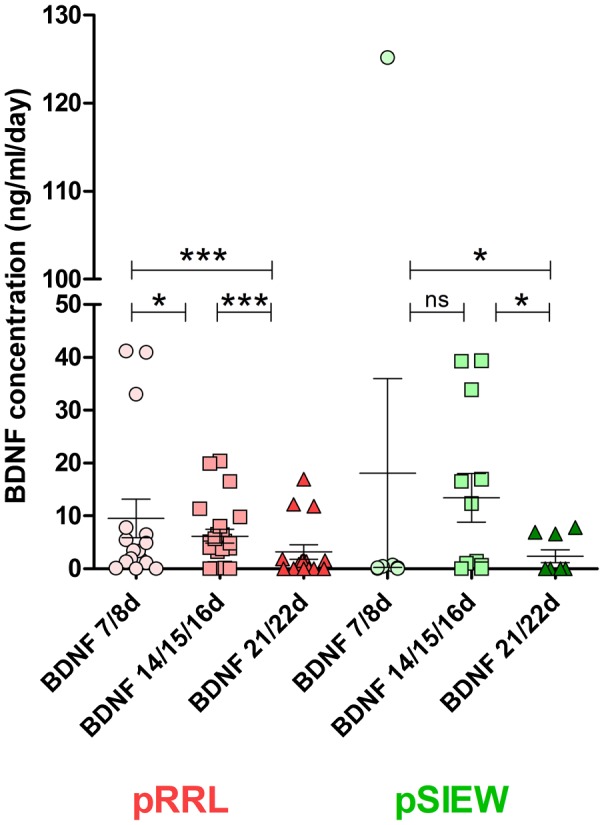
BDNF concentration in the supernatants of pRRL‐ and pSIEW‐infected hMSCs during chondrogenic differentiation. The protein expression did not differ between the lentiviral expression systems used. Production of BDNF was maximal after one week of differentiation and decreased afterwards. pRRL = plasmid with tdTomato‐red marker protein; pSIEW = plasmid with green fluorescent protein marker
Figure 5.
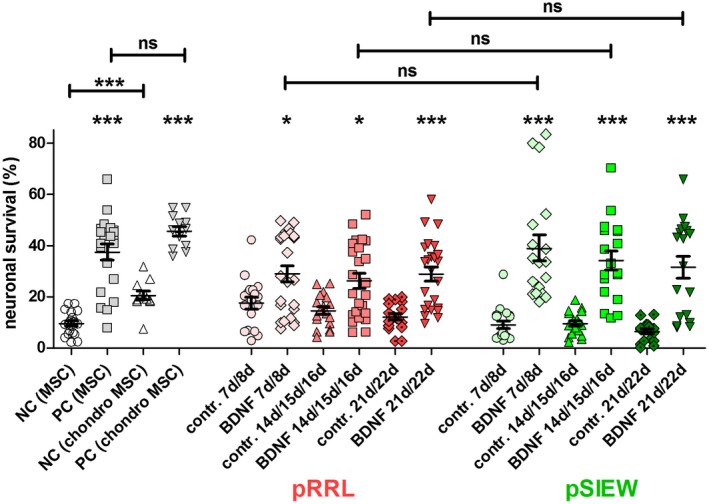
Neuroprotection of SGN by supernatants of pRRL‐ and pSIEW‐ infected hMSCs during chondrogenic differentiation. The mean and SEM SGN survival rate (in % related to the seeding control) of controls and of three MSC‐donors infected either with pRRL (red) or pSIEW (green) and subsequently differentiated to chondrocytes are shown. Differences between a BDNF containing (exogenous or endogenous) group and the relevant control (lacking BDNF) are depicted directly above the relevant bar. Differences between experimental conditions are indicated by horizontal line above the respective bars. The control data are depicted in gray with MSC growth medium (MSC, including 5% FCS) without any growth factor addition (negative control [NC MSC]) or with addition of recombinant BDNF (positive control [PC MSC]). A second set of positive and negative controls was added with chondrogenic differentiation medium metabolized by native MSCs (NC [chondro MSC] and PC [chondro MSC]). The positive controls significantly protected the neurons from degeneration. The metabolized chondrogenesis medium without BDNF addition (NC (chondro MSC) increased the neuronal survival significantly compared to the control using MSC‐medium. At all time points the infection with the BDNF‐lentiviruses (BDNF) achieved a BDNF‐induced neuronal protection compared to the relevant control (contr.: lentivirus without BDNF for pSIEW, luciferase instead of BDNF for pRRL), independently from the fluorescence‐marker gene used. When comparing the neuroprotective effect of the produced BDNF at a specific time point between pRRL and pSIEW‐infected hMSC no differences were observed. *p < 0.05; **p < 0.01; ***p < 0.001; ns = not significant
Figure 6.
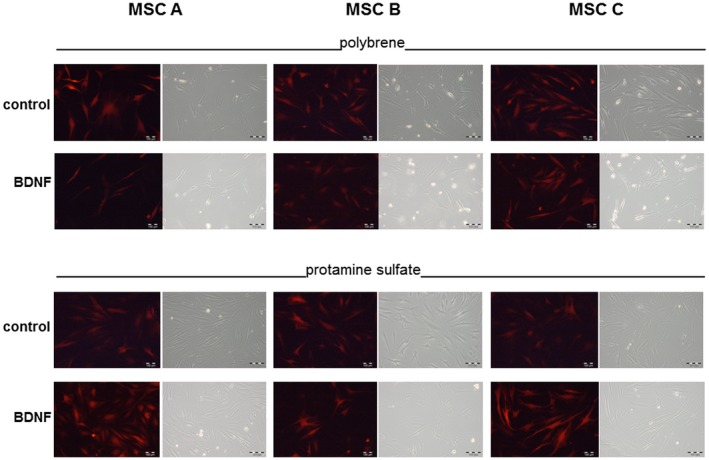
Microscopic images of MSCs using either polybrene or protamine sulfate with the pRRL vector system. Red fluorescence of the tdTomato marker protein expression and corresponding phase contrast microscopic images 6 days after lentiviral infection of the three different MSC populations A, B, C with the pRRL system. Control virus (Luc/pRRL) and the BDNF virus (BDNF/pRRL) were used for lentiviral modification of the MSCs either in the presence of 8 μg/ml polybrene or 50 μg/ml protamine sulfate. There are no notable differences in fluorescence intensity between viruses, polycations (polybrene and protamine sulfate) and MSC donors. Bar: 100 μm. Native hMSCs before lentiviral infection do not exhibit any red autofluorescence (data not shown)
Figure 7.
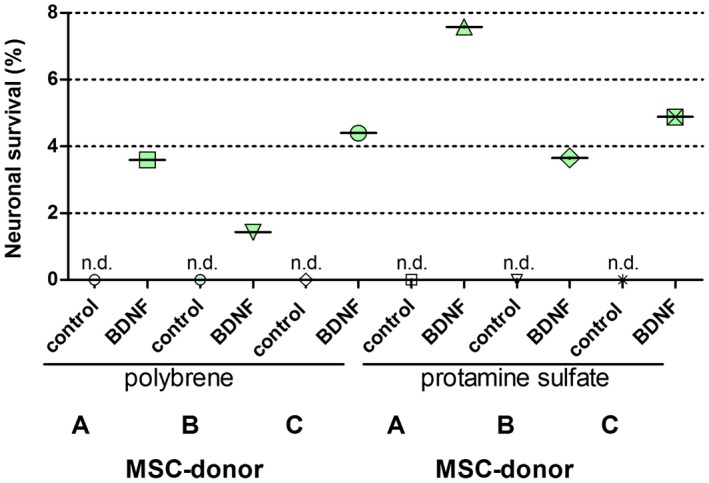
BDNF secretion per hMSC (in ng/cell) after polybrene‐ or protamine sulfate‐ mediated lentiviral transduction with the pRRL vector system (without subsequent chondrogenic differentiation). BDNF secretion was determined by ELISA. The cell count of the MSCs (a mixture of infected and uninfected cells) was determined by means of Fuchs‐Rosenthal counting chamber. In the presence of the control virus (Luc/pRRL) no endogenous BDNF secretion of the MSC populations is detectable. The BDNF secretion is generally higher when infected with protamine sulfate than with polybrene. n.d.: not detectable
Figure 8.
