Abstract
Severe infections (SI) significantly impact on non‐relapse mortality after hematopoietic stem cell transplantation (HSCT). We assessed 432 children and adolescents with acute lymphoblastic leukemia (ALL) after total body irradiation based myeloablative HSCT within the multicenter ALL‐BFM‐SCT 2003 trial for SI grade 3 or higher according to common terminology criteria for adverse events. A total 172 patients experienced at least one SI. Transplantation from matched unrelated donors (MUD) was associated with any type of SI in the pre‐engraftment period (hazard ratio [HR]: 2.57; P < .001), and with any SI between day +30 and + 100 (HR: 2.91; P = .011). Bacterial (HR: 2.24; P = .041) and fungal infections (HR: 4.06; P = .057) occurred more often in the pre‐engraftment phase and viral infections more often before day +30 (HR: 2.66; P = .007) or between day +30 and + 100 (HR: 3.89; P = .002) after HSCT from MUD as compared to matched sibling donors. Chronic GvHD was an independent risk factor for any type of SI after day +100 (HR: 2.57; P < .002). We conclude that allogeneic HSCT from MUD in children and adolescents with pediatric ALL is associated with higher infection rates, which seems attributable to an intensified GvHD prophylaxis including serotherapy and methotrexate.
1. INTRODUCTION
Severe infections have remained an important cause of non‐relapse mortality (NRM) after allogeneic hematopoietic stem cell transplantation (HSCT).1, 2, 3 Infections account for almost 30% of all hospital re‐admissions within the first 100 days after HSCT using myeloablative or reduced intensity conditioning.4, 5
A patients' individual risk for infections after HSCT is influenced by multiple factors including patient characteristics, transplant modalities and post‐transplant immune reconstitution. For instance, achieving a sustained neutrophil engraftment is paramount to limit the occurrence of infections caused by gram‐positive and gram‐negative bacteria or fungi, which are usually observed in the early neutropenic period.6, 7 Moreover, children and adults transplanted from unrelated donors and those experiencing acute graft‐vs‐host disease (GvHD) are at higher risk for bacterial and fungal infections.8, 9, 10, 11, 12, 13
Viral infections or reactivations are typically observed in the post‐engraftment phase, in particular in patients with delayed T‐cell reconstitution or sustained immunosuppression due to acute or chronic GvHD.7, 14, 15, 16 The most relevant viral infections after HSCT include cytomegalovirus (CMV), adenovirus (AdV) or Ebstein‐Barr virus (EBV).
CMV reactivation and ‐disease is influenced by recipient and donor pre‐transplant serostatus and by the presence of CMV‐specific T‐cells post‐transplant.17, 18, 19 Young age, unrelated or HLA‐mismatched donor transplantation, GvHD and delayed T‐cell reconstitution are associated with an increased incidence of ADV infections.20, 21
The incidence of late opportunistic infections occurring beyond day +100 increases after HSCT from unrelated donors and in patients with advanced GvHD.22 In particular, older patients, individuals with CMV reactivation or patients suffering from severe GvHD are affected by late fungal infections.23, 24
The multicenter ALL‐SCT BFM 2003 trial revealed comparable event‐free survival and relapse rates in children with acute lymphoblastic leukemia (ALL). This was after transplantation from HLA‐matched sibling (MSD) or matched unrelated donors (MUD), defined by HLA match in at least nine of ten loci. However, there was a significantly higher cumulative incidence of NRM after HSCT from MUD, which was primarily attributable to severe infections (16% vs 39%; P < .001).25 To further elucidate the complex interplay between contributing factors and infection risk after HSCT for pediatric high‐risk ALL, a cohort of 432 children and young adults with ALL, in first or second complete remission (CR) after total body irradiation, (TBI)‐based myeloablative conditioning was analyzed. This was done retrospectively for clinically severe bacterial, viral or fungal infections at defined time points after transplantation. All patients were registered in the multicenter ALL‐SCT‐BFM 2003 trial.
2. METHODS
This study was conducted as supplementary research project of the international multicenter ALL‐SCT‐BFM 2003 trial,25 which recruited patients prospectively between 2003 and 2011. The recruitment was thereafter extended until 2013.
An approval of the local institutional review board has been obtained at each participating site and the study was performed in accordance with the Declaration of Helsinki. A written informed consent signed by the patients or their legal guardians was obtained before enrolment into the study.
Children in first or subsequent CR of ALL were eligible for study enrolment if an allogeneic HSCT was indicated according to the risk‐adapted stratification criteria of the Berlin‐Frankfurt‐Münster (BFM) Study Group.26, 27, 28 According to the concept of the BFM study group, patients in CR1 were transplanted after minimum of three high‐risk (HR) chemotherapy‐blocks, aiming to ensure a sound molecular remission with targeted minimal residual disease (MRD) levels below 10E‐3. Patients with standard‐risk relapsed ALL were eligible for HSCT after at least five chemotherapy blocks, and patients with high‐risk relapses after at least three blocks, according to the BFM concept, aiming MRD levels below 10E‐3 before HSCT respectively. Detailed information on study design, criteria for patient and donor selection and conditioning regimens has been published previously.25
The GvHD‐prophylaxis for patients transplanted from MSD consisted of cyclosporin A (CsA) only, starting at day −1, and given at a dosage of 2 x 1.5 mg/kg BW as infusion over 2 hours each. The GvHD‐prophylaxis in patients transplanted from MUD included administration of anti‐thymocyte globulin (ATG Fresenius, given at a dose of 20 mg/kg/d on day −3, −2 and − 1), CsA as mentioned for MSD, and short‐course methotrexate (MTX 10 mg/m2; administered on days +1, +3, and + 6, followed by leucovorin rescue 24 hours later, at a dose of 15 mg/m2).
2.1. Definition of infection‐related complications/microbiological methods
The cohort was evaluated for severe infections (SI) graded III or higher, according to the common terminology criteria for adverse events (CTCAE).29 All reported SI were then analyzed for the respective contributing pathogens, and grouped according to their appearance as follows: Infections occurring within the first 30 days after HSCT, infections between day +30 and day +100, or infections beyond day +100 after HSCT.
In symptomatic patients, an infection was classified as confirmed when at least one positive microbiological testing result was obtained. Positive results from surveillance cultures due to colonization or contamination in otherwise asymptomatic patients and asymptomatic viremias were not considered.
