Abstract
Much data implicates the amygdala in the expression and learning of fear. Yet, few studies have examined the neuronal correlates of fear in the amygdala. This study aimed to determine whether fear is correlated to particular activity patterns in the lateral amygdaloid (LA) nucleus. Cats, chronically implanted with multiple microelectrodes in the LA and a catheter in the femoral artery, learned that a series of tones interrupted by a period of silence (5 sec) preceded the administration of a footshock. During the silent period, their blood pressure increased, indicating that they anticipated the noxious stimulus. In parallel, the firing rate of LA neurons doubled, and the discharges of simultaneously recorded cells became more synchronized. Moreover, cross-correlation of focal LA waves revealed a significant increase in synchrony restricted to the theta band. In keeping with this, perievent histograms of neuronal discharges revealed rhythmic changes in the firing probability of LA neurons in relation to focal theta waves. Finally, the responsiveness of LA cells to the stimuli predicting the footshock (the tones) increased during the trials, whereas responses to unrelated stimuli (perirhinal shocks) remained stable. Thus, during the anticipation of noxious stimuli, a state here defined anthropomorphically as fear, the firing rate of LA neurons increases, and their discharges become more synchronized through a modulation at the theta frequency. The presence of theta oscillations in the LA might facilitate cooperative interactions between the amygdala and cortical areas involved in memory.
Keywords: amygdala, fear, multisite recording, lateral amygdala, learning, memory, cat
Much evidence implicates the amygdaloid complex in fear and anxiety (for review, see Aggleton, 1992). For instance, amygdala lesions decrease the response of animals to various fear-inducing stimuli (Klüver and Bucy, 1938;Weiskrantz, 1956; Blanchard and Blanchard, 1972) and prevent the acquisition of classically conditioned fear responses (Kapp et al., 1979; Gentile et al., 1986; Iwata et al., 1986; Hitchcock and Davis, 1987; LeDoux et al., 1990; Killcross et al., 1997). However, these changes do not result from sensory deficits, as humans with relatively selective amygdala lesions can identify faces but are unable to recognize facial expressions of fear (Adolphs et al., 1994). Consistent with these results, it was reported that amygdala stimulation can elicit the behavioral, visceral and, in humans, the subjective concomitants of fear (Kaada, 1967; Gloor et al., 1982; Kapp et al., 1982; Iwata et al., 1987).
So far, most physiological investigations have focused on the sensory responsiveness of amygdala neurons (Machne and Segundo, 1956; Sawa and Delgado, 1963; O'Keefe and Bouma, 1969; Jacobs and McGinty, 1971;Cain and Bindra, 1972; Nishijo et al., 1988a,b; Bernard et al., 1992;Bordi and LeDoux, 1992; Maeda et al., 1993; Muramoto et al., 1993) and how it changed as a result of various conditioning procedures (Applegate et al., 1982; Ono et al., 1983; Pascoe and Kapp, 1985a;Maren et al., 1991; Quirk et al., 1995, 1997; Uwano et al., 1995; Rogan et al., 1997). Surprisingly, few studies have attempted to examine the cellular correlates of fear in the amygdala (however, see Adamec, 1991) independently of the sensory events that triggered it.
To investigate the neuronal correlates of fear in the amygdala, we devised an experimental paradigm in which cats learned that a series of six 1 sec tones, interrupted by a period of silence (four tones, 5 sec silence, two tones), predicted the administration of a noxious stimulus. Examining the activity of multiple, simultaneously recorded lateral amygdala (LA) neurons during the period of silence allowed us to determine whether the anticipation of the noxious stimulus or, in anthropomorphic terms, fear is correlated to particular activity patterns in the LA, independent of the sensory events that triggered it. In some experiments, perirhinal shocks were applied in between the tones to compare the responsiveness of LA cells to synaptic inputs of differing significance, some predicting the administration of a noxious stimulus (the tones) and irrelevant ones (the perirhinal stimuli).
Our results suggest that during fear, the firing rate of LA neurons increases, and their activity tends to synchronize in a rhythmic population event at the theta frequency range. Moreover, within each trial, the responsiveness of LA cells to the tones progressively increased, whereas that to perirhinal shocks remained stable.
MATERIALS AND METHODS
Electrode implantation
Experiments were performed in four adult cats (2.5–3.5 kg) that were chronically implanted in a stereotaxic position under deep barbiturate anesthesia. This species was chosen because the large size of cat brains facilitates the placement of multiple microelectrodes within the amygdala. The anesthesia was induced with ketamine (15 mg/kg, i.m.), and atropine sulfate (0.05 mg/kg, i.m.) was administered to prevent secretions. Then, sodium pentobarbital was injected gradually (Somnotol; 15–25 mg/kg, i.v.). The bone overlying the amygdaloid complex was removed on one side, and the dura mater was opened. Then, an array of 10–21 tungsten electrodes (2–6 MΩ at 1 kHz; outer diameter of 80 μm; Frederick Haer & Co., Brunswick, ME) was lowered until the electrodes reached the dorsal aspect of the amygdala (for details, see Collins and Paré, 1999). To construct the array, small holes were drilled in a circular Teflon block, and the electrodes were inserted into them. The Teflon block was inserted in a tightly fitting Delrin sleeve, which was cemented to the bone. During the recording sessions, the electrodes could be lowered as a group by means of a micrometric screw. The electrodes were arranged in various configurations (two or three rows of five to seven electrodes) with fixed intervals between the electrodes (0.4 mm in the mediolateral and 1 mm in the rostrocaudal axes).
To identify LA projection cells physiologically, stimulating electrodes were stereotaxically inserted in the perirhinal cortex. Electrical stimuli consisted of 0.05–0.2 msec pulses of 0.1–1 mA delivered at various frequencies. Cells that could be antidromically activated by perirhinal stimuli were formally identified as projection neurons (Paré and Gaudreau, 1996). Neurons had to meet at least two of the following criteria to be considered antidromically identified: fixed response latency, ability to follow high-frequency stimulation, and collision with spontaneous action potentials. These physiological identifications were complemented with histological controls as described below.
For chronic monitoring of blood pressure, a cannula was inserted in the femoral artery, sutured to the adjacent tissues, and run subcutaneously to a connector fixed to the skin on the back of the neck. It was flushed daily with a sterile saline containing the anticoagulant heparin (20 IU/ml). In one cat, the cannula blocked before the beginning of the experiment.
Finally, four screws were cemented to the skull. These screws were later used to fix the cat's head in a stereotaxic position without pain or pressure. Bicillin (intramuscular daily for 3 d) and buprenorphine (0.03 mg/kg, i.m. every 12 hr for 24 hr) were administered postoperatively. Recording sessions began 6–8 d after the surgery. Between experimental sessions, the animals slept, ate, and drank ad libitum.
Recording and stimulating methods
The experiments proceeded in three phases, as detailed below.
Habituation. For the first 3–4 d, the animals were gradually accustomed to the head restraint and repeatedly presented a series of six 1 sec tones (6 kHz at 70 dB) at a frequency of 0.5 Hz (Fig. 1A). This series of tones was interrupted by a 5 sec period of silence (four tones, silence, two tones; Fig. 1A). In this and subsequent experimental phases, intertrial intervals ranged between 3 and 30 min. The tones could be presented with or without interleaved perirhinal stimuli (at 0.5 Hz).
Fig. 1.