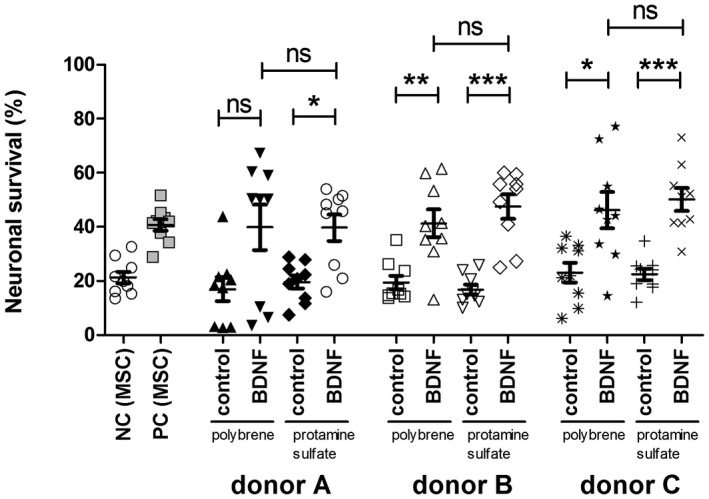
Neuronal survival rate using either polybrene or protamine sulfate with the pRRL vector system (without subsequent chondrogenic differentiation). Neuronal survival rate relative to the seeding control (=100%) of supernatants collected from different donors (donor A, donor B, donor C) with control‐vector infection (control) or BDNF infection using either polybrene or protamine sulfate. In all donors the infection with BDNF did result in increased neuronal survival rates with exclusion of donor A infected with polybrene. No difference in polybrene and protamine sulfate infection was observed. Even though compared to polybrene‐infected MSCs, the protamine sulfate treatment resulted in a higher rate of neuronal survival than the relevant control. *p < 0.05; **p < 0.01; ***p < 0.001; ns = not significant
Figure 9.
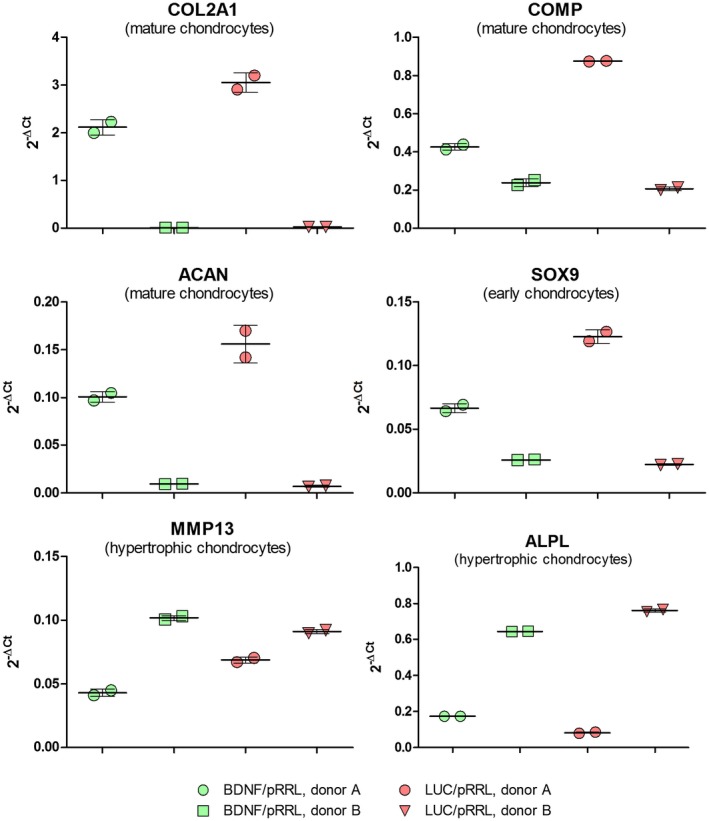
Gene expressions after chondrogenic differentiation of pRRL‐infected MSCs from two donors. The gene expressions of all chondrogenic marker genes investigated for MSC C are notably lower than for MSC A although the experiments were conducted in parallel for three weeks. Markers for mature chondrocytes: COL2A1 (collagen type II alpha I), COMP (cartilage oligomeric protein), ACAN (aggrecan); marker of early chondrogenesis: SOX9 (sex determining region Y‐box 9); marker for hypertrophic, mineralized chondrocytes: MMP13 (matrix metalloprotease 13), ALPL (Alkaline Phosphatase). Luc: control vector with luciferase and BDNF expression (vector with BDNF expression, either in the plasmid system with tdTomato as marker gene [pRRL] or with GFP as marker gene [pSIEW])
Figure 10.
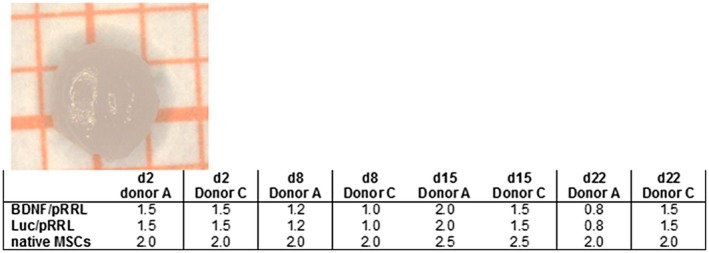
Pellet diameters (in mm) of hMSCs (lentivirally modified and native, respectively) differentiated to chondrocytes from donors A and C. Image: representative macroscopic view of one pellet. The grid size is 0.5 × 0.5 mm. The table gives the pellet diameters on different experimental days (d) measured for single representative pellets
2.1. Isolation of MSCs from human bone marrow
For studies involving human bone marrow ethical approval from the ethical committee of Hannover Medical School, Hannover, Germany, was obtained (565–2009, 565–2016). Samples were collected in accordance with the Declaration of Helsinki after written informed consent of all donors. For the present study, cells from three donors aged between 21 and 23 years (one male, two female) were selected since studies have shown that cells from younger donors perform better than cells from older donors in many functional aspects (Stolzing, Jones, McGonagle, & Scutt, 2008). All personal information was made anonymous and is denoted “A,” “B,” “C” in the present publication when reference is made to experiments with individualized data. Bone marrow samples were obtained during resection of the femoral head for implantation of a total hip arthroplasty in otherwise healthy donors. Human MSCs were isolated from these samples by density gradient centrifugation and subsequent plastic adhesion of mononuclear cells and cultured as previously described (Hoffmann, Floerkemeier, Melzer, & Hass, 2017; Jungwirth et al., 2018).