The onset of an infection was defined as the day when the first positive testing result was recorded. The applied methods included direct culture (bacteria and fungi), or PCR‐based methods to detect pathogen DNA (viruses) from otherwise sterile materials (whole blood, plasma, urine, cerebrospinal fluid). A central review of reported infections was not performed.
Bacterial infections were categorized as gram‐positive (GP), gram‐negative (GN) or mixed infections if different species were isolated. Confirmed invasive fungal infections were categorized as either Aspergillus‐ or Candida spp related infections or as other fungal infections including mucormycetes. For virus infections, only patients with infections graded III or IV according to CTCAE, in combination with a proven viral pathogen, were considered as clinically relevant virus infections. The evaluated viral infections included Cytomegalovirus (CMV), Ebstein‐Barr virus (EBV), Adenoviruses (AdV), Herpes simplex virus (HSV), Varicella Zoster virus (VZV), BK Virus (BKV), or other viruses.
Only the first infection of the respective pathogen in a given patient within the pre‐defined time periods was considered for the calculation of transplant outcomes, per previous publications.22, 30 Also, transplant‐related variables considered relevant to impact on infection risk were retrieved from our database, and put in the univariate and multivariate analysis models. The following potential risk factors were considered: Patient age, donor and recipient gender, ALL immunophenotype, remission status, time between diagnosis of ALL or relapse and HSCT, donor type, serotherapy, stem cell source, graft CD34+ cell content, recipient and donor serostatus for CMV and EBV, acute GvHD and chronic GvHD.
2.2. Supportive care and infection prophylaxis
Detailed information on supportive care recommendations is provided elsewhere.25 In brief, all patients were nursed in rooms with reverse isolation and high‐efficiency particulate air filters during the neutropenic period, and received a low bacterial diet.
The protocol defined no general recommendation for antibacterial or antifungal prophylaxis. However, most centers provided an antifungal prophylaxis either with azoles or amphotericin B according to their local in‐house policies.
Prophylaxis against HSV and VZV usually consisted of acyclovir starting at day +1. A CMV IgG seropositive recipient transplanted from a CMV IgG seronegative donor, thus implicating the highest risk for CMV reactivation, indicated the administration of prophylactic ganciclovir. A prospective virus‐screening for CMV, AdV and EBV in the peripheral blood usually performed on a weekly basis was available to most participating centers. If screening tests were positive, the patients received a pre‐emptive therapy with ganciclovir, cidofovir or rituximab, respectively.
Prophylaxis against pneumocystis jirovecii primarily consisted of trimethoprim/sulfamethoxazole starting at day +28.
All prophylactic therapies were carried on until the immunosuppression was stopped or adequate immunologic recovery was reached.
The institution and choice of empirical first‐line treatment of febrile neutropenia with broad‐spectrum antibiotics was left at the discretion of the attending physicians and was carried out according to local center policies.
2.3. Statistical analysis
For non‐time to event variables, the chi‐Square and Fisher's exact test were used to compare groups of categorical variables. Wilcoxon rank‐sum test (Kruskal‐Wallis test for more than two populations) was used for continuous variables. The cumulative incidence (CI) of infection was analyzed for the whole cohort of patients. This was also done separately for the time periods before day +30, between day +30 and + 100, and beyond day +100 after HSCT, and for the different types of infections. The CI was calculated for first episode of infection of respective type and for the respective time period. The univariate analysis was conducted according to the method of Kalbfleisch and Prentice,31 groups were compared using the Gray test.32 The role of risk factors for the CI of infections was further analyzed in a multivariate proportional sub‐distribution hazards model by Fine and Gray for censored data subject to competing risks.33 Relapse and death without infection were defined as competing events. Acute and chronic GvHD were considered time dependent, while for non‐relapse mortality (NRM), relapses and secondary malignancies were considered competing events. All P values below .05 were considered significant. The statistical analyses were performed by means of the SAS version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
In total, 432 children and adolescents with ALL in first (n = 233) or second (n = 199) CR, and available data on infection‐related toxicity after myeloablative conditioning with TBI (12Gy administered in 6 fractions), and intravenous etoposide (60 mg/kg), were available for evaluation. Median age at HSCT was 10.3 years (range: 2.0‐18.5), and the donors were MSD in 129 cases, and MUD in 303 cases. Allogeneic HSCT was performed with a median at 6.6 months (range: 4.0‐17.2 months), after first diagnosis of ALL in patients with CR1 and at a median of 5.2 months (range: 2.7‐9.1 months), after diagnosis of relapse in patients with CR2. The median follow‐up from HSCT until last follow‐up evaluation was 5.4 years (range 0.04‐11.7). An overview of clinical and transplant characteristics is shown in Table 1.
Table 1.
Transplant characteristics
| Characteristics | Number (%) |
|---|---|
| Diagnosis | |
| ALL CR1 | 233 (54%) |
| ALL CR2 | 199 (46%) |
| Recipient age | |
| ≤10 y | 202 (47%) |
| 10‐15 y | 144 (33%) |
| ≥15 y | 86 (20%) |
| Recipient gender | |
| Female | 151 (35%) |
| Male | 278 (64%) |
| Data not available | 3 (1%) |
| Donor/Recipient gender constellation | |
| Male/Male | 186 (43%) |
| Male/Female | 84 (19%) |
| Female/Male | 92 (21%) |
| Female/Female | 67 (16%) |
| Data not available | 3 (1%) |
| Donor | |
| Matched sibling donor (MSD)a | 129 (30%) |
| Matched unrelated donor (MUD) | 303 (70%) |
| Serotherapy | |
| ATG Fresenius | 300 (69%) |
| Not administered | 130 (30%) |
| Data not available | 2 (1%) |
| Graft type | |
| Bone Marrow (BM) | 327 (76%) |
| Peripheral Blood Stem Cells (PBSC) | 105 (24%) |
| Graft composition | |
| CD34+ <3 × 106/kg BW | 118 (27%) |
| CD34+ >3 × 106/kg BW | 305 (71%) |
| Data not available | 9 (2%) |
| ALL phenotype | |
| B‐cell precursor ALL | 307 (71%) |
| T‐ALL | 98 (23%) |
| Biphenotypic ALL | 4 (1%) |
| Data not available | 23 (5%) |
Abbreviations: ALL, acute lymphoblastic leukemia; ATG, Anti‐thymocyte globulin; CR, complete remission.
Patients transplanted from MSD did not receive serotherapy with ATG.
