Abstract
Anxiety is a common psychiatric illness often treated by benzodiazepines (BZs). BZs, such as Valium, bind to the α subunit of the pentameric GABAA receptor and increase inhibition in the CNS. There is considerable evidence for abnormal GABAAreceptor function in anxiety, and a significant proportion of anxiety patients has a reduced sensitivity to BZs. Here, we show that serotonin1A (5-HT1A) receptor knock-out mice display BZ-resistant anxiety. Consistent with this finding, binding of both BZ and non-BZ GABAA receptor ligands were reduced and GABAergic inhibition was impaired in mutant mice. These changes were reflected by abnormal α subunit expression in the amygdala and hippocampus, two important limbic regions involved in fear and anxiety. These data suggest a pathological pathway, initiated by a 5-HT1A receptor deficit, leading to abnormalities in GABAA receptor composition and level, which in turn result in BZ-insensitivity and anxiety. This model mechanistically links together the 5-HT and GABA systems, which both have been implicated in anxiety. A related mechanism may underlie reduced BZ sensitivity in certain forms of anxiety.
Keywords: 5-HT1A receptor, GABAA receptor, subunit, knock-out, benzodiazepine, anxiety, sedation, anxiolytic
Brain 5-HT is implicated in the etiology of neuropsychiatric disorders, such as anxiety and depression (Murphy, 1990; Andrade, 1992). Recent work from our and other laboratories revealed that mice lacking the 5-HT1A receptor display marked anxiety (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998). Other lines of evidence also support the correlation between 5-HT1A receptor hypofunction and anxiety.McKittrick et al. (1995) reported that subordinate rats in a dominance hierarchy show severe stress and anxiety accompanied by a reduced 5-HT1A receptor level. Other stressors have also been associated with the downregulation of the 5-HT1A receptor (Watanabe et al., 1993; Flugge, 1995; Lopez et al., 1998). In human studies, Lesch (1991) reported the association of 5-HT1A receptor hypofunction (measured as an attenuated endocrine response to receptor agonists) with panic, a form of anxiety disorders. Together, these data indicate that 5-HT1A receptor knock-out (KO) mice could provide a useful model to study a 5-HT-related pathogenic pathway leading to anxiety.
The 5-HT1A receptor is expressed both presynaptically and postsynaptically. Presynaptic 5-HT1A receptors are expressed on the soma and dendrites of 5-HT neurons located in the dorsal and medial raphe nuclei (Blier et al., 1988; Hamon, 1997). Activation of these autoreceptors reduces the firing rate of serotonergic neurons (Aghajanian and Lakoski, 1984; Blier et al., 1988; Jolas et al., 1993) and suppresses 5-HT synthesis, turnover, and release (Kennett et al., 1987; Bohmaker et al., 1993). Postsynaptic 5-HT1A receptors are found in the terminal fields of the 5-HT neurons that include hippocampus, lateral septum, cortex, and amygdala (Pazos and Palacios, 1985; Jacobs, 1997). It has been suggested that abnormalities in 5-HT release and presynaptic 5-HT1A receptor function could lead to anxiety (Lucki et al., 1994; De Vry, 1995). However, in vivomicrodialysis studies showed that the absence of presynaptic receptors does not alter 5-HT dynamics in receptor KO mice (M. He, E. Sibille, T. Shippenberg , and M. Toth, unpublished observations). This indicates that the anxiety phenotype of the receptor KO mice is probably attributable to the lack of receptors at the postsynaptic sites.
Surprisingly, when injected with the classical benzodiazepine (BZ) diazepam to relieve anxiety, 5-HT1A receptor KO mice appeared to be insensitive to the anxiolytic effect of the drug. The known interaction of BZs with GABAAreceptors prompted us to investigate these receptors in 5-HT1A receptor KO mice. GABAA receptors are ligand-gated chloride channels. Currently, there are at least 19 related GABAA receptor subunits in mammals (six α, four β, three γ, three ρ, one δ, one ε, and one π subunits) (Barnard et al., 1998). Generally, pentameric CNS GABAA receptors are combinations of at least one α and one β, with one or more γ, δ, or ε subunit (Sieghart, 1995). Here, we show that inactivation of the 5-HT1A receptor in mice results in alterations in the expression of GABAA receptor subunits in amygdala, cerebral cortex, and hippocampus. We propose that these GABAA receptor subunit changes are responsible for the reduced BZ responsiveness and anxiety of 5-HT1A receptor KO mice.
MATERIALS AND METHODS
Animals. 5-HT1Areceptor-deficient mice were generated by targeted gene disruption (Parks et al., 1998). First, the 5-HT1A receptor gene was inactivated by homologous recombination in embryonic stem (ES) cells derived from 129sv mice. Targeted ES cells were injected into blastocysts, which were then implanted into pseudopregnant females. Because the 129sv genetic background is not particularly suitable for behavioral testing, ES cell chimeras were bred with Swiss-Webster mice to obtain heterozygotes (129sv × Swiss-Webster). Homozygous F2 mutants were obtained by crossbreeding F1 animals (Parks et al., 1998). A similar breeding scheme was followed with wild-type (WT) 129sv and Swiss-Webster mice to generate genetically matching control animals. To avoid a disequilibrium of genes that are linked to the 5-HT1A receptor locus, WT F2 progeny with two WT 129sv5-HT1A receptor alleles were selected by single-strand-length polymorphism (Parks et al., 1998). By using this method, we generated control mice that matched the homozygous mice in background, but their 5-HT1Areceptor gene was not inactivated.
Behavioral studies. The elevated plus maze was performed using a cross maze with 12 × 2 inch arms. The percentage of entries into or time spent in the open arm versus total entries into or time spent in open and closed arms were calculated for a period of 10 min as markers of anxiety behavior. The open field test used a 15 × 21 inch black box, divided into 12 even-sized (4 × 3 inch) rectangles. The time spent in and number of entries into the two rectangles at the center of the field were recorded for 10 min to evaluate anxiety. Diazepam (Research Biochemicals, Natick, MA and Sigma, St. Louis, MO) was injected intraperitoneally 30 min before the test. For the “loss of righting reflex,” mice were injected with an intraperitoneal dose of pentobarbital (65 mg/kg) and monitored for the duration of the loss of reflex. Mice were placed on their back and were judged to have regained the reflex when able to turn themselves three times within 30 sec. For deep anesthesia, mice were injected with a 65 mg/kg bolus of pentobarbital, followed by 6.5 mg/kg increments of the drug every 10 min, until they failed to respond to a deep pain (hindpaw squeeze).