2.2. Determination of endogenous gene expressions in native MSCs by quantitative real‐time PCR
During the expansion (37°C, 5% CO2) of the MSC populations (i.e., without infection and chondrogenic induction), the basal gene expression of several genes was investigated in order to assess the cells' putative chondrogenic potential. MSC growth medium consisting of Dulbecco's Modified Eagle's Medium (1 g/l glucose, Biochrom, FG0415) supplemented with 10% (v/v) fetal calf serum (FCS, not heat‐inactivated, Thermo Fisher Scientific, Schwerte, Germany, “HyClone,” SV30160.03), 25 mM HEPES (Biochrom, Berlin, Germany), 1% (100 U/ml/ 100 µg/ml) penicillin/streptomycin (Biochrom, Berlin, Germany), and 2 ng/ml human recombinant FGF 2 (from Escherichia coli, PeproTech, Hamburg, Germany) was used for expansion. MSCs from three different donors (A, B, and C) were used in passages 2 and 4. Briefly, cells cultured in T25‐Roux flasks (Greiner) were harvested using 1 ml TRIzol reagent (Ambion) and stored at −80°C at least overnight. The thawed solution was mixed with 100 µl 1‐bromo‐3‐chloropropane (Merck) and centrifuged for 15 min at 20,000 × g and 4°C. The upper layer was carefully collected in a new tube, treated with 500 µl 2‐propanol for 5 min at RT and centrifuged for 30 min at 20,000 × g and 4°C. The supernatant was discarded and the RNA pellet was washed with ice‐cold 80% ethanol, dried, and subsequently dissolved in 20 µl deionized water. RNA concentration was calculated by absorption at 260–280 nm. Synthesis of cDNA was performed with 1 µg of RNA after treatment with DNase I and using oligo‐dT18 primers according to the manufacturer's protocol (Fermentas). Quantitative real‐time PCR based on the housekeeping gene RPS29 was performed for AGGRECAN (gene name: ACAN, representative for mature chondrocytes), endoglin (gene name: ENG, protein also called CD105, co‐receptor for Transforming Growth Factor‐Beta that induces chondrogenic differentiation [Fan et al., 2016]), and sex determining region Y‐Box 9 (gene name: SOX9, upregulated during early chondrogenic differentiation). Quantitative real‐time PCR analyses were performed using the Applied Biosystems® StepOnePlus instrument (Life Technologies). The gene‐specific assays as well as the Fast Advanced Mastermix were purchased from Life Technologies: RPS29 (Hs03004310_g1), ACAN (Hs00153936_m1), ENG (Hs00923996_m1), SOX9 (Hs00165814_m1). Analyses were implemented according to the manufacturer's instructions. Each sample was measured in duplicate (with standard deviations shown in Figure 2) with the following conditions: 20 s at 95°C and 40 cycles 1 s at 95°C and 20 s at 60°C. The level of gene expression is indicated by the 2−ΔCT value (referencing gene of interest versus housekeeping gene [Schmittgen & Livak, 2008]).
2.3. Infection of human MSCs with lentiviruses for BDNF overexpression
In order to achieve a constitutive expression of human BDNF, lentiviral constructs were prepared in two different vectors containing a ribosomal entry site with expression of a fluorescent marker gene. The expression of human BDNF (entire coding sequence including signal peptide please see [Warnecke et al., 2012]) was under control of a SFFV (spleen focus‐forming virus) promoter in both systems (Figure 1b1, b3). The marker was either tdTomato red (plasmid pRRL.PPT.SFFV (pRRL), or green fluorescent protein (GFP; plasmid pHR‐SIN‐cppt‐SFFV‐IEW (pSIEW). For the pRRL‐system, a control vector expressing firefly luciferase (Luc) instead of BDNF was constructed (Figure 1b2). For the pSIEW‐system, a control vector without BDNF transgene was generated (Figure 1b4).
From these 2 × 2 vectors, the respective lentivirus stocks were produced by transient transfection of HEK 293T cells (1 * 107 cells per transduction) with 50 µg of the respective lentiviral vector plus 12.5 µg pMD.G (encoding the VSV‐G envelope glycoprotein for pseudotyping of the virus) and 50 µg pCMVÄR8.2 as helper plasmids. The viral supernatants were harvested 48 and 72 hr after transfection and pooled. The virus stocks were titrated with the Lenti‐XTM (qRT‐PCR Titration Kit, TaKaRa). Trial infections were performed in HEK 293T cells with these virus stocks and the resulting expression of the fluorescent marker proteins was confirmed by fluorescence microscopy.
Subsequently, the hMSCs (seeded at 3,000 cells/cm2, passage 4 or 5) were infected with the BDNF‐ or control lentiviruses using the appropriate amount of virus diluted in fresh MSC growth medium. To investigate whether polybrene or protamine sulfate addition leads to a higher secretion of BDNF including an enhancement of spiral ganglion neuron survival, 8 μg/ml polybrene or 50 μg/ml protamine sulfate (Lin et al., 2012) were applied and MSCs from three different donors (A, B, C) were compared. For a subsequent downgrading of the cells to S1 level, the cells were cultured and expanded for 11 days before investigating the BDNF secretion by ELISA and the neuroprotective effect.
2.4. Chondrogenic differentiation of lentivirally modified hMSCs
Prior to cell seeding for chondrogenic induction, MSCs from the two individual donors A and C were lentivirally modified for BDNF‐secretion or luciferase expression (control cells) in the presence of 50 µg/ml protamine sulfate. Enough cells were infected to allow for chondrogenic differentiation using a three‐dimensional pellet culture system without further passaging. Each experiment was conducted once per donor. For each pellet, six days after lentiviral infection, cell suspensions containing 1.25 × 105 MSCs were aliquoted into wells of a 96‐well plate (polypropylene, Corning #3879 via Sigma‐Aldrich) and centrifuged for 5 min at 300 × g. The supernatant was removed and replaced by induction medium (chondrogenic differentiation medium) based on Dulbecco's modified Eagle's medium with 4.5 g/l glucose (Biochrom FG 0345) and supplemented with additives as follows: 20 mM HEPES, 1% (100 U/ml/ 100 µg/ml) penicillin/streptomycin, 100 nM dexamethasone, 170 µM ascorbate‐2‐phosphate, 1 mM sodium pyruvate, 0.35 mM proline, 6.25 µg/ml human insulin, 6.25 µg/ml human transferrin, 6.25 ng/ml selenic acid, 5.35 µg/ml linoleic acid, 1.25 mg/ml bovine serum albumin (as “100× ITS + supplements,” Corning # 354352), and 10 ng/ml recombinant human TGF‐β3 (Peprotech, from E. coli, dissolved in 4 mM HCl with 1 mg/ml bovine serum albumin). Medium changes were performed twice a week within a time period of up to 21 or 22 days. The supernatants collected during media changes (at time points 2–3, 7–8, 14–16, and 21–22 days after induction of chondrogenic differentiation, cf next paragraph) were frozen for later determination of the secreted BDNF amount and the neuroprotective effect. RNA was isolated from the cell pellets as follows: Per each analysis, two to three pellets (dependent on total cell numbers available for the entire experiment) were pooled. Despite the high cell numbers necessary for formation of individual cell pellets, due to the desired anaerobic and low metabolic conditions within each pellet, they are highly compact, hard to destroy and contained low amounts of RNA. Therefore, the pellets were homogenized with the Precellys® 24 tissue homogenizer (Peqlab) using the Total RNA Kit (S‐Line) + Precellys Ceramic Kit (beads with diameters of 1.4 and 2.8 mm, Peqlab) with the following program: 2× 30 s, 6.000 rpm, 40 s break. Subsequent RNA purification was performed according to the manufacturer's protocol.
Gene expression analyses of chondrogenic marker genes were conducted by quantitative real‐time PCR as described above. To follow the course of chondrogenesis and to assess the efficacy of the differentiation the following genes were investigated: COL2A1 (collagen type II alpha I; Hs01064869_m1), COMP (cartilage oligomeric protein; Hs00164359_m1), ACAN (aggrecan; Hs00153936_m1) (all three are markers for mature chondrocytes), SOX9 (sex determining region Y‐Box 9; Hs00165814_m1 (marker of early chondrogenesis), MMP13 (Matrix Metalloprotease 13; Hs00233992_m1), ALPL (Alkaline Phosphatase, tissue non‐specific; Hs00758162_m1) (both are marker for hypertrophic, mineralized chondrocytes, indicative of progressive chondrogenesis). Each sample was pipetted in duplicates.
2.5. In vitro measurement of BDNF overexpression and neuronal protection
To investigate the neuroprotective effect of native hMSC‐conditioned medium (possibly including released neurotrophic factors as synergistic activators) in comparison to non‐conditioned medium, cells from donor A and donor C were subjected to chondrogenic differentiation for 21 days. The conditioned supernatants were collected and added to dissociated SGN, alone and in combination with exogenously produced BDNF (cf Table 1). The effect was evaluated after 48 hr of cultivation and compared to SGN cultured with the same MSC supernatant plus addition of 50 ng/ml recombinant human BDNF. Pure MSC growth medium (without cell exposure) was supplemented with 50 ng/ml recombinant human BDNF (positive control, PC) or applied to the cells without any factor addition (negative control, NC). These experiments were repeated in triplicates in five independent tests.
Table 1.