3.1. Neutrophil engraftment
Neutrophil engraftment (NE) defined as absolute neutrophil count (ANC) of >500 per microliter peripheral blood, was observed in 424 of 432 (98%) patients after a median time of 21 days (range: 8‐108 days). Six patients died before achieving NE and two patients never reached 500 ANC after HSCT. The median time to NE in patients transplanted from MSD was 18 days (range: 9‐45) vs 23 days for the MUD group (range: 8‐108) (P < .001).
3.2. Cumulative incidences of infections at pre‐defined time periods
Among 172 of 432 patients (40%) with at least one single SI reported, 464 SI were registered in total, including bacteria in 122 (26%) cases, viruses in 274 (59%), fungi in 29 cases (6%) and protozoa in one case (0.2%) respectively (Table S1). In 38 cases (8%) a pathogen was not confirmed despite an overt clinical presentation suggesting a SI. The majority of SI (56%) was classified as systemic infection due to positive testing results from blood samples. In cases of severe localized infections, the affected sites included the gastrointestinal tract (26%), the urinary tract (17%), the lungs (12%), skin or soft tissues (11%), the central nervous system (4%) or various other sites (30%). The median time to the first SI caused by any pathogen was 19 days (range: 0‐1441).
Bacterial infections were caused by GP strains in 62% (76/122); 26% (32/122) had GN infections, and 12% (14/122) had mixed or atypical bacterial infections. Gram‐positive strains included Staphylococcus epidermidis (22%), Staphylococcus haemolyticus (11%) or other coagulase‐negative Staphylococci (9%), Enterococcus spp. (16%) or Streptococcus viridans group (7%). The most frequently isolated GN pathogens included Escherichia spp. (25%), Pseudomonas spp. (19%), Enterobacter spp. (9%) and Klebsiella spp. (6%). Infections by GP bacteria were encountered earlier than GN infections (median onset: 15 days (range: 0‐1441) vs 87 days (range: 0‐533)). Gram‐positive infections outnumbered GN infections over all time periods as shown in Figure S1A.
Viral infections were caused by EBV in 58 (21%), CMV in 45 (17%), BKV in 56 (20%) and AdV in 38 (14%) of reported events respectively. In 77 (28%) cases, other viruses such as VZV or different Herpesviruses were found. The median onset for any viral infection was 46 days (range: 1‐1776), with infections by BKV appearing earlier than all other viruses investigated (median onset: 22 days; range: 3‐405). Figure S1B demonstrates the distribution of viral infections.
Of 29 reported fungal infections, 14 (48%) were caused by Aspergillus spp., 11 (38%) by Candida spp. and 4 (14%) by other fungi including mucormycetes. The median onset of fungal infections was 18 days (range: 1‐306). Infections by Candida spp. appeared earlier than infections by Aspergillus spp., with a median onset of 17 days (range: 1‐306) and 29 days (range: 5‐285) respectively. Fungal infections are shown in Figure S1C.
The cumulative incidence (CI) of the first SI by any pathogen was 0.27 ± 0.02 at day +30, 0.33 ± 0.02 at day +100 and 0.39 ± 0.02 1 year after HSCT. The CIs of the first reported severe bacterial, viral and fungal infections were 0.12 ± 0.02, 0.15 ± 0.02, and 0.04 ± 0.01 at day +30; 0.16 ± 0.02, 0.24 ± 0.02; 0.05 ± 0.01 at day +100 and 0.21 ± 0.02; 0.29 ± 0.02 and 0.06 ± 0.01 1 year after HSCT. The CI of infections are shown in Figures 1 and 2.
Figure 1.
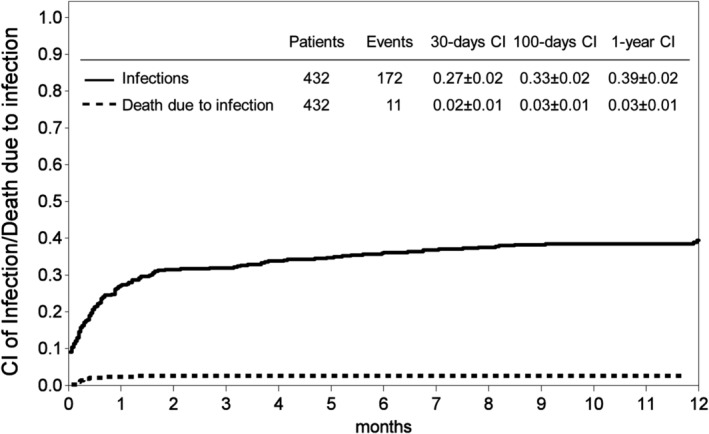
Cumulative incidences (CI) of the first severe infection and CI of death due to infection at day +30, +100 and 1 year after transplant for any type of infections
Figure 2.
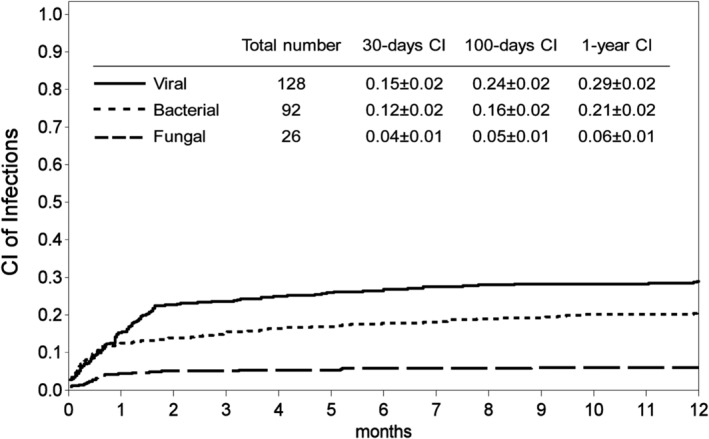
Cumulative incidences (CI) of bacterial, fungal and viral infections at day +30, +100 and 1 year after transplant
3.3. Impact of transplant‐specific parameters on infections after HSCT: Univariate and multivariate analysis
The donor type correlated significantly with the incidence of SI. Transplantation from MSD, compared to MUD, was associated with lower rates for any SI occurring within the first 30 days (16% vs 32%; P < .001), or between day +30 and day +100 (6% vs 23%; P < .001), while SI beyond day +100 were not different among the donor groups. Comparable results were obtained for different pathogens. The infection incidences within 30 days after HSCT were lower after HSCT from MSD, compared to the MUD group (8% vs 15% (P = .057) for bacterial infections, 8% vs 18% (P < .005) for viral and 2% vs 6% (P = .073) for fungal infections). The incidence of viral infections in the post‐engraftment period was significantly lower in the MSD group (5% vs 19%; P < .001).
