Abstract
Somatic sensation can be localized precisely, whereas localization of visceral sensation is vague, possibly reflecting differences in the pattern of somatic and visceral input to the cerebral cortex. We used functional magnetic resonance imaging to study the cortical processing of sensation arising from the proximal (somatic) and distal (visceral) esophagus in six healthy male subjects. Esophageal stimulation was performed by phasic distension of a 2 cm balloon at 0.5 Hz. For each esophageal region, five separate 30 sec periods of nonpainful distension were alternated with five periods of similar duration without distension. Gradient echoplanar images depicting bold contrast were acquired using a 1.5 T GE scanner. Distension of the proximal esophagus was localized precisely to the upper chest and was represented in the trunk region of the left primary somatosensory cortex. In contrast, distension of the distal esophagus was perceived diffusely over the lower chest and was represented bilaterally at the junction of the primary and secondary somatosensory cortices. Different activation patterns were also observed in the anterior cingulate gyrus with the proximal esophagus being represented in the right midanterior cingulate cortex (BA 24) and the distal esophagus in the perigenual area (BA32). Differences in the activation of the dorsolateral prefrontal cortex and cerebellum were also observed for the two esophageal regions. These findings suggest that cortical specialization in the sensory-discriminative, affective, and cognitive areas of the cortex accounts for the perceptual differences observed between the two sensory modalities.
Keywords: cerebral cortex, esophagus, somatic, visceral, functional magnetic resonance imaging, sensation
The perception of somatic and visceral sensation is fundamentally different. Somatic sensation is localized precisely to the site of origin, whereas visceral sensation is vague, often referred to somatic structures and radiates to one or other side of the body (Polland and Bloomfield, 1931; Cervero, 1985;Ness and Gebhart, 1990).
Some of these differences can be explained on the basis of our current knowledge of the projections of somatic and visceral spinal afferents. For instance, the poor localization of visceral sensation can be partially explained by the fact that visceral afferents, which constitute <10% of the total afferent input to the spinal cord have a much greater rostrocaudal distribution within the spinal cord than somatic afferents (Polland and Bloomfield, 1931; Foreman et al., 1984; Cervero, 1985; Ness and Gebhart, 1990; Sengupta and Gebhart, 1994). In addition, referral of visceral sensation to somatic structures has been explained by the fact that most visceral afferents synapse on spinal neurons that also receive projections from somatic afferents (Polland and Bloomfield, 1931; Foreman et al., 1984; Sengupta and Gebhart, 1994).
However, the perceptual differences between somatic and vis- ceral sensation cannot be explained on the basis of spinal innervation, because perception is largely dependent on cortical processing. Animal data indicate differential cortical processing of somatic and visceral sensation (Bruggemann et al., 1994), but comparisons between the cortical processing of visceral and somatic sensation in man remain unexplored.
The human esophagus is virtually unique in that it develops as both a somatic and a visceral structure. It consists of striated muscle in its proximal one-third and smooth muscle in its distal two-thirds and differs in a number of ways in the innervation of the two regions (Christensen and De Carle, 1974; Kahrilas, 1992). First, the proximal (striated muscle) region has a denser spinal innervation than the distal (smooth muscle) region. Second, vagal afferents from the proximal region are largely myelinated, whereas those from the distal region are unmyelinated (Christensen, 1984). Third, intramural myenteric and submucous plexi are almost exclusive to the distal esophagus allowing it a degree of autonomy from supraspinal control (Christensen and De Carle, 1974; Christensen, 1984; Kahrilas, 1992). Fourth, the quality of sensation experienced from these two regions is different. Sensation arising from the proximal esophagus is localized precisely to the upper sternum, in contrast, that arising from the lower esophagus is perceived diffusely over the chest.
The human esophagus therefore provides a convenient neural system to compare the cortical processing of somatic and visceral sensation from the same anatomical organ. We therefore compared the cortical representation of the proximal and distal regions of the human esophagus using functional magnetic resonance imaging (fMRI).
MATERIALS AND METHODS
Subjects. Six right-handed healthy male subjects (mean age of 33 years; range, 25–48 years) were studied. All were free of esophageal symptoms, and all refrained from alcohol for at least 24 hr before the studies. Each subject underwent esophageal manometry to identify the distance of the proximal and distal esophageal sphincters from the incisors to accurately position the probe.
Informed, written consent was obtained from all subjects, and approval from the local ethics committee was obtained before all studies.
Esophageal stimulation was performed by distending a 2-cm-long silicone balloon with air. The balloon was mounted 15 cm from the tip of a 4-mm-diameter multilumen polyvinyl catheter (Wilson Cook; Letchworth, Herts, UK). For proximal esophageal stimulation, the center of the balloon was placed 3 cm distal to the upper esophageal sphincter, whereas for the distal esophageal stimulation, its center was placed 5 cm proximal to the lower esophageal sphincter. During each study, the balloon was repeatedly inflated with air using a purpose built pump (Medical Physics Department, Hope Hospital, Salford, UK), which was designed to operate in the strong magnetic field of the MR scanner. The flow of air produced by the inflation pump was 12 l/min. The volume of distension was adjusted for each subject to ensure that each inflation produced a clearly perceptible but nonpainful sensation. The balloon inflation frequency was 0.5 Hz.