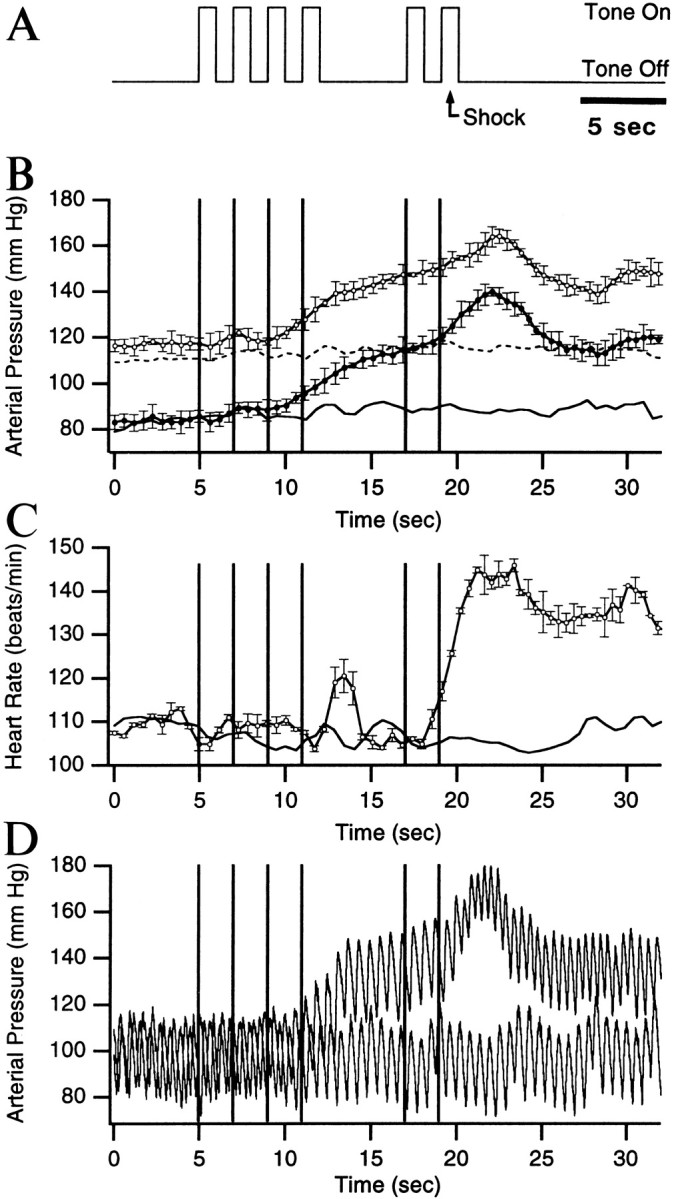
Anticipatory increases in blood pressure and heart rate in conditioned animals. A, Experimental paradigm. A series of 1 sec tones, interrupted by a period of silence (5 sec), was presented to the animals (4 tones, silence, 2 tones). In control conditions, no noxious stimuli were administered. In later sessions, an electrical shock to the front paws (0.5 sec, 1.5 mA) was administered, 0.5 sec after the onset of the last tone (A, arrow). The vertical lines in B–D indicate the tone onsets. The changes in arterial blood pressure (B) and heart rate (C) observed during the tones are depicted for naive animals (B, C, lines without symbols) and for conditioned animals (B, C, lines with symbols; average of three experiments, in three cats). In B, the systolic (top curves) and diastolic (bottom curves) blood pressure are shown. The average interbeat interval, as estimated in C, was used to scale the blood pressure and heart rate data in time. For clarity, only the SE of the data obtained after conditioning is shown in B and C. D depicts two superimposed trials obtained in the same cat before and after conditioning.
Control sessions. Once the cats were habituated, the recording sessions began (see below). In the control phase, the animals were presented with the series of tones five times. In only one of the trials were the tones presented with interleaved perirhinal stimuli. In such cases, perirhinal stimuli began ∼20 sec after the conclusion of the preceding trial and continued for the entire intertrial epoch. Three control sessions were performed in each animal, and a different set of cells was recorded during each session. Trials with perirhinal stimuli were analyzed independently of the others.
Conditioning sessions. In the first conditioning session, six trials were performed. The first trial included perirhinal stimuli, but no noxious stimulus. In the four subsequent trials, a footshock (0.5 sec, 1.5 mA) was administered 0.5 sec after the onset of the sixth tone (Fig. 1A), and no perirhinal stimuli were delivered. In the sixth trial, the animals received the six tones, interleaved perirhinal stimuli, and a footshock. All tested cats developed anticipatory increases in blood pressure by the fourth trial of the first conditioning session (see Results). The data obtained in the first conditioning session was treated separately from that obtained on subsequent days. In the following conditioning sessions, five trials were performed, all included the footshock, but only one with interleaved perirhinal stimuli. Five conditioning sessions were performed in each animal. Unless otherwise stated, the expression “conditioning sessions” excludes the first one.
The recording methods used in control and conditioning sessions were identical. At the beginning of each session, the electrode array was lowered 80–160 μm. Thirty minutes later, each recording site was examined for units with a high signal-to-noise ratio (≥3). After documenting the anti- and orthodromic responsiveness of the selected cells, the minimal perirhinal stimulation intensity required to elicit reliable field and unit responses was determined. Thereafter, the stimulation intensity was kept constant for the entire session. The spontaneous and evoked activity of the selected neurons was observed on a digital oscilloscope, printed on a chart recorder, digitized, and stored on tape. Data from neurons that were not held for the duration of the sessions was discarded.
Identification of recording sites
At the end of the experiments, the animals were deeply anesthetized with sodium pentobarbital, and selected recording sites were marked with electrolytic lesions (0.5 mA for 5 sec). After this, the animals were perfused with 500 ml of a cold saline solution (0.9%) followed by 1 l of a fixative containing 2% paraformaldehyde and 1% glutaraldehyde in 0.1 m PBS, pH 7.4. The brains were later sectioned on a vibrating microtome (at 80 μm) and stained with thionin to verify the position of the recording and stimulating electrodes. The microelectrode tracks were reconstructed by combining micrometer readings with the histological controls. Despite the high number of electrodes, it was easy to determine the position of recorded neurons as the relative position of the electrodes was known. The data were only included in the analyses after histological determination of the recording sites.
Analysis
Analyses were performed off-line with the software Igor (Wavemetrics, Oswego, OR) and homemade software running on Macintosh microcomputers. Spikes were detected with a window discriminator after digital filtering (0.3–10 kHz) of the data. The auditory and cortical responsiveness of recorded neurons was studied by computing peristimulus histograms. In addition, we computed cross-correlation matrices for all sets of simultaneously recorded neurons. Focal waves were analyzed by means of fast Fourier transforms (FFT), auto-, and cross-correlograms. In Results, values are expressed as mean ± SE unless otherwise stated.
RESULTS
Changes in heart rate and blood pressure produced by conditioning
Figure 1A illustrates the experimental paradigm used in the present study (see details in Materials and Methods). Cats were presented a series of tones interrupted by a period of silence. In control sessions, the tones were presented alone whereas, in conditioning sessions, a footshock was applied 500 msec after the onset of the last tone. The changes in arterial blood pressure and heart rate observed in naive (lines without symbols) and conditioned (lines with symbols) animals during the presentation of the tones are shown in Figure 1, B and C. Figure 1Dshows examples of blood pressure recordings obtained before (lower wave) and after (upper wave) conditioning.
In control conditions, the tone presentations did not elicit significant changes in systolic and diastolic blood pressure (Fig.1B, lines without symbols) or in heart rate (Fig.1C, line without symbol). However, after the introduction of the footshocks, the animals developed anticipatory increases in blood pressure by the fourth trial of the first conditioning session (Fig.1B, lines with symbols). On average, the systolic and diastolic pressure increased from 117.0 ± 0.26 and 84.0 ± 0.34 mmHg in the pretone period to 139.7 ± 1.96 and 106.9 ± 2.08 mmHg during the period of silence and to 164.3 ± 3.07 and 140.1 ± 1.83 mmHg immediately after the noxious stimulus.
To determine when the changes in blood pressure became statistically significant, measurements obtained in three cats after conditioning were averaged. Then, the data point of the pretone period (Fig.1B, 0–5 sec) with the largest variability was determined. Its SD was then used to establish when the blood pressure increase reached statistical significance in a one-tailedt test (p < 0.05; i.e., increasing by >1.64 times the SD). Using this method, it was found that the changes in systolic and diastolic blood pressure became statistically significant by the end of the fourth tone. At the end of the silent period, the anticipatory increase in systolic and diastolic blood pressure increased by 4.87 and 4.18 times the SD, respectively.