Conditions tested for BDNF production and neuroprotection
| Genes | Fluorescence | Polycation during lentiviral transduction | |
|---|---|---|---|
| MSCs | |||
| Native | – | – | – |
| Modification | Luc/pRRL | Red | Polybrene or protamine sulfate |
| Modification | No insert/pSIEW | Green | Polybrene |
| Modification | BDNF/pRRL | Red | Polybrene or protamine sulfate |
| Modification | BDNF/pSIEW | Green | Polybrene |
| Controls | |||
| MSC growth medium | |||
| Chondrogenic differentiation medium metabolized by MSCs | |||
| Negative control: MSC growth or MSC‐metabolized chondrogenic differentiation medium without growth factor addition (NC) | |||
| Positive control: MSC growth or MSC‐metabolized chondrogenic differentiation medium with 50 ng/ml recombinant BDNF (PC) | |||
Abbreviations: MSCs, mesenchymal stem cells; Luc/pRRL, luciferase/tdTomato red (plasmid pRRL.PPT.SFFV); pSIEW, green fluorescent protein (GFP; plasmid pHR‐SIN‐cppt‐SFFV‐IEW); BDNF: brain‐derived neurotrophic factor.
To investigate if the polycation used for lentiviral modification has an impact on the amount of BDNF synthesis MSCs lentivirally modified using polybrene or protamine sulfate were examined for their BDNF production and neuroprotective effect during the course of chondrogenic differentiation. For this purpose, the conditioned media of the hMSC pellets were harvested after induction of chondrogenic differentiation. To investigate the neuroprotective effect of the produced BDNF, the conditioned supernatant (collected from 2 to 6 days after lentiviral infection) was added to SGN and the surviving SGN were analyzed. Experiments were performed in triplicate with threefold independent repetition. All conditions examined are listed in Table 1.
The biological effect of the BDNF was determined on cultured SGN. Neonatal Sprague‐Dawley rats of both sexes (2–5 days old) were used for SGN dissection in accordance with the German “Law on protecting animals” and the European Communities Council Directive 86/609/EEC for the protection of animals used for experimental purposes. The preparation of SGN and the dissection of the cochleae were performed as previously described (Schwieger et al., 2015). Poly‐D/L‐ornithine (0.1 mg/ml, Sigma Aldrich) and laminin (0.01 mg/ml Naturel Mouse Laminin; Invitrogen) coated wells of a 96‐multiwell culture plate (TPP Techno Plastic Products AG, Trasadingen, Switzerland) were prepared. Panserin 401 (Pan Biotech) supplemented with insulin (8.7 µg/ml; Biochrom), penicillin (30 U/ml; Biochrom), glucose (0.15%; B.Braun Melsungen), PBS (0.172 mg/ml; Gibco by life‐technologies), HEPES‐buffer solution (23.43 µM; Invitrogen), and N2‐supplement (0.1 µl/ml; Invitrogen) was used as culture medium (SGN‐medium). 1 × 104 SGN suspended in 50 µl SGN‐medium were seeded into each well. Culture was performed in a humidified (95%) atmosphere of 5% CO2 at 37°C. Fifty microliters of supernatant of the hMSC that had been incubated for different time points (2–3, 7–8, 14–16 and 21–22 days), as well as relevant control media (Table 1) were added to the SGN culture at time of seeding. Pure chondrogenic differentiation medium had a negative impact on the cultured SGN and could therefore not serve as control medium. For that reason, MSC‐medium and metabolized chondrogenic differentiation medium were chosen as controls. Finally, serum‐free SGN‐medium and the tested supernatants or control media were mixed 1:1 in each well. SGN cultivated in control media (Table 1) with addition of 50 ng/ml BDNF (human recombinant BDNF, from E. coli; Invitrogen), which is the concentration former studies proved to induce best SGN survival, served as positive control (Wefstaedt et al., 2005). Negative control contained no additional BDNF. The seeding density was determined after 4 hr of cultivation. Fixation was performed with 1:1 acetone/methanol solution after 4 hr in case of wells used to determine the seeding density and after 48 hr of cultivation in case of experimental groups.
The SGN were labeled by immunocytochemistry using mouse monoclonal neurofilament—200 kDa primary antibody (NCL‐L‐NF200‐N52, Novocastra, Leica Biosystems) and stained with the Vectastain®Elite® ABC kit (VEC‐PK‐6102, Vector Laboratories) followed by peroxidase substrate kit (DAB; VEK‐SK‐4100, Vector Laboratories). For more details please see Wefstaedt et al., 2005.
The number of surviving SGN was determined microscopically. SGN showing a positive immunostaining and an axon length thrice as long as the somas' diameter were defined as surviving neurons and counted. The observed SGN number was set into ratio with the number of cells observed 4 hr after seeding, giving the survival rate as percentage of the seeding control. Temporally independent cultures (numbers of replicates are indicated in the results section because they differ between the performed experiments) with 3 wells each for each experimental group were analyzed. Data were tested for normal distribution using the D'Agostino & Pearson omnibus normality test. To compare the SGN survival rates between experimental groups (N = 6 for chondrogenically differentiated native hMSCs (Figure 3); N = 16 for infected and differentiated hMSCs (Figure 5) the Mann–Whitney U test was used. For comparison of data between different time points within one experimental group, the Wilcoxon signed rang test was performed.
BDNF protein levels in culture supernatants were measured by ELISA to examine secretion of growth factor produced by transfected and differentiated cells. ELISAs were performed using the BDNF ELISA kit (Human BDNF PicoKine ELISA Kit; Boster Biological Technology) according to the manufacturer's instructions and the previously described protocol (Schulze et al., 2017). The BDNF expression (ng ml−1 day−1) of pRRL‐ or pSIEW‐infected MSCs was tested for normal distribution using the D'Agostino & Pearson omnibus normality test and Wilcoxon‐Test was performed for comparison of BDNF production within infection method over time in both groups whereas the Mann–Whitney U test was used for comparison of BDNF concentrations between the two infection methods at the relevant experimental days.
3. RESULTS
For the establishment of hMSCs from different donors to stably overexpress and secrete human BDNF with the aim of providing continuous trophic support to neuronal cells different infection protocols and differentiation methods were compared with respect to the resulting BDNF secretion and the biological effect on neurons in vitro.
3.1. Native hMSCs—Gene expression and neuroprotection
During the expansion of the native hMSC populations the basal gene expression of selected genes was investigated. Figure 2 demonstrates a notable higher expression of ACAN for the donors B and C in passage 2 compared to donor A. At the later passage, however, ACAN expression is comparable for all three donors. In contrast to the behavior of ACAN, the expressions of END and SOX9 increased between passage 2 and 4 for all three hMSC populations. Without any treatment (besides the 5% FCS contained in the mixed media from hMSCs and SGN, NC) 14.15% ± 2.17% (mean ± SEM) of neurons survived (Figure 3). The addition of 50 ng/ml recombinant BDNF (PC) resulted in a significantly improved neuronal survival (33.80% ± 3.02%; p < 0.0001). Conditioned medium from the chondrogenically differentiated native hMSCs of both donors did have a neuroprotective effect compared to the negative control (Mann–Whitney test, p = 0.0287 (MSC A) and p = 0.0329 (MSC C) (Figure 3). The neuroprotective effect for supernatants from both donors was nearly equal (mean ± SEM: MSC A: 21.70% ± 2.70% versus MSC C: 22.14% ± 2.83%). In comparison to treatment with recombinant BDNF this effect was significantly smaller (p < 0.01 compared to MSC A and p < 0.05 compared to MSC C). Conditioned media from MSC A supplemented with exogenous recombinant BDNF protected SGN more effectively from degeneration, resulting in a survival rate of 44.45% ± 5.36%. Compared to the negative control (p < 0.001) and to medium from native hMSCs (p < 0.01) this effect was statistically significant. In contrast to SGN treated with recombinant BDNF (PC) there was no difference. In contrast to this, conditioned medium from MSC C supplemented with recombinant BDNF did have a significantly higher neuroprotective effect than the positive control (45.22% ± 5.15% versus 33.80% ± 3.02%; p = 0.0133). Additionally, the neuronal survival of MSC C + BDNF was significantly increased compared to NC (p < 0.001) and to the native hMSCs from the same donor (p < 0.01; Figure 3). Indeed, the hypothesis that the combination of hMSC‐conditioned medium with exogenous recombinant BDNF can induce a higher neuronal survival than each condition alone was confirmed by these data.