Serotherapy with anti‐thymocyte globulin (ATG) as administered for HSCT from MUD was associated with higher overall infection rates within the first 30 days (31% vs 17%; P = .002), and between day +30 and + 100 (23% vs 7%; P < .001), as compared to transplantation without ATG in the MSD group. Similar results were found for bacterial (14% vs 8%, P = .113), viral (18% vs 9%, P = .001) and fungal infections (6% vs 2%, P = .072) within the first 30 days. Between day +30 and day +100, significantly more viral infections were observed in the serotherapy group (19% vs 6%; P = .001).
The ALL phenotype, the remission status, recipient age, donor/recipient gender constellation, the graft type, graft CD34+ content, CMV‐ or EBV donor/recipient serostatus, or the time interval from diagnosis to HSCT, did not significantly impact infection rates for the defined time periods. Table 2 summarizes the univariate analysis (infection subtypes not included).
Table 2.
Risk factors associated with severe infections—univariate analysis
| Infections < day 30 | Infections day 30‐100 | Infections > day 100 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Infec‐tions | % | P value | Patients | Infec‐tions | % | P value | Patients | Infec‐tions | CI ± SE | P value | |
| Patients total | 432 | 117 | 27 | 429 | 77 | 18 | 344 | 60 | 0.13 ± 0.02 | |||
| Remission status | ||||||||||||
| CR1 | 233 | 58 | 25 | .279 | 232 | 38 | 16 | .379 | 184 | 29 | 0.12 ± 0.02 | .364 |
| CR2 | 199 | 59 | 30 | 197 | 39 | 20 | 160 | 31 | 0.14 ± 0.03 | |||
| Recipient age | ||||||||||||
| ≤10 y | 202 | 52 | 26 | .819 | 200 | 37 | 19 | .797 | 171 | 30 | 0.15 ± 0.03 | .989 |
| 10‐15 y | 144 | 40 | 28 | 144 | 27 | 19 | 116 | 20 | 0.10 ± 0.03 | |||
| >15 y | 86 | 25 | 29 | 85 | 13 | 15 | 57 | 10 | 0.12 ± 0.04 | |||
| Donor/Recipient gender | ||||||||||||
| Female/Male | 92 | 28 | 30 | .428 | 92 | 22 | 24 | .086 | 74 | 18 | 0.15 ± 0.04 | .069 |
| Other | 337 | 88 | 26 | 334 | 52 | 16 | 266 | 40 | 0.12 ± 0.02 | |||
| Data missing | 3 | 1 | 3 | 3 | 4 | 2 | ||||||
| Donor type | ||||||||||||
| MSDa | 129 | 20 | 16 | <.001 | 129 | 8 | 6 | <.001 | 113 | 18 | 0.11 ± 0.03 | .560 |
| MUD | 303 | 97 | 32 | 300 | 69 | 23 | 231 | 42 | 0.14 ± 0.02 | |||
| CMV status | ||||||||||||
| Patient+ /Donor‐ | 63 | 17 | 27 | 1.0 | 63 | 14 | 22 | .377 | 52 | 8 | 0.14 ± 0.05 | .704 |
| Other | 364 | 98 | 27 | 361 | 63 | 17 | 287 | 52 | 0.13 ± 0.02 | |||
| Data missing | 5 | 2 | 5 | 5 | 0 | |||||||
| EBV status | ||||||||||||
| Patient+ /Donor‐ | 49 | 6 | 12 | .015 | 48 | 6 | 13 | .323 | 45 | 7 | 0.16 ± 0.05 | .702 |
| Other | 348 | 99 | 28 | 346 | 67 | 19 | 264 | 48 | 0.12 ± 0.02 | |||
| Data missing | 35 | 12 | 35 | 4 | 35 | 5 | ||||||
| Serotherapy | ||||||||||||
| No serotherapy | 130 | 22 | 17 | .002 | 130 | 9 | 7 | <.001 | 113 | 19 | 0.11 ± 0.03 | .743 |
| ATG Fresenius | 300 | 94 | 31 | 297 | 67 | 23 | 229 | 14 | 0.14 ± 0.02 | |||
| Data missing | 2 | 1 | 50 | 2 | 1 | 2 | 0 | |||||
| Graft composition | ||||||||||||
| ≤3 × 106/kg | 118 | 32 | 27 | 1.0 | 116 | 19 | 16 | .775 | 91 | 15 | 0.13 ± 0.04 | .779 |
| >3 × 106/kg | 305 | 82 | 27 | 304 | 55 | 18 | 247 | 44 | 0.13 ± 0.02 | |||
| Data missing | 9 | 3 | 9 | 3 | 6 | 1 | ||||||
| Graft type | ||||||||||||
| BM | 327 | 87 | 27 | .706 | 324 | 55 | 17 | .381 | 267 | 48 | 0.14 ± 0.02 | .643 |
| PBSC | 105 | 30 | 29 | 105 | 22 | 21 | 77 | 12 | 0.10 ± 0.04 | |||
| ALL phenotype | ||||||||||||
| B‐cell precursor | 307 | 85 | 28 | .549 | 304 | 59 | 19 | .105 | 254 | 42 | 0.11 ± 0.02 | .386 |
| T‐ALL | 98 | 26 | 27 | 98 | 14 | 14 | 73 | 15 | 0.16 ± 0.04 | |||
| Other/n.a. | 27 | 6 | 22 | 27 | 4 | 15 | 17 | 3 | ||||
| Time to HSCT | ||||||||||||
| <6 mo | 201 | 50 | 25 | .730 | 200 | 32 | 16 | .787 | 166 | 32 | 0.15 ± 0.03 | .227 |
| ≥6 mo | 194 | 52 | 27 | 193 | 33 | 17 | 152 | 22 | 0.11 ± 0.03 | |||
| Data missing | 37 | 15 | 36 | 12 | 26 | 6 | ||||||
Abbreviations: ALL, acute lymphoblastic leukemia; ATG, Anti‐thymocyte globulin; BM, bone marrow; CMV, cytomegalovirus; CR, complete remission; EBV, Ebstein‐Barr virus; GvHD, graft‐vs‐host disease; MSD, matched sibling donor; MUD, matched unrelated donor; n.a., not available; PBSC, peripheral blood stem cells.