In control conditions, the series of tones did not elicit significant changes in heart rate (Fig. 1C, line without symbols). However, after conditioning, two phases of heart rate increases were seen. The first one was short-lived and occurred after the offset of tone 4 (peak rate of 121 ± 3.74 beats/min). The second began toward the end of the trial and continued well after the noxious stimulus (peak rate of 146 ± 1.5 beats/min). Both phases reached statistical significance in a one-tailed t test (p < 0.05), using the approach described above for the blood pressure.
LA firing rates increase during the anticipation of noxious stimuli
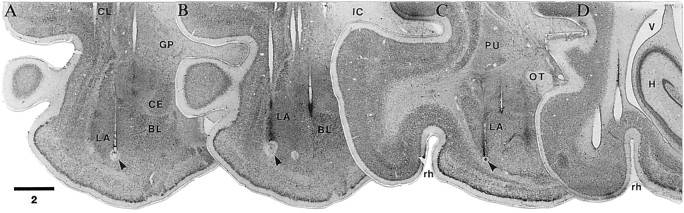
A total of 98 spontaneously active neurons, histologically determined to be located in the LA (Fig.2A–C), were recorded from four cats in this study. Of these cells, 36 neurons were recorded in control sessions, 7 in the first conditioning session, and 49 in the ensuing conditioning sessions. Data from control and conditioning sessions was obtained in each cat. To ensure sample homogeneity, six additional neurons with tonically elevated firing rates (>5 Hz) were excluded from the present analysis, because much data suggests that such cells are local-circuit GABAergic neurons, whereas neurons with little or no spontaneous activity are projection cells (for review, seeCollins and Paré, 1999).
Fig. 2.
Histological determination of recording and stimulating sites. Thionin-stained coronal sections arranged from rostral in A to caudal in D.Arrowheads in A–C point the electrolytic lesions (0.5 mA for 5 sec) made at the end of the experiments to mark selected recording sites in the LA.D, Stimulating electrodes in the perirhinal cortex. Scale bar in millimeters. BL, Basolateral nucleus of the amygdala; CE, central nucleus; CL, claustrum; GP, globus pallidus; H, hippocampal formation; IC, internal capsule;LA, lateral nucleus of the amygdala; OT, optic tract; PU, putamen; rh, rhinal sulcus; V, ventricle.
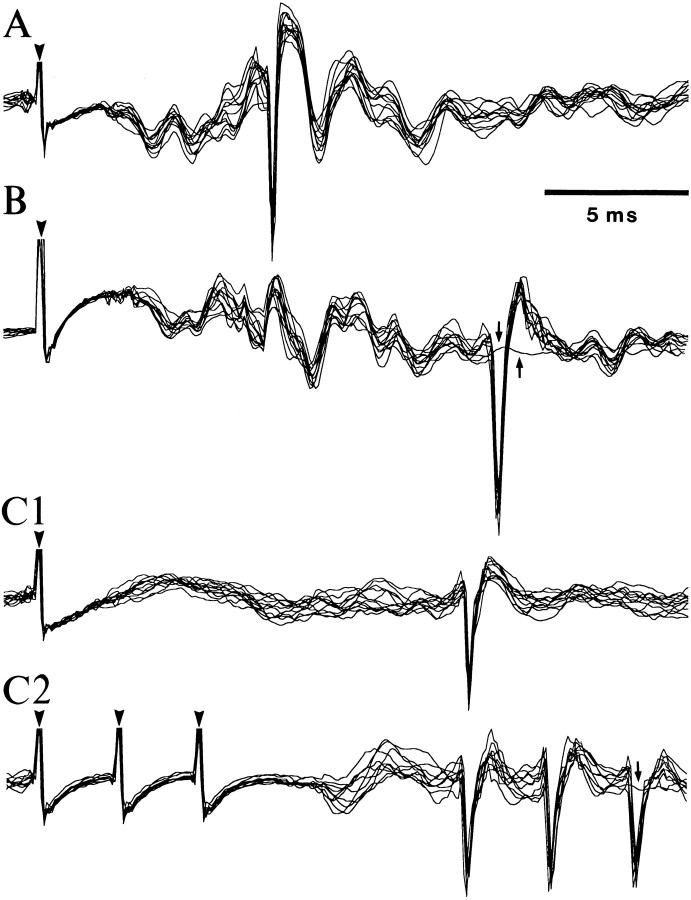
Among the LA cells with low spontaneous firing rates (see below), 16% were activated othodromically by perirhinal stimulation at latencies ranging from 4 to 25 msec (average, 9.5 ± 0.33 msec), and 19% could be backfired from the perirhinal cortex (latency range, 6–17; average, 9.3 ± 0.19 msec). A lucky example of three simultaneously recorded LA cells that were antidromically responsive to perirhinal stimuli is illustrated in Figure3. Taking into account the distance between the stimulating (Fig. 2D) and recording sites (Fig. 2A–C), this yields a conduction velocity of ∼0.5–1 m/sec, consistent with previous estimates (Paré et al., 1995).
Fig. 3.
Physiological identification of LA projection cells by antidromic invasion from the perirhinal cortex. (A, B, C1) Three simultaneously recorded LA neurons that generated antidromic spikes in response to electrical stimuli applied in the perirhinal cortex.Arrowheads point to stimulation artifacts. In each case, several responses are superimposed. Note fixed response latencies.C2, Response of the cell shown in C1 to three perirhinal stimuli delivered at 300 Hz. Note the ability of the antidromic spikes to follow high-frequency stimulation. Small arrows in B and C2 point to traces where the antidromic spike failed. The neuron inA was located in the caudolateral part of the LA, whereas those in B and C were located 2 mm more rostrally, and at different lateromedial levels (C 0.8 mm more lateral than B). Data were digitally filtered (100 Hz to 10 kHz).
Figure 4 illustrates how the firing rate of LA cells changed during the presentation of the tones in the control phase (Fig. 4A) and after conditioning (Fig.4B). A population histogram of the instantaneous firing rate of all LA cells recorded in the control and conditioning sessions (excluding the first one) was computed (for details, see Fig.4, legend). The two groups of LA cells did not fire at significantly different rates before the tone presentations (control, 0.82 ± 0.149 Hz; conditioned, 0.86 ± 0.110 Hz).
Fig. 4.
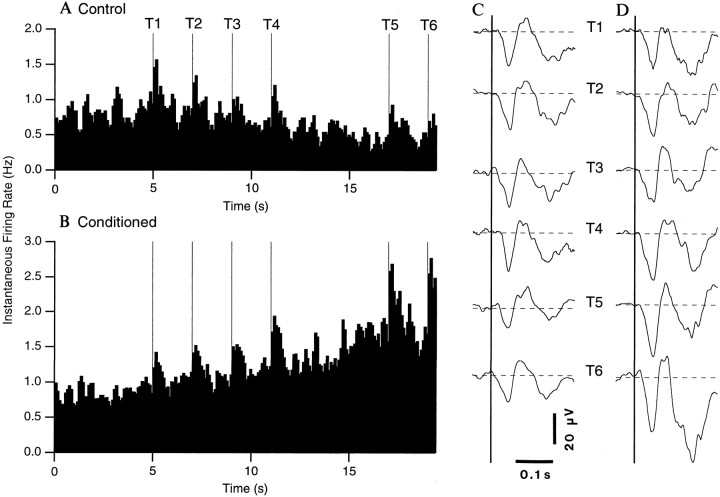
Increases in the firing rate and auditory responsiveness of LA neurons during the anticipation of noxious stimuli. Instantaneous firing rate of LA neurons in naive (A, n = 36) and conditioned (B, n = 49) animals (bins of 100 msec) during the presentation of the tones (T1–T6, thin vertical lines). The tone series was presented four times. For each cell, the spike counts in the four trials were averaged. Then, the activity of all the cells within a group (control or conditioned) was averaged and converted into instantaneous firing rates. Theright part of the figure shows the average field potentials evoked by the six tones in naive (C) and conditioned (D) animals. Each wave represents the average of 144 responses (36 sites times 4 trials) inC and 196 responses (49 sites times 4 trials) inB. Negative, downward. The amplitude of tone-evoked field potentials was measured by subtracting the peak voltage (within a SE of the average latency) from the average voltage value of the 50 msec preceding the tone onset (dashed lines).
