Abstract
Polysialic acid–neural cell adhesion molecule (PSA-NCAM) expression in the adult nervous system is restricted to regions retaining a capacity for morphological plasticity. For the female rat hypothalamoneurohypophysial system (HNS), we have previously shown that lactation induces a dramatic decrease in PSA-NCAM, while leaving the level of total NCAM protein unchanged. Here, we wanted to elucidate the molecular mechanisms leading to a downregulation of PSA, thereby stabilizing newly established synapses and neurohemal contacts that accompany the increased activity of oxytocinergic neurons.
First, we show that the overall specific activity of polysialyltransferases present in tissue extracts from supraoptic nuclei decreases by ∼50% during lactation. So far, two polysialyltransferase enzymes, STX and PST, have been characterized for their capacity to transfer PSA onto NCAM in vitro. Using a competitive RT-PCR on RNA extracts from the HNS, we demonstrate furthermore a significant decrease in the expression levels of both STX and PST mRNAs in lactating versus virgin animals. Interestingly, this downregulation of NCAM polysialylation is not correlated with the post-transcriptional regulation of variable alternative spliced exon splicing, in contrast to neural development. The control of polysialylation via a regulation of both enzyme activity and expression underlines the important role of this post-translational modification of NCAM in morphofunctional plasticity in adult brain.
Keywords: PSA-NCAM, PST, STX, competitive RT-PCR, enzymatic activity, lactation
Development of the nervous system, and its structural remodeling in the adult, rely on site- and time-dependent expression of specific combinations of adhesion molecules. In addition, adhesive properties of individual cell adhesion molecules can be modified both on post-transcriptional and post-translational levels. For the neural cell adhesion molecule (NCAM), addition of polysialic acid (PSA) to the fifth Ig-like domain facilitates events such as cell migration, neurite growth, and synaptic reorganization (for review, see Rutishauser and Jessell, 1988; Rougon, 1993; Rutishauser, 1993; Seki and Arai, 1993; Fryer and Hockfield, 1996). Postnatal loss of PSA is generally associated with stabilization of NCAM-mediated cell–cell interactions and synapse formation (Szele et al., 1994).
PSA is a large, negatively charged homopolymer of α-2,8 sialic acid. Two characterized polysialyltransferases, STX (ST8SiaII) and PST (ST8SiaIV) (Livingston and Paulson, 1993; Nakayama et al., 1995), have been shown to add PSA onto NCAM in vitro; however, there is no direct evidence of the exact role of these enzymes in vivo. Highly expressed during development, their expression is downregulated with maturation of the nervous system, in close correlation with PSA-NCAM expression (Kurosawa et al., 1997; Wood et al., 1997; Hildebrandt et al., 1998b; Ong et al., 1998). STX and PST messenger expression persists, however, in restricted areas of the adult brain (Kurosawa et al., 1997; Phillips et al., 1997), i.e., in the olfactory system, and in certain areas capable of structural reorganization, such as the hippocampus and, in particular, the hypothalamoneurohypophysial system (HNS).
The HNS, comprising the hypothalamic magnocellular nuclei [supraoptic nuclei (SON) and paraventricular nuclei (PVN)] that project into the neurohypophysis (NH), undergoes specific morphological modifications in association with certain physiological changes, e.g., prolonged dehydration and lactation (Hatton, 1990). In the hypothalamus of a lactating female, the astrocytic coverage of oxytocinergic neurons is reduced, thereby increasing membrane appositions and synaptic contacts, and in the NH, the interactions of oxytocinergic axon terminals with blood vessels are enhanced. This morphological state, which is reversible after weaning, is associated with increased, highly synchronous electrical activity of oxytocinergic neurons (Lincoln and Wakerley, 1975). We have shown previously that during lactation, subsequent to the observed morphofunctional changes in the HNS, PSA-NCAM expression is dramatically decreased in both SON and NH. In contrast, no modifications in the total amount of NCAM protein or in its mRNA were observed. The HNS may therefore represent a good in vivo model for morphological plasticity affecting the polysialylation of NCAM.
To elucidate the molecular mechanisms underlying the changes in PSA-NCAM expression, we evaluated the overall activity of polysialyltransferases in the HNS of virgin and lactating females. We also analyzed, using a competitive RT-PCR, any potential lactation-induced changes in expression in mRNAs of both STX and PST. Finally, we analyzed the regulation of NCAM expression on a post-translational level by examining the alternative splicing of the 30 bp variable alternative spliced exon (VASE) on NCAM mRNA. In contrast to PSA, VASE peptide presence on the fourth NCAM Ig-like domain, which is developmentally regulated, appears to reduce neurite outgrowth and enhance NCAM-mediated adhesion (Small et al., 1988).
MATERIALS AND METHODS
Animals. Virgin adult and lactating (at 10 d of lactation) Wistar rats (IFFA Credo, L'Arbresle, France) were used. For enzyme activity tests and RT-PCR, animals were killed by decapitation, and the brains and neurohypophysis were quickly removed, frozen with isopentane at −40°C and stored at −80°C. SON were dissected by punching them out from frozen brain slices.
Immunohistochemistry. Animals were prepared for brain sectioning as described (Nothias et al., 1997). Coronal sections (30 μm) were incubated with primary antibody directed against polysialic acid [IgM antibody, anti-Men-B (Rougon et al., 1986), followed by peroxidase-labeled secondary antibody (Cappel/Flobio)]. Peroxidase reactivity was revealed with 0.05% diaminobenzidine tetrahydrochloride and 0.01% hydrogen peroxide in 0.05 m Tris buffer, pH 7.6. Care was taken to treat sections from virgin and lactating rats in parallel under exactly the same conditions. To visualize both cell bodies and fibers, adjacent sections were treated by Kluver and Barrera staining.
Polysialyltransferase activity test. From SON of virgin and lactating rats, a protein extract enriched for polysialyltransferases was prepared according to Oka et al. (1995). The enzyme activity test reaction was performed in a final volume of 50 μl, containing 10 μg purified NCAM-Fc chimera (generously provided by P. Doherty, Guy's Hospital, London, UK) (Saffell et al., 1997), 20 mmCMP-14C-neuraminic acid (2.8 × 105 cpm), 10 mm2-N-morpholino ethanesulfonic acid (MES), 20 mmMnCl2, 2.5 mm ATP, and the enzyme-enriched cell extract. After incubation for 3 hr at 37°C, the reaction was terminated by addition of EDTA (50 mm). One-half of the sample was digested with EndoN (generously provided by G. Rougon, CNRS Luminy, Marseille, France) for 4 hr at 37°C. Treated and untreated samples were then spotted onto Whatman GF/C paper disks, rinsed three times in MES buffer (20 mm) containing NaCl 250 mm, rinsed once with ethanol 95%, and air-dried. Radioactivity on the disks was measured using a scintillation counter, and the difference between EndoN-treated and untreated samples was taken as a measure of the amount of sialic acid incorporated. Total protein concentration was determined by the Lowry method, and specific enzymatic activity was calculated and compared for each case using an ANOVA test.