3.2. Comparison of two different lentiviral systems with respect to BDNF‐overexpression and neuronal survival
The project rationale was to use chondrogenically differentiated hMSCs for envisioned long‐term in vivo survival and BDNF secretion. However, differentiation might impact BDNF secretion. We therefore compared the BDNF secretion achieved by lentiviral modification of hMSCs in the presence of the widely used polycation polybrene with both lentiviral systems pRRL and pSIEW at different time points during chondrogenic differentiation. The BDNF expression (ng ml−1 day−1) of pRRL‐ or pSIEW‐infected hMSCs did decrease over time. Using pRRL for infection hMSCs produced on day 7 a mean ± SEM of 9.55 ± 3.64 ng BDNF−1 ml−1 day and on day 21 after infection 3.17 ± 1.33 ng BDNF−1 ml−1 day, p < 0.0001. Using pSIEW the BDNF amount declined from 18.11 ± 17.84 ng BDNF−1 ml−1 day on day 7 to 2.36 ± 1.18 ng BDNF−1 ml−1 day on experimental day 21 (p < 0.01). The BDNF production of pSIEW‐infected hMSCs did not differ from pRRL‐infected ones on the respective experimental days tested (Figure 4). An infection with the control vectors lacking the BDNF‐gene resulted in no detectable amounts of BDNF (data not shown).
The neuroprotective effect of the produced BDNF was tested on dissociated SGN. The highly significant neuronal protection in PC compared to NC demonstrates the success of the experiment (Figure 5). Pellets containing 125.000 hMSCs modified to overexpress BDNF by infection with either the pRRL‐ or pSIEW‐based lentiviruses and subsequently differentiated to chondrocytes did release human BDNF which significantly protected the neurons compared to the relevant control (without BDNF overexpression). This effect was detectable during the entire period of chondrogenic differentiation, i.e., 7–8, 14–16, and 21–22 days (Figure 5). When comparing the neuroprotective effect of the released BDNF at a specific time point no differences were observed between pRRL‐ and pSIEW‐modified hMSC. Due to the finding that the guinea pig cochlea exhibits notable green autofluorescence but the comparable BDNF secretion of both lentiviral systems all subsequent experiments were conducted with the pRRL lentiviral system only.
4. OPTIMIZATION OF LENTIVIRAL INFECTION FOR MAXIMAL BDNF SECRETION
Efficient lentiviral transduction necessitates use of a polycation species. Polybrene and protamine sulfate are in widespread use, yet, polybrene may severely impede proliferation of hMSCs after successful transduction (Lin et al., 2012). Therefore, both polycations were compared for their efficiency of lentiviral modification of hMSCs from three donors A, B, and C (without subsequent chondrogenic differentiation). Three parameters were assessed: the fluorescent marker expression (determined by fluorescence microscopy, Figure 6), BDNF secretion (determined by ELISA, Figure 7), and the biological effect of the released BDNF (determined by the SGN assay, Figure 8) 6 days after lentiviral infection.
Cell numbers of MSCs (manually counted in a Fuchs‐Rosenthal cell chamber, data not shown) were similar in all cases as evidenced by the microscopic sample images in Figure 6. There were no notable differences in fluorescence intensity between viruses, polycations, and MSC donors.
The transduced MSCs from donor B expressed the lowest BDNF amount per cell both after transduction in the presence of polybrene and protamine sulfate compared to the other two donors (Figure 7), despite the fluorescence intensity of the marker protein being similar (Figure 6). For all three MSC donors, the BDNF secretion was higher when hMSCs were infected with protamine sulfate than with polybrene (Figure 7). No BDNF secretion could be measured from the control hMSCs that had been infected with the luciferase control virus.
To investigate the neuroprotective effect of the produced BDNF, the conditioned supernatant was added to dissociated SGN. In comparison to the seeding control 21.30% ± 2.17% (mean ± SEM) of untreated neurons cultivated in MSC growth medium (NC [MSC]) survived (Figure 8). An addition of recombinant BDNF (PC [MSC]) significantly increased the number of surviving SGN to 40.73% ± 2.18% (mean ± SEM). BDNF‐infection of hMSCs compared to control‐infected hMSCs resulted in significantly increased neuronal survival except for hMSC A after infection with polybrene. In all cases, the mean SGN‐survival rate was twice as high as in native hMSCs or in control‐modified hMSCs. No statistically relevant difference in neuronal survival of protamine sulfate‐ and polybrene‐treated hMSCs was observed.
4.1. Chondrogenic differentiation of lentivirally modified hMSCs
Chondrogenic differentiation was subsequently performed after lentiviral modification of hMSC in the presence of protamine sulfate as described in Materials and Methods. At the end of the differentiation period (i.e., after 21 days), the gene expression analyses of lentivirally modified and differentiated hMSCs clearly demonstrated substantial donor‐dependent differences in in vitro differentiation efficiency: Donor A showed a considerably better chondrogenic differentiation than donor C (Figure 9). This is evidenced by the notably higher expression of all chondrogenic marker genes for MSCs from donor A compared to donor C that were investigated including the marker of early chondrogenesis, SOX9 (sex determining region Y‐box 9), and the markers for mature chondrocytes, COL2A1 (collagen type II alpha I), COMP (cartilage oligomeric protein), and ACAN (aggrecan). Interestingly, markers for hypertrophic, mineralized chondrocytes represented by MMP13 (matrix metalloprotease 13) and ALPL (Alkaline Phosphatase, tissue non‐specific) were expressed more prominently in MSC donor C. Since mineralized chondrocytes have inferior tissue regenerating properties (although this criterion is not relevant for the secretory functions exploited in the present project) all gene expressions support the conclusion that MSCs from donor A are much better suited for in vitro‐chondrogenic differentiation than MSCs from donor C.
During chondrogenic differentiation, the diameter of the cell pellets from 125,000 genetically modified hMSCs per pellet was >1 mm in all tested conditions already after 2 days of differentiation and remained stable during further differentiation. For donor A with the notably higher chondrogenic differentiation capacity, the pellet diameter decreased at late stages of differentiation (day 22), in contrast to donor B with lower chondrogenic differentiation potential. The diameter of pellets from 125,000 native hMSC‐pellets is substantially larger (Figure 10).
5. DISCUSSION
Using three different human donors for isolation of MSCs from bone marrow, different transduction methods and gene expression analyses we were able to differentiate hMSC to chondrocytes overexpressing the neurotrophic factor BDNF in a sufficient amount to increase neuronal survival significantly in vitro.
This may be one first step toward the individualized chronic therapy for neuronal disorders using autologous cells.
5.1. Native hMSCs
During the expansion of the native hMSC populations, the basal gene expression was investigated in order to assess the cells' putative chondrogenic potential. In passage 2, a notable higher expression of ACAN for donors B and C compared to donor A was detected but at the later passage ACAN expression was comparable for all three donors. In contrast to the behavior of ACAN, the expressions of END and SOX9 increased between passage 2 and 4 for all three MSC populations. Influences of culture conditions on gene expressions have been observed for other genes before (Schäck et al., 2013). In the present study, additional experiments including chondrogenic differentiation of cells (Figure 9), however, demonstrated adequate chondrogenic differentiation only for donor A who exhibited the lowest relative ACAN expression in passage 2. Therefore, a high expression of ACAN in an early passage (here MSCs B, C) may possibly predict poor in vitro‐chondrogenesis. This hypothesis would, however, need to be verified by further investigations; it was not the focus of the present investigation to closer investigate reasons and predictors of poor chondrogenic differentiation.