Diazepam measurements. Trunk blood was collected 30 min after diazepam (3 mg/kg) from four WT and KO mice (Azzam et al., 1998). Other groups of WT and KO mice were injected with saline before collecting blood. Organic compounds were chloroform-extracted from serum, dehydrated under a stream of nitrogen, and resuspended in ethanol. Samples (10 μl) were injected on a Vydac reverse-phase C18 column (250 × 4.6 mm) in a Water 600 HPLC system with a photodiode array detector set at 232 nm. The mobile phase was methanol–acetonitrile–dihydrogenphosphate buffer, 0.05m (50:10:40, v/v), with a pH of 3.5 and a flow rate of 1.0 ml/min.
Autoradiography. GABAA receptor autoradiography was performed on 20 μm coronal sections in the presence of 6.5 nm3H-SR 95531 (DuPont NEN, Boston, MA) in 50 mm TRIS–citrate (Ashworth-Preece et al., 1997). Nonspecific binding was determined in the presence of 10 mm GABA. BZ sites on the GABAA receptor were measured with 2 nmmethyl-3H-flunitrazepam (DuPont NEN) (Thielen et al., 1997). Nonspecific binding was determined in the presence of 2 mm diazepam. Sections were exposed to Hyperfilm (Amersham, Arlington Heights, IL) for 4 weeks. Computerized densitometry was performed with the NIH Image program. Quantification was based on a series of [3H] autoradiographic internal standards (Amersham).
Electrophysiology. Transverse hippocampal slices (300 μm) were obtained on a McIllwain tissue chopper and kept submerged in artificial CSF (in mm: 124.0 NaCl, 5.0 KCl, 2.4 CaCl2, 1.3 MgSO4, 10 NaHCO3, 1.25 NaH2PO4, and 10.0 glucose) for 1 hr at room temperature. Extracellular field potentials were recorded on an interface chamber maintained at 32°C with glass micropipettes filled with 3 m NaCl with a 2–3 MΩ tip resistance. The field potentials were amplified with an AC differential amplifier with low-pass filter set at 3 kHz and high-pass at 30 Hz and stored using the Labview program on a Apple Computers (Cupertino, CA) MacIntosh computer for analysis. An input–output curve was taken between minimum and maximum responses. The test stimulus was chosen at approximately half maximum response. The stimulation and recording positions were determined by mapping the slice for optimal stimulus response. For the paired-pulse experiments, two stimuli were applied to the slice with a delay ranging from 10 to 90 msec.
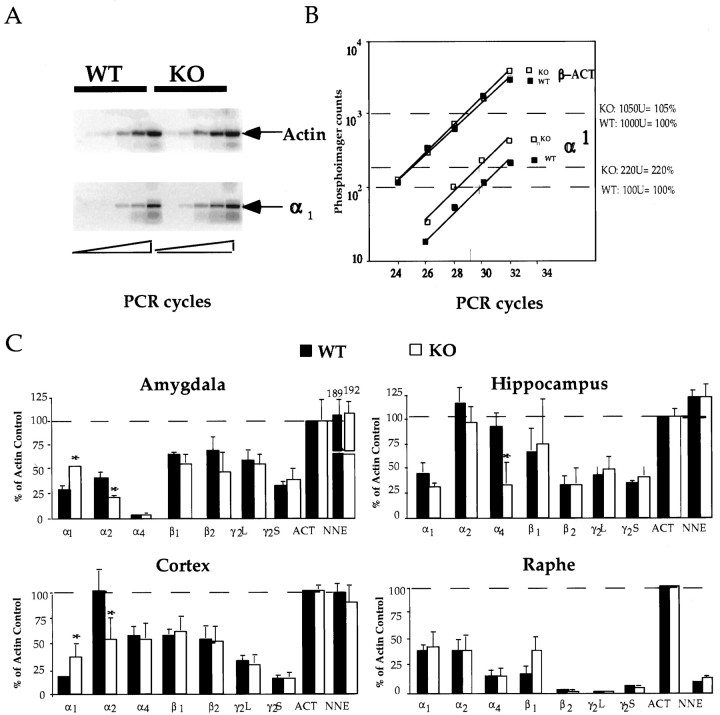
Kinetic quantitative reverse transcription-PCR. Total RNA was isolated from micropunches (two per mouse, three mice per sample) by using TRIZOL reagent (Life Technologies, Gaithersburg, MD). The isolated RNA was DNase I-treated and reverse transcribed by reverse primers (Liu and Burt, 1998) [α1, 5′-CGGGCTGGCTCTCTGGTCCACTC-3′; α2, 5′-AAATTGTTAAGTCGAAGGATATTC-3′; α4, 5′-TGCCATTTCTCATAATTCTAA-3′; β1, 5′- TGCTCCCTCTCCTCCATTCCA-3′; β2, 5′- GTCTCCAAGTCCCATTACTGCTTC-3′; γ2L (and S), 5′-CAAAAGGCGGTAGGGAAGAAGATCCGAGCA-3′; β-actin, 5′-ATTTGCGGTGCACGATGGAGGGGCCGGACT-3′; and non-neuronal enolase (NNE), 5′-AGGTGCGAATCCACCCTCATCA-3′]. PCR amplification was performed in the presence of reverse and forward primers (α1, 5′-ATCTTTGGGCCTGGACCCTCATTCT-3′; α2, 5′-GAAGACAAAATTGAGCACATGCA-3′; α4, 5′-TTTAAACGAATCCCCAGGACAGAA-3′; β1, 5′-ACAGCTCCAATGAACCCAGCAA-3′; β2, 5′-GGAGTGACAAAGATTGAGCTTCCT-3′; γ2, 5′-GTGGAGTATGGCACCCTGCATTATTTTGTC-3′; β-actin, 5′-CACCACAGCTGAGAGGGAAATCGTGCGTGA-3′; and NNE, 5′- ACTCCGAGACAATGATAAGACCC-3′) with the Advantage cDNA Polymerase mix (Clontech, Palo Alto, CA). PCR products were trace-labeled with32P-dCTP [α1, 580 bp; α2, 345 bp; α4, 389 bp; β1, 521 bp; β2, 564 bp; γ2L (and S), 335 (311); β-actin, 517 bp; and NNE, 504 bp) and quantified on a STORM 860 Phosphorimager (Molecular Dynamics, Sunnyvale, CA). Efficacy of amplification was similar for the actin and subunit mRNAs (see similar slopes for α2 subunit and actin mRNAs in Fig.3B). Subunit RNA levels were normalized to the expression level of actin.