Image acquisition. Gradient-echo echoplanar MR images were acquired using a 1.5 T GE Signa System (General Electric, Milwaukee, WI) fitted with Advanced Nuclear Magnetic Resonance (Woburn, MA) hardware and software at the Maudsley Hospital (London, UK). A quadrate birdcage head coil was used for radio frequency transmission and reception. In each of 16 noncontiguous planes parallel to the intercommissural anterior commissure-posterior commissure (AC-PC) plane, 100 T2*-weighted MR images depicting bold contrast were acquired with an echo time (TE) of 40 msec and a repetition time (TR) of 3600 msec. The slices had an in-plane resolution of 3.1 mm, with a slice thickness of 7 mm and interslice interval of 0.7 mm. Head movement was limited by foam padding within the head coil and a restraining band across the forehead. During the same session, a 43 slice, high-resolution inversion recovery echoplanar image of the whole brain was acquired in the AC-PC plane with TE of 73 msec, inversion time (TI) of 180 msec, and TR of 16,000 msec. The in-plane resolution was 1.5 mm, and the slice thickness was 3 mm with a 0.3 mm slice gap. The higher resolution image allowed subsequent superimposition of area of activation from the lower resolution bold images.
Image artifacts caused by subject movement during the course of the functional study were minimized using a combination of cushions and head straps, whereas those identified by subsequent postprocessing were minimized using locally developed image realignment software (Friston et al., 1996).
Generic brain activation mapping. The power of periodic signal change at the (fundamental) ON-OFF frequency of stimulation was estimated by iterated least squares fitting a sinusoidal regression model to the motion-corrected time series at each voxel of all images. The fundamental power quotient (FPQ) i.e., fundamental power divided by its SE was estimated at each voxel and represented in a parametric map. Each observed fMRI time series was then randomly permuted 10 times, and FPQ was re-estimated after each permutation. This resulted in 10 parametric maps (for each subject at each plane) of FPQ estimated under the null hypothesis that FPQ is not determined by experimental design (Bullmore et al., 1996). All parametric maps were then registered in the standard space of Talairach and Tournoux (1988), as described elsewhere (Brammer et al., 1997). This was achieved in two stages, using realignment algorithms similar to those previously used for movement correction. First, the set of FPQ maps observed in each subject is registered with that subject's high-resolution echo planar imaging dataset, then registered and rescaled relative to a Talairach template image.
Identical transformations were applied to the randomized FPQ maps obtained for each subject. After spatial normalization, the observed and permuted FPQ maps from each subject were identically smoothed with a Gaussian filter (full-width, half-maximum, 7 mm) to accommodate variability in gyral anatomy and error of voxel displacement during normalization. Generic activation was then robustly determined by computing the median value of FPQ at each voxel of the observed parametric maps and comparing it to a null distribution of median FPQ values computed from the randomized parametric maps. If the observed median FPQ exceeded the critical value of randomized median FPQ, then that voxel was considered generically activated with probability of false positive activation, p < 0.0008. This conservative threshold for activation was chosen to achieve acceptable control over type 1 error despite the large number of tests conducted. Generically activated voxels were colored and superimposed on the gray scale Talairach template, to create generic brain activation maps (GBAM) (Brammer et al., 1997). The coordinates for the activated regions were then identified on the Talairach and Tournoux (1988) atlas in sagittal, coronal, and axial sections. To determine the relationship of esophageal representation over the primary somatosensory cortex with the somatic homunculus, a comparison was made with the somatic homunculus mapped in a recent human magnetoencephalography study (Nakamura et al., 1998).
Comparison of proximal and distal esophageal brain activation maps. To identify those voxels that demonstrated significant difference in standardized power of response to proximal and distal esophageal stimulation, the observed difference in the median FPQ between the two experimental conditions was computed at each voxel. Subjects were then randomly reassigned to one of two equal-sized groups, and the difference in median FPQ between randomized groups was computed at each voxel. This process was repeated 64 times, and the results were pooled over voxels to generate a null distribution for the difference in median FPQ. For a two-tailed test of sizep < 0.01, the critical values were the 100*p/2th and 100*(1 − p/2)th percentiles of the randomization distribution. Note that this more lenient probability threshold was used to test for a differential power of response between experiments only at those voxels that were significantly activated in one or both of the GBAMs separately computed for each experiment (Phillips et al., 1997; Curtis et al., 1998).
Protocol. At the start of each study, the balloon catheter was passed perorally into the esophagus, and the balloon was positioned either in the proximal or the distal esophagus. The catheter was then connected to the pump and the balloon repeatedly inflated, increasing the volume of inflation in 1 ml increments to determine both the sensory and pain thresholds for each individual. A value was then determined that represented 50% of the difference between the sensory and the pain threshold. For instance, if the sensory threshold was 5 ml and the pain threshold 11 ml, then the volume used for the study was 8 ml. This method has been validated and described in detail in our previous study (Hobson et al., 1998). It was confirmed that this calculated volume produced a clearly perceptible but nonpainful sensation in each subject. The subjects were also asked to mark the site of radiation of esophageal sensation over the chest wall, and this area was then measured. The subjects were then comfortably positioned in the scanner, and a 5 min study was performed that was divided into 10 separate, 30 sec periods, of alternating nonpainful esophageal sensation (ON) and no-sensation (OFF) (where the balloon remained completely deflated). The order in which the two esophageal regions were stimulated was randomized between subjects. After completion of the process in one esophageal region, the balloon was repositioned in the other esophageal region, and the procedure was repeated.