To analyze the changes in firing rate induced by the tones, we compared the spontaneous activity of LA cells before the tones and during the silent period. In the control phase (Fig. 4A), a significant decrease in firing rate was observed during the period of silence (0.53 ± 0.126 Hz) compared to pretone values (pairedt test; p < 0.05). In contrast, an increased firing rate was observed during the silent period in conditioned animals (1.49 ± 0.245 Hz; paired t test;p < 0.05; Fig. 4B). In fact, the firing rate of LA cells reached 1.74 ± 0.311 Hz by the end of the silent period (last second).
Progressive increase in auditory responsiveness during the anticipation of the noxious stimulus
In previous studies on the auditory responsiveness of LA cells in cats, a close temporal correspondence was found between the fluctuations in firing probability of LA cells and the initial components of auditory-evoked field potentials (Collins and Paré, 1999). This correlation suggested that the field potentials were not volume-conducted from neighboring structures but that they reflected local extracellular currents associated with synaptic activity in the LA. Consequently, we considered both auditory-evoked single-unit discharges and field potentials when analyzing how conditioning affected the responsiveness of LA cells. These signals were recorded simultaneously by the same microelectrodes and dissociated off-line by digital filtering (fields, 3–300 Hz; unit activity, 0.3–10 kHz).
Tone-evoked single-unit discharges
In control and conditioning sessions, LA cells responded to the tones with an increase in firing probability that was assessed in the following manner. For the six tones, the difference between the first three 100 msec bins of tone-evoked activity and the five prestimulus bins was averaged and normalized for both the control and conditioning sessions. This approach revealed that the increase in firing probability produced by the tones ranged between 0.31 and 0.96 of their prestimulus activity (average of 0.53 ± 0.032 and 0.42 ± 0.014 in control and conditioning sessions, respectively).
Visual inspection of the population histograms of Figure 4 suggests that the anticipation of the noxious stimulus produced marked changes in LA responses to the tones. Indeed, the magnitude of the tone-evoked LA responses appeared to increase in conditioning sessions, especially toward the end of the trials (T4–6; Fig. 4B), but not in control sessions (Fig. 4A). To determine if these changes were statistically significant, a two-factor ANOVA was performed, using the experimental phase (control, conditioned) as the “between” factor and the tones (T1–T6) as the “within” factor. A significant interaction was found between the experimental phase and the tone variables using both the raw data (F= 31.17; p < 0.05) or after normalization to prestimulus values (F = 11.76; p < 0.05).
Post hoc analyses (paired, two-tailed t tests) on the normalized data confirmed the impression gained by visual inspection of the histograms (Fig. 4A,B), namely that the responsiveness of LA cells to the tones increased during the conditioning sessions (independently of changes in pretones firing rates), but not in control sessions, in comparison to pretone values. In conditioning sessions, the difference between the responsiveness to the first and subsequent tones became significant by the fourth tone (p < 0.05), and the level of statistical significance increased as the trials progressed (from 0.02 at T4 to 0.0001 at T6). Although different groups of cells were analyzed in control and conditioning sessions, their response to the first tone was not significantly different, suggesting similar tone-response properties in the two groups.
Auditory-evoked field potentials
Fluctuations in the amplitude of auditory-evoked field potentials largely paralleled the variations in firing probability. In keeping with previous work in rats (Rogan and LeDoux, 1995) and cats (Collins and Paré, 1999), tone presentations evoked multiphasic field potentials in the LA (Fig. 4C,D). The latency to peak of the initial negative wave, the most reliable component of these potentials, did not change significantly from control to conditioning sessions (47.9 ± 1.52 and 48.8 ± 1.33 msec, respectively).
To determine whether the anticipation of the noxious stimulus affected these responses, an ANOVA was performed on the amplitude of the initial negative wave evoked by individual tones (T1–T6), for all available LA sites and trials (for details, see Fig. 4, legend), using the experimental phase (control, conditioned) as the between factor and the tones (T1–T6) as the within factor. A significant interaction was found between the experimental phase and the tone variables (F = 2.977; p < 0.05).
Post hoc analyses (paired, two-tailed t tests; T1 vs T2-T6) revealed that the auditory-evoked field potentials gradually increased in amplitude during the conditioning trials (T1, 24.2 ± 2.92 μV; T6, 34.9 ± 3.07 μV), with the p values decreasing progressively from the fourth to the sixth tone (T1 vs T4, 0.08; T1 vs T5, 0.02; T1 vs T6, 0.014). By contrast, none of the amplitude differences reached statistical significance in control sessions (paired t tests, p > 0.05).
Changes in cortical responsiveness produced by conditioning
The progressive increases in auditory responsiveness taking place during the trials after conditioning lend themselves to two different interpretations. One possibility is that the increased responsiveness is specific to the stimulus predicting the footshock (i.e., the tones). Alternatively, the auditory responses might increase simply because the animals are aroused. If this was the case, any excitatory synaptic input would elicit a larger response in the late phase of the trials, irrespective of its predictive relation to the noxious stimulus. To address this issue, we compared unit and field potential responses to perirhinal stimuli delivered before or during the tones series, in the naive and conditioned states.
In all tested animals (n = 3), the unit (n = 8) and field potential (n = 54) responses to perirhinal stimuli applied before versus during individual trials (each trial containing six tones) did not change in both the naive and conditioned states. On the other hand, marked variations in perirhinal-evoked responses were observed when the same sites were compared at the beginning and end of the first conditioning session (n = 7).
These two points are illustrated in Figure5, which compares the unit and field responses recorded at the same site in trials 1 (Fig. 5A) and 6 (Fig. 5B) of the first conditioning session. In Figure5, A1 and B1, note that the tones are marked on the left, and that brackets and arrows (right) indicate what portion of the data were used to construct the histograms. In the naive state (Fig. 5A; trial 1 of first conditioning session), the unit and field responses to perirhinal stimuli applied before (Fig.5A2) compared to during (Fig. 5A3) the trial did not change significantly (t test, p > 0.05; see precise values in figure legend). The same phenomenon was seen after conditioning (trial 6 of first conditioning session; Fig. 5, compare B2, B3). However, perirhinal-evoked field potentials increased dramatically (by ∼40%) from the first to the sixth trial of the first conditioning session (Fig. 5, compare A2,3, B2,3; t test,p < 0.05). When unit responses to perirhinal stimuli applied before and during the trials were pooled together, the difference in unit responsiveness was also found to be statistically significant (1.61 ± 0.475 vs 2.33 ± 0.512; pairedt test, p < 0.05).
Fig. 5.