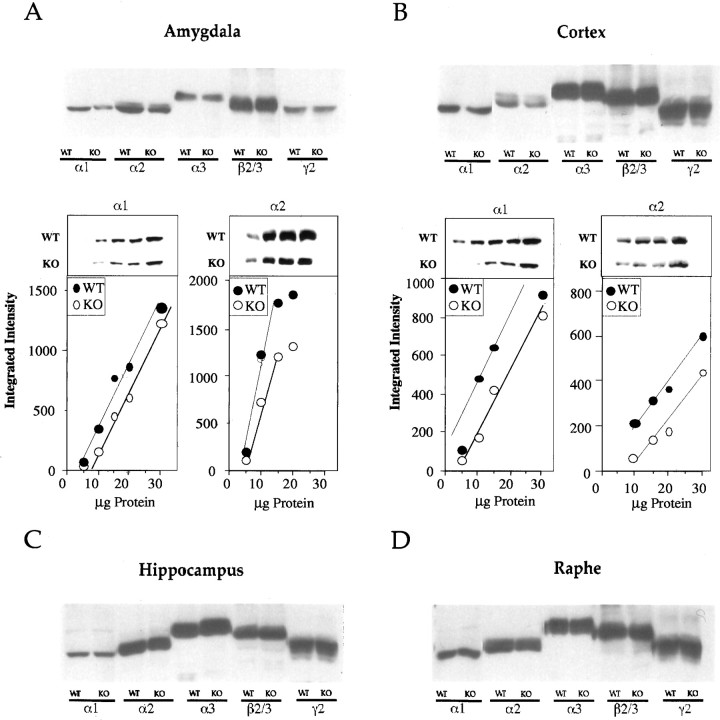
Fig. 3.
Expression of GABAA receptor subunits in amygdala (A), cortex (B), hippocampus (C), and raphe (D), measured by Western blotting. Downregulation of the α1 and α2 subunits in amygdala and cortex were calculated by using six independent blots with increasing protein concentrations (see graphs in A andB). In these experiments, a total of three pools of tissues (3 mice per pool) were analyzed for each genotype. Representative blots with serial dilutions of samples are also shown for α1 and α2 subunits in amygdala and cortex.
Western blots. Aliquots of crude membrane samples were subjected to SDS-PAGE. Proteins were transferred onto nitrocellulose membranes in a semidry electroblotting apparatus (Trans Blot; Bio-Rad, Hercules, CA). For immunodetection, the blots were blocked for 1–2 hr in 0.1% v/v Tween 20 in TBS containing 5% nonfat dry milk at room temperature, followed by incubation with affinity-purified antisera overnight at 4°C in TBST–5% nonfat dry milk. Incubation with secondary antibodies (horseradish peroxidase-conjugated goat anti-rabbit IgG diluted 1:5000 in TBST–5% nonfat dry milk; Promega, Madison, WI) was performed for 1 hr at room temperature. Immunoreactivity was detected by the chemiluminescence method (Western Blot Chemiluminescence Reagent Plus; DuPont NEN). Quantification of immunoreactive bands was performed with a high-resolution computer-based image analysis system (MCID M2; Imaging Research, Ontario, Canada). To ensure an analysis in the linear ranges, x-ray films were exposed to Western blots of increasing protein concentrations (5–30 μg) for various times.
RESULTS
5-HT1A receptor KO mice are insensitive to the anxiolytic effect of BZ
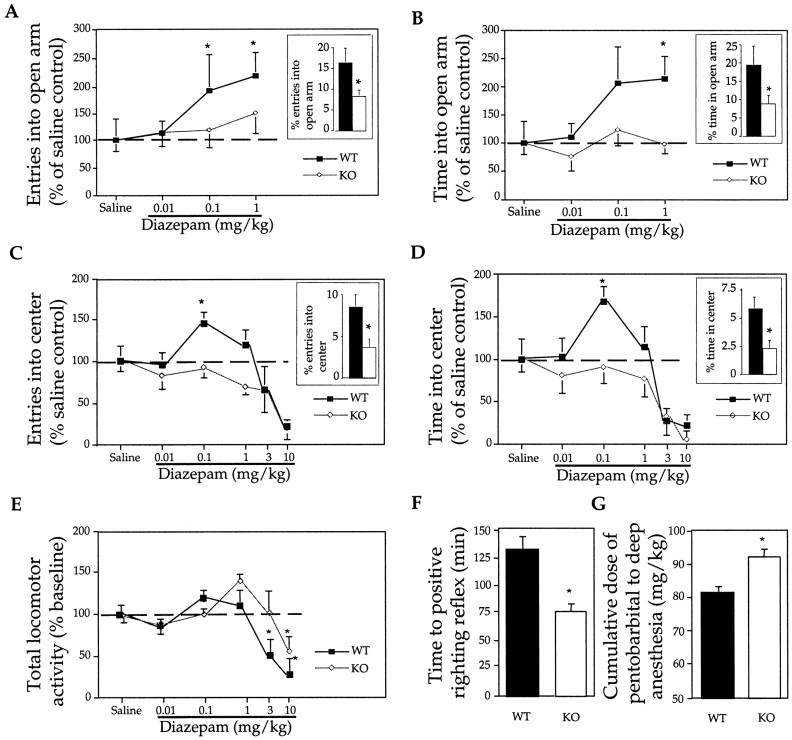
We and others have shown previously that inactivation of the 5-HT1A receptor gene results in anxiety in mice (Heisler et al., 1998; Parks et al., 1998; Ramboz et al., 1998) (Fig.1A–D,insets). Surprisingly, we found that 5-HT1A receptor KO mice are insensitive to the anxiolytic effect of diazepam, a classical BZ. Experiments in the elevated plus maze, a highly reliable test to identify anxiolytic BZ compounds, showed that 0.1 and 1 mg/kg diazepam significantly increased the number of entries into and the time spent in the open arm of the WT, but not KO, mice (Fig. 1A,B). These doses of diazepam had no effect on the total locomotor activity, measured as total number of entries, of either WT or KO mice (data not shown). In the open field test of anxiety, 0.1 mg/kg diazepam increased the number of entries into and the time spent in the center of the field of WT, but not KO, mice (Fig. 1C,D). Diazepam, up to 1 mg/kg, had no effect on the total locomotor activity, measured as total number of crosses, of WT mice (Fig. 1E). Diazepam (1 mg/kg) caused a moderate increase in total locomotor activity of receptor KO mice (Fig. 1E). However, this effect was not reproducible because another group of 10 KO mice displayed no increased locomotor activity after the injection of 1 mg/kg diazepam. We concluded that 5-HT1A receptor KO mice are insensitive to the anxiolytic effect of diazepam.