RESULTS
All subjects tolerated the study without difficulty. Proximal esophageal sensation was localized precisely by each subject at a site 1–2 cm in area, in the midline over the lower neck and the upper chest, whereas distal esophageal sensation was perceived as a vague poorly localized sensation over an area of at least 5 cm in the lower chest. The balloon volumes required to produce a definite sensation in proximal and distal esophagus were 6 ± 2 and 9 ± 3 ml, respectively.
Brain activations
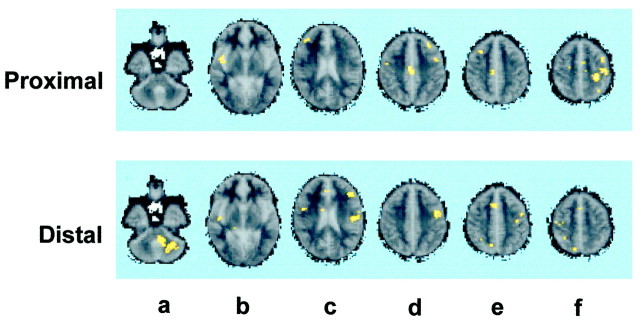
Proximal esophagus (Fig. 1)
Fig. 1.
Brain activation after esophageal stimulation. The figure shows the brain regions activated after stimulation of the proximal (somatic) and the distal (visceral) regions of the esophagus.a shows activation of the left cerebellum for the distal but not for the proximal esophagus. b shows activation of the right insula for both the proximal and the distal esophagus.c shows activation of the right inferior frontal gyrus for the proximal esophagus and the inferior part of the left primary somatosensory cortex, the left secondary somatosensory cortex, the left inferior frontal gyrus, and the right premotor cortex for the distal esophagus. d shows activation of the right anterior midcingulate gyrus, the left dorsolateral prefrontal cortex, the left premotor and supplementary motor cortices, and the right premotor cortex for the proximal esophagus, and the left premotor and supplementary motor cortices for the distal esophagus. eshows activation of the right dorsolateral prefrontal cortex, right anterior midcingulate gyrus for the proximal esophagus and right anterior cingulate gyrus, the right precuneus, left motor, premotor, and supplementary cortices for the visceral esophagus. Fshows activation of the left motor and supplementary motor cortices, the left primary somatosensory cortex, left precuneus and the right premotor cortex, and supplementary cortex for the proximal esophagus, and that of the right precuneus, the right premotor, and supplementary cortices for the visceral esophagus.
The largest spatial extent of activation occurred over the upper arm and chest area of the left primary sensory (S1) and motor cortices (BA 1 and BA 4), the right insula, the right anterior midcingulate gyrus (BA 24), the right dorsolateral prefrontal cortex (BA9), and the left supplementary motor cortex (BA 6). Smaller clusters of activated voxels were also seen bilaterally over inferior frontal gyrus (Broca's area BA 44), the left dorsolateral prefrontal cortex (BA 9), bilaterally over the premotor and supplementary motor cortices (BA 6), and the left precuneus (BA 7), Table1.
Table 1.
Brain regions activated after proximal esophageal stimulation
| Activated voxels | Max (FPQ) | Prob-Max FPQ | Tal(x) | Tal(y) | Tal(z) | Side | BA/region |
|---|---|---|---|---|---|---|---|
| 17 | 2.0 | 0.000004 | −26 | −28 | 48 | L | 4/Precentral gyrus (M1) (upper arm and chest) |
| 19 | 1.8 | 0.000035 | 3 | −19 | 37 | R | 24/Anterior-midcingulate gyrus |
| 14 | 1.8 | 0.000004 | 43 | −3 | −2 | R | Insula |
| 14 | 1.8 | 0.000004 | −40 | −17 | 48 | L | 1/Postcentral gyrus (S1) (upper arm and chest) |
| 13 | 1.8 | 0.000027 | −38 | 3 | 48 | L | 4/Motor cortex (forearm/arm/thorax) |
| 13 | 1.7 | 0.000222 | 32 | 17 | 42 | R | 9/Dorsolateral prefrontal cortex |
| 9 | 1.7 | 0.000142 | −46 | 0 | 31 | L | 6/Supplementary motor cortex |
| 7 | 1.8 | 0.000035 | −49 | 8 | 26 | L | 44/Inferior frontal gyrus (Broca's) |
| 6 | 1.7 | 0.000226 | −43 | 0 | 37 | L | 6/Premotor cortex and SMA |
| 5 | 1.7 | 0.000470 | 43 | 28 | 20 | R | 45/Inferior frontal gyrus (Broca's) |
| 5 | 1.7 | 0.000195 | −29 | 25 | 37 | L | 9/Dorsolateral prefrontal cortex |
| 2 | 1.6 | 0.000559 | −29 | −50 | 48 | L | 7/Precuneus (parietal association cortex) |
| 2 | 1.6 | 0.000541 | 0 | −3 | 48 | R | 6/Premotor cortex and SMA |
Abbreviations for Tables 1-3: FPQ, fundamental power quotient; SMA, supplementary motor cortex; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; M1, primary motor cortex.