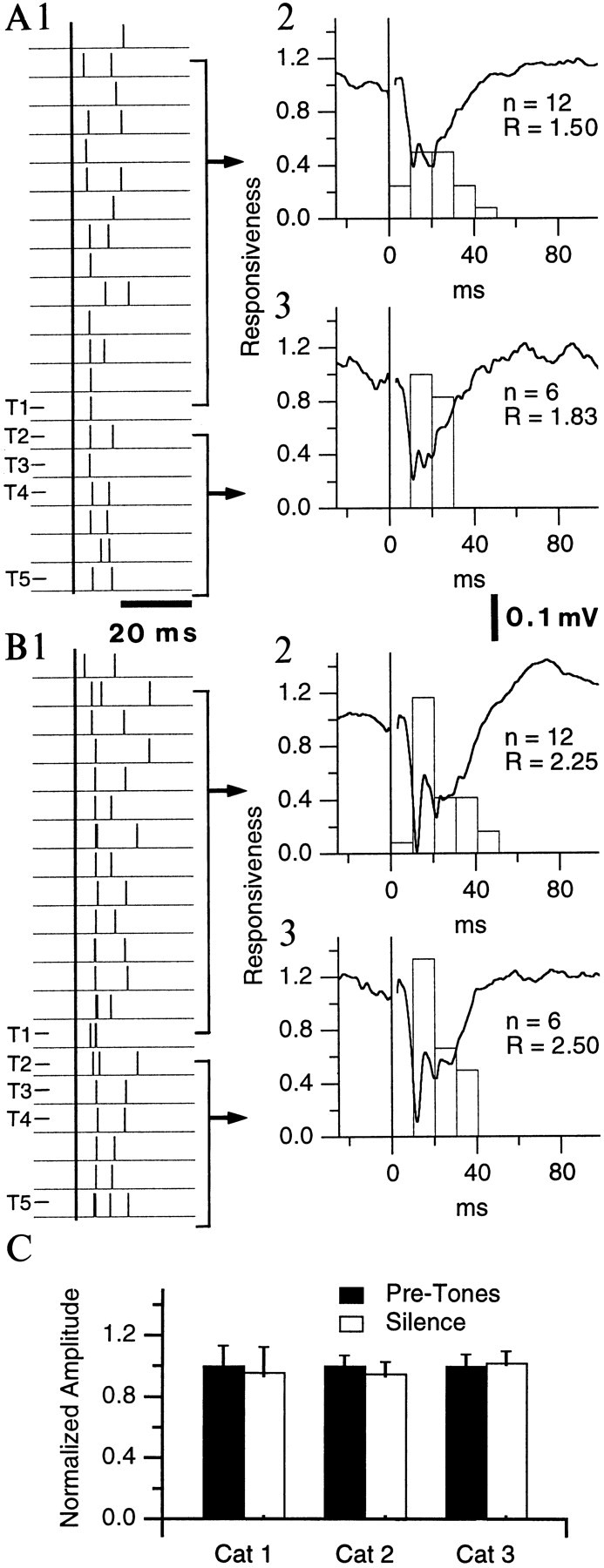
LA responsiveness to perirhinal stimuli does not increase during the anticipation of noxious stimuli. Perirhinal-evoked LA responses in the same cat before (A) and after (B) introduction of the noxious stimulus (trials 1 and 6 of the first conditioning session, respectively). Same recording site, neuron, and stimulation intensity in Aand B. 1, Window discriminator output.Thick vertical line indicates perirhinal stimuli.2, 3, Peristimulus histogram of neuronal discharges and simultaneously recorded evoked potential (averages of 12 and 6 in2 and 3, respectively). R, Responsiveness (number of spikes divided by number of shocks). Average unit responsiveness and field potential amplitude were 1.50 ± 0.151 and 218.2 ± 16.86 μV in A2, 1.83 ± 0.167 and 245 ± 22.53 μV in A3, 2.25 ± 0.131 and 327.3 ± 18.11 μV in B2, and 2.5 ± 0.342 and 336.4 ± 27.43 μV in B3.C, Normalized amplitude of field potentials evoked by perirhinal stimuli in three conditioned cats in quiescent periods (black bars) and during the anticipation of noxious stimuli (white bars). Whereas the amplitude of the field potentials and neuronal responsiveness did not change significantly within a trial (A2 vs A3 orB2 vs B3), it increased from the naive to the conditioned state (A vs B).
The lack of time-dependent changes in cortical responsiveness was observed in all tested animals (n = 3). Figure5C illustrates the averaged normalized amplitude of perirhinal-evoked field potentials recorded before the tones versus during the silent period in conditioned animals. Note that the changes in field potential amplitudes did not even approach significance, in sharp contrast with tone-evoked responses.
In contrast, the amplitude changes observed from the naive to the conditioned states at the same sites were more variable. Highly significant decreases and increases in response amplitudes were observed (57 and 43% of the sites, respectively) in the first conditioning session (trial 1 vs trial 6). These statistically significant, yet inconsistent changes in response amplitudes were sometimes observed in the same session, at simultaneously recorded sites.
Synchronized and rhythmic LA firing during the anticipation of noxious stimuli
To determine whether the anticipation of the painful stimulus produced changes in the probability of synchronized firing among LA cells, cross-correlograms were computed for all pairs of neurons recorded simultaneously in conditioning sessions. For each cell pair (n = 59), individual cross-correlograms were computed separately for the data acquired immediately before the trial onsets and during the silent period. The resulting correlograms were then normalized to the number of spikes generated by the reference cells and averaged.
The result of this analysis is illustrated in Figure6. Consistent with the increased firing rate observed during the silent period, with respect to pretone values, the average bin value augmented from 0.81 ± 0.0384 before the trial onset (Fig. 6A) to 2.07 ± 0.149 in the silent period (Fig. 6B). The slight discrepancy between these figures and the average rates reported above reflect the necessarily different sample compositions used for the two analyses (many LA cells were not recorded in couples).
Fig. 6.
Increased synchrony in the activity of LA neurons during the anticipation of noxious stimuli. In conditioned animals, cross-correlograms of neuronal discharges were computed for 59 pairs of simultaneously recorded LA neurons before the tones (A) or during the silent period preceding the noxious stimulus (B). Before averaging, the individual cross-correlograms were normalized to the number of spikes generated by the reference cell. The number of cell couples (n) as well as the number of spikes generated by the reference (nR) and test (nT) cells are indicated on the top right of the histograms.
To determine whether the differing appearance of the cross-correlograms reflected increases in firing rates from the pretone epochs to the silent periods, we computed the ratio of the maximal bin (closest to time 0) to the average value for both histograms. The ratio was 1.78 for pretone epochs (Fig. 6A) compared to 3.43 for silent periods (Fig. 6B), suggesting that the probability of synchronized firing is higher in the LA during the anticipation of the noxious stimulus than during pretone epochs. In addition, the presence of distinct peaks and troughs at intervals ranging from 160 to 190 msec in Figure 6B suggests that the activity of LA cells is also more rhythmic in the silent period.
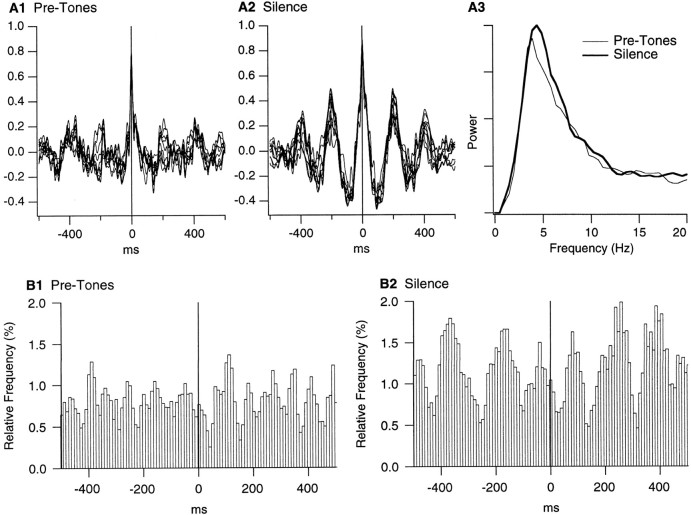
If, as suggested by Figure 6B, LA cells fire rhythmically at the theta frequency during silent periods, this should be apparent in the focal waves that were recorded simultaneously by the same electrodes used for unit activity. In addition, because LA cells exhibited gradual increases in firing rate and auditory responsiveness during the trials, the theta rhythmicity might develop progressively during the silent period. To verify this, focal waves were digitally filtered from 2 to 55 Hz and divided in 1 sec segments sliding in steps of 100 msec over the epoch preceding the tones or the silent period. Then, the wave segments simultaneously recorded at different LA sites were cross-correlated. Figure7A shows the result of this analysis for a particular pretone epoch and the matching silent period. In this striking example, the theta rhythmicity was much more pronounced in the silent period (Fig. 7A2) than before the trial onset (Fig. 7A1).
Fig. 7.