Patients transplanted from MSD did not receive serotherapy with ATG Fresenius.
In our multivariate regression model, the donor type significantly correlated with SI. Transplantation from MUD was associated with any SI during the pre‐engraftment period (HR: 2.57; 95% CI: 1.52‐4.33; P < .001), and with any SI between day +30 and + 100 (HR: 2.91; 95% CI: 1.28‐6.61; P = .011).
Patients with documented SI before day +30, or day +100, carried a significant risk of experiencing another SI in the subsequent observation period (HR: 7.94; 95% CI: 4.55‐13.84; P < .001 for infections between day +30 and + 100; HR: 1.96; 95% CI: 1.09‐3.53; P = .024 for infections after day +100).
Chronic GvHD was an independent risk factor for any SI beyond day +100 (HR: 2.57; 95% CI: 1.43‐4.63; P = .002), and we found a non‐significant trend for any SI occurring between day +30 and + 100 in patients with acute GvHD grade 3 or 4 (HR: 1.56; 95% CI: 0.91‐2.69; P = .108). The multivariate analysis is shown in Table 3.
Table 3.
Risk factors associated with severe infections—multivariate analysis
| Infections < day 30 | Infections day 30‐100 | Infections > day 100 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%‐CI | P value | HR | 95%‐CI | P value | HR | 95%‐CI | P value | ||||
| Remission status | .162 | .800 | .558 | |||||||||
| CR2 vs CR1 | 1.31 | 0.90 | 1.90 | .162 | 1.07 | 0.65 | 1.74 | .800 | 1.18 | 0.67 | 2.08 | .558 |
| Recipient age | .363 | .972 | .972 | |||||||||
| 10‐15 y vs ≤10 y | 1.24 | 0.82 | 1.89 | .307 | 1.02 | 0.61 | 1.72 | .939 | 0.95 | 0.53 | 1.73 | .871 |
| >15 y vs ≤10 y | 1.41 | 0.85 | 2.33 | .188 | 0.94 | 0.46 | 1.90 | .856 | 0.91 | 0.39 | 2.12 | .827 |
| Donor/Recipient gender | .230 | .140 | .138 | |||||||||
| Female/Male vs other | 1.31 | 0.85 | 2.02 | .230 | 1.52 | 0.87 | 2.64 | .140 | 1.54 | 0.87 | 2.74 | .138 |
| Donor | <.001 | .011 | .574 | |||||||||
| MUD vs MSD | 2.57 | 1.52 | 4.33 | <.001 | 2.91 | 1.28 | 6.61 | .011 | 1.20 | 0.64 | 2.24 | .574 |
| CMV status donor/patient | .799 | .416 | .731 | |||||||||
| Negative/Positive vs Other | 0.93 | 0.53 | 1.63 | .799 | 1.32 | 0.67 | 2.61 | .416 | 1.15 | 0.53 | 2.51 | .731 |
| Graft composition | .755 | .446 | .479 | |||||||||
| >3 × 106/kg vs ≤3 × 106/kg | 1.07 | 0.70 | 1.62 | .755 | 1.26 | 0.70 | 2.26 | .446 | 1.24 | 0.69 | 2.22 | .479 |
| Graft type | .376 | .708 | .412 | |||||||||
| BM vs PB unmanipulated | 1.22 | 0.79 | 1.89 | .376 | 1.12 | 0.62 | 2.01 | .708 | 1.38 | 0.64 | 2.97 | .412 |
| Infection prior day 30 | <.001 | |||||||||||
| yes vs no | n.a. | n.a. | n.a. | n.a. | 7.94 | 4.55 | 13.84 | <.001 | n.a. | n.a. | n.a. | n.a. |
| Infection prior day 100 | .024 | |||||||||||
| yes vs no | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1.96 | 1.09 | 3.53 | .024 |
| Acute GVHD grade 3‐4 | .108 | |||||||||||
| yes vs no | n.a. | n.a. | n.a. | n.a. | 1.56 | 0.91 | 2.69 | .108 | n.a. | n.a. | n.a. | n.a. |
| Chronic GVHD | .002 | |||||||||||
| yes vs no | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2.57 | 1.43 | 4.63 | .002 |
Abbreviations: ALL, acute lymphoblastic leukemia; ATG, Anti‐thymocyte globulin; BM, bone marrow; CMV, cytomegalovirus; CR, complete remission; GvHD, graft‐versus‐host disease; MSD, matched sibling donor; MUD, matched unrelated donor; PB, peripheral blood stem cells; n.a. not available.
A subgroup analysis for different pathogens (detailed data not shown) revealed similar results: Viral infections were encountered more often in the MUD group, both early (HR: 2.66; 95% CI: 1.30‐5.41; P = .007) and between day +30 and + 100 after HSCT (HR: 3.89; 95% CI: 1.62‐9.32; P = .002). Furthermore, bacterial infections (HR: 2.24; 95% CI: 1.03‐4.85; P = .041) and fungal infections (HR: 4.06; 95% CI: 0.96‐17.18; P = .057), were observed more frequently in the pre‐engraftment period after HSCT from MUD.
3.4. Lethal infections/impact on non‐relapse mortality
Eleven patients died due to severe infections, one of them after HSCT from MSD and 10 after MUD transplantation. The reported primary causes of death were bacteria in three cases, viruses in seven cases, and a fungal pathogen in one case, respectively. Lethal infections occurred within the pre‐engraftment phase in six cases, between day +30 and + 100 in four cases, and after day +100 in one patient. The calculated 1‐year cumulative incidence of death due to infection was 0.03 ± 0.01 (Figure 1).
4. DISCUSSION
Severe infections (SI) are encountered in up to 60% of patients receiving HSCT. They remain a major cause of non‐relapse mortality after allografting for malignant and non‐malignant diseases in adults.1, 34, 35, 36 However, published data are difficult to compare. The investigated cohorts often differ significantly in terms of transplant modalities, underlying disease, age or prophylactic measures. We evaluated a cohort of 432 children and young adults with ALL after HSCT for the occurrence of SI. They received a TBI‐based myeloablative allogeneic transplantation from MSD or MUD within the multicenter ALL‐BFM SCT 2003 trial. The SI with positive microbiologic testing results were reviewed. A total of 172 patients (40%) experienced at least one SI.