Distal esophagus (Fig. 1)
The largest spatial extent of activation occurred over the left cerebellum, right anterior midcingulate gyrus (BA 24), left premotor and supplementary motor cortex (BA 6), left inferior primary sensory (gustatory) and secondary sensory (S2) cortices (BA 43), the left inferior frontal gyrus (BA45), the perigenual part of the right anterior cingulate gyrus (BA 32), and the right precuneus. Smaller clusters of activated voxels were also seen at the right inferior primary sensory and motor cortices (BA 1 and BA 4), left dorsolateral prefrontal cortex (BA 46), right insula, (BA 7), and the premotor and supplementary cortices bilaterally (Table2).
Table 2.
Brain-activated regions after distal esophageal stimulation
| Activated voxels | Max(FPQ) | Prob-Max FPQ | Tal(x) | Tal(y) | Tal(z) | Side | BA/Region |
|---|---|---|---|---|---|---|---|
| 54 | 2.0 | 0.000004 | −26 | −58 | −29 | L | Cerebellum |
| 12 | 1.9 | 0.000004 | −23 | −44 | −18 | L | Cerebellum |
| 8 | 1.7 | 0.000040 | −6 | −47 | −18 | L | Cerebellum |
| 20 | 1.9 | 0.000004 | 0 | 11 | 26 | R | 24/Anterior-midcingulate gyrus |
| 16 | 1.9 | 0.000004 | −40 | −3 | 37 | L | 6/Premotor cortex and SMA |
| 16 | 1.7 | 0.000057 | −49 | −17 | 20 | L | 3 and 43/Inferior part of S1 (gustatory cortex) and S2 |
| 11 | 1.8 | 0.000026 | −38 | 25 | 20 | L | 45/Inferior frontal gyrus |
| 9 | 1.7 | 0.000167 | −52 | −8 | 4 | L | 43/S2 |
| 8 | 1.7 | 0.000167 | 6 | 11 | 42 | R | 32/Anterior cingulate gyrus |
| 8 | 1.7 | 0.000167 | 14 | −58 | 48 | R | 7/Precuneus (parietal association cortex) |
| 6 | 1.7 | 0.000141 | −43 | −3 | 31 | L | 4/Precentral gyrus (M1) and premotor cortex (BA4 and 6) |
| 4 | 1.7 | 0.000141 | −40 | 22 | 26 | L | 46/Dorsolateral prefrontal cortex |
| 4 | 1.6 | 0.000203 | 46 | −14 | −2 | R | Insula |
| 3 | 1.6 | 0.000511 | −40 | −6 | 42 | L | 6/Premotor cortex and SMA |
| 3 | 1.7 | 0.000154 | 29 | −28 | 53 | R | 4/1 Inferior part of S1 and M1 |
| 2 | 1.6 | 0.000546 | 3 | 11 | 48 | R | 6/Premotor cortex and SMA |
Comparison of activation maps from proximal and distal esophagus (Fig. 2)
Fig. 2.
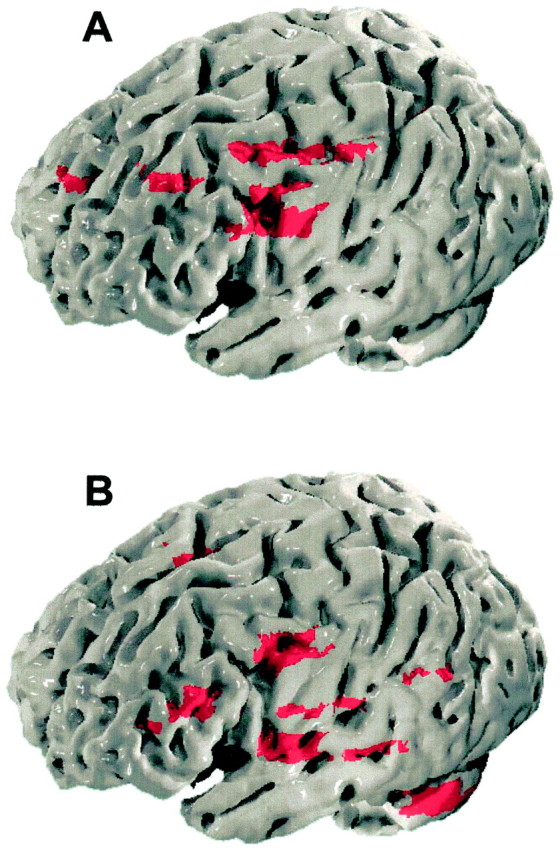
Three-dimensional brain representation of esophageal sensation. The figure shows the brain loci that process proximal (A) and the distal (B) esophageal sensation, superimposed on three-dimensionally rendered images of the brain. In comparison to the distal esophagus, the proximal esophagus shows representation in the more rostral regions of the primary somatosensory and motor cortex. Furthermore, differential representation of the two esophageal regions over the secondary somatosensory cortex, premotor, frontal, and prefrontal cortices and the cerebellum is notable.