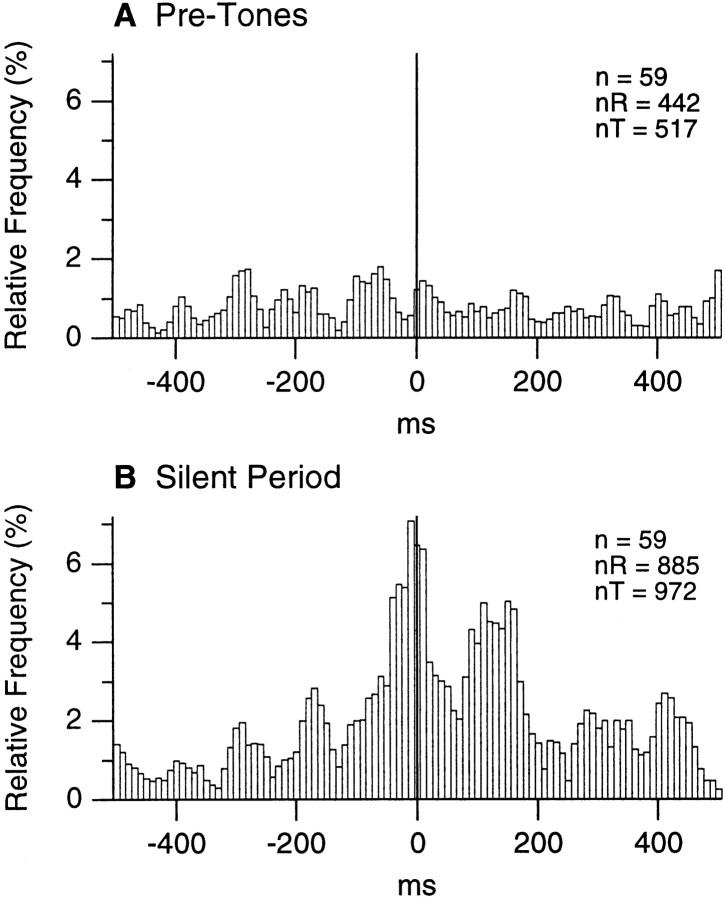
Simultaneously recorded LA sites exhibit increased correlation in the theta band during the anticipation of noxious stimuli. A, Cross-correlograms of local field potentials recorded simultaneously in two LA sites (distance, 0.8 mm) before the tones (A1) and during the silent period (A2). Focal waves were digitally filtered from 2 to 55 Hz and divided in 1 sec segments sliding in steps of 100 msec over the 5 sec epoch preceding the tones or the silent period. Then, the corresponding wave segments were cross-correlated. Only 10 of the resulting cross-correlograms are shown in A1 andA2. A3, Power spectrum of cross-correlograms before the tones (thin line) and during the silent period (thick line). To compute this, the analysis described in A1 and A2 was repeated for all pairs of simultaneously recorded LA sites (n = 59), and the FFTs of the resulting cross-correlograms were averaged. B, Population perievent histograms of LA firing using the positive peaks of focal theta waves as reference times, before the tones (B1) or during the silent period (B2). Average of 49 perievent histograms. To compute these histograms, focal waves were digitally filtered (4–7 Hz), and the positive peaks of theta waves exceeding 1.5 times the SD of the 5 sec segments were detected. The number of theta peaks and spikes were 803 and 652 for B1 compared to 1076 and 1986 for B2.
However, in other cases, the difference between the cross-correlograms of the pretones and silence epochs was not so clear-cut. Moreover, from trial to trial, the dominant frequency varied from 4 to 7 Hz. To address this issue quantitatively, we computed cross-correlograms for all available pairs of simultaneously recorded LA sites and examined the spectral content of the resulting cross-correlograms. Figure7A3 illustrates the average power spectrum of cross-correlograms for pretones (thin lines) and silence (thick lines) epochs. A paired t test revealed that the power in the 4–7 Hz band increased significantly (by 13.4%; p < 0.05) during the silent period. However, analysis of serial cross-correlograms failed to reveal progressive changes in theta rhythmicity.
Yet, the possibility remained that the theta rhythmicity present in the focal waves resulted from volume conduction of hippocampal activity to the LA. To address this possibility, LA focal waves were digitally filtered between 4 and 7 Hz, and positive theta peaks were detected for pretones and silent periods (for details, see Fig. 7, legend). Using these positive theta peaks as time 0, perievent histograms of neuronal firing were constructed for each cell, normalized, and averaged. The result of this analysis is shown in Figure7B.
To assess the statistical significance of these perievent histograms, we determined the modulation of firing probability required to reach statistical significance in a one-tailed t test (mean + 1.64 times the SD, as described in Collins et al., 1999). Briefly, spike trains of various lengths were repeatedly shuffled with respect to a series of theta peaks. The resulting perievent histograms were smoothed with a moving average of 3 and normalized so that the average bin height was 100. Then, the maximal difference in bin height found at half a theta cycle within 200 msec of the origin was determined. This difference in bin height and that found on either side of the central peak were averaged. Repeating this procedure numerous times allowed us to estimate the average firing modulation ± the corresponding SD that can be expected by chance. See Collins et al. (1999) for the exact distribution. Comparing the perievent histograms of Figure7B to this random distribution revealed that in both cases, the firing modulation was statistically significant (p < 0.05; numbers of spikes and theta peaks in figure legend).
However, it is obvious that the theta-related modulation is much more pronounced during the silent periods (Fig. 7B2) than during the pretone epochs (Fig. 7B1). Not only are the differences between the peak and troughs higher in Figure 7B2 but they are more numerous on either side of the origin.
DISCUSSION
Although much evidence implicates the amygdaloid complex in fear (Aggleton, 1992), little data is available regarding the neuronal correlates of this emotion in the amygdala. The present study shows that during the anticipation of noxious stimuli, a state tentatively equated to fear, the firing rates of LA neurons increase, and their discharges become more synchronized through a rhythmic modulation in the theta frequency band. In addition, LA neurons displayed a time-dependent increase in responsiveness to sensory stimuli holding a predictive relationship to the footshocks, but not to inputs that did not.
Modification of the firing rate of LA cells during the anticipation of noxious stimuli
Compared to most neurons of the CNS (for review, seeSteriade and McCarley, 1990), amygdala neurons (Jacobs and McGinty, 1971; Pascoe and Kapp, 1985b), and LA projection cells in particular (Gaudreau and Paré, 1996; Paré and Gaudreau, 1996), stand out because of their extremely low levels of spontaneous activity. Indeed, in a previous study where LA cells were searched with a hunting perirhinal stimulus, it was shown that most LA cells remain undetected when using extracellular recording methods because they have no spontaneous activity (Gaudreau and Paré, 1996). Consistent with this, we observed that the average firing rate of LA cells was <1 Hz in control conditions, despite the fact that our recording method was biased for cells with higher levels of spontaneous activity (no hunting stimuli was used).
During the anticipation of the noxious stimuli, we observed that LA firing rates doubled. Considering that arousal is correlated to increased firing rates in much of the brain (Steriade and McCarley, 1990), this result might not seem surprising. However, it takes on a particular significance because it was obtained in the amygdala, a structure whose lesion interferes with the expression and learning of fear (for review, see Davis, 1992; LeDoux, 1995).
Nevertheless, given the important autonomic changes that accompanied the anticipation of noxious stimuli, the fear-related increases in LA firing rates (from 0.8 to 1.7 Hz) appear modest. Although this may be partly compensated for by the increased synchrony of LA cells (see below), the following question arises: have we failed to identify the conditions where LA cells become active or is the impact of LA axons on their targets so important that even modest elevations in firing rate have dramatic consequences?
Origin and significance of the firing modulation at the theta frequency
As suggested above, the increased probability of synchronous firing among LA cells might compensate for their low discharge rates. This was evidenced in the population cross-correlation analysis where, after normalization for changes in firing rates, the probability of synchronous firing was found to double in the silent period compared to pretone levels. This analysis also revealed that the activity of LA neurons became more rhythmic during the anticipation of noxious stimuli. Consistent with this, FFT analyses of focal cross-correlograms disclosed an increased rhythmicity in the theta frequency range during the same period. Finally, the possibility that this phenomenon was an artifact caused by volume conduction of hippocampal activities was ruled out because perievent histograms of neuronal discharges, performed using the positive peak of focal theta waves as a reference, revealed significant firing modulations at the theta frequency in the LA.
