Abstract
After peripheral nerve lesions, some axotomized afferent neurons develop ongoing discharges that originate in the dorsal root ganglion (DRG). We investigated in vivo which functional types of afferent neurons contributed to this ectopic activity. Six to twelve days after the gastrocnemius soleus (GS) nerve supplying skeletal muscle and the sural (SU) nerve supplying skin had been transected (experimental group E1), 20.4% of afferent neurons with myelinated axons projecting into the GS nerve produced ongoing discharges of irregular or bursting pattern. In contrast, all SU neurons were silent. Additional transection of peroneal and tibial nerves (group E2) induced ongoing activity in a similar percentage of GS neurons (22.1%), but their mean discharge frequency was higher (6.0 vs 2.7 Hz), and more of them exhibited bursting discharges (63 vs 17%). When the GS nerve had been left intact while tibial, peroneal, and SU nerve had been transected (group E3), 18.8% of unlesioned GS neurons developed ongoing discharges at a mean frequency of 6.1 Hz; most of them exhibited a bursting pattern. Without a preceding nerve lesion, almost no GS neuron (1.1%) fired spontaneously. Most afferent neurons with ongoing activity had an axonal conduction velocity of 5–30 m/sec indicating that some of these neurons may have had nociceptive function. These findings provide the first evidence that after peripheral nerve injury both axotomized as well as intact afferent neurons supplying skeletal muscle but not skin afferents generate ongoing activity within the DRG, probably because of a yet unknown signal in the DRG triggered by axotomy.
Keywords: dorsal root ganglion, ectopic firing, paresthesia, neuropathic pain, muscle pain, referred pain, rat
After peripheral nerve injuries, many axotomized primary afferent neurons start to generate ongoing discharges of ectopic origin. Possible consequences of these discharges are twofold: first, they may directly evoke ongoing paresthesias and pain; second, it is believed that ectopic activity triggers and maintains so-called central sensitization. This state of increased excitability of dorsal horn neurons in the spinal cord may pathologically augment peripheral sensory input resulting in hyperalgesia and allodynia (for review, see Bennett, 1994; Devor and Seltzer, 1999).
Ectopic activity after nerve lesion may originate at the lesion site (neuroma), in the dorsal root ganglion (DRG) or even elsewhere along the lesioned nerve. While the neuroma is well known as a source of ectopic discharges (for review, see Jänig et al., 1996; Devor and Seltzer, 1999), the DRG came first into focus as a prominent site of ectopic impulse generation by work done by Wall and Devor (1983). It has been demonstrated that ectopic discharges of DRG origin are present in two important animal models of neuropathic pain—the chronic constriction injury model (Kajander et al., 1992; Xie et al., 1995;Petersen et al., 1996; Study and Kral, 1996; Zhang et al., 1997) and the spinal nerve lesion model (Lee et al., 1999; Liu et al., 2000a,b). Because in these models onset of ectopic discharges originating in the DRG occurred during the same time when hyperalgesia- and allodynia-like behavior appeared, it has been assumed that these ectopic discharges are an important causative factor for onset and maintenance of the neuropathic symptoms (Kajander et al., 1992; Liu et al., 2000b).
It is to date unknown which functional types of afferent neuron preferentially develop ectopic activity of DRG origin after peripheral nerve lesion. In the aforementioned animal models, mixed nerves (sciatic nerve, spinal nerve) are lesioned, which makes functional identification of afferents with ongoing activity impossible. Here, we report about experiments in which we determined the ectopic activity generated in the DRG by recording from afferent fibers in distally cut microfilaments isolated from muscle and skin nerves of the rat hindlimb. Recordings were made in rats with a small nerve lesion, in rats with a large nerve lesion, and in control rats without a preceding nerve lesion. We found that ectopic activity of DRG origin occurred only in muscle afferents but not in cutaneous afferents. Muscle afferents that had been axotomized by the nerve lesion as well as unlesioned muscle afferents generated this ectopic ongoing activity. Discharging afferents had medium-sized myelinated axons, whereas ectopic activity was almost absent in large diameter myelinated and unmyelinated afferents. Rate and pattern of ectopic activity significantly depended on the size of the nerve lesion.