Fig. 1.
Lack of anxiolytic-like effect of diazepam in 5-HT1A receptor KO mice demonstrated in elevated plus maze (A, B) and in open field (C, D). Number of animals for each dose and treatment were as follows: WT, n = 8; KO,n = 8 for the elevated plus maze; and WT,n = 7; KO, n = 7 for the open field. Insets display the increased anxiety of KO mice (open bars) compared with WT animals (filled bars) measured as a decrease in the number of entries into the open arm (inset inA) and time spent in the open arm (B), as well as a decrease in the number of entries into the center (C) and time spent in the center (D). Total locomotor activity, measured in open field, is represented by the total number of crosses (E). Decreased sensitivity to the sedative effect of pentobarbital, measured as time to regain righting reflex after a 65 mg/kg dose (F) and as a cumulative dose of the drug required for deep anesthesia (G). Number of WT and KO animals per group in the experiments displayed inF and G were n = 9 and n = 14, respectively. *p < 0.05 represents significant difference between KO and WT animals.
5-HT1A receptor KO mice are less sensitive to the sedative effect of BZ and barbiturate
When higher doses of diazepam (3 and 10 mg/kg) were tested on open field behavior, total locomotor activity was reduced in both groups of animals, indicating a sedative effect (Fig.1E). However, KO mice were less sensitive than WT animals to the sedative effect of diazepam. Whereas the locomotor activity of WT mice was already reduced by 3 mg/kg diazepam, only the larger 10 mg/kg dose was sedative in receptor KO mice (Fig.1E). Generally, sedated animals, after placing them into the center, moved to the periphery of the field and stayed there immobile. This effect led to a reduction in the number of entries into and the time spent in the center of the field as shown in Figure1C, D.
We also tested the sedative–anesthetic effect of pentobarbital, a non-BZ compound, on WT and 5-HT1A receptor KO mice. The sedative–anesthetic effect of pentobarbital was measured by monitoring the duration of the “loss of righting reflex” after a single drug injection (65 mg/kg) and also by measuring the cumulative dose required to achieve deep anesthesia (loss of pain reaction). Receptor KO mice showed a significant reduction in the duration of the loss of righting reflex and required more pentobarbital to reach deep anesthesia (Fig. 1F,G). We concluded that 5-HT1A receptor KO mice have a reduced sensitivity to the sedative effect of diazepam. KO mice were also less sensitive to the sedative–anesthetic effect of pentobarbital.
BZ receptor binding is reduced in the amygdala of 5-HT1A receptor KO mice
Insensitivity to the anxiolytic and reduced sensitivity to the sedative effect of BZ could be based on several mechanisms. For example, an increased drug metabolism in KO mice may cause reduced responses to BZ. However, this is not likely because plasma drug levels in WT and KO mice, after a 3 mg/kg diazepam injection, were not different (WT, 65.8 ± 3.9 μm; KO, 66.9 ± 0.6 μm). It was more likely that the insensitivity of KO mice to the anxiolytic action of diazepam was attributable to a reduction in some of the BZ-sensitive GABAA sites. The reduced pentobarbital sensitivity could also be explained by such a mechanism because this drug also binds to GABAAreceptors.
As Table 1 shows, binding of the BZ-specific ligand methyl-3H-flunitrazepam was reduced ∼16% in amygdala. The reduced flunitrazepam binding in this region is particularly interesting because amygdala has been shown to be the main site of action for the anxiolytic effect of BZs in conflict-based behavioral assays, such as the elevated plus maze (Kataoka et al., 1987). Cortical regions showed smaller reductions (8%) in flunitrazepam binding. Total GABAAreceptor binding (BZ- and non-BZ-sensitive sites), as measured by3H-SR95531, was not reduced in the amygdala and cortex of KO mice, suggesting that receptor changes in these regions are limited to the BZ-sensitive GABAA receptors and that the reduction in BZ-sensitive GABAA receptors is undetectable in the larger total GABAA receptor pool. Conversely, the reduced total GABAA receptor (measured by3H-SR95531) and normal BZ-specific receptor binding (measured by methyl-3H-flunitrazepam) in the CA1 region and dentate gyrus of the hippocampus of KO mice indicated a change in BZ-insensitive but not in BZ-sensitive GABAAreceptors in these brain regions (Table 1).
Table 1.
BZ-sensitive and total GABAA receptor binding in different brain regions of 5-HT1A KO and WT mice
| Binding (fmol/mg wet tissue) | ||||||
|---|---|---|---|---|---|---|
| BZ (3H-Flunitrazepam) | GABA (3H-SR 95531) | |||||
| WT | KO | Changes (pvalue) | WT | KO | Changes (p value) | |
| Parietal cortex | ||||||
| Layer II–IV | 72.77 ± 2.25 | 66.41 ± 3.05 | −8.71% | 65.59 ± 9.76 | 63.88 ± 11.78 | ns |
| ns (0.06) | ||||||
| Layer V–VI | 45.95 ± 1.26 | 42.70 ± 1.19 | −8.10% | 28.79 ± 5.72 | 31.1 ± 8.77 | ns |
| (0.04) | ||||||
| Hippocampus | ||||||
| Dentate gyrus | 48.69 ± 1.01 | 47.60 ± 1.38 | ns | 82.99 ± 5.6 | 62.08 ± 8.52 | −25.20% |
| (0.03) | ||||||
| CA1 | 46.86 ± 0.8 | 44.48 ± 1.49 | ns | 93.33 ± 7.19 | 71.71 ± 8.52 | −23.20% |
| (0.04) | ||||||
| CA3 | 45.00 ± 1.38 | 43.71 ± 1.35 | ns | 60.04 ± 6.82 | 47.24 ± 5.48 | −23.30% |
| ns (0.09) | ||||||
| Amygdala Central nucleus | 48.85 ± 1.52 | 40.78 ± 1.67 | −16.50% | 46.48 ± 7.02 | 42.36 ± 8.30 | ns |
| (0.02) | ||||||
| Basolateral nucleus | 30.12 ± 1.09 | 25.71 ± 1.04 | −15.50% | 62.95 ± 4.04 | 51.49 ± 9.40 | ns |
| (0.02) | ||||||
| Hypothalamus | ||||||
| Paraventricular nucleus | 28.88 ± 1.28 | 27.49 ± 1.90 | ns | 21.62 ± 5.27 | 24.14 ± 3.64 | ns |
| Ventral Hypothalamus | 41.97 ± 2.46 | 41.29 ± 1.70 | ns | 40.74 ± 7.09 | 34.13 ± 5.12 | ns |
| Dorsal raphe | 27.13 ± 0.38 | 26.37 ± 0.7 | ns | 53.76 ± 3.85 | 48.79 ± 7.12 | ns |
| Median raphe | 30.75 ± 0.98 | 28.64 ± 1.39 | ns | 38.98 ± 4.74 | 38.63 ± 5.0 | ns |
| Central gray | 48.69 ± 1.01 | 47.60 ± 1.38 | ns | 55.02 ± 4.76 | 54.96 ± 6.44 | ns |
ns, Not significant; p > 0.1 are not displayed and labeled only as ns.