Native undifferentiated hMSCs protect neurons in vitro from degeneration. This effect is already described for native undifferentiated hMSCs (Schulze et al., 2017) and is based on their potency to synthesize and secrete neurotrophic factors such as NGF, BDNF, and GDNF (Arnhold et al., 2006; Wilkins et al., 2009). The SGN survival rates in the present study were 22.14% ± 2.83% (donor C) and 21.70% ± 2.70% (donor A) mediated by native hMSCs during chondrogenesis and are comparable for both hMSC preparations that were investigated (Figure 3). This effect is already significant compared to untreated negative controls but not as successful as the positive control. The positive control used within our study is 50 ng/ml recombinant human BDNF. This is a concentration of human recombinant BDNF already presented to significantly increase the SGN survival rate compared to relevant controls (Schmidt et al., 2018; Schulze et al., 2017; Schwieger et al., 2015; Wefstaedt et al., 2005). An additional substitution of the native hMSC supernatant with 50 ng/ml recombinant BDNF could further increase the neuroprotective effect of the hMSCs. This finding implicated an even higher potential for neuroprotection when hMSCs would be induced to produce higher amounts of BDNF. A combination of these two neuroprotective effects has not been described before.
5.2. Genetically modified hMSCs
Our study for the first time shows that the neuroprotective effect of native (and chondrogen differentiated) hMSCs can significantly be increased by genetically modifying the MSCs to overexpress BDNF. This effect is equal or—in case of donor C—significantly better than the neuroprotective effect achieved using recombinant human BDNF (50 ng/ml, PC). Studies investigating the optimal in vitro concentration of recombinant BDNF for protection of auditory neurons examined a wide range of concentration as optimal, e.g., 25 ng/ml (Ramku, Ramku, Spanca, & Zhjeqi, 2017), 50 ng/ml (Wefstaedt et al., 2005), or 100 ng/ml (Hegarty, Kay, & Green, 1997), i.e., all in the nanogram‐scale. Interestingly, the BDNF concentration produced by and secreted into the supernatant of genetically modified hMSCs is mainly tremendously lower than the concentration used as positive control (under 1 ng ml−1 day−1 to about 40 ng ml−1 day−1 except one transfection with 125 ng ml−1 day−1 versus 50 ng/ml). Despite this, the supernatant including the cell‐delivered BDNF did have an equal or even better neuroprotective effect than the recombinant BDNF. A similar finding was observed when investigating the neuroprotective effect of BDNF overexpressed from genetically modified NIH3T3 fibroblasts (Hütten et al., 2013). There, fibroblasts produced BDNF in concentrations in the picogram range but the neuroprotective effect was significantly increased compared to the relevant control (recombinant human BDNF 50 ng/ml). This highly significant biological protectiveness of cell‐delivered BDNF was additionally proven in vivo by adding ~230–700 pg BDNF/ml produced from transfected fibroblasts. The applied BDNF concentration protected auditory neurons from degeneration in deafened guinea pigs (Rejali et al., 2007). Another study with deafened guinea pigs could also detect a significant protection of SGN from degeneration by a treatment with encapsulated and for BDNF‐production genetically modified Schwann cells. They detected an endogenously produced BDNF amount of ~570 pg−1 day−1 106 cells, later (4 weeks) decreasing to about 150 pg−1 day−1 106 cells. Therefore, low concentrations of endogenous BDNF (i.e., produced by mammalian cells) result in highly significant neuronal protection. This neuroprotective effect of genetically modified BDNF‐secreting hMSCs was confirmed in the present study for the three MSC‐donors A, B, and C (Figure 8) and therefore seems to be largely donor‐independent pointing to the notion that donor‐specific (autologous) therapy might be an option.
One can speculate that this effect is due to an increased bioactivity of the endogenously produced BDNF. A study on comparison of endogenous and recombinant EPO (erythropoietins) presented data supporting the assumption that there indeed exists a profound structural difference between human endogenous erythropoietins and all pharmaceutical preparations of human recombinant erythropoietins, regardless of whether they are produced by animal (CHO, BHK) or human (HT‐1080) cell lines (Reichel, 2011). Likewise, similar effects cannot be excluded for endogenous and recombinant BDNF. In addition, BDNF is synthesized as a pro‐protein in eukaryotic cells and needs to be processed correctly for optimal biological activity. Consequently, such processes may result indifferent bioavailability and activity of the drugs leading to a much higher neuroprotection in cell‐secreted BDNF than recombinant BDNF (especially if the latter is produced in prokaryotic cell systems including E. coli).
Another reason for the significantly increased neuroprotective effect of endogenously produced BDNF may be the simultaneous production of factors by MSCs which promote the BDNF effect, leading to an increased neuronal survival. Over the past decade, numerous studies have appeared supporting the neuroprotective and neurotrophic effects of the MSC‐secretome (Caseiro, Pereira, Ivanova, Luis, & Mauricio, 2016; Luarte, Batiz, Wyneken, & Lafourcade, 2016; Ratajczak et al., 2016). Increasing evidence indicates that MSCs produce massive amounts of exosomes and that many of the regenerative properties previously credited to stem cells are being shown to be mediated through these secreted exosomes (Basu & Ludlow, 2016; Vizoso, Eiro, Cid, Schneider, & Perez‐Fernandez, 2017).
5.3. Chondrogenic differentiation
We investigated the capacity of BDNF‐secreting hMSCs for in vitro chondrogenic differentiation. This might be interesting insofar as chondrocytes and chondrocyte‐like cells are capable of surviving in low oxygen and low nutrient‐surroundings as they are present in target tissues like the inner ear, especially when encapsulated into biomaterials that are designed to cover neuronal electrodes. During our comparison of donors A and C with respect to their capacity for in vitro‐chondrogenic differentiation, the present study found that the expression and secretion of BDNF did not significantly influence the outcome of chondrogenic differentiation and that MSCs from donor A are much better suited for in vitro‐chondrogenic differentiation than hMSCs from donor C (Figure 8). Such highly donor‐dependent variabilities in differentiating capacities of hMSCs from bone marrow have been found also by other groups. This is the reason why many studies try to identify predictive endogenous hMSC “markers” for in vitro‐chondrogenesis like mRNA expressions of SOX9, ACAN, and ENG as tested here or additional markers like surface proteins including the receptor protein ROR2 (Regeneron Orphan Receptor 2 [Dickinson et al., 2017]). This highly donor‐dependent in vitro‐differentiation capacity challenges the notion that donor‐specific (autologous) therapy might be an option, as stated for the neuroprotective effect of genetically modified BDNF‐secreting hMSCs. Since to our knowledge no such predictive marker for chondrogenic capacity of hMSCs has convincingly been identified this calls for further studies.
We detected an increased BDNF‐production in transfected and differentiated hMSCs during the time period observed within this study (4 weeks). Since with chondrogenic differentiation the cell metabolism decreases the BDNF secretion may be decreased as well. It will be necessary to find out in further studies whether the BDNF‐secretory function of encapsulated but not differentiated hMSCs might be feasible or whether chondrogenic differentiation is necessary or at least prolongs survival of encapsulated and differentiated MSCs. This will help to decide whether autologous or allogeneic hMSCs (the latter with optimized chondrogenic potential) can be used. Additionally, prolonged experiments have to show how long the MSCs survive and keep their secretory activity.
The chondrogenic differentiation method used in this study is based on three‐dimensional pellet culture. The pellets released an amount of BDNF that was sufficient to achieve a neuroprotective effect in vitro (Figure 8) but regardless of the donor and his chondrogenic differentiation potential the diameter of these pellets were too large (≥1.0 mm at the end of the differentiation period) for implantation into or near neuronal tissue or for embedding into an implant coating. To overcome this obstacle, differentiation of cells after encapsulation within an implant coating as described in some studies might present an alternative (Goren, Dahan, Goren, Baruch, & Machluf, 2010). At all time points tested (1, 2, and 3 weeks of chondrogenic differentiation), the infection of the hMSCs with the BDNF‐lentivirus and subsequent BDNF production (Figure 4) resulted in a higher neuronal protection compared to the relevant control (Figure 5). Therefore, it can be concluded that the infection and transfection used in this study are feasible methods to induce a significant neuronal protection in vitro, which moreover is superior compared to the neuroprotective effect of native hMSCs.