Proximal esophageal stimulation produced greater activation over the left upper arm and chest area of the primary somatosensory/motor cortex, the right dorsolateral prefrontal cortex (BA 9), and left inferior frontal gyrus (BA 44). In contrast, distal esophageal stimulation produced greater activation of the left cerebellum, the left dorsolateral prefrontal cortex (BA 46), inferior (gustatory) part of the left primary sensory and motor cortex, and the right anterior cingulate cortex (perigenual part, BA 32), (Table3).
Table 3.
Comparison of brain regions activated after proximal and distal esophageal stimulation
| Activated voxels | Max(FPQ) | Prob Max FPQ | Tal(x) | Tal(y) | Tal(z) | Side | BA/Region |
|---|---|---|---|---|---|---|---|
| Proximal esophagus | |||||||
| 12 | 0.9 | 0.000184 | −40 | −17 | 48 | L | 1/Postcentral gyrus (S1) (upper arm-chest area) |
| 10 | 0.7 | 0.002250 | −26 | −25 | 48 | L | 4/Precentral gyrus (M1) (upper arm-chest area) |
| 8 | 0.7 | 0.002231 | 29 | 14 | 42 | R | 9/Dorsolateral prefrontal cortex |
| 7 | 0.9 | 0.000161 | −52 | 8 | 26 | L | 44/Inferior frontal gyrus (Broca's) |
| Distal esophagus | |||||||
| 27 | −0.5 | 0.012036 | −23 | −56 | −29 | L | Cerebellum |
| 10 | −0.3 | 0.065842 | −40 | 28 | 20 | L | 46/Dorsolateral prefrontal cortex |
| 9 | −0.5 | 0.010404 | −52 | −11 | 20 | L | 43/Inferior postcentral gyrus (gustatory) |
| 8 | −0.2 | 0.003279 | 9 | 11 | 42 | R | 32/Anterior cingulate gyrus |
| 6 | −0.0 | 0.001216 | −46 | −8 | 37 | L | 4/Precentral gyrus (M1) |
DISCUSSION
Our study shows fundamental differences in the cortical representation of sensation arising from the proximal (somatic) and distal (visceral) esophagus, which correlate with perceptual differences in sensation arising from the two regions. The significantly greater activation of the chest area of the primary somatosensory cortex during proximal in comparison to distal esophageal stimulation could explain the better localization of sensation from the somatic region of the esophagus.
This difference in somatosensory cortical representation may reflect different cortical projections from the lateral thalamic neurons that receive somatic and visceral afferent input. Animal electrophysiological studies show that thalamic neurons that receive somatotopically organized projections from the spinal cord also receive nontopographically organized visceral spinal projection (Bruggemann et al., 1994). It is therefore possible in humans that the thalamocortical projections maintain this distribution, with somatic neurons projecting somatotopically to the sensory homunculus, whereas visceral afferents project either nontopographically or in a crudely topographic manner to the caudal primary and secondary somatosensory cortices. It should be noted, however, that a representation has been found in the rostral hand region of primary somatosensory cortex of the squirrel monkey in response to nociceptive stimulation of the distal esophagus, colon, and urinary bladder (Bruggeman et al., 1997). In this study, cortical activation to non-nociceptive stimulation was not consistently seen probably because the animals were anesthetized and hence the cortical excitability was diminished. In our current study, we have used nonpainful esophageal stimulation, and it is possible therefore that activation of additional more rostral regions of the primary somatosensory cortex may have occurred if we had used a higher stimulation intensity. This is, however unlikely, because in our previous PET study (Aziz et al., 1997), we found a similar distribution of cortical activation in the caudal primary somatosensory cortex for both nonpainful and painful distal esophageal stimulation. It is likely, therefore, that differences between our current study and that performed by Bruggeman et al. (1997) are attributable to differences in the species studied and the methods used.
It is of relevance to note that the proximal esophagus was represented unilaterally over the left primary somatosensory cortex. In contrast, the distal esophagus was represented bilaterally, although a larger number of voxels were activated on the left in comparison to the right hemisphere. Unilateral cortical representation of proximal esophagal sensation has also been demonstrated in a study using magnetoencephalography (Furlong et al., 1998), whereas a PET study of distal esophageal sensation (Aziz et al., 1997) has shown bilateral but asymmetric representation. Although the significance of this asymmetric cortical representation of the esophagus remains unclear, it may explain why esophageal pain, like that of myocardial pain, often radiates to one or other side of the chest, mimicking angina pectoris (Kramer and Hollander, 1955).