GABAergic inhibition is reduced in the hippocampus of 5-HT1A receptor KO mice
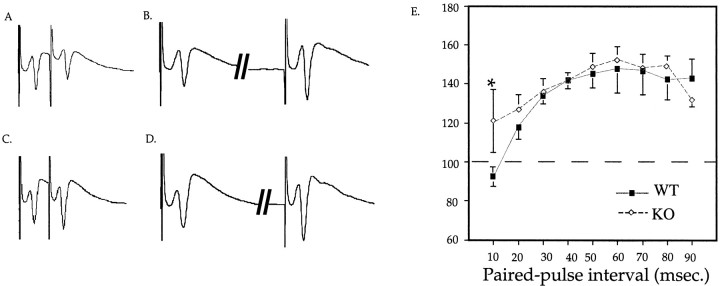
The reduction in GABAA receptor binding in the hippocampus prompted us to asses GABAergic inhibition in this brain region. In WT animals, a single electrical pulse, followed within 10 msec by a pulse of an equal intensity (paired-pulse), induces a response of decreased amplitude (Rock and Taylor, 1986) (Fig.2). This inhibition was impaired in the CA1 region of the hippocampus of KO animals (Fig. 2). Because paired-pulse inhibition of pyramidal cell activity is believed to reflect the strength of GABAergic transmission and because this inhibition may be mediated predominantly by GABAAreceptors (Rock and Taylor, 1986), the impaired paired-pulse inhibition in the CA1 region of the hippocampus of KO mice suggested a functional GABAA receptor deficit.
Fig. 2.
Impaired paired-pulse inhibition in the CA1 region of the hippocampus of 5-HT1A receptor KO mice.A–D, Representative field potentials induced by paired-pulse stimuli presented at 10 (A,C) and 50 (B, D) msec intervals of WT (A, B) and KO (C, D) hippocampal slices. Paired-pulse inhibition–facilitation was measured as a percentage of the second to the first stimulus. E, Paired-pulse inhibition–facilitation in the CA1 hippocampal field of WT and KO mice, as a function of interpulse interval. The number of slices investigated per group were as follows: 10 mice/group; 2–3 slices per mouse; and 30 slices for WT and 22 for KO mice. *p< 0.05 represents significant differences between KO and WT animals.
Expression of GABAA receptor α1 and α2 subunits is downregulated in the amygdala of 5-HT1A KO mice
Reduced BZ binding in amygdala could be caused by structural changes in GABAA receptors. These receptors are pentamers and are assembled mostly from α, β, and γ subunits (MacDonald and Olsen, 1994; Sieghart, 1995). We measured subunits that are highly expressed and that participate in the assembly of BZ-sensitive GABAA receptors (thus could explain the reduced BZ sensitivity of KO mice). Among the α subtypes, α1, α2, and α3 subunits were measured by Western blotting. The α5 subtype, which may also be considered, was not included in these studies because α5-containing receptors generally contribute to a small subset of GABAA receptors. We also measured the expression of the γ2 subunit because this subunit is an essential component of the BZ-sensitive GABAA receptor (Gunther et al., 1995). In addition, expression of β subunits was followed by using antibody recognizing both the β2 and β3 subunits. We analyzed these subunits in four different brain regions (amygdala, hippocampus, parietal cortex, and raphe) in WT and KO mice (Fig. 3).
Western blotting indicated that both α1 and α2 subunits were downregulated in amygdala and cortex (Fig.3A,B, top panels). Levels of other subunits, such as α3, β2/3, and γ2, were unchanged in these regions in KO mice. Western blotting with serial dilutions of protein samples confirmed the downregulation of the α1 and α2 subunits in amygdala and cortex of KO mice (α1 subunit levels in KO amygdala and cortex were 56 ± 16 and 68 ± 14% of the WT level, respectively, and α2 subunit levels in KO amygdala and cortex were 59 ± 18 and 47 ± 17% of the WT level, respectively) (Fig.3A,B, bottom panels). None of the investigated subunits showed changes in hippocampus and raphe of KO mice (Fig. 3C, D).
Levels of GABAA receptor α subunit mRNAs are altered in 5-HT1A KO mice
Levels of a number of GABAA receptor subunit mRNAs were also analyzed in amygdala, hippocampus, parietal cortex, and raphe in WT and KO mice (Fig. 4). Subunit mRNA levels were measured by kinetic quantitative reverse-transcription PCR (QRT-PCR) using endogenous actin and NNE mRNAs as internal standards (Freeman et al., 1999) (Fig.4A,B). Again, only α subunit mRNAs showed changes in KO mice (Fig. 4C). Whereas the α1 subunit mRNA level was increased, the level of the α2 subunit mRNA was decreased in both amygdala and cortex of KO mice. Specifically, α1 mRNA levels were 185 ± 4% (n = 5) and 225 ± 66% (n = 5) of the WT level in KO amygdala and cortex, respectively. α2 subunit mRNA levels were 54 ± 9% (n = 5) and 54 ± 21% (n = 5) of the WT level in KO amygdala and cortex, respectively. In addition, the level of the α4 subunit mRNA was decreased in the hippocampus of KO mice.
Fig. 4.
Expression of GABAA receptor subunit mRNAs in amygdala, hippocampus, cortex, and raphe measured by kinetic QRT-PCR. Top left, Comparison of actin and α2 subunit RNA levels in the amygdala of WT and KO mice in a phosphorimager scan. PCR products from amplification cycles 24, 26, 28, 30, and 32, resolved on a 1.5% agarose gel, are shown.Top right, Radioactivity in bands (displayed inA) are plotted as a function of cycle numbers.Bottom, Relative subunit mRNA levels in amygdala, hippocampus, cortex, and raphe. *p < 0.05 represents significant difference between KO and WT animals.