Quantification of STX and PST mRNAs by competitive RT-PCR.Total RNA was extracted with Triaxis reagent according to the manufacturer's specification (Genaxis) and treated with DNase (0.02 U/μl; Promega). To clone STX and PST, 5 μg aliquots of hypothalamic RNA were incubated for 5 min at 70°C with 1 μg of random and 1 μg of oligo-dT primers in a final volume of 25 μl, and reverse transcription was performed with Moloney murine leukemia virus-RT (Promega) according to the manufacturer's recommendations. For selective amplification of STX and PST, primers were carefully chosen from nonoverlapping sequences. The primer sequence for STX was from mouse (GenBank accession number X83562; 5′-GGG TCT TGC TGA ACA GCG GC-3′ and 5′-GTG TAG CCA TAC TTG AGG CTG-3′; nucleotides 658–1186); the primer sequence for PST was from rat (GenBank accession numberU90215; 5′-ACT GAG GAG CAC CAA GAG ACG C-3′ and 5′-CCA TGA AGG CAG GAA TCC AAA GG-3′; nucleotides 31–637). PCR was performed with Tfl DNA polymerase (Promega) according to instructions of the manufacturer. After an initial step of 3 min at 93°C, 40 cycles were performed with 1 min at 92°C, 1 min at 58°C for STX and 62°C for PST, then 1 min at 72°C. Amplified products (528 bp for STX and 606 bp for PST) were subcloned in pGEM-T Easy (Promega) plasmid, and authenticity was verified by sequencing (Genome Express) and comparison with respective GenBank data. Standard STXΔ and PSTΔ were constructed by digesting STX and PST cDNA (2 μg) with AvaI and Tth111, respectively, for 2 hr at 37°C, eliminating a fragment of 156 bp for STX and 110 bp for PST. Digested cDNAs were purified on a microspin column (Pharmacia Biotech) and ligated with T4 DNA ligase (Promega), and the size of the standards (372 bp for STXΔ and 496 bp for PSTΔ) was verified on an agarose gel, after amplification by PCR using the same primers as above. Plasmids were then linearized withSalI and transcribed into cRNA by T7 RNA polymerase.
For the competitive PCR, varying amounts (from 0.5 to 0.1 pg for STX and from 10 to 1 pg for PST) of the internal standard cRNAs were added to a constant amount (2 μg for PST and 5 μg for STX) of total RNA, extracted from virgin or lactating SON and NH. Each mixture was reverse-transcribed, and 2 μl of the product was subjected to PCR as described above, except that primers were 5′-radiolabeled with γ-32P-ATP. Briefly, 1 nmol of each primer was incubated for 30 min at 37°C with 50 μCi of γ-32P-ATP and T4-polynucleotide kinase (20 U; Promega) in the appropriate buffer in 50 μl final volume. For the radioactive PCR (25 μl final volume), each template was used in duplicate for either 35 or 40 cycles. Seven microliters of PCR products were loaded on an 8% polyacrylamide gel, run at 150 V, dried, and exposed on a phosphorimaging screen (PhosphorImager, Molecular Dynamics) for further quantification. A competitive PCR linear regression curve was obtained by plotting the ratio of radioactivity [expressed as log(cpm)] incorporated into the respective amplification products of target mRNA and cRNA internal standards against the quantities of internal standard used in each reaction. The relative amounts of STX and PST target RNAs present in the different tissues were directly calculated from this curve and reported to the total amount of RNA used in each RT-PCR (Liu et al., 1997).
Semiquantitative RT-PCR of NCAM-VASE mRNAs.γ-32P-ATP radiolabeled oligonucleotides 5′-ACC TGG AGA ACG TCC ACC CGA AAC ATC-3′ and 5′-AGG ACA CAC GAG CAT GGC TGC GTA CCA CCA-3′ covering both flanking regions of the VASE sequence were chosen as primers for amplification of NCAM mRNAs with or without the VASE exon (Small and Akeson, 1990). Hot PCR was performed on SON and NH cDNA from virgin and lactating (at 1 and 10 d) rats. An initial step of 3 min at 93°C was followed by 1 min at 92°C, 1 min at 55°C, then 1 min at 72°C for extension, for 25, 30, and 35 cycles. Seven microliters of each reaction product were loaded on a 12% polyacrylamide gel that was run at 150 V, dried, and exposed to a phosphorimaging screen (PhosphorImager; Molecular Dynamics) for quantification. The PCR reaction gives rise to two bands, the top corresponding to NCAM-VASE+ mRNA (120 bp) and the bottom (90 bp) to NCAM-VASE−mRNA. Data are presented as the ratio of radioactivity [expressed as log(cpm)] incorporated into NCAM-VASE+mRNA and NCAM-VASE− mRNA and compared for virgin versus lactating conditions using an ANOVA test.
RESULTS
We have recently shown that the morphofunctional modifications in the hypothalamus during lactation are accompanied by a significant decrease in PSA-NCAM, an isoform of NCAM strongly expressed by the SON of virgin females (Nothias et al., 1997). This decrease in PSA staining becomes obvious at the end of the first week of lactation and is most prominent by day 10 (Fig.1b,c) [see also Nothias et al. (1997)], whereas the levels of total NCAM protein remain unchanged. Here, we demonstrate that the PVN exhibit the same progressive decline in PSA-NCAM staining (Fig.1d,e). It is noteworthy that in contrast to other hypothalamic nuclei, this change in PSA-NCAM during lactation is exclusively observed in the magnocellular nuclei (SON and PVN) that project into the NH.
Fig. 1.