Our results also indicate that processing of visceral and somatic sensation occurs differently in the anterior cingulate cortex. Although the anterior midcingulate cortex (BA24) was activated in response to both proximal and distal esophageal stimulation, the more rostral perigenual part of the cingulate cortex (BA 32) was activated only in response to distal stimulation. Perigenual cingulate cortical activation has been reported in previous studies of visceral (Channer et al., 1994; Aziz et al., 1997; Silverman et al., 1997) and somatic pain (Derbyshire et al., 1997). Our study now shows that perigenual cingulate activation also accompanies nonpainful visceral stimulation. Whereas, the midcingulate cortex is largely responsible for response selection, attention, and preparatory motor functions (Devinksy et al., 1995; Vogt et al., 1996), the perigenual part of the cingulate cortex is known to have direct connections with brainstem autonomic nuclei and is involved in visceromotor control and regulation of autonomic and emotional responses to external stimuli (Devinksy et al., 1995; Vogt et al., 1996). Because the autonomic and affective responses to visceral stimulation are more intense than those to somatic stimulation (Cervero, 1985), it seems plausible that these differences relate to greater visceral afferent projection to the perigenual cingulate cortex. This explanation, however, remains speculative as autonomic responses to esophageal stimulation were not measured in our study.
We also found that the dorsolateral prefrontal cortex was activated both by proximal and by distal esophageal stimulation. This region is generally considered to be responsible for cognitive evaluation, self-awareness, and attention (Dias et al., 1996; Frith and Dolan, 1996). It interacts closely with the anterior cingulate cortex and is involved in behavioral control (Devinksy et al., 1995; Vogt et al., 1996). Activation of this area is seen during somatic stimulation (Hsieh et al., 1996; Derbyshire et al., 1997), and it has been implicated in cognitive appraisal of the stimulus. Through its connections to the limbic and other association cortices (Devinsky et al., 1995; Vogt et al., 1996), it may also be responsible for integrating motor responses to the stimulus.
The different activation patterns of the dorsolateral prefrontal cortex to proximal and distal stimulation is intriguing. Functional differences between the left and right dorsolateral prefrontal areas have been observed in modulating emotions, motor behavior, and attention (Ross et al., 1994), so it is possible that functional specialization also exists for processing somatic and visceral sensation.
Activation of similar regions of the right insular cortex were seen after both proximal and distal esophageal stimulation. The insula is well recognized from both human and animal studies as an important visceral sensory and motor area (Augustine 1996). Animal studies suggest that the viscera are organized viscerotopically within the insular cortex with gastrointestinal neurons located most rostrally and cardiovascular neurons most caudally within the granular region. While in our study, a small difference was seen in the representation of the two esophageal regions in the insular cortex, the current resolution of functional MRI is not sufficient to allow us to determine whether this difference represents a true viscerotopic representation.
In addition to activating sensory areas, stimulation of either esophageal region also activated motor, premotor, supplementary motor cortices and the sensory associative cortex. This is not surprising as sensory feedback from the esophagus is well known to modulate the motor control of swallowing (Jean and Car, 1979; Hamdy et al., 1998). Furthermore, esophageal motility was not recorded in our study because of the technical limitations posed by using manometry equipment within the MRI environment. Although it is possible that esophageal distension may have evoked secondary peristaltic activity, which contributed to the activation seen in the motor, premotor, and supplementary motor cortices, the role of the cortex in mediating secondary peristaltic activity remains hitherto unconfirmed.
The most robust difference seen between the proximal and the distal esophagus was cerebellar activation that followed distal but not proximal esophageal stimulation. The cerebellum forms an important part of the sensorimotor integratory network but is not directly involved in perception of sensation (Roland, 1993). In previously reported studies of somatic sensation, cerebellar activation was observed only in response to pain (Casey et al., 1994; Hsieh et al., 1996; Svensson et al., 1997). Cerebellar activation, which overlaps with the activity seen in our study, was also observed in a recent PET study of somatic pain induced by capsaicin (Iadarola et al., 1998), a potent stimulator of nonmyelinated C Fibers (Torebjork et al., 1992), whereas nonpainful somatic sensation induced by activating myelinated A β fibers (Woolf and Doubell, 1994) failed to produce cerebellar activation. The viscera are innervated predominantly by nonmyelinated C fibers (Sengupta and Gebhart, 1994), and these fibers mediate both painful and nonpainful sensation (Cervero and Tattersall, 1986). Animal studies have shown that distension of the smooth muscle esophagus in the non-noxious range predominantly activates C fibers, (Sengupta et al., 1990). In contrast, similar distension of the striated muscle esophagus predominantly activates myelinated fibers (Satchell, 1984). It is possible therefore to attribute differences in cerebellar activation for the two esophageal regions in our study to a greater cerebellar projection of C-fibers from the visceral than from the somatic esophagus.
Schnitzler et al. (1999) have recently used magnetoencephalography to compare the cortical representation of the distal esophagus, median nerve, and the lip. They observed that while median nerve and lip stimulation activated both the primary and secondary somatosensory cortices, esophageal stimulation activated only the secondary somatosensory cortex. No differences in other cortical and subcortical areas were reported. They concluded that lack of esophageal afferent projection to the primary somatosensory cortex explains the poor spatial localization of visceral sensation.
Our results are in partial agreement with those of Schnitzler et al. (1999), however, we have shown that not only do esophageal afferents have a projection to the caudal part of the primary somatosensory cortex, but also that there are differences in the processing of somatic and visceral sensation in other cortical and subcortical areas. It can be argued that with the current spatial resolution of fMRI, it may not be possible to categorically differentiate between primary and secondary somatosensory cortical activation and that the signal observed in our study represents composite activation of the two regions. However MEG studies (Furlong et al., 1998; Hecht et al., 1999) suggest that esophageal stimulation activates two distinct dipoles located in the primary and secondary somatosensory cortices. It is likely therefore that the activation seen in our current study represents two distinct foci in the primary and secondary somatosensory cortices.