DISCUSSION
Lack of 5-HT1A receptor elicits the downregulation of the α1 and α2 GABAA subunits in amygdala
A major finding of this report is that inactivation of the 5-HT1A receptor in mice results in alterations in the expression of the α1 and α2 subunits of GABAAreceptor in amygdala. The ∼50% reduction in both mRNA and protein of the α2 subunit in KO mice in amygdala and cortex indicates that the downregulation of this subunit is primarily attributable to a transcriptional and/or post-transcriptional mechanism. The increased steady-state level of α1 subunit mRNA in amygdala and cortex is also attributable to a transcriptional–post-transcriptional mechanism. However, the reduced α1 subunit levels in the presence of increased mRNA levels in amygdala and cortex of KO mice indicates an additional, presumably translational and/or post-translational perturbation in the expression of this subunit in mutant animals.
These data show that expression of certain GABAA receptor subunits are under serotonergic control exerted by 5-HT1A receptors in amygdala, cortex, and hippocampus. The 5-HT1Areceptor-mediated regulation of α1 and α2 subunit expression in amygdala is particularly interesting given the BZ-insensitive anxiety of receptor KO mice and because the amygdala is believed to serve as an interface between the environment and effector organs generating behavioral responses associated with fear and anxiety. How is the expression of the α1 and α2 subunits regulated by the 5-HT1A receptor in amygdala? The basic neuronal network and its modulation by 5-HT in amygdala has been described recently (Stutzmann et al., 1998; Rainnie, 1999; Stutzmann and LeDoux, 1999). Glutamatergic afferents impinge on projection neurons in amygdala, and activation from these afferents is inhibited by GABA interneurons. 5-HT exerts an additional inhibitory input on projection neurons by directly activating 5-HT1A receptor. We suggest that lack of 5-HT1A receptors in these cells in the amygdala eliminates an important 5-HT input, which is otherwise necessary to maintain a proper expression of the α1 and α2 subunits. 5-HT modulation of hippocampal circuits is similar (Gulyas et al., 1999); thus, a comparable 5-HT-mediated regulation of the α4 subunit could also be proposed.
The 5-HT1A receptor is coupled to inward-rectifying K+ channels through Gβγ (Andrade, 1992), indicating that genetic inactivation of the receptor could alter the cellular membrane potential and the frequency and duration of electrical impulses (Aghajanian and Lakoski, 1984; Corradetti et al., 1996; Ehrengruber et al., 1997) leading to depolarization and an increase in depolarization-evoked Ca2+ influx (Cheng et al., 1998). In addition, the 5-HT1A receptor is negatively coupled to adenyl cyclase (via Gαi and/or Gαo), raising the possibility that genetic inactivation of the receptor leads to a rise in cAMP and activation of the linked protein kinase A pathway above a normal physiological level. Also, Gαiactivates the mitogen-activated protein kinase cascade; thus, in the absence of the 5-HT1A receptor, this signaling could also be altered. We propose that lack of the 5-HT1A receptor, by altering these signaling pathways, results in changes in the expression of genes, including the GABAA receptor α1, α2, and α4 subunits in amygdala and hippocampus.
Decreased BZ binding in amygdala of 5-HT1A receptor KO mice is consistent with the reduced expression of the α1and α2 GABAA subunits
The reduced α1/α2 subunit levels can explain the attenuated BZ binding in amygdala and cortex. GABAA receptor subunit composition influences the sensitivity of binding sites to BZ (Belzung et al., 1987; MacDonald and Olsen, 1994). The BZ recognition site is located predominantly in the α subunit, and the different α subtypes confer major pharmacological differences with respect to BZ. The α1 and α2 subunits are major components of the BZ-specific GABAAreceptors (MacDonald and Olsen, 1994; Sieghart, 1995); thus, a loss in α1 and α2 subunits can lead to the assembly of less BZ receptors. However, the reduction in α1/α2 subunit expression (∼50%) was larger than the reduction in BZ binding (∼16%) in the amygdala of KO mice. One possibility to explain this difference is that α1 and α2 subunit-containing receptors represent only part of the total BZ–GABAA receptor pool (α3 and α5 subunits also participate in assembly). Also, changes in intracellular subunit levels may not be directly proportional with changes in binding because subunits in assembled receptors represent only a fraction of the total intracellular subunit pool.
The decline in BZ binding was less pronounced in cortex than in amygdala, despite a comparable reduction in α1/α2 subunit expression in these brain regions. This indicates that downregulation of the α1/α2 subunit expression can differentially affect overall BZ binding in amygdala and cortex. It is possible that, depending on the size of the intracellular pool of unassembled subunits, downregulation of the subunits affects the number of assembled receptors more or less profoundly. Also, other α subunits may compensate for the loss of the α1 and α2 subunits differently in amygdala and cortex.
Hippocampus, in contrast to amygdala–cortex, showed a change in the expression of the α4 subunit mRNA in KO mice. Downregulation of this subtype mRNA is consistent with the reduced GABAA and unchanged BZ receptor binding in this region, because α4 subunit forms BZ-insensitive GABAA receptors. However, to support this notion, it will be necessary to measure the expression of the α4 subunit by Western blotting.
Changes in GABAA receptor subunit expression and BZ binding can explain the lack of anxiolytic effect of BZ in 5-HT1A receptor KO mice
Because α1/α2 subunits represent major α subtypes in amygdala and because amygdala is believed to be the site of the anxiolytic action of BZ, we propose that the downregulation of the α1/α2 subunits in the amygdala of KO mice is important and probably essential for the development of the BZ insensitivity. Recently, it was proposed that GABAA receptors containing α2, α3, and/or α5 subunits are responsible for the anxiolytic activity of BZs (Rudolph et al., 1999). The downregulation of the α2 subunit in 5-HT1Areceptor KO mice and their insensitivity to the anxiolytic action of diazepam are consistent with this notion. Because the α3 subunit is unchanged in 5-HT1A receptor KO mice and because the α5 is a minor subtype in amygdala, we propose that the anxiolytic action of BZ is specifically mediated by GABAA receptors containing α2 subunits.