The CI of the first bacterial SI was 0.12 ± 0.02 at day +30, 0.16 ± 0.02 at day+100, and 0.21 ± 0.02 one year after HSCT. Gram‐positive infections (62%) dominated over GN infections (26%) across all time periods, and were predominantly encountered in the pre‐engraftment period, as shown by others.34, 35, 37 Coagulase‐negative staphylococci, accounting for 42% of all GP infections, were the most frequent isolates in our cohort, in line with previous observations.9, 12, 37 However, a contamination or colonization seems conceivable in several of these cases. Gram‐negative infections were primarily caused by Escherichia spp. or Pseudomonas spp., accounting for approximately 20% of all GN isolates each. Other investigators reported infection rates up to 37% for E. coli and 26% for Pseudomonas.22, 35, 37 Of note, our cohort did not routinely receive prophylactic fluoroquinolones, which was a common practice among many adult transplant centers during the last decade, to lower the burden of GN strains.38 Severe kinds of bacterial infections accounted for 27% of all lethal infections, and occurred within the first 100 days in most cases.
The early neutropenic period is not only critical for the development of bacterial but also for fungal infections, although a shift towards later fungal infections has been reported during recent years.7, 11, 39, 40 The CI of the first fungal SI in our cohort was 0.04 ± 0.01 at day +30, 0.05 ± 0.01 at day+100 and 0.06 ± 0.01 1 year after HSCT. The majority of severe fungal infections were caused by Aspergillus spp. (48%) and Candida (38%), both primarily observed in the pre‐engraftment phase. Other fungal pathogens, such as mucormycetes, were rarely detected. Infections by Aspergillus spp. were fatal in 7% of reported cases. Altogether, fungal infections accounted for 10% of the eleven reported fatalities, which is in contrast to Srinivasan et al., who reported more than 50% of all fatal infections being caused by molds in a similar pediatric cohort.22
The comparably low overall rate of invasive fungal infections in our study might have various reasons. Most centers provided a primary antifungal prophylaxis until adequate immune reconstitution was reached. The immunosuppression in patients without acute GvHD was tapered as soon as day +60 on, to minimize the relapse risk in our cohort, which might have prevented late fungal infections. Also, the low overall acute GvHD rates within the study population (acute GvHD grade 2 or 3: 11% for MSD and 10% for MUD). Could have limited fungal infections after the engraftment period. It has been shown by others that GvHD per se is associated with invasive aspergillosis, primarily due to substantial immunosuppression including corticosteroids.11
Systemic viral infections have been reported in up to 60% of children after HSCT.30 Viremia is most frequently detected after the engraftment period.41 Although not exclusively confined to the post‐engraftment period, viral infections in our cohort were mainly observed during this phase. As shown in Figure S1B, severe EBV and CMV infections usually occurred after day +30, whereas AdV infections were equally distributed between pre‐engraftment and post‐engraftment. Most viral infections were observed approximately 35 to 40 days after HSCT, except BKV, which was detected earlier. Severe viral infections were reported as the primary cause of death in 64% of all patients with fatal infections.
The donor type has been identified as an important risk factor for SI in both adults9, 10, 34 and children.42 So, HSCT from alternative donors conveys a higher risk for bacterial or fungal infections, as compared to transplantation from siblings. In line with these observations, we found more SI in our cohort of children with ALL after HSCT from MUD, compared to MSD. Transplantation from MUD was associated with any type of SI in the pre‐engraftment period, and with any SI between day +30 and + 100. Furthermore, fatal infections were more often encountered after HSCT from MUD.
The observed increase of infections after MUD HSCT could be explained by differences in transplant modalities. In our cohort, almost all patients transplanted from MUD received a GvHD prophylaxis with ATG, and short‐course methotrexate in addition to CsA, both of which impact on hematopoietic engraftment and immune reconstitution.
Methotrexate is well known to interfere with granulocyte recovery after HSCT.43 A prolonged neutropenia increases the risk, especially for severe bacterial or fungal infections.7 Patients transplanted from MSD recovered their neutrophils significantly faster, compared to recipients of MUD grafts, as reported previously.44 This might have influenced infection rates in the pre‐engraftment period after HSCT from unrelated donors. Both, bacterial and fungal infections were observed more often after HSCT from MUD.
In‐vivo T‐cell depletion with ATG additionally seems to increase the infection risk. It has been demonstrated, that ATG impairs the T‐cell reconstitution,45, 46 which in turn might facilitate virus reactivations.47 In line, significantly more viral infections were observed before day +30, or between day +30 and +100 after HSCT from MUD, compared to transplantation from MSD, who did not receive ATG. Although it is impossible for our cohort to prove that the observed effect in patients transplanted from MUD is directly related to the administration of ATG, an association seems at least conceivable. Unfortunately, data on T‐cell immune reconstitution is not available.
Patients with GvHD often require an intensified immunosuppression, including high‐dose corticosteroids, which make them susceptible to opportunistic infections.48, 49 Children in our cohort with acute GvHD grade 3 or 4, had a non‐significant trend towards SI occurring beyond day +30, as reported by others.22, 50, 51 Moreover, children with chronic GvHD particularly were at risk for any type of SI occurring beyond day +100. That confirms the data by Srinivasan, who identified chronic GvHD as an independent risk factor for bacterial and fungal infections beyond day +100 after HSCT in a similar cohort.22
We are aware that our study has several limitations, which are the heterogeneous practice for antimicrobial prophylaxis, pre‐emptive or empiric treatment of infections, the lack of standardized microbiological testing methods for all participating centers, and missing information on antimicrobial resistance of pathogens. Detailed information on pre‐transplant chemotherapy, or on asymptomatic viral reactivations after HSCT, was not available. Furthermore, the cohort was too small for a thorough subgroup analysis.
In conclusion, our study confirms the correlation between donor type and infection rates in a cohort of 432 children and adolescents, after TBI‐based myeloablative HSCT for ALL. Transplantation from MUD was associated with an increased risk for any type of SI in the pre‐engraftment period, and for viruses between day +30 and + 100 after HSCT. This could be explained by an intensified GvHD prophylaxis consisting of serotherapy with ATG and administration of methotrexate for recipients of MUD transplants.