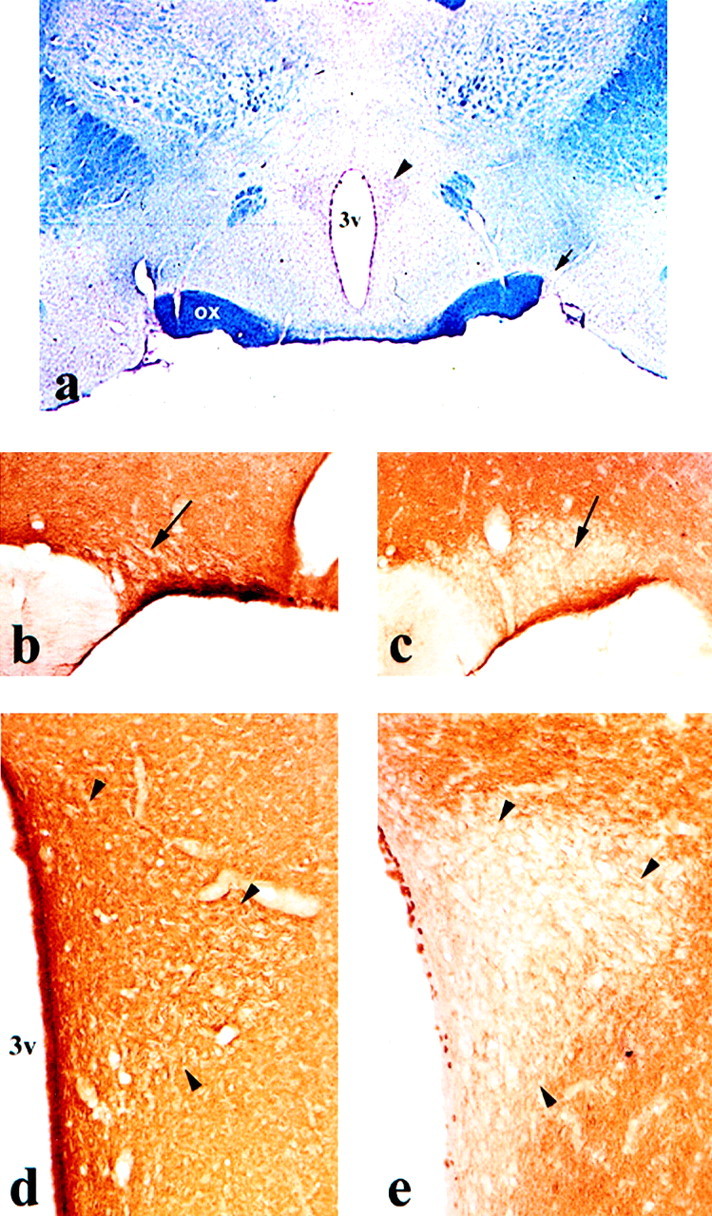
PSA-NCAM expression in SON and PVN of virgin and lactating rats. a, Photomicrograph of a coronal section from adult rat brain, at the level of SON (arrow) and PVN (arrowhead) hypothalamic nuclei; Kluver and Barrera staining. b–e, Photomicrographs of PSA-NCAM immunostaining in SON (b, c) and PVN (d, e) nuclei of virgin (b, d) and 10 d lactating (c, e) rats. During lactation, polysialic acid immunoreactivity is dramatically decreased in both nuclei, whereas the rest of the hypothalamus remains highly stained. Magnifications:a, 17×; b, 67×; c, 80×;d–e, 120×.
This decrease in PSA immunoreactivity during lactation could in principle be caused by a change in the specific activity of polysialyltransferases, a change in the expression level of sialyltransferase mRNAs, or both. In all experiments that follow, virgin rats were always compared with animals at day 10 of lactation, the time point at which the decrease in PSA levels is most obvious (see above).
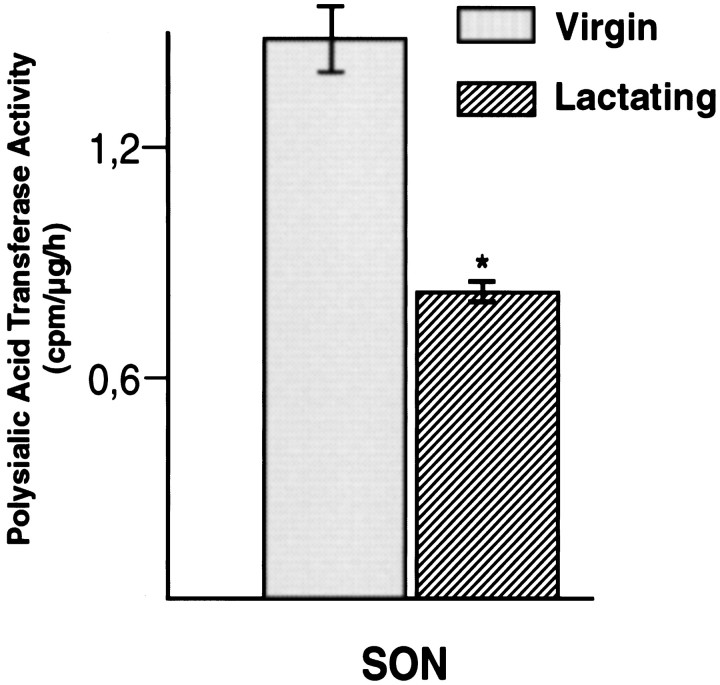
To analyze the overall polysialyltransferase activity, polysialyltransferase-enriched tissue extracts were prepared from the SON of virgin and lactating rats (Oka et al., 1995), and enzymatic activity was determined by the incorporation of CMP-14C-sialic acid into NCAM-Fc chimeras. As shown in Figure 2, SON extracts of lactating rats exhibited a specific polysialyltransferase activity that was reduced by ∼50% in comparison to that of nonlactating rats. Similar values were obtained for the PVN (data not shown). Thus, the observed decrease of PSA-NCAM in both hypothalamic nuclei during lactation appears to be closely correlated with a specific downregulation of polysialyltransferase activity.
Fig. 2.
Decrease in overall polysialyltransferase activity in the SON during lactation. Polysialyltransferase-enriched tissue extracts obtained from virgin and 10 d lactating animals were used to catalyze incorporation of 14C-labeled sialic acid into PSA chains on NCAM-Fc chimeras of a given concentration. The radioactivity incorporated under each condition, in relation to the total protein content of the corresponding tissue extract, was taken as a measure for the specific enzyme activity (*p < 0.05; ANOVA test).
The reduction in specific enzymatic activity observed in the SON during lactation (Fig. 2) could be attributable, at least in part, to a downregulation of enzyme expression itself. Thus, we have analyzed the relative mRNA expression levels of the two well characterized vertebrate polysialyltransferases STX and PST in SON and NH of virgin and lactating animals. With respect to the limited amounts of RNA to be extracted from these tissues, a competitive RT-PCR appeared to be the adequate approach for quantification. By carefully choosing nonoverlapping regions from the known coding sequences for both enzymes, we amplified a 528 bp stretch of STX and a 606 bp stretch of PST cDNA, from which corresponding internal standards were constructed (372 bp for STXΔ and 496 bp for PSTΔ), to be used as competitors in the RT-PCR.