The impaired sedative effect of diazepam in 5-HT1A receptor KO mice could also be explained by alterations in GABAA receptor subunits.Rudolph et al. (1999) have found that a point mutation in the BZ binding site of the α1 subunit results in a reduced sensitivity to the sedative but not the anxiolytic action of BZs in mice. Thus, the reduced level of this subtype in 5-HT1A receptor KO mice could be specifically responsible for the impaired sedative effect of diazepam. Effects of barbiturates are less subtype-specific, and it is more likely that the reduced sedative–anesthetic effect of pentobarbital in receptor KO mice is attributable to the downregulation of both the α1 and α2 subunits.
Downregulation of the α1 and α2GABAA subunits may also underlie the anxiety of 5-HT1A receptor KO mice
The relevance of the GABA system and GABAA receptors in anxiety disorders has long been implicated. Competitive and noncompetitive GABAA receptor antagonists and BZ inverse agonists elicit anxiety (Belzung et al., 1987; Dalvi and Rodgers, 1996). The GABAA receptors themselves have been implicated in the pathogenesis of anxiety. Indeed, reduced BZ receptor binding was found in anxiety (Marczynski and Urbancic, 1988; Sundstrom et al., 1997). Importantly, altered GABAAreceptor binding in the limbic system has been correlated with increased anxiety in both humans (Schlegel et al., 1994; Kaschka et al., 1995; Sundstrom et al., 1997) and animals (Rainnie et al., 1992). Currently, it is believed that certain forms of anxiety, such as that associated with drug withdrawal, can be linked to changes in the subunit composition of the BZ receptor (Mahmoudi et al., 1997; Smith et al., 1998). We propose that the downregulation of the α1/α2 subunits in amygdala is responsible, at least partly, for the expression of the anxiety phenotype in the 5-HT1A receptor KO mice.
A link between the 5-HT and GABA systems and its implication in anxiety disorders
A novel feature of the 5-HT1A receptor KO mouse anxiety model is that it mechanistically links together two important neurotransmitter systems, the 5-HT and GABA systems. Both of these systems have been implicated in anxiety disorders. Specifically, the 5-HT1A receptor KO mice provides a model to study a pathogenic pathway leading to BZ-insensitive forms of anxiety. A significant portion of patients suffering from generalized anxiety have a reduced sensitivity to the anxiolytic action of BZs. Patients with panic anxiety have a reduced BZ binding and a reduced responsiveness to BZs (Schlegel et al., 1994; Kaschka et al., 1995;Roy-Byrne et al., 1996). However, BZ insensitivity may not be specific for panic disorder (Roy-Byrne et al., 1996; Sundstrom et al., 1997) but rather could reflect a more general aspect of anxiety disorders.
Footnotes
We thank Drs. J. Buck (Weill Medical College of Cornell University) and D. Benjamin (Rutgers University) for their help with the HPLC measurement of serum diazepam levels and the BZ autoradiography, respectively.
Correspondence should be addressed to Miklos Toth, Department of Pharmacology, Weill Medical School of Cornell University, 1300 York Avenue, LC 522, New York, NY 10021. E-mail:mtoth@mail.med.cornell.edu.
REFERENCES
- 1.Aghajanian G, Lakoski JM. Hyperpolarization of serotonergic neurons by serotonin and LSD: studies in brain slices showing increased K+ conductance. Brain Res. 1984;305:181–185. doi: 10.1016/0006-8993(84)91137-5. [DOI] [PubMed] [Google Scholar]
- 2.Andrade R. Electrophysiology of 5-HT1A receptors in the rat hippocampus and cortex. Drug Dev Res. 1992;26:275–286. [Google Scholar]
- 3.Ashworth-Preece M, Krstew E, Jarrott B, Lawrence AJ. Functional GABAA receptors on rat vagal afferent neurones. Br J Pharmacol. 1997;120:469–475. doi: 10.1038/sj.bjp.0700909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzam RM, Notarianni LJ, Ali HM. Rapid and simple chromatographic method for the determination of diazepam and its major metabolites in human plasma and urine. J Chromatogr B Biomed Sci Appl. 1998;708:304–349. doi: 10.1016/s0378-4347(98)00004-8. [DOI] [PubMed] [Google Scholar]
- 5.Barnard E, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 6.Belzung C, Misslin R, Vogel E, Dodd RH, Chapouthier G. Anxiogenic effects of methyl-beta-carboline-3-carboxylate in a light/dark choice situation. Pharmacol Biochem Behav. 1987;28:29–33. doi: 10.1016/0091-3057(87)90006-2. [DOI] [PubMed] [Google Scholar]
- 7.Blier P, de Montigny C, Chaput Y. Electrophysiological assessment of the effects of antidepressant treatments on the efficacy of 5-HT neurotransmission. Clin Neuropharmacol. 1988;11:S1–S10. [PubMed] [Google Scholar]
- 8.Bohmaker K, Eison AS, Yocca FD, Meller E. Comparative effects of chronic 8-OH-DPAT, gepirone and ipsapirone treatment on the sensitivity of somatodendritic 5-HT1A autoreceptors. Neuropharmacology. 1993;32:527–534. doi: 10.1016/0028-3908(93)90048-8. [DOI] [PubMed] [Google Scholar]
- 9.Cheng LL, Wang SJ, Gean PW. Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci. 1998;10:2163–2172. doi: 10.1046/j.1460-9568.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- 10.Corradetti R, Le Poul E, Laaris N, Hamon M, Lanfumey L. Electrophysiological effects of N-(2-(4-(2-methoxyphenyl)-1-piperazinyl(ethyl)-N-(2-pyridinyl) cyclohexane carboxamide (WAY 100635) on dorsal raphe serotonergic neurons and CA1 hippocampal pyramidal cells in vitro. J Pharmacol Exp Ther. 1996;278:679–688. [PubMed] [Google Scholar]
- 11.Dalvi A, Rodgers RJ. GABAergic influences on plus-maze behaviour in mice. Psychopharmacology. 1996;128:380–397. doi: 10.1007/s002130050148. [DOI] [PubMed] [Google Scholar]
- 12.De Vry J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology. 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- 13.Ehrengruber MU, Doupnik CA, Xu Y, Garvey J, Jasek MC, Lester HA, Davidson N. Activation of heteromeric G protein-gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons. Proc Natl Acad Sci USA. 1997;94:7070–7075. doi: 10.1073/pnas.94.13.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flugge G. Dynamics of central nervous 5-HT1A-receptors under psychosocial stress. J Neurosci. 1995;15:7132–7140. doi: 10.1523/JNEUROSCI.15-11-07132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: pitfalls and potential. Biotechniques. 1999;26:112–125. doi: 10.2144/99261rv01. [DOI] [PubMed] [Google Scholar]