5.4. Lentiviral infection with different vector systems
Additionally, to constitutive overexpression of the human BDNF transgene, hMSCs were functionalized to express a fluorescence marker to allow a tracking of the cells without staining. pRRL was used for induction of tdTomato red fluorescence protein expression or pSIEW for green fluorescent protein production. Since the fluorescence genes were coupled to the BDNF gene under the same promotor with an internal ribosomal entry site the fluorescence was directly related to BDNF expression. The neuronal protection efficiency of the fluorophore‐functionalized hMSCs did not differ between the pRRL‐ and pSIEW‐systems even though there was a tendency of increased neuronal survival in the pSIEW‐system compared to pRRL. For future studies which may include in vivo research on the neuroprotective potential of the herein developed cells we recommend to use red fluorescence markers as tdTomato in pRRL if the target tissue, for example the inner ear, may exhibit a green autofluorescence which interferes with detection of the green fluorescence as in pSIEW in subsequent histological analysis.
6. CONCLUSION
Supernatants of native as well as genetically modified and chondrogenically differentiated hMSCs of the different donors significantly protected neurons from degeneration (Figures 3 and 5) despite exhibiting notable donor‐dependent variations in the efficiency of the chondrogenic differentiation itself (Figure 9). This neuroprotective effect by conditioned media from native chondrogenically differentiated hMSCs extends data from previous studies using undifferentiated hMSCs. However, native hMSCs performed less well in neuronal protection than pure recombinant BDNF added to the medium. In contrast, hMSCs genetically modified to overexpress and secrete BDNF performed tremendously better than conditioned medium from native hMSCs. Therefore, with respect to neuronal protection, autologous application of hMSCs seems a feasible approach for individualized therapy of neuronal degeneration. Future investigations will need to show whether or not the chondrogenic differentiation of the genetically modified BDNF overexpressing hMSCs is necessary for long‐term survival and BDNF delivery in cell matrices implanted into the relevant target tissue.
DECLARATION OF TRANSPARENCY
The authors, reviewers, and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
CONFLICT OF INTEREST
The author reports no conflicts of interest in this work.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization: V.S. and A.Ho.; Methodology: V.S., J.S. A.Ho.; Investigation: J.S., A.Ha and A.Ho.; Formal Analysis: V.S., J.S., A.Ha., and A.Ho.; Resources: V.S., T.L., A.Ho.; Writing – Original Draft: V.S.; Writing – Review & Editing: V.S., J.S., A.Ha., T.L., A.Ho.; Visualization: V.S., J.S., T.L. A.Ho.; Funding Acquisition: V.S., A.Ho.
Supporting information
[Correction added on July 30, 2019 after first online publication: The heading ‘Transparent Peer Review Report.’ was added and Peer review communication document was uploaded online.]
Transparent Science Questionnaire for Authors.
Transparent Peer Review Report.
ACKNOWLEDGMENTS
We acknowledge funding by the DFG (Deutsche Forschungsgemeinschaft, German Research Foundation) by projects HO 2058/13‐1 to A.H. and SCHE 1663/2‐1 to V. S. Plasmid pHR‐SIN‐cppt‐SFFV‐IEW (pSIEW) was kindly provided by Prof. Dr. Michaela Scherr, Hannover Medical School. Plasmid pRRL.PPT.SFFV (pRRL) was kindly provided by Prof. Dr. Axel Schambach, Hannover Medical School. We highly appreciate provision of bone marrow samples by PD Dr. Thilo Flörkemeier, Hannover Medical School.
Scheper V, Schwieger J, Hamm A, Lenarz T, Hoffmann A. BDNF‐overexpressing human mesenchymal stem cells mediate increased neuronal protection in vitro . J Neuro Res. 2019;97:1414–1429. 10.1002/jnr.24488
Edited by Stephane Lefrancois. Reviewed by Chengbiao Wu.
All peer review communications can be found with the online version of the article.
Funding information
Support or grant information: DFG (Deutsche Forschungsgemeinschaft, German Research Foundation) by projects HO 2058/13‐1 to A.H. and SCHE 1663/2‐1 to V. S
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Arnhold, S. , Klein, H. , Klinz, F. J. , Absenger, Y. , Schmidt, A. , Schinkothe, T. , … Addicks, K. (2006). Human bone marrow stroma cells display certain neural characteristics and integrate in the subventricular compartment after injection into the liquor system. European Journal of Cell Biology, 85(6), 551–565. 10.1016/j.ejcb.2006.01.015 [DOI] [PubMed] [Google Scholar]
- Balaratnasingam, S. , & Janca, A. (2012). Brain derived neurotrophic factor: A novel neurotrophin involved in psychiatric and neurological disorders. Pharmacology & Therapeutics, 134(1), 116–124. 10.1016/j.pharmthera.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Basu, J. , & Ludlow, J. W. (2016). Exosomes for repair, regeneration and rejuvenation. Expert Opinion on Biological Therapy, 16(4), 489–506. 10.1517/14712598.2016.1131976 [DOI] [PubMed] [Google Scholar]
- Benussi, L. , Binetti, G. , & Ghidoni, R. (2017). Loss of neuroprotective factors in neurodegenerative dementias: The end or the starting point? Frontiers in Neuroscience, 11, 672 10.3389/fnins.2017.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdjian, A. , Kraaijenga, V. J. , Ramekers, D. , Versnel, H. , Thomeer, H. G. , Klis, S. F. , & Grolman, W. (2016). Towards clinical application of neurotrophic factors to the auditory nerve; assessment of safety and efficacy by a systematic review of neurotrophic treatments in humans. International Journal of Molecular Sciences, 17(12). 10.3390/ijms17121981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj, R. , & Deshmukh, R. (2018). Neurotrophic factors and Parkinson's disease. Clinical Investigation, 7(4), 53–62. [Google Scholar]
- Caseiro, A. R. , Pereira, T. , Ivanova, G. , Luis, A. L. , & Mauricio, A. C. (2016). Neuromuscular regeneration: Perspective on the application of mesenchymal stem cells and their secretion products. Stem Cells International, 2016, 9756973 10.1155/2016/9756973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, M. V. (2003). Neurotrophins and their receptors: A convergence point for many signalling pathways. Nature Reviews Neuroscience, 4(4), 299–309. 10.1038/nrn1078 [DOI] [PubMed] [Google Scholar]
- Dickinson, S. C. , Sutton, C. A. , Brady, K. , Salerno, A. , Katopodi, T. , Williams, R. L. , … Hollander, A. P. (2017). The Wnt5a receptor, receptor tyrosine kinase‐like orphan receptor 2, is a predictive cell surface marker of human mesenchymal stem cells with an enhanced capacity for chondrogenic differentiation. Stem Cells, 35(11), 2280–2291. 10.1002/stem.2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, W. , Li, J. , Wang, Y. , Pan, J. , Li, S. , Zhu, L. , … Yan, Z. (2016). CD105 promotes chondrogenesis of synovium‐derived mesenchymal stem cells through Smad2 signaling. Biochemical and Biophysical Research Communications, 474(2), 338–344. 10.1016/j.bbrc.2016.04.101 [DOI] [PubMed] [Google Scholar]
- Geral, C. , Angelova, A. , & Lesieur, S. (2013). From molecular to nanotechnology strategies for delivery of neurotrophins: Emphasis on brain‐derived neurotrophic factor (BDNF). Pharmaceutics, 5(1), 127–167. 10.3390/pharmaceutics5010127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren, A. , Dahan, N. , Goren, E. , Baruch, L. , & Machluf, M. (2010). Encapsulated human mesenchymal stem cells: A unique hypoimmunogenic platform for long‐term cellular therapy. FASEB Journal, 24(1), 22–31. 10.1096/fj.09-131888 [DOI] [PubMed] [Google Scholar]
- Hegarty, J. L. , Kay, A. R. , & Green, S. H. (1997). Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. Journal of Neuroscience, 17(6), 1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. , Floerkemeier, T. , Melzer, C. , & Hass, R. (2017). Comparison of in vitro‐cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord. Journal of Tissue Engineering and Regenerative Medicine, 11(9), 2565–2581. [DOI] [PubMed] [Google Scholar]