In conclusion, differences occur in the processing of sensation arising from somatic and visceral regions of the esophagus, particularly in the primary somatosensory, limbic, and prefrontal cortices and in the cerebellum. These findings indicate that the perception of somatic and visceral sensations differs not only as a result of differences in the spinal innervation of the two structures, but also because of differences in the mode of cortical processing. This information may help to explain differences in perception and autonomic responses between visceral and somatic structures.
Footnotes
Dr. Q. Aziz is a Medical Research Council Clinician Scientist, Dr. V. Ng is a Wellcome Clinical Research Fellow, and Dr. S. Hamdy is a Medical Research Council Research Training Fellow. We thank J. Suckling (Institute of Psychiatry, London) for image analysis and Helen Navarro (University of Manchester) for secretarial support.
Correspondence should be addressed to Dr. Qasim Aziz, Clinical Sciences Building, Department of Gastroenterology, Hope Hospital, Stott Lane, Salford M6 8HD, UK. E-mail: qaziz@fs1.ho.man.ac.uk.
REFERENCES
- 1.Aine CJ. A conceptual overview and critique of functional neuroimaging in humans: I. MRI/fMRI and PET. Crit Rev Neurobiol. 1995;9:229–309. [PubMed] [Google Scholar]
- 2.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 3.Aziz Q, Andersson JLR, Valind S, Sundin A, Hamdy S, Jones AKP, Foster ER, Langstrom B, Thompson DG. Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology. 1997;113:50–59. doi: 10.1016/s0016-5085(97)70079-9. [DOI] [PubMed] [Google Scholar]
- 4.Brammer MJ, Bullmore ET, Simmons A, Williams SCR, Grasby PM, Howard RJ, Woodruff PWR, Rabe-Hesketh S. Generic brain activation mapping in fMRI: a nonparametric approach. Magn Reson Imaging. 1997;15:763–770. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- 5.Bruggemann J, Shi T, Apkarian AV. Squirrel monkey lateral thalamus. II. Viscerosomatic convergent representation of urinary bladder, colon, and esophagus. J Neurosci. 1994;14:6796–814. doi: 10.1523/JNEUROSCI.14-11-06796.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruggemann J, Shi Ting, Apkarian AV. Viscero-somatic neurons in the primary somatosensory cortex (S1) of the squirrel monkey. Brain Res. 1997;756:297–300. doi: 10.1016/s0006-8993(97)00296-5. [DOI] [PubMed] [Google Scholar]
- 7.Bullmore ET, Brammer MJ, Williams SCR, Rabe-Hesketh S, Janot N, David AS, Mellers JDC, Howard R, Sham P. Statistical methods of estimation and inference for functional MR image analysis. Magn Reson Med. 1996;35:261–277. doi: 10.1002/mrm.1910350219. [DOI] [PubMed] [Google Scholar]
- 8.Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- 9.Cervero F. Visceral nociception: peripheral and central aspects of visceral nociceptive systems. Philos Trans R Soc Lond B Biol Sci. 1985;308:325–337. doi: 10.1098/rstb.1985.0033. [DOI] [PubMed] [Google Scholar]
- 10.Cervero F, Tattersall JEH. Somatic and visceral sensory integration in the thoracic spinal cord. In: Cervero F, Morrison JFB, editors. Progress in brain research. Visceral sensations, Vol 67. Elsevier; Amsterdam: 1986. pp. 189–205. [DOI] [PubMed] [Google Scholar]
- 11.Channer KS, Cronin CC, Rosen SD. Central nervous system pathways mediating angina pectoris. Lancet. 1994;344:964–965. [PubMed] [Google Scholar]
- 12.Christensen J. Origin of sensation in the esophagus. Am J Physiol. 1984;246:G221–G225. doi: 10.1152/ajpgi.1984.246.3.G221. [DOI] [PubMed] [Google Scholar]
- 13.Christensen J, De Carle DJ. Comparative anatomy of the esophagus. Gastroenterology. 1974;67:407–408. [PubMed] [Google Scholar]
- 14.Curtis VA, Bullmore ET, Brammer MJ, Wright IC, Williams SCR, Morris RG, Sharma TS, Murray RM, McGuire PK. Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry. 1998;155:1056–1063. doi: 10.1176/ajp.155.8.1056. [DOI] [PubMed] [Google Scholar]
- 15.Derbyshire SWG, Jones AKP, Gyulai F, Clark S, Townsend D, Firestone LL. Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- 16.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 17.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 18.Foreman RD, Blair RW, Webber RM. Viscerosomatic convergence on T-T spinoreticular, spinoreticular-spinothalamic and spinothalamic tract neurons in the cat. Exp Neurol. 1984;85:597–619. doi: 10.1016/0014-4886(84)90034-7. [DOI] [PubMed] [Google Scholar]
- 19.Friston KJ, Williams SCR, Howard R, Frackowiak RSJ, Turner R. Movement-related effects in fMRI time series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 20.Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain Res Cogn Brain Res. 1996;5:175–81. doi: 10.1016/s0926-6410(96)00054-7. [DOI] [PubMed] [Google Scholar]