- 16.Gulyas AI, Acsady L, Freund TF. Structural basis of the cholinergic and serotonergic modulation of GABAergic neurons in the hippocampus. Neurochem Int. 1999;34:359–372. doi: 10.1016/s0197-0186(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 17.Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, Bluethmann H, Mohler H, Luscher B. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamon M. The main features of the central 5-HT1A receptors. in serotoninergic neurons and 5-HT receptors in the CNS (Baumgarten HG, Gotner M, eds), pp 238–268. Springer; New York: 1997. [Google Scholar]
- 19.Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Nat Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs BL. Physiology and pharmacology of brain serotoninergic neurons. In: Van de Kar LD, editor. Serotoninergic neurons and 5-HT receptors in the CNS. Springer; New York: 1997. pp. 91–116. [Google Scholar]
- 21.Jolas T, Haj-Dahmane S, Lanfumey L, Fattaccini CM, Kidd EJ, Adrien J, Gozlan H, Guardiola-Lemaitre B, Hamon M. (−) Tertatolol is a potent antagonist at pre- and postsynaptic serotonin 5-HT1A receptors in the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:453–463. doi: 10.1007/BF00166735. [DOI] [PubMed] [Google Scholar]
- 22.Kaschka W, Feistel H, Ebert D. Reduced benzodiazepine receptor binding in panic disorders measured by iomazenil SPECT. J Psychiatr Res. 1995;29:427–434. doi: 10.1016/0022-3956(95)00019-2. [DOI] [PubMed] [Google Scholar]
- 23.Kataoka Y, Shibata K, Yamashita K, Ueki S. Differential mechanisms involved in the anticonflict action of benzodiazepines injected into the central amygdala and mammillary body. Brain Res. 1987;416:243–247. doi: 10.1016/0006-8993(87)90903-6. [DOI] [PubMed] [Google Scholar]
- 24.Kennett GA, Marcou M, Dourish CT, Curzon G. Single administration of 5-HT1A agonists decreases 5-HT1A presynaptic, but not postsynaptic receptor-mediated responses: relationship to antidepressant-like action. Eur J Pharmacol. 1987;138:53–60. doi: 10.1016/0014-2999(87)90336-0. [DOI] [PubMed] [Google Scholar]
- 25.Lesch KP. 5-HT1A receptor responsivity in anxiety disorders and depression. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:723–733. doi: 10.1016/0278-5846(91)90001-h. [DOI] [PubMed] [Google Scholar]
- 26.Liu ZF, Burt DR. A synthetic standard for competitive RT-PCR quantitation of 13 GABA receptor type A subunit mRNAs in rats and mice. J Neurosci Methods. 1998;85:89–98. doi: 10.1016/s0165-0270(98)00125-3. [DOI] [PubMed] [Google Scholar]
- 27.Lopez JF, Chalmers DT, Little KY, Watson SJ. A. E. Bennett Research Award. Regulation of serotonin1A: glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 28.Lucki I, Singh A, Kreiss DS. Antidepressant-like behavioral effects of serotonin receptor agonists. Neurosci Biobehav Rev. 1994;18:85–95. doi: 10.1016/0149-7634(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald RL, Olsen RW. GABA A receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABA(A) receptor alpha4-subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- 31.Marczynski TJ, Urbancic M. Animal models of chronic anxiety and “fearlessness.”. Brain Res Bull. 1988;21:483–490. doi: 10.1016/0361-9230(88)90163-3. [DOI] [PubMed] [Google Scholar]
- 32.McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37:383–393. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- 33.Murphy DL. Neuropsychiatric disorders and the multiple human brain serotonin receptor subtypes and subsystems. Neuropsychopharmacology. 1990;3:457–471. [PubMed] [Google Scholar]
- 34.Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- 36.Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82:69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- 37.Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Kindling-induced long-lasting changes in synaptic transmission in the basolateral amygdala. J Neurophysiol. 1992;67:443–454. doi: 10.1152/jn.1992.67.2.443. [DOI] [PubMed] [Google Scholar]
- 38.Ramboz S, Oosting R, Ait Amara D, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: An animal model of anxiety-related disorder. Proc Nat Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rock DM, Taylor CP. Effects of diazepam, pentobarbital, phenytoin and pentylenetetrazol on hippocampal paired-pulse inhibition in vivo. Neurosci Lett. 1986;65:265–270. doi: 10.1016/0304-3940(86)90272-7. [DOI] [PubMed] [Google Scholar]
- 40.Roy-Byrne P, Wingerson DK, Radant A, Greenblatt DJ, Cowley DS. Reduced benzodiazepine sensitivity in patients with panic disorder: comparison with patients with obsessive-compulsive disorder and normal subjects. Am J Psychiatry. 1996;153:1444–1449. doi: 10.1176/ajp.153.11.1444. [DOI] [PubMed] [Google Scholar]
- 41.Rudolph U, Crestani F, Benke D, Brünig I, Benson J, Fritschy JM, Martin J, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 42.Schlegel S, Steinert H, Bockisch A, Hahn K, Schloesser R, Benkert O. Decreased benzodiazepine receptor binding in panic disorder measured by IOMAZENIL-SPECT. A preliminary report. Eur Arch Psychiatry Clin Neurosci. 1994;244:49–51. doi: 10.1007/BF02279812. [DOI] [PubMed] [Google Scholar]
- 43.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 44.Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 45. Stutzmann GE, LeDoux JE. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J Neurosci 19 1999. RC8, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stutzmann GE, McEwen BS, LeDoux JE. Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. J Neurosci. 1998;18:9529–9538. doi: 10.1523/JNEUROSCI.18-22-09529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sundstrom I, Ashbrook D, Backstrom T. Reduced benzodiazepine sensitivity in patients with premenstrual syndrome: a pilot study. Psychoneuroendocrinology. 1997;22:25–38. doi: 10.1016/s0306-4530(96)00035-2. [DOI] [PubMed] [Google Scholar]
- 48.Thielen RJ, McBride WJ, Chernet E, Lumeng L, Li TK. Regional densities of benzodiazepine sites in the CNS of alcohol-naive P and NP rats. Pharmacol Biochem Behav. 1997;57:875–882. doi: 10.1016/s0091-3057(96)00464-9. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe Y, Sakai RR, McEwen BS, Mendelson S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]