- Hütten, M. , Ehrhart, F. , Zimmermann, H. , Reich, U. , Esser, K. H. , Lenarz, T. , & Scheper, V. (2013). UHV‐alginate as matrix for neurotrophic factor producing cells‐a novel biomaterial for cochlear implant optimization to preserve inner ear neurons from degeneration. Otology & Neurotology, 34(6), 1127–1133. 10.1097/MAO.0b013e3182804949 [DOI] [PubMed] [Google Scholar]
- Jungwirth, N. , Salinas Tejedor, L. , Jin, W. , Gudi, V. , Skripuletz, T. , Stein, V. M. , … Hansmann, F. (2018). Mesenchymal stem cells form 3D clusters following intraventricular transplantation. Journal of Molecular Neuroscience, 65(1), 60–73. 10.1007/s12031-018-1070-x [DOI] [PubMed] [Google Scholar]
- Konerding, W. S. , Janssen, H. , Hubka, P. , Tornoe, J. , Mistrik, P. , Wahlberg, L. , … Scheper, V. (2017). Encapsulated cell device approach for combined electrical stimulation and neurotrophic treatment of the deaf cochlea. Hearing Research, 350, 110–121. 10.1016/j.heares.2017.04.013 [DOI] [PubMed] [Google Scholar]
- Korte, M. , Carroll, P. , Wolf, E. , Brem, G. , Thoenen, H. , & Bonhoeffer, T. (1995). Hippocampal long‐term potentiation is impaired in mice lacking brain‐derived neurotrophic factor. Proceedings of the National Academy of Sciences, 92(19), 8856–8860. 10.1073/pnas.92.19.8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake, P. A. , Hradek, G. T. , Hetherington, A. M. , & Stakhovskaya, O. (2011). Brain‐derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. The Journal of Comparative Neurology, 519(8), 1526–1545. 10.1002/cne.22582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, P. , Lin, Y. , Lennon, D. P. , Correa, D. , Schluchter, M. , & Caplan, A. I. (2012). Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Translational Medicine, 1(12), 886–897. 10.5966/sctm.2012-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, P. , Jones, L. L. , & Tuszynski, M. H. (2005). BDNF‐expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Experimental Neurology, 191(2), 344–360. 10.1016/j.expneurol.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Luarte, A. , Batiz, L. F. , Wyneken, U. , & Lafourcade, C. (2016). Potential therapies by stem cell‐derived exosomes in CNS diseases: Focusing on the neurogenic niche. Stem Cells International, 2016, 5736059 10.1155/2016/5736059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim, R. W. (1991). Cell death during development of the nervous system. Annual Review of Neuroscience, 14, 453–501. 10.1146/annurev.ne.14.030191.002321 [DOI] [PubMed] [Google Scholar]
- Poduslo, J. F. , & Curran, G. L. (1996). Permeability at the blood‐brain and blood‐nerve barriers of the neurotrophic factors: NGF, CNTF, NT‐3, BDNF. Molecular Brain Research, 36(2), 280–286. 10.1016/0169-328X(95)00250-V [DOI] [PubMed] [Google Scholar]
- Ramku, E. , Ramku, R. , Spanca, D. , & Zhjeqi, V. (2017). Functional pattern of increasing concentrations of brain‐derived neurotrophic factor in spiral ganglion: Implications for research on cochlear implants. Open Access Macedonian Journal of Medical Sciences, 5(2), 121–125. 10.3889/oamjms.2017.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak, J. , Bronckaers, A. , Dillen, Y. , Gervois, P. , Vangansewinkel, T. , Driesen, R. B. , … Hilkens, P. (2016). The Neurovascular properties of dental stem cells and their importance in dental tissue engineering. Stem Cells International, 2016, 9762871 10.1155/2016/9762871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel, C. (2011). The overlooked difference between human endogenous and recombinant erythropoietins and its implication for sports drug testing and pharmaceutical drug design. Drug Testing and Analysis, 3(11–12), 883–891. 10.1002/dta.388 [DOI] [PubMed] [Google Scholar]
- Rejali, D. , Lee, V. A. , Abrashkin, K. A. , Humayun, N. , Swiderski, D. L. , & Raphael, Y. (2007). Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hearing Research, 228(1–2), 180–187. 10.1016/j.heares.2007.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäck, L. M. , Noack, S. , Weist, R. , Jagodzinski, M. , Krettek, C. , Buettner, M. , & Hoffmann, A. (2013). Analysis of surface protein expression in human bone marrow stromal cells: New aspects of culture‐induced changes, inter‐donor differences and intracellular expression. Stem Cells and Development, 22(24), 3226–3235. [DOI] [PubMed] [Google Scholar]
- Schmidt, N. , Schulze, J. , Warwas, D. P. , Ehlert, N. , Lenarz, T. , Warnecke, A. , & Behrens, P. (2018). Long‐term delivery of brain‐derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro. PLoS One, 13(3), e0194778 10.1371/journal.pone.0194778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen, T. D. , & Livak, K. J. (2008). Analyzing real‐time PCR data by the comparative C(T) method. Nature Protocols, 3(6), 1101–1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Schulze, J. , Kaiser, O. , Paasche, G. , Lamm, H. , Pich, A. , Hoffmann, A. , … Warnecke, A. (2017). Effect of hyperbaric oxygen on BDNF‐release and neuroprotection: Investigations with human mesenchymal stem cells and genetically modified NIH3T3 fibroblasts as putative cell therapeutics. PLoS One, 12(5), e0178182 10.1371/journal.pone.0178182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieger, J. , Warnecke, A. , Lenarz, T. , Esser, K.‐H. , & Scheper, V. (2015). Neuronal survival, morphology and outgrowth of spiral ganglion neurons using a defined growth factor combination. PLoS One, 10(8), e0133680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzing, A. , Jones, E. , McGonagle, D. , & Scutt, A. (2008). Age‐related changes in human bone marrow‐derived mesenchymal stem cells: Consequences for cell therapies. Mechanisms of Ageing and Development, 129(3), 163–173. 10.1016/j.mad.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Vizoso, F. J. , Eiro, N. , Cid, S. , Schneider, J. , & Perez‐Fernandez, R. (2017). Mesenchymal stem cell secretome: Toward cell‐free therapeutic strategies in regenerative medicine. International Journal of Molecular Sciences, 18(9). 10.3390/ijms18091852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg, L. U. , Lind, G. , Almqvist, P. M. , Kusk, P. , Tornoe, J. , Juliusson, B. , … Linderoth, B. (2012). Targeted delivery of nerve growth factor via encapsulated cell biodelivery in Alzheimer disease: A technology platform for restorative neurosurgery. Journal of Neurosurgery, 117(2), 340–347. 10.3171/2012.2.JNS11714 [DOI] [PubMed] [Google Scholar]
- Warnecke, A. , Sasse, S. , Wenzel, G. I. , Hoffmann, A. , Gross, G. , Paasche, G. , … Wissel, K. (2012). Stable release of BDNF from the fibroblast cell line NIH3T3 grown on silicone elastomers enhances survival of spiral ganglion cells in vitro and in vivo. Hearing Research, 289(1–2), 86–97. [DOI] [PubMed] [Google Scholar]
- Wefstaedt, P. , Scheper, V. , Lenarz, T. , & Stover, T. (2005). Brain‐derived neurotrophic factor/glial cell line‐derived neurotrophic factor survival effects on auditory neurons are not limited by dexamethasone. NeuroReport, 16(18), 2011–2014. 10.1097/00001756-200512190-00008 [DOI] [PubMed] [Google Scholar]
- Weissmiller, A. M. , & Wu, C. (2012). Current advances in using neurotrophic factors to treat neurodegenerative disorders. Translational Neurodegeneration, 1(1), 14 10.1186/2047-9158-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins, A. , Kemp, K. , Ginty, M. , Hares, K. , Mallam, E. , & Scolding, N. (2009). Human bone marrow‐derived mesenchymal stem cells secrete brain‐derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Research, 3(1), 63–70. 10.1016/j.scr.2009.02.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Correction added on July 30, 2019 after first online publication: The heading ‘Transparent Peer Review Report.’ was added and Peer review communication document was uploaded online.]
Transparent Science Questionnaire for Authors.
Transparent Peer Review Report.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