Consistent with previous in situ hybridization data (Phillips et al., 1997), electrophoretic analysis of the competitive RT-PCR reaction products confirmed the presence of mRNA transcripts encoding for STX (Fig. 3) and PST (Fig.4) in adult SON, as well as in PVN (data not shown) and NH. Incorporation of radioactivity into the target RNA amplification products (top band) depended on the relative amount of competing internal standard cRNAs (bottom band). Quantification was performed by calculating the ratio between the log of the cpm of the two bands (STX/STXΔ or PST/PSTΔ) across the series of concentrations of internal standards. For both STX (three independent experiments) and PST (four independent experiments), the point of equivalence [i.e., the ratio log(cpm STX)/log(cpm STXΔ) = 1] is obtained at a concentration of internal standards that differs considerably between virgin versus lactation conditions. Indeed, in the case of STX (see graph in Fig. 3), the equivalence point corresponds to ∼0.5 pg of STXΔ for the SON of virgin and ∼0.2 pg for that of lactating rats. The content in STX mRNA per microgram of total RNA is ∼0.1 pg for virgin SON and decreases by 60% to ∼0.04 pg during lactation. For PST mRNA expression (see graph in Fig. 4), the point of equivalence lies at ∼4.5 pg of PSTΔ for the SON of virgin rats and ∼3 pg for the SON of lactating rats. This corresponds to a decrease of 35%, from ∼2.3 pg PST mRNA per microgram total RNA for the SON of a virgin rat to ∼1.5 pg of a lactating one. Thus, in the SON, mRNAs encoding for PST are more abundant than those encoding for STX. Most important, however, these data demonstrate that downregulation of the expression of both polysialyltransferase mRNAs is at least one of the molecular mechanisms involved in the decrease of polysialylation of NCAM during lactation.
Fig. 3.
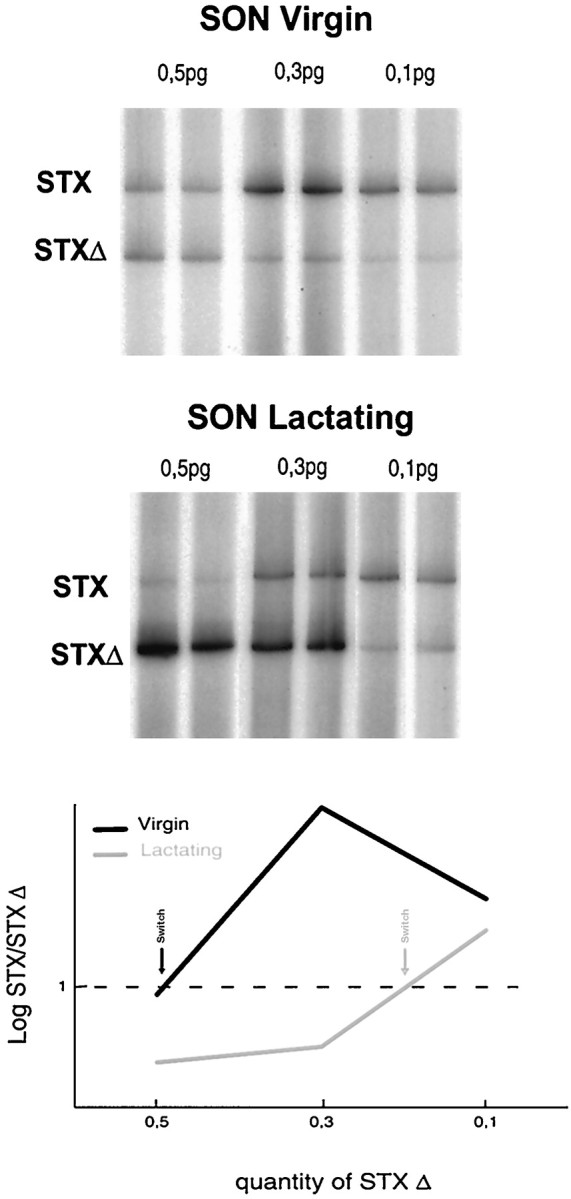
STX expression in the SON of virgin and lactating rats. Top, Total RNA was extracted from the SON of virgin rats and at day 10 of lactation. Competitive RT-PCRs were performed using γ-32P-ATP-labeled STX-specific primers with different amounts of STXΔ internal standard cRNA (0.5, 0.3, and 0.1 pg) and a constant amount of total SON RNA (5 μg). The reaction products were separated on an acrylamide gel, and the radioactivity incorporated into each band was determined using a Phosphorimager.Graph, Phosphorimager quantitative analysis of the above gels: to evaluate the relative STX mRNA levels in virgin and lactating SON, a competitive RT-PCR linear regression curve is constructed by plotting for each concentration of internal standard cRNA, and the ratios of log(cpm) are incorporated into STX versus STXΔ reaction products. The point of equivalence [log(cpm STX)/log(cpm STXΔ) = 1; indicated by arrows] switches from 0.5 pg of STXΔ for virgin SON to 0.2 pg for lactating SON, corresponding to a decrease of 60%.
Fig. 4.
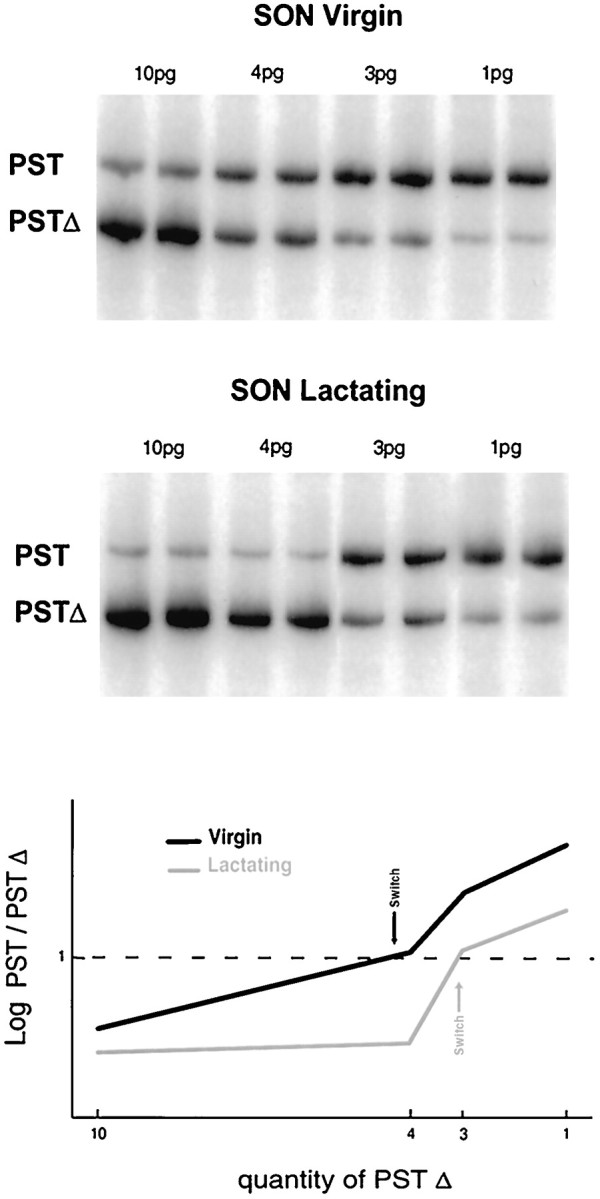
PST expression in the SON of virgin and lactating rats. Gels, The radioactive RT-PCR was performed as detailed in Figure 3, using PST-specific primers with different amounts of PSTΔ internal standard cRNA (10, 4, 3, and 1 pg) and 2 μg of total SON RNA. Graph, Phosphorimager quantitative analysis of the above gels. Competitive RT-PCR linear regression plot of log(cpm PST)/log(cpm PSTΔ) against the concentrations of internal standard used in each experiment. The point of equivalence [log(cpm PST)/log(cpm PSTΔ) = 1; indicated by arrows] switches from 4.5 pg for the virgin SON to 3 pg for lactating SON, corresponding to a decrease in PST mRNA levels of 35%.