Two nonexclusive factors probably contribute to the appearance of theta oscillations in the LA during the anticipation of noxious stimuli. First, LA neurons are endowed with intrinsic membrane properties that predispose them to oscillate or reverberate in this range of frequencies (Paré et al., 1995; Pape et al., 1998). Second, the LA receives synaptic inputs from the rhinal cortices and hippocampal formation (for review, see McDonald, 1998) where rhythmic neuronal activity in the theta range has been observed (Mitchell and Ranck, 1980; Buzsáki et al., 1983; Alonso and García-Austt, 1987; Collins et al., 1999). The perirhinal cortex should be regarded as the most likely source of synaptic inputs for this LA oscillation. Indeed, of the aforementioned areas, the perirhinal cortex contributes the most powerful projection to the LA (Russchen, 1982; Witter and Groenewegen, 1986; for review, see McDonald, 1998). Finally, it should be noted that the propensity of rhinal and hippocampal areas to generate theta activity increases during EEG-activated states and arousal (Green and Arduini, 1954; Collins et al., 1999).
The occurrence of theta activity during fear probably generates short recurring time windows where excitatory synaptic interactions are facilitated because the membrane potential and electrotonic structure of the cells favor the genesis of orthodromic spikes. Considering the essential role that coincident neuronal activity is believed to play in synaptic plasticity and the hypothesized involvement of the amygdala and perirhinal cortex in memory (Zola-Morgan et al., 1989; Suzuki, 1996; Cahill et al., 1999; Fanselow and LeDoux, 1999), the presence of coherent theta oscillations in the LA and related cortices might be very important.
Whereas the literature has emphasized the distinct contributions of the amygdala and hippocampal regions to learning (Phillips and LeDoux, 1992; Gaffan, 1994; Maren, 1999), the dominant presence of a similar EEG rhythm within these interconnected structures (Witter et al., 1989) suggests that cooperative interactions may take place between them, in agreement with recent findings (Cahill and McGaugh, 1998; Sacchetti et al., 1999). In this context, it is interesting to note that rats subjected to an auditory fear-conditioning paradigm display hippocampal theta in the interval between a tone and a footshock (Whishaw, 1972).
Differential regulation of LA synaptic responsiveness as a function of input significance
The origin of the differential modulation of LA synaptic responses to inputs holding a predictive relationship to noxious stimuli (the tones) compared to those that did not (the perirhinal stimuli) is unclear. One possible explanation, perhaps the most parsimonious, is that auditory neurons located upstream from the LA gradually increase their responsiveness as the occurrence of the noxious stimulus approaches. In agreement with this possibility, it was reported that the tone-evoked responses of thalamic and cortical auditory neurons increases during various conditioning procedures (Weinberger, 1995). Moreover, a huge body of evidence indicates that the synaptic excitability of dorsal thalamic neurons augments with behavioral arousal (Steriade and McCarley, 1990). To a large extent, this results from the modulatory influence of mesopontine cholinergic neurons (for review, see Steriade and McCarley, 1990) projecting to the entire dorsal thalamus (Paré et al., 1988; Smith et al., 1988; Steriade et al., 1988). In this context, it should be noted that the central amygdaloid nucleus projects heavily to this brainstem region (Hopkins and Holstege, 1978) and that this projection has been implicated in emotional arousal (Kapp et al., 1992; Silvestri and Kapp, 1998). Irrespective of the underlying mechanism, the possibility that tone-evoked activity is regulated upstream of the LA would explain why perirhinal responses did not increase during the silent period.
A second, nonexclusive possibility is that an extrinsic or intrinsic mechanism somehow “informs” the LA of the differing significance of stimuli, perhaps through an action on intrinsic inhibitory neurons. This speculative scenario implies that the intrinsic inhibitory pressures that maintain LA cells virtually silent (Lang and Paré, 1997a,b) can be modulated selectively depending on the input significance as established through experience. Recent findings (Mahanty and Sah, 1998) suggest that this possibility might not be so far-fetched. In this context, it should be mentioned that some work suggests that thalamically relayed tone inputs can be potentiated in the LA nucleus (Rogan and LeDoux, 1995).
To address these issues, future studies should compare the fate of LA responses to synaptic inputs that bypass, or are relayed by, the dorsal thalamus when their significance is manipulated.
Footnotes
This work was supported by Medical Research Council Grant MT-11562. We thank Dr. Eric J. Lang for comments on an earlier version of this manuscript and P. Giguère and D. Drolet for their technical assistance.
Correspondence should be addressed to Denis Paré, Laboratoire de Neurophysiologie, Département de Physiologie, Faculté de Médecine, Université Laval Québec, Canada G1K 7P4. E-mail: denis.pare@phs.ulaval.ca.
REFERENCES
- 1.Adamec RE. Individual differences in temporal lobe sensory processing of threatening stimuli in the cat. Physiol Behav. 1991;49:455–464. doi: 10.1016/0031-9384(91)90264-o. [DOI] [PubMed] [Google Scholar]
- 2.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 3.Aggleton JP. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. [Google Scholar]
- 4.Alonso A, García-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. Exp Brain Res. 1987;67:493–501. doi: 10.1007/BF00247282. [DOI] [PubMed] [Google Scholar]
- 5.Applegate CD, Frysinger RC, Kapp BS, Gallagher M. Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Res. 1982;238:457–462. doi: 10.1016/0006-8993(82)90123-8. [DOI] [PubMed] [Google Scholar]
- 6.Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard DC, Blanchard RJ. Innate and conditioned reaction to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 8.Bordi F, LeDoux J. Sensory tuning beyond the sensory system: An initial analysis of auditory response properties of neurons in the lateral amygdaloid nucleus and overlying areas of the striatum. J Neurosci. 1992;12:2493–2503. doi: 10.1523/JNEUROSCI.12-07-02493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res Rev. 1983;6:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 10.Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 11.Cahill L, Weinberger NM, Roozendaal B, McGaugh JL. Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron. 1999;23:227–228. doi: 10.1016/s0896-6273(00)80774-6. [DOI] [PubMed] [Google Scholar]
- 12.Cain DP, Bindra D. Responses of amygdala single units to odors in the rat. Exp Neurol. 1972;35:98–110. doi: 10.1016/0014-4886(72)90062-3. [DOI] [PubMed] [Google Scholar]
- 13.Collins DR, Paré D. Reciprocal changes in the firing probability of lateral and central medial amygdala neurons. J Neurosci. 1999;19:836–844. doi: 10.1523/JNEUROSCI.19-02-00836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins DR, Lang EJ, Paré D. Spontaneous activity of the perirhinal cortex in behaving cats. Neuroscience. 1999;89:1025–1039. doi: 10.1016/s0306-4522(98)00396-0. [DOI] [PubMed] [Google Scholar]
- 15.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 16.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 17.Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Exp Brain Res. 1994;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- 18.Gaudreau H, Paré D. Projection neurons of the lateral amygdaloid nucleus are virtually silent throughout the sleep-waking cycle. J Neurophysiol. 1996;75:1301–1305. doi: 10.1152/jn.1996.75.3.1301. [DOI] [PubMed] [Google Scholar]
- 19.Gentile CG, Jarrell TW, Teich AH, McCabe PM, Schneiderman N. The role of amygdaloid central nucleus in differential Pavlovian conditioning of bradycardia in rabbits. Behav Brain Res. 1986;20:263–276. doi: 10.1016/0166-4328(86)90226-3. [DOI] [PubMed] [Google Scholar]
- 20.Gloor P, Olivier A, Quesney LF, Andermann F, Horowitz S. The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol. 1982;12:129–144. doi: 10.1002/ana.410120203. [DOI] [PubMed] [Google Scholar]