CONFLICT OF INTEREST
PB has received consulting fees from Amgen, Cellgene and Novartis; institutional research funding from Medac, Neovii Biotech, and RIEMSER; he is a patent holder with Medac. CP has received honoraria from EUSA Pharma, Medac Pharma; consulting fees from Medac Pharma, EUSA Pharma, Pfizer; Speakers' Bureau: Amgen, Novartis, Medac Pharma, Fresenius Biotech; Research Funding: Amgen, Fresenius Biotech, Genzyme, Medac, RIEMSER Pharma. MA has received honoraria from MSD, CSL Behring and Octapharma; consulting fees from GSK; and research funding from GSK. BS has received consulting fees from Novartis. TL has received research grants from Gilead Sciences, is a consultant to Astellas, Basilea, Gilead Sciences, and Merck/MSD, and served at the speaker's bureau of Astellas, Gilead Sciences, Merck/MSD, and Pfizer. All other authors have no conflicts of interests to disclose.
AUTHOR CONTRIBUTIONS
H.P. and C.P. designed the research; E.G. and U.P. performed statistical analyses; A.L., A.M.W., I.v.L., T.L., S.M., P.L., P.B., K.W.S., J.S., B.K., K.E., M.H.A., M.K., R.M., T.G., B.S., B.G., A.S. and W.W. provided case information and important clinical data; H.P. drafted the manuscript with important contributions from C.P., S.M., P.B. and T.L. All authors reviewed and approved the final version of the manuscript.
Supporting information
Figure S1 Numbers and subtypes of the first reported bacterial (A), viral (B) and fungal infections (C) within defined time periods after HSCT.
Table S1. Reported infections
Pichler H, Lawitschka A, Glogova E, et al. Allogeneic hematopoietic stem cell transplantation from unrelated donors is associated with higher infection rates in children with acute lymphoblastic leukemia—A prospective international multicenter trial on behalf of the BFM‐SG and the EBMT‐PDWP. Am J Hematol. 2019;94:880–890. 10.1002/ajh.25511
The copyright line for this article was changed on 23 July 2019 after original online publication.
REFERENCES
- 1. Gratwohl A, Brand R, Frassoni F, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005;36(9):757‐769. [DOI] [PubMed] [Google Scholar]
- 2. Leather HL, Wingard JR. Infections following hematopoietic stem cell transplantation. Infect Dis Clin North am. 2001;15(2):483‐520. [DOI] [PubMed] [Google Scholar]
- 3. Tanaka Y, Kurosawa S, Tajima K, et al. Analysis of non‐relapse mortality and causes of death over 15 years following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51(4):553‐559. [DOI] [PubMed] [Google Scholar]
- 4. Spring L, Li S, Soiffer RJ, Antin JH, Alyea EP 3rd, Glotzbecker B. Risk factors for readmission after allogeneic hematopoietic stem cell transplantation and impact on overall survival. Biol Blood Marrow Transplant. 2015;21(3):509‐516. [DOI] [PubMed] [Google Scholar]
- 5. Maher OM, Silva JG, Huh WW, et al. Etiologies and impact of readmission rates in the first 180 days after hematopoietic stem cell transplantation in children, adolescents, and young adults. J Pediatr Hematol Oncol. 2017;39:609‐613. [DOI] [PubMed] [Google Scholar]
- 6. Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin Infect Dis. 2001;33(7):947‐953. [DOI] [PubMed] [Google Scholar]
- 7. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castagnola E, Bagnasco F, Faraci M, et al. Incidence of bacteremias and invasive mycoses in children undergoing allogeneic hematopoietic stem cell transplantation: a single center experience. Bone Marrow Transplant. 2008;41(4):339‐347. [DOI] [PubMed] [Google Scholar]
- 9. Blennow O, Ljungman P, Sparrelid E, Mattsson J, Remberger M. Incidence, risk factors, and outcome of bloodstream infections during the pre‐engraftment phase in 521 allogeneic hematopoietic stem cell transplantations. Transpl Infect Dis. 2014;16(1):106‐114. [DOI] [PubMed] [Google Scholar]
- 10. Girmenia C, Raiola AM, Piciocchi A, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant. 2014;20(6):872‐880. [DOI] [PubMed] [Google Scholar]
- 11. Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909‐917. [DOI] [PubMed] [Google Scholar]
- 12. Ninin E, Milpied N, Moreau P, et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis. 2001;33(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 13. Castagnola E, Bagnasco F, Bandettini R, et al. Role of acute graft‐versus‐host disease in the risk of bacteremia and invasive fungal disease after allogeneic hemopoietic stem cell transplantation in children. Results from a single‐center observational study. Biol Blood Marrow Transplant. 2014;20(7):1068‐1073. [DOI] [PubMed] [Google Scholar]
- 14. Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T‐cell immunity. Blood. 2003;101(2):407‐414. [DOI] [PubMed] [Google Scholar]
- 15. Bruno B, Gooley T, Hackman RC, Davis C, Corey L, Boeckh M. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9(5):341‐352. [DOI] [PubMed] [Google Scholar]
- 16. Ljungman P, Perez‐Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91(1):78‐83. [PubMed] [Google Scholar]
- 17. Ciaurriz M, Beloki L, Zabalza A, et al. Functional specific‐T‐cell expansion after first cytomegalovirus reactivation predicts viremia control in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2017;19(6). 10.1111/tid.12778. Epub 2017 Oct 25. [DOI] [PubMed] [Google Scholar]
- 18. Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C. Donor CMV serologic status and outcome of CMV‐seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood. 2003;102(13):4255‐4260. [DOI] [PubMed] [Google Scholar]
- 19. Matthes‐Martin S, Lion T, Aberle SW, et al. Pre‐emptive treatment of CMV DNAemia in paediatric stem cell transplantation: the impact of recipient and donor CMV serostatus on the incidence of CMV disease and CMV‐related mortality. Bone Marrow Transplant. 2003;31(9):803‐808. [DOI] [PubMed] [Google Scholar]
- 20. Feuchtinger T, Lang P, Handgretinger R. Adenovirus infection after allogeneic stem cell transplantation. Leuk Lymphoma. 2007;48(2):244‐255. [DOI] [PubMed] [Google Scholar]
- 21. Leen AM, Bollard CM, Myers GD, Rooney CM. Adenoviral infections in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(3):243‐251. [DOI] [PubMed] [Google Scholar]