MATERIALS AND METHODS
Male adult Wistar rats (n = 18; body weight 250–370 gm) were used. All experimental procedures had been approved by the local animal care committee of the state administration and were conducted in accordance with the German Federal Law.
Experimental groups. Most animals (n= 16) were subjected to a nerve lesion before the electrophysiological experiments (Fig. 1B). Under pentobarbital anesthesia (Nembutal; 60 mg/kg), the sural nerve supplying skin as well as the medial and lateral gastrocnemius soleus (GS) nerves supplying skeletal muscle of the hindlimb were exposed on the left side and cut across near the ankle followed by excision of ∼3 mm of the distal stump (group E1; n = 7). In animals of group E2 (n = 6), additionally to the sural and GS nerves the left common peroneal and tibial nerve were transected. In rats of group E3 (n = 3), the left common peroneal, tibial, and sural nerves were transected, but the GS nerves were left intact. The incision was closed, and recovery was uneventful in all animals. Terminal electrophysiological experiments were performed 6–12 d later. Two animals (group E4) received no nerve lesion before the terminal experiment.
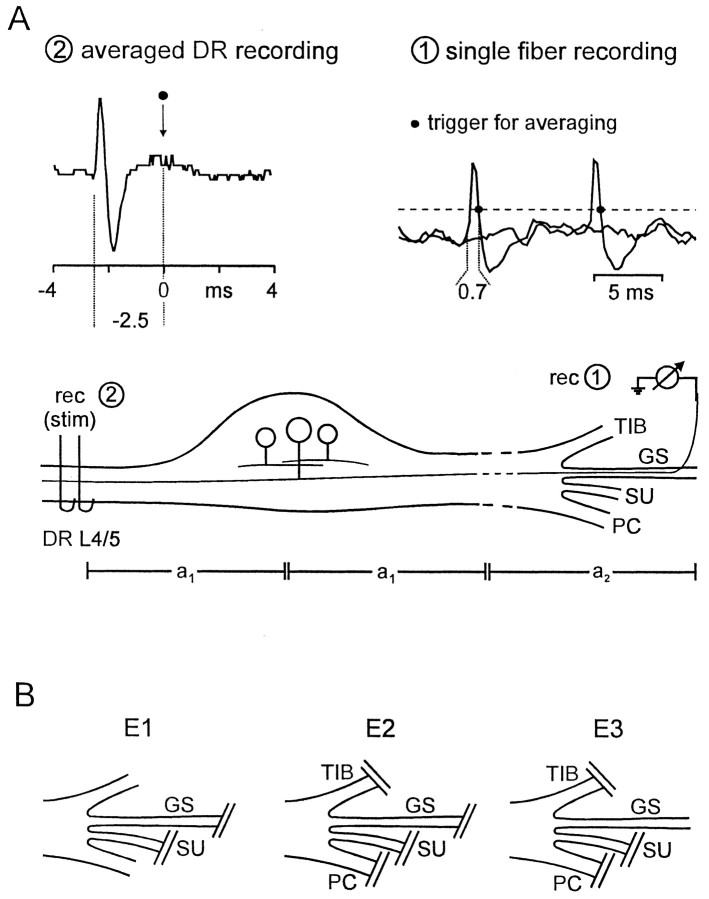
Fig. 1.