- 21.Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 22.Hitchcock JM, Davis M. Fear-potentiated startle using an auditory conditioned stimulus: effect of lesions of the amygdala. Physiol Behav. 1987;39:403–408. doi: 10.1016/0031-9384(87)90242-3. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–547. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- 24.Iwata J, Chida K, LeDoux JE. Cardiovascular responses elicited by stimulation of neurons in the central amygdaloid nucleus in awake but not anesthetized rats resemble conditioned emotional responses. Brain Res. 1987;418:183–188. doi: 10.1016/0006-8993(87)90978-4. [DOI] [PubMed] [Google Scholar]
- 25.Iwata J, LeDoux JE, Meeley MP, Arneric S, Reis DJ. Intrinsic neurons in the amygdaloid field projected to by the medial geniculate body mediate emotional responses conditioned to acoustic stimuli. Brain Res. 1986;383:195–214. doi: 10.1016/0006-8993(86)90020-x. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs BL, McGinty DJ. Amygdala unit activity during sleep and waking. Exp Neurol. 1971;33:1–15. doi: 10.1016/0014-4886(71)90097-5. [DOI] [PubMed] [Google Scholar]
- 27.Kaada BR. Brain mechanisms related to aggressive behavior. In: Clemente DC, Lindsley DB, editors. Aggression and defense. Neural mechanisms and social patterns. University of California; Berkeley: 1967. pp. 95–133. [PubMed] [Google Scholar]
- 28.Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: Effects on heart rate conditioning in the rabbit. Physiol Behav. 1979;23:1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- 29.Kapp BS, Gallagher M, Underwood MD, McNall CL, Whitehorn D. Cardiovascular responses elicited by electrical stimulation of the amygdala central nucleus in the rabbit. Brain Res. 1982;234:251–262. doi: 10.1016/0006-8993(82)90866-6. [DOI] [PubMed] [Google Scholar]
- 30.Kapp BS, Whalen PJ, Supple WF, Pascoe JP. Amygdaloid contributions to conditioned arousal and sensory information processing. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 229–254. [Google Scholar]
- 31.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. [DOI] [PubMed] [Google Scholar]
- 32.Klüver H, Bucy PC. An analysis of certain effects of bilateral lobectomy in the rhesus monkey, with special reference to “psychic blindness”. J Psychol. 1938;5:33–54. [Google Scholar]
- 33.Lang EJ, Paré D. Similar inhibitory processes dominate the responses of cat lateral amygdaloid projection neurons to their various afferents. J Neurophysiol. 1997a;77:341–352. doi: 10.1152/jn.1997.77.1.341. [DOI] [PubMed] [Google Scholar]
- 34.Lang EJ, Paré D. Synaptic and synaptically activated intrinsic conductances underlie inhibitory potentials in cat lateral amygdaloid projection neurons in vivo. J Neurophysiol. 1997b;77:353–363. doi: 10.1152/jn.1997.77.1.353. [DOI] [PubMed] [Google Scholar]
- 35.LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- 36.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machne X, Segundo JP. Unitary responses to afferent volleys in amygdaloid complex. J Neurophysiol. 1956;19:232–240. doi: 10.1152/jn.1956.19.3.232. [DOI] [PubMed] [Google Scholar]
- 38.Maeda H, Morimoto H, Yanagimoto K. Response characteristics of amygdaloid neurons provoked by emotionally significant environmental stimuli in cats, with special reference to response durations. Can J Physiol Pharmacol. 1993;71:374–378. doi: 10.1139/y93-057. [DOI] [PubMed] [Google Scholar]
- 39.Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- 40.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditioned fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Res. 1991;549:311–316. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- 42.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell S, Ranck JB. Generation of theta rhythm in medial entorhinal cortex of freely moving rats. Brain Res. 1980;189:49–66. doi: 10.1016/0006-8993(80)90006-2. [DOI] [PubMed] [Google Scholar]
- 44.Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- 45.Nishijo H, Ono T, Nishino H. Topographic distribution of modality-specific amygdalar neurons in alert monkey. J Neurosci. 1988a;8:3556–3569. doi: 10.1523/JNEUROSCI.08-10-03556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishijo H, Ono T, Nishino H. Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. J Neurosci. 1988b;8:3570–3583. doi: 10.1523/JNEUROSCI.08-10-03570.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Keefe J, Bouma H. Complex sensory properties of certain amygdala units in the freely moving cat. Exp Neurol. 1969;23:384–398. doi: 10.1016/0014-4886(69)90086-7. [DOI] [PubMed] [Google Scholar]
- 48.Ono T, Fukuda M, Nishino H, Sasaki K, Muramoto KI. Amygdaloid neuronal responses to complex visual stimuli in an operant feeding situation in the monkey. Brain Res Bull. 1983;11:515–518. doi: 10.1016/0361-9230(83)90123-5. [DOI] [PubMed] [Google Scholar]
- 49.Pape HC, Paré D, Driesang RB. Two types of intrinsic oscillations in neurons of the lateral and basolateral nuclei of the amygdala. J Neurophysiol. 1998;79:205–216. doi: 10.1152/jn.1998.79.1.205. [DOI] [PubMed] [Google Scholar]
- 50.Paré D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paré D, Smith Y, Parent A, Steriade M. Projections of brainstem core cholinergic and non-cholinergic neurons of cat to intralaminar and reticular thalamic nuclei. Neuroscience. 1988;25:69–86. doi: 10.1016/0306-4522(88)90007-3. [DOI] [PubMed] [Google Scholar]
- 52.Paré D, Pape HC, Dong JM. Bursting and oscillating neurons of the cat basolateral amygdaloid complex in vivo: electrophysiological properties and morphological features. J Neurophysiol. 1995;74:1179–1191. doi: 10.1152/jn.1995.74.3.1179. [DOI] [PubMed] [Google Scholar]
- 53.Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res. 1985a;16:117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- 54.Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons in the awake rabbit. Brain Res Bull. 1985b;14:331–338. doi: 10.1016/0361-9230(85)90194-7. [DOI] [PubMed] [Google Scholar]
- 55.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 56.Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 57.Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 58.Rogan MT, LeDoux JE. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 59.Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 60.Russchen FT. Amygdalopetal projections in the cat. I. Cortical afferent connections. A study with retrograde and anterograde tracing techniques. J Comp Neurol. 1982;206:159–179. doi: 10.1002/cne.902060206. [DOI] [PubMed] [Google Scholar]
- 61.Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci. 1999;19:9570–9578. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawa M, Delgado JMR. Amygdala unitary activity in the unrestrained cat. Electroencephalogr Clin Neurophysiol. 1963;15:637–650. doi: 10.1016/0013-4694(63)90115-9. [DOI] [PubMed] [Google Scholar]
- 63.Silvestri AJ, Kapp BS. Amygdaloid modulation of mesopontine peribrachial neuronal activity: implications for arousal. Behav Neurosci. 1998;112:571–588. doi: 10.1037//0735-7044.112.3.571. [DOI] [PubMed] [Google Scholar]
- 64.Smith Y, Paré D, Deschênes M, Parent A, Steriade M. Cholinergic and non-cholinergic projections from the upper brainstem core to the visual thalamus in the cat. Exp Brain Res. 1988;70:166–180. doi: 10.1007/BF00271858. [DOI] [PubMed] [Google Scholar]
- 65.Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. Plenum; New York and London: 1990. [Google Scholar]
- 66.Steriade M, Paré D, Parent A, Smith Y. Projections of cholinergic and non-cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience. 1988;25:47–67. doi: 10.1016/0306-4522(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki WA. The anatomy, physiology and functions of the perirhinal cortex. Curr Opin Neurobiol. 1996;6:179–186. doi: 10.1016/s0959-4388(96)80071-7. [DOI] [PubMed] [Google Scholar]
- 68.Uwano T, Nishijo H, Ono T, Tamura R. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience. 1995;68:339–361. doi: 10.1016/0306-4522(95)00125-3. [DOI] [PubMed] [Google Scholar]
- 69.Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;49:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- 71.Whishaw IQ. Hippocampal electorencephalographic activity in the Mongolian gerbil during natural behaviors and wheel running and in the rat during wheel running and conditioned immobility. Can J Psychol. 1972;26:219–239. doi: 10.1037/h0082431. [DOI] [PubMed] [Google Scholar]
- 72.Witter MP, Groenewegen HJ. Connections of the parahippocampal cortex in the cat. IV. Subcortical efferents. J Comp Neurol. 1986;251:51–77. doi: 10.1002/cne.902520104. [DOI] [PubMed] [Google Scholar]
- 73.Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- 74.Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]