- 21.Furlong PL, Aziz Q, Singh KD, Thompson DG, Hobson A, Harding GFA. Cortical localisation of magnetic fields evoked by esophageal distension. Electroencephalogr Clin Neurophysiol. 1998;108:234–243. doi: 10.1016/s0168-5597(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 22.Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term re-organisation of human motor cortex driven by short term sensory stimulation. Nat Neurosci. 1998;1:64–68. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- 23.Hecht M, Kober H, Claus D, Hilz M, Veith J, Neundorfer B. The electrical and magnetical cerebral responses evoked by electrical stimulation of the esophagus and the location of their cerebral sources. Clin Neurophysiol. 1999;110:1435–1444. doi: 10.1016/s1388-2457(99)00072-3. [DOI] [PubMed] [Google Scholar]
- 24.Hobson A, Aziz-Q, Furlong PL, Barlow JD, Bancewicz J, Thompson DG. Identification of the optimal parameters for recording cortical evoked potentials to human oesophageal electrical stimulation. Neurogastroenterol Motil. 1998;10:421–430. doi: 10.1046/j.1365-2982.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh JC, Stahle-Backdahl M, Hagermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996;64:303–314. doi: 10.1016/0304-3959(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 26.Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, Bennett GJ. Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain. 1998;121:931–947. doi: 10.1093/brain/121.5.931. [DOI] [PubMed] [Google Scholar]
- 27.Jean A, Car A. Inputs to the swallowing medullary neurons from the peripheral afferent fibers and the swallowing cortical area. Brain Res. 1979;178:567–572. doi: 10.1016/0006-8993(79)90715-7. [DOI] [PubMed] [Google Scholar]
- 28.Kahrilas PJ. Functional anatomy and physiology of the esophagus. In: Castell DO, editor. The esophagus, Ed 1. Little Brown; Philadelphia: 1992. pp. 1–27. [Google Scholar]
- 29.Kramer P, Hollander W. Comparison of experimental oesophageal pain with clinical pain of angina pectoris and esophageal disease. Gastroenterology. 1955;29:719–743. [PubMed] [Google Scholar]
- 30.Nakamura A, Yamada T, Goto A, Kato T, Ito K, Abe Y, Kachi T, Kakigi R. Somatosensory homunculus as drawn by MEG. NeuroImage. 1998;7:377–386. doi: 10.1006/nimg.1998.0332. [DOI] [PubMed] [Google Scholar]
- 31.Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- 32.Phillips ML, Young AW, Senior C, Brammere M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SCR, Gray JA, David AS. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- 33.Polland WS, Bloomfield AL. Experimental referred pain from the gastrointestinal tract. Part 1: The esophagus. J Clin Invest. 1931;10:435–452. doi: 10.1172/JCI100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roland PE. Partition of the human cerebellum in sensory-motor activities, learning and cognition. Can J Neurol Sci. 1993;20:S75–S77. doi: 10.1017/s0317167100048563. [DOI] [PubMed] [Google Scholar]
- 35.Ross ED, Homan RW, Buck R. Differential hemispheric lateralisation of primary and social emotions: implications for developing a comprehensive neurology for emotions, repression, and the subconscious. Neuropsychiatry Neuropsychol Behav Neurol. 1994;7:1–19. [Google Scholar]
- 36.Satchell PM. Canine esophageal mechanoreceptors. J Physiol (Lond) 1984;346:287–300. doi: 10.1113/jphysiol.1984.sp015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnitzler A, Volkmann J, Enck P, Frieling T, Wittle OW, Freund HJ. Different cortical organization of visceral and somatic sensation in humans. Eur J Neurosci. 1999;11:305–315. doi: 10.1046/j.1460-9568.1999.00429.x. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta JN, Gebhart GF. Gastrointestinal afferent fibres and sensation. In: Johnson LJ, editor. Physiology of the gastrointestinal tract, Ed 3. Raven; New York: 1994. pp. 483–519. [Google Scholar]
- 39.Sengupta JN, Saha JK, Goyal RK. Stimulus-response function studies of esophageal mechanosensitive nociceptors in sympathetic afferents of opossum. J Neurophysiol. 1990;64:796–812. doi: 10.1152/jn.1990.64.3.796. [DOI] [PubMed] [Google Scholar]
- 40.Silverman DHS, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/s0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 41.Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL. Cerebral processing of acute skin and muscle pain in humans. J Neurophysiol. 1997;78:450–460. doi: 10.1152/jn.1997.78.1.450. [DOI] [PubMed] [Google Scholar]
- 42.Talairach J, Tournoux P. A coplanar stereotactic atlas of the human brain. Theme; New York: 1988. [Google Scholar]
- 43.Torebjork HE, Lundberg LER, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol (Lond) 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vogt BA, Derbyshire S, Jones AK. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. Eur J Neurosci. 1996;8:1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 45.Woolf CJ, Doubell TP. The pathophysiology of chronic pain–increased sensitivity to low threshold A beta-fibre inputs. Curr Opin Neurobiol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]