- 22. Srinivasan A, Wang C, Srivastava DK, et al. Timeline, epidemiology, and risk factors for bacterial, fungal, and viral infections in children and adolescents after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(1):94‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcia‐Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47(8):1041‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrison N, Mitterbauer M, Tobudic S, et al. Incidence and characteristics of invasive fungal diseases in allogeneic hematopoietic stem cell transplant recipients: a retrospective cohort study. BMC Infect Dis. 2015;15:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peters C, Schrappe M, von Stackelberg A, et al. Stem‐cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors‐the ALL‐SCT‐BFM‐2003 trial. J Clin Oncol. 2015;33(11):1265‐1274. [DOI] [PubMed] [Google Scholar]
- 26. Schrauder A, Reiter A, Gadner H, et al. Superiority of allogeneic hematopoietic stem‐cell transplantation compared with chemotherapy alone in high‐risk childhood T‐cell acute lymphoblastic leukemia: results from ALL‐BFM 90 and 95. J Clin Oncol. 2006;24(36):5742‐5749. [DOI] [PubMed] [Google Scholar]
- 27. Schrauder A, von Stackelberg A, Schrappe M, et al. Allogeneic hematopoietic SCT in children with ALL: current concepts of ongoing prospective SCT trials. Bone Marrow Transplant. 2008;41(Suppl 2):S71‐S74. [DOI] [PubMed] [Google Scholar]
- 28. von Stackelberg A, Volzke E, Kuhl JS, et al. Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non‐response to salvage protocol therapy: a retrospective analysis of the ALL‐REZ BFM study group. Eur J Cancer. 2011;47(1):90‐97. [DOI] [PubMed] [Google Scholar]
- 29. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176‐181. [DOI] [PubMed] [Google Scholar]
- 30. Satwani P, Baldinger L, Freedman J, et al. Incidence of viral and fungal infections following busulfan‐based reduced‐intensity versus myeloablative conditioning in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2009;15(12):1587‐1595. [DOI] [PubMed] [Google Scholar]
- 31. Prentice RL, Kalbfleisch JD, Peterson AV Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541‐554. [PubMed] [Google Scholar]
- 32. Gray RJ. A class of k‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141‐1154. [Google Scholar]
- 33. Fine JG, Robert J. A proportional hazards model for the subdistribution of a competing risk. J am Stat Assoc. 1999;94(446):496‐509. [Google Scholar]
- 34. Cappellano P, Viscoli C, Bruzzi P, Van Lint MT, Pereira CA, Bacigalupo A. Epidemiology and risk factors for bloodstream infections after allogeneic hematopoietic stem cell transplantion. New Microbiol. 2007;30(2):89‐99. [PubMed] [Google Scholar]
- 35. Gjærde LI, Moser C, Sengeløv H. Epidemiology of bloodstream infections after myeloablative and non‐myeloablative allogeneic hematopoietic stem cell transplantation: a single‐center cohort study. Transpl Infect Dis. 2017;19(5). 10.1111/tid.12730. Epub 2017 Jul 12. [DOI] [PubMed] [Google Scholar]
- 36. Mikulska M, Del Bono V, Bruzzi P, et al. Mortality after bloodstream infections in allogeneic haematopoietic stem cell transplant (HSCT) recipients. Infection. 2012;40(3):271‐278. [DOI] [PubMed] [Google Scholar]
- 37. Kikuchi M, Akahoshi Y, Nakano H, et al. Risk factors for pre‐ and post‐engraftment bloodstream infections after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2015;17(1):56‐65. [DOI] [PubMed] [Google Scholar]
- 38. Mikulska M, Del Bono V, Raiola AM, et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of gram‐negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009;15(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 39. Grow WB, Moreb JS, Roque D, et al. Late onset of invasive aspergillus infection in bone marrow transplant patients at a university hospital. Bone Marrow Transplant. 2002;29(1):15‐19. [DOI] [PubMed] [Google Scholar]
- 40. Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001‐2006: overview of the transplant‐associated infection surveillance network (TRANSNET) database. Clin Infect Dis. 2010;50(8):1091‐1100. [DOI] [PubMed] [Google Scholar]
- 41. Schonberger S, Meisel R, Adams O, et al. Prospective, comprehensive, and effective viral monitoring in children undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(10):1428‐1435. [DOI] [PubMed] [Google Scholar]
- 42. Castagnola E, Faraci M, Moroni C, et al. Bacteremias in children receiving hemopoietic SCT. Bone Marrow Transplant. 2008;41(Suppl 2):S104‐S106. [DOI] [PubMed] [Google Scholar]
- 43. Storb R, Deeg HJ, Pepe M, et al. Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft‐versus‐host disease in patients given HLA‐identical marrow grafts for leukemia: long‐term follow‐up of a controlled trial. Blood. 1989;73(6):1729‐1734. [PubMed] [Google Scholar]
- 44. Pichler H, Witt V, Winter E, et al. No impact of total or myeloid Cd34+ cell numbers on neutrophil engraftment and transplantation‐related mortality after allogeneic pediatric bone marrow transplantation. Biol Blood Marrow Transplant. 2014;20(5):676‐683. [DOI] [PubMed] [Google Scholar]
- 45. de Koning C, Plantinga M, Besseling P, Boelens JJ, Nierkens S. Immune reconstitution after allogeneic hematopoietic cell transplantation in children. Biol Blood Marrow Transplant. 2016;22(2):195‐206. [DOI] [PubMed] [Google Scholar]
- 46. de Koning C, Admiraal R, Nierkens S, Boelens JJ. Immune reconstitution and outcomes after conditioning with anti‐thymocyte‐globulin in unrelated cord blood transplantation; the good, the bad, and the ugly. Stem Cell Investig. 2017;4:38 10.21037/sci.2017.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Admiraal R, de Koning CCH, Lindemans CA, et al. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol. 2017;140:1643‐1650.e9. [DOI] [PubMed] [Google Scholar]
- 48. Sayer H, Longton G, Bowden R, Pepe M, Storb R. Increased risk of infection in marrow transplant patients receiving methylprednisolone for graft‐versus‐host disease prevention. Blood. 1994;84(4):1328‐1332. [PubMed] [Google Scholar]
- 49. Hol JA, Wolfs TF, Bierings MB, et al. Predictors of invasive fungal infection in pediatric allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2014;49(1):95‐101. [DOI] [PubMed] [Google Scholar]
- 50. Almyroudis NG, Fuller A, Jakubowski A, et al. Pre‐ and post‐engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2005;7(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 51. Poutsiaka DD, Munson D, Price LL, Chan GW, Snydman DR. Blood stream infection (BSI) and acute GVHD after hematopoietic SCT (HSCT) are associated. Bone Marrow Transplant. 2011;46(2):300‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Numbers and subtypes of the first reported bacterial (A), viral (B) and fungal infections (C) within defined time periods after HSCT.
Table S1. Reported infections