A, Sketch of the experimental set-up used to determine that the origin of spontaneous firing was within the DRG. Single fiber activity was recorded from centrally connected microfilaments teased from the gastrocnemius soleus (GS) nerve (rec 1). At first, electrical stimuli were delivered through the dorsal root (DR) electrode (stim 2), and the evoked single fiber activity was recorded at rec 1, so that the response latency (t = 3.0 msec) could be measured. After determination of propagation distancesa1 = 11 mm anda2 = 47 mm, conduction velocity (v) was calculated as v = (2a1 +a2)/t = 23 m/sec. Next, the DR electrode was used as recording electrode (rec 2). Amplitude window discrimination of spontaneous action potentials, recorded at rec 1, triggered averaging of dorsal root activity recorded at rec 2, from −4 to 4 msec with the trigger point at 0 msec. Onset of the averaged potential was measured 2.5 msec before the trigger. The time between onset of action potentials recorded by electrode 1 and the point at the falling phase of these action potentials that served as trigger (marked with dot) was 0.7 msec. Thus, onset of single action potentials at rec 1 followed 2.5–0.7 = 1.8 msec after onset of the averaged potential at rec 2. This time difference between onset of the potentials recorded at rec 1 and 2 is termed t2 (sot2 = 1.8 msec). The propagation timet1 of action potentials from their site of origin to rec 2 could now be calculated: because t= 2t1 +t2, we gett1 = 0.6 msec. Therefore, the estimated site of ectopic spike generation wast1 × v = 13.8 mm distal to rec 2. B, Schematic drawings illustrate the lesions used in the different experimental groups. TIB, Tibial nerve; SU, sural nerve; PC, common peroneal nerve. Experimental groups: E1, GS and SU nerve cut; E2, TIB, PC, SU, and GS nerve cut;E3, GS nerve intact, TIB, PC, and SU nerve cut.
Animal maintenance. Anesthesia was induced by intraperitoneal injection of pentobarbital sodium (Nembutal; 60 mg/kg). Tail artery and jugular vein catheters were inserted for continuous blood pressure recording and for application of drugs and fluid, respectively. The animals were paralyzed with Pancuronium (Organon; 1 mg/kg, i.v.) and artificially ventilated through a cannula that was inserted into the trachea, with a gas mixture of 40% O2 and 60% N2. Additional doses of pentobarbital (10–20 mg · kg−1 · hr−1, i.v., as needed) kept the anesthesia at a sufficient level as judged from the absence of corneal reflexes, withdrawal reflexes, and spontaneous gross blood pressure fluctuations. Throughout the experiments, mean arterial blood pressure exceeded 80 mmHg. Blood gases were regularly measured (ABL30; Radiometer, Copenhagen, Denmark). Rectal temperature was kept close to 37.0°C using a servo-controlled heating blanket. At the end of the experiments, the animals were killed by intravenous injection of a saturated potassium chloride solution under deep anesthesia.
Electrophysiological recordings. The sural and GS nerves were exposed, and their distal ends together with the neuromata were isolated from connective tissue and placed on a rigidly fixed small black Perspex platform (groups E1, E2). In other experiments, the GS nerve (group E3) or sural and GS nerves (group E4) were exposed and acutely transected at comparable location. By a lumbar laminectomy, the dorsal roots L4 and L5, to which >95% of all afferents in the GS nerve and >90% of all afferents in the sural nerve project (Baron et al., 1988), were exposed. The nerves and roots together with surrounding tissues were covered with warm (37°C) paraffin oil in two pools made from the skin flaps. After resection of the neuromata, the distal ends of the sural nerve and the GS nerve were split with the aid of fine jewelers' forceps for single fiber recording. Nerve filaments were placed on a platinum wire electrode referred to an indifferent electrode connected to nearby tissue. The roots were cut near the dorsal root entry zone and mounted on a bipolar electrode either for electrical stimulation or for recording (Fig.1A).
Experimental procedure. In each filament the number of afferent fibers was counted while the dorsal roots were electrically stimulated with square wave pulses of 0.1–0.5 msec duration at 0.3 Hz at variable intensities up to 30 V. All fibers were identified as myelinated (A) or unmyelinated (C) afferent fibers according to their conduction velocity, which was estimated from the propagation time of their action potentials evoked by electrical stimulation and the distance between stimulation and recording electrode (A fibers > 2 m/sec; C fibers < 2 m/sec). Once a nerve fiber fired spontaneously, the ongoing action potential activity was recorded for a period of at least 5 min.