A similar competitive RT-PCR analysis was also performed for the NH. In contrast to the SON, however, we did not detect any changes in STX and PST mRNA expression during lactation (data not shown), although PSA levels are decreasing in the NH (Nothias et al., 1997). Furthermore, the expression of PST mRNA in the NH was comparatively low, whereas the amount of STX mRNA was as high as in SON. For the SON and the NH, results of the competitive RT-PCR experiments are summarized in Table1.
Table 1.
Competitive RT-PCR analysis of STX and PST mRNA expression in SON and NH of virgin and lactating rats
| Tissue extracts | STX mRNA | PST mRNA | ||||
|---|---|---|---|---|---|---|
| Point of equivalence | (pg mRNA/μg total RNA) | Point of equivalence | (pg mRNA/μg total RNA) | |||
| SON virgin | 0.5 pg | 0.1 | Decrease of 60% | 4.5 pg | 2.3 | Decrease of 35% |
| SON lactating | 0.2 pg | 0.04 | 3 pg | 1.5 | ||
| NH virgin | ∼0.4 pg | ∼0.1 | No change | ∼0.8 pg | ∼0.4 | No change |
| NH lactating | id | id | id | id | ||
PhosphorImager data on radioactivity incorporated into the reaction products of the different RT-PCRs were transformed into log (cpm); equivalence points correspond to log[cpm (target cRNA/standard RNA)] = 1. Absolute values for target STX and PST mRNAs were then calculated by reporting the respective equivalence points to the amount of total RNA used in each reaction. See also Figures 3 and 4. id, Identical.
In parallel, we wanted to determine whether lactation would affect NCAM expression in the HNS, not only on the post-translational but also on the post-transcriptional level. In particular, we have studied the expression of VASE on NCAM in SON and NH isolated from virgin or lactating rats. VASE is a 30 bp spliced exon that codes for a 10 amino acid peptide in the fourth Ig-like domain on a certain percentage of NCAM molecules, depending on the tissue and the state of maturation. By semiquantitative RT-PCR, we compared the relative expression levels of mRNAs encoding for NCAM with (NCAM-VASE+) or without (NCAM-VASE−) the VASE exon. Primers were chosen to co-amplify mRNAs containing or not containing the VASE exon (Small and Akeson, 1990), yielding a 120 and a 90 bp PCR amplification product, respectively (Fig.5).
Fig. 5.
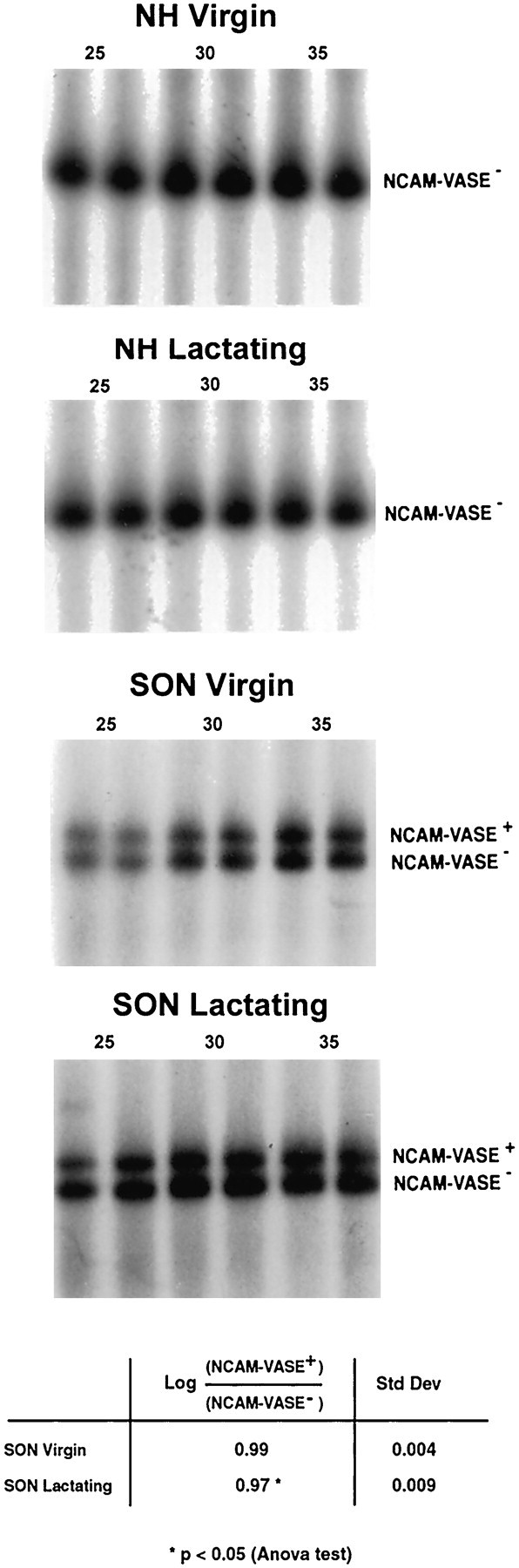
VASE-NCAM expression in NH and SON of virgin and lactating rats. γ-32P-ATP-labeled primers were constructed from both flanking regions of the VASE sequence to amplify a stretch of NCAM cDNA containing or not containing the VASE exon. Semiquantitative PCRs (25, 30, and 35 cycles) were performed on cDNA reverse-transcribed from total RNA extracted from NH and SON of virgin and lactating (day 10) rats. Amplification products were separated on acrylamide gels, and the radioactivity of the bands representing NCAM-VASE+ (top band) and NCAM-VASE− mRNA (bottom band) was evaluated using a PhosphorImager. The table summarizes the respective values of the ratio log(cpm NCAM-VASE+/NCAM-VASE−) together with standard deviations. Note that although NCAM-VASE+ is completely absent from NH, for both hypothalamic nuclei the ratio NCAM-VASE+/NCAM-VASE− is significantly higher in virgin than in lactating rat.
For the neurohypophysis of both virgin and lactating rats, PCR amplification products corresponded only to the bottom band, indicating that the NH expresses uniquely NCAM lacking the VASE exon. In the hypothalamic nuclei, however, NCAM mRNAs with and without the VASE exon are present. As shown in Figure 5 for the SON (similar results were obtained for the PVN; data not shown), the NCAM-VASE+/NCAM-VASE−ratio in virgin versus lactating rats is slightly decreased, but in a reproducible manner, during lactation. This decrease is observed from the first day of lactation (data not shown). Thus, in addition to the post-translational regulation of its polysialylation, NCAM expression is regulated also on the post-transcriptional level. In contrast to the downregulation of polysialylation, however, VASE exon splicing occurs at the beginning of the lactation-induced morphological remodeling.
DISCUSSION
The present study demonstrates for the first time a clear link between the modulation of PSA levels on the NCAM molecule, coinciding with morphological plasticity in the adult brain, and the regulation of gene expression for the STX and PST polysialyltransferases. The change in STX and PST mRNA levels may be responsible in part for the observed decrease in the overall activity of polysialyltransferases in the hypothalamic magnocellular nuclei. However, similar changes in PSA levels in the neurohypophysis during lactation are not accompanied by changing STX and PST gene expression levels.
Among the six sialyltransferases characterized, only STX and PST have been found to exhibit a considerable degree of homology in their genomic structure, exceeding 60% within the catalytic domain (Eckhardt and Gerardy-Schahn, 1998). They are the only enzymes capable of polymerizing sialic acid, and they have a substrate specificity that is restricted to NCAM (Kojima et al., 1996). Despite these similarities, certain differences in their activities have also been noted: mouse STX activity is stimulated in the presence of Mn2+ or Mg2+and that of PST is stimulated by Ca2+; the efficiency of PST to polysialylate NCAM in vitro seemed much higher than that of STX (Kojima et al., 1996; Angata et al., 1998). Furthermore, their differential expression with respect to cell type and developmental stages (Angata et al., 1997; Kurosawa et al., 1997;Phillips et al., 1997), and the analysis of genomic structure and promoter activities of the corresponding genes (Yoshida et al., 1996;Takashima et al., 1998), suggested the regulation of PST and STX by distinct transcription factors and probably also distinct signal transduction pathways (Kojima et al., 1996). Little is known about the regulation of polysialyltransferase activity. Electrical activity of neurons, or contractile activity in cultured myotubes (Rafuse and Landmesser, 1996) appears to stimulate NCAM polysialylation; this may be based on the effect of changes in intracellular Ca2+ concentrations (Bruses and Rutishauser, 1998) and/or inhibition of protein kinase C (PKC) on the activity of polysialyltransferases. Furthermore, it has recently been shown that PKCδ, and the 29 kDa acidic protein 14-3-3, copurify with immunoprecipitated PST (H. Gallagher and C. M. Regan, personal communication); thus, control of PST activity may be effectuated by a mutual regulation of the activity of PKCδ by 14-3-3 protein, and vice versa.
Several comparative studies were undertaken to determine the expression pattern of PST and STX in developing and adult nervous systems. In early stages of development, PST and STX mRNAs are relatively abundant and usually coexpressed in most of the tissues examined (Phillips et al., 1997), the level of STX mRNA being several times higher than that of PST (Angata et al., 1997). Through postnatal developmental stages, expression of STX and PST is increasingly heterogeneous: STX is substantially downregulated, to become almost undetectable in the adult, whereas the decline in PST expression is moderate (Hildebrandt et al., 1998a; Ong et al., 1998). In the present study, both polysialyltransferase mRNAs were detected in the SON and PVN, the mRNA encoding for PST being more abundant than STX mRNA, consistent with previous in situ hybridization data (Phillips et al., 1997). Apparent contradictions between the different studies concerning the relative expression levels of the STX and PST genes might be attributable to the various species and brain regions analyzed, rendering a direct comparison difficult. Nevertheless, all of these studies, including ours, agree in that the amount of PSA found in developing or adult brain is closely correlated with the levels of PST and STX mRNA (Kurosawa et al., 1997; Phillips et al., 1997; Ong et al., 1998).
In certain restricted regions of the adult brain that maintain detectable levels of PSA, such as the hypothalamic nuclei, STX and PST expression remains relatively high. Here, we demonstrate that changes in NCAM-PSA levels in both SON and PVN during lactation coincide precisely with a change in STX and PST gene expression. This suggests that the in vivo polysialylation of NCAM is at least partially regulated at the level of polysialyltransferase gene expression. Thus, these enzymes may play an important role in neuronal plasticity in the adult.
Transfection of cells in vitro with either enzyme, STX or PST, enhances NCAM-mediated neurite outgrowth in a similar way. When both PST and STX are cotransfected, synthesis of polysialic acid is more efficient than with PST or STX alone, suggesting a synergistic rather than competitive action (Angata et al., 1997, 1998). In fact, it has been shown that PST adds PSA preferentially at the sixthN-glycosylation site, closest to the NCAM transmembrane domain (Angata et al., 1998), which in turn may facilitate polysialylation at the fifth site by STX. Although direct in vivo evidence is still lacking, such a cooperative mechanism may well be active in the HNS system, given that PST and STX are always coexpressed in brain regions in which extensive polysialylation is required even in the adult (Phillips et al., 1997; present study).
Independently from the analysis of STX and PST mRNA expression, we also determined the overall enzymatic activity of polysialyltransferases in tissue extracts from rat SON. Thus, the observed decrease of PSA-NCAM during lactation appears to be closely related to a reduction of polysialyltransferase activity by ∼50%. It has already been noted that in the chick, the downregulation of PSA on ciliary ganglion motoneurons during synaptogenesis precisely coincides with a decrease in polysialyltransferase activity levels (Bruses et al., 1995; Oka et al., 1995). It may be interesting that in this latter study the developmental regulation of PSA synthesis was not reflected by a change in the PST mRNA. In case of the rat SON, we present the first example of a tissue examined under two different physiological conditions in which changing polysialyltransferase activities can be correlated with changing expression levels of PST and STX genes. This does not preclude that also in the SON, specific polysialyltransferase activity could be affected independently from gene expression, as seems to be the case for the NH. In this target organ of the oxytocinergic neurons, PSA-NCAM levels are also decreasing during the period of lactation (Nothias et al., 1997); however, we did not detect any changes in PST or STX mRNA expression. The decrease in PSA immunoreactivity in the NH may be caused by reduced PSA on the hypothalamic afferents and by a reduced specific activity of the pituicyte polysialyltransferases. Thus, as had been shown previously for chick ciliary ganglion motoneurons (Bruses and Rutishauser, 1998), the loss of PSA on NCAM in the HNS of lactating rats is not generally coupled to a decrease in polysialyltransferase mRNA expression.
Finally, adhesivity of NCAM can be modified not only on the post-translational but also, via differential splicing, on the post-transcriptional level. The alternative splicing of the 30 bp VASE exon is developmentally regulated, and NCAM-VASE+ mRNAs are highly expressed in adulthood (Small et al., 1988). Insertion of the VASE peptide increases NCAM adhesivity, and some reports have noticed a negative correlation between the presence of VASE and the degree of NCAM polysialylation (Small and Akeson, 1990; Doherty et al., 1992), at least for certain brain areas (Oka et al., 1995). Although the fourth Ig-like domain, which eventually carries the VASE peptide, has previously been implicated in NCAM polysialylation (Nelson et al., 1995), insertion of VASE into the NCAM sequence in vitro did not affect the affinity between PST and NCAM, nor PSA synthesis in general (Oka et al., 1995).
Using semiquantitative PCR, we examined a potential regulation of VASE expression in the HNS during lactation. In fact, NCAM-VASE+ and NCAM-VASE− mRNA were found in SON; the NH contained only NCAM-VASE− mRNA. Surprisingly, the downregulation of polysialylation was not paralleled by an increase in VASE expression; rather, the ratio of NCAM-VASE+ to NCAM-VASE− was slightly decreased in SON during lactation. In addition, this decrease was already detectable on the first day of lactation and thus seems to precede the downregulation of polysialylation. This demonstrates that VASE expression and polysialylation are two events that are controlled independently, in agreement with the findings of Oka et al. (1995). Reduced expression of the VASE peptide may well facilitate the initiation of the lactation-induced morphological changes taking place in the HNS. The new state of equilibrium that will finally be attained at 10 d of lactation would then be progressively stabilized by a downregulation of polysialylation.
In summary, the reversible modifications of synaptic, neuroglial, and neurohemal contacts taking place in the HNS during lactation offer an excellent possibility for studying the molecular mechanisms that are at the basis of neuronal plasticity in the adult CNS. Our results suggest that two enzymes that have been shown to polysialylate NCAM in vitro, STX and PST, may exert the same function in vivo. The lactation-induced downregulation of the degree of NCAM polysialylation in the SON may be a result, at least in part, of the observed downregulation of these two enzymes. Thus, post-translational modifications of the NCAM molecule are regulated under stringent control by at least two mechanisms, underlining the important role of a precise fine tuning of cell adhesion for plasticity of the nervous system.
Footnotes
This work was supported by Centre National de la Recherche Scientifique and European Community Grant BM-H4-CT95-0524. We thank Dr. Alain Prochiantz for critical reading of this manuscript, Dr. Geneviève Rougon for providing us with PSA-NCAM antibodies (anti-Men-B) and EndoN enzyme, and Dr. Pat Doherty and Dr. Jane Saffell for the NCAM-Fc chimera. We also thank Stéphane Père and Jean-Paul Bouillot for technical help.
Correspondence should be addressed to Fatiha Nothias, Institut Alfred Fessard—Centre National de la Recherche Scientifique, 1 Avenue de la Terrasse, 91198 Gif sur Yvette, France. E-mail:nothias@iaf.cnrs-gif.fr.
REFERENCES
- 1.Angata K, Nakayama J, Fredette B, Chong K, Ranscht B, Fukuda M. Human STX polysialyltransferase forms the embryonic form of the neural cell adhesion molecule. Tissue-specific expression, neurite outgrowth, and chromosomal localization in comparison with another polysialyltransferase, PST. J Biol Chem. 1997;272:7182–7190. doi: 10.1074/jbc.272.11.7182. [DOI] [PubMed] [Google Scholar]
- 2.Angata K, Suzuki M, Fukada M. Differential and cooperative polysialylation of the neural cell adhesion molecule by two polysialyltransferases, PST and STX. J Biol Chem. 1998;273:28524–28532. doi: 10.1074/jbc.273.43.28524. [DOI] [PubMed] [Google Scholar]
- 3.Bruses JL, Rutishauser U. Regulation of neural cell adhesion molecule polysialylation to an intracellular poll of calcium. J Cell Biol. 1998;140:1177–1186. doi: 10.1083/jcb.140.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruses JL, Oka S, Rutishauser U. NCAM-associated polysialic acid on ciliary ganglion neurons is regulated by polysialytransferase levels and interaction with muscle. J Neurosci. 1995;15:8310–8319. doi: 10.1523/JNEUROSCI.15-12-08310.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty P, Moolenaar CE, Ashton SV, Michalides RJ, Walsh FS. The VASE exon downregulates the neurite growth-promoting activity of NCAM 140. Nature. 1992;356:791–793. doi: 10.1038/356791a0. [DOI] [PubMed] [Google Scholar]
- 6.Eckhardt M, Gerardy-Schahn R. Genomic organization of the murine polysialyltransferase gene ST8SiaIV (PST-1). Glycobiology. 1998;8:1165–1172. doi: 10.1093/glycob/8.12.1165. [DOI] [PubMed] [Google Scholar]
- 7.Fryer HJ, Hockfield S. The role of polysialic acid and other carbohydrate polymers in neural structural plasticity. Curr Opin Neurobiol. 1996;6:113–118. doi: 10.1016/s0959-4388(96)80016-x. [DOI] [PubMed] [Google Scholar]
- 8.Hatton GI. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurobiol. 1990;34:437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- 9.Hildebrandt H, Becker C, Gluer S, Rosner H, Gerardy-Schahn R, Rahmann H. Polysialic acid on neural cell adhesion molecule correlates with expression of polysialyltransferases and promotes neuroblastoma cell growth. Cancer Res. 1998a;58:779–784. [PubMed] [Google Scholar]
- 10.Hildebrandt H, Becker C, Murau M, Gerardy-Schahn R, Rahmann H. Heterogeneous expression of the polysialyltransferases ST8SiaII and ST8SiaIV during postnatal rat brain development. J Neurochem. 1998b;71:2339–2348. doi: 10.1046/j.1471-4159.1998.71062339.x. [DOI] [PubMed] [Google Scholar]
- 11.Kojima N, Tachida Y, Yoshida Y, Tsuji S. Characterization of mouse ST8SiaII (STX) as a neural cell adhesion molecule-specific polysialic acid synthase. J Biol Chem. 1996;271:19457–19463. doi: 10.1074/jbc.271.32.19457. [DOI] [PubMed] [Google Scholar]
- 12.Kurosawa N, Yoshida Y, Kojima N, Tsuji S. Polysialic acid synthase (ST8Sia II/STX) mRNA expression in developing mouse central nervous system. J Neurochem. 1997;69:494–503. doi: 10.1046/j.1471-4159.1997.69020494.x. [DOI] [PubMed] [Google Scholar]
- 13.Lincoln DW, Wakerley JB. Neurosecretory activation in the rat: correlation of the suckling stimulus with the pulsatile release of oxytocin. J Physiol (Lond) 1975;245:42P–43P. [PubMed] [Google Scholar]
- 14.Liu J, Morrow AL, Devaud L, Grayson DR, Lauder JM. GABAA receptors mediate trophic effects of GABA on embryonic brainstem monoamine neurons in vitro. J Neurosci. 1997;17:2420–2428. doi: 10.1523/JNEUROSCI.17-07-02420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingston BD, Paulson JC. Polymerase chain reaction cloning of a developmentally regulated member of the sialyltransferase gene family. J Biol Chem. 1993;268:11504–11507. [PubMed] [Google Scholar]
- 16.Nakayama J, Fukuda MN, Fredette B, Ranscht B, Fukuda M. Expression cloning of a human polysialyltransferase that forms the polysialylated neural cell adhesion molecule present in embryonic brain. Proc Natl Acad Sci USA. 1995;92:7031–7035. doi: 10.1073/pnas.92.15.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson RW, Bates PA, Rutishauser U. Protein determinants for specific polysialylation of the neural cell adhesion molecule. J Biol Chem. 1995;270:17171–17179. doi: 10.1074/jbc.270.29.17171. [DOI] [PubMed] [Google Scholar]
- 18.Nothias F, Vernier P, von Boxberg Y, Mirman S, Vincent JD. Modulation of NCAM polysialylation is associated with morphofunctional modifications in the hypothalamo-neurohypophysial system during lactation. Eur J Neurosci. 1997;9:1553–1565. doi: 10.1111/j.1460-9568.1997.tb01513.x. [DOI] [PubMed] [Google Scholar]
- 19.Oka S, Bruses JL, Nelson RW, Rutishauser U. Properties and developmental regulation of polysialyltransferase activity in the chicken embryo brain. J Biol Chem. 1995;270:19357–19363. doi: 10.1074/jbc.270.33.19357. [DOI] [PubMed] [Google Scholar]
- 20.Ong E, Nakayama J, Angata K, Reyes L, Katsuyama T, Arai Y, Fukuda M. Developmental regulation of polysialic acid synthesis in mouse directed by two polysialyltransferases, PST and STX. Glycobiology. 1998;8:415–424. doi: 10.1093/glycob/8.4.415. [DOI] [PubMed] [Google Scholar]
- 21.Phillips GR, Krushel LA, Crossin KL. Developmental expression of two rat sialyltransferases that modify the neural cell adhesion molecule, N-CAM. Brain Res Dev Brain Res. 1997;102:143–155. doi: 10.1016/s0165-3806(97)00069-2. [DOI] [PubMed] [Google Scholar]
- 22.Rafuse VF, Landmesser L. Contractile activity regulates isoform expression and polysialylation of NCAM in cultured myotubes: involvement of Ca2+ and protein kinase C. J Cell Biol. 1996;132:969–983. doi: 10.1083/jcb.132.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rougon G. Structure, metabolism and cell biology of polysialic acids. Eur J Cell Biol. 1993;61:197–207. [PubMed] [Google Scholar]
- 24.Rougon G, Dubois C, Buckley N, Magnani JL, Zollinger W. A monoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult N-CAM. J Cell Biol. 1986;103:2429–2437. doi: 10.1083/jcb.103.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutishauser U. Regulation of cell-cell interactions by NCAM and its polysialic acid moiety. In: Roth J, Rutishauser U, Troy FA II, editors. Polysialic acid. Birkhauser Verlag; Basel: 1993. pp. 215–227. [Google Scholar]
- 26.Rutishauser U, Jessell TM. Cell adhesion molecules in vertebrate neural development. Physiol Rev. 1988;68:819–857. doi: 10.1152/physrev.1988.68.3.819. [DOI] [PubMed] [Google Scholar]
- 27.Saffell J, Williams E, Mason I, Walsh F, Doherty P. Expression of a dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron. 1997;18:231–242. doi: 10.1016/s0896-6273(00)80264-0. [DOI] [PubMed] [Google Scholar]
- 28.Seki T, Arai Y. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci Res. 1993;17:265–290. doi: 10.1016/0168-0102(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 29.Small SJ, Akeson R. Expression of the unique NCAM VASE exon is independently regulated in distinct tissues during development. J Cell Biol. 1990;111:2089–2096. doi: 10.1083/jcb.111.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small SJ, Haines SL, Akeson RA. Polypeptide variation in an N-CAM extracellular immunoglobulin-like fold is developmentally regulated through alternative splicing. Neuron. 1988;1:1007–1017. doi: 10.1016/0896-6273(88)90158-4. [DOI] [PubMed] [Google Scholar]
- 31.Szele FG, Dowling JJ, Gonzales C, Theveniau M, Rougon G, Chesselet MF. Pattern of expression of highly polysialylated neural cell adhesion molecule in the developing and adult rat striatum. Neuroscience. 1994;60:133–144. doi: 10.1016/0306-4522(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 32.Takashima S, Yoshida Y, Kanematsu T, Kojima N, Tsuji S. Genomic structure and promoter activity of the mouse polysialic acid synthase (mST8SiaIV/PST) gene. J Biol Chem. 1998;273:7675–7683. doi: 10.1074/jbc.273.13.7675. [DOI] [PubMed] [Google Scholar]
- 33.Wood GK, Liang JJ, Flores G, Ahmad S, Quirion R, Srivastava LK. Cloning and in situ hybridization analysis of the expression of polysialyltransferase mRNA in the developing and adult rat brain. Brain Res Mol Brain Res. 1997;51:69–81. doi: 10.1016/s0169-328x(97)00209-x. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida Y, Kurosawas N, Kanematsu T, Kojima N, Tsuji S. Genomic structure and promoter activity of the mouse polysialic acid synthase gene (mST8SiaII). J Biol Chem. 1996;271:30167–30173. doi: 10.1074/jbc.271.47.30167. [DOI] [PubMed] [Google Scholar]