Spike-triggered averaging. In seven experiments the site of ectopic impulse generation was localized using a spike-triggered averaging procedure. The falling phase of spontaneously occurring action potentials served as a trigger. Activity in that dorsal root through which the discharging afferent neuron projected was averaged by superimposing 750–2700 time periods −4 to +4 msec with respect to the trigger (Fig. 1A). At the end of the experiments, the distances between the dorsal root activity recording site and the DRG (a1; Fig. 1A) as well as the distance between the DRG and the peripheral recording electrode (a1 + a2; Fig.1A) were measured. The site of origin of the spontaneous activity was estimated as described in legend of Figure1A.
Data processing. Neural activity and arterial blood pressure were stored on a digital tape recorder (DTR-2602; Biological, Claix, France) for construction of interspike intervals and further analysis [custom data acquisition software (CARDS by S. Tiedemann) and template-matching program (Forster and Handwerker, 1990)]. Statistical evaluations are based on ANOVA, t test, or χ2 test, as appropriate (CSS statistic software package; StatSoft, Tulsa, OK).
RESULTS
Ectopic ongoing activity originates within dorsal root ganglia
A total of 1660 afferent nerve fibers projecting to the dorsal roots L4 or L5 was analyzed in this study. Among these, 81 exhibited ongoing action potential activity. To identify the location where the ectopic activity was generated, averaging of dorsal root activity triggered by spontaneously occurring action potentials was used in 15 of 81 units (Fig. 1A). In all 15 cases, the origin of ectopic ongoing activity was estimated to be inside or very close to the DRG.
Ectopic ongoing activity develops in axotomized DRG neurons that supply skeletal muscle but not in those which supply skin
We examined the occurrence of ongoing activity in DRG neurons that projected either into a nerve supplying skin (sural nerve) or into a nerve supplying skeletal muscle (gastrocnemius-soleus n.). Axotomized cutaneous DRG neurons never exhibited ongoing activity: all 293 myelinated and 638 unmyelinated units were silent (Table1, E1–E3). The same result was obtained from DRG neurons projecting to skin in controls without preceding nerve lesion (Table 1, E4). In contrast, DRG neurons with myelinated axons that supplied muscle before axotomy did show spontaneous activity (59 of 278, 21.2%; Table 1, E1,E2). The prevalence of spontaneous activity in these neurons was similar no matter whether the preceding nerve lesion was small (sural and gastrocnemius-soleus nerve cut; Table 1, E1) or whether it was large (additionally tibial and peroneal nerve cut; p > 0.7, χ2 test; Table 1, E2). The prevalence of spontaneous activity varied across animals in group E1 [23.2 ± 15.5% (mean ± SD), range 3.8–45.8%] and to similar extent in group E2 (24.5 ± 14.3%; range 10.5–42.3%). Ongoing activity in axotomized DRG neurons with unmyelinated axons was rare and was found exclusively in group E2 (Table 1). In controls, with one exception ongoing discharges were absent in DRG neurons supplying muscle (Table 1, E4).
Table 1.
Spontaneous activity in afferent neurons supplying muscle or skin
| Exp. group | Afferent neurons projecting into GS nerve | Afferent neurons projecting into sural nerve | ||
|---|---|---|---|---|
| A neurons | C neurons | A neurons | C neurons | |
| E1 | 29/142 (20.4%) | 0/32 | 0 /126 | 0 /140 |
| E2 | 30/136 (22.1%) | 2/59 (3.4%) | 0 /152 | 0 /487 |
| E3 | 19/101 (18.8%) | 0/17 | 0 /15 | 0 /11 |
| E4 | 1/92 (1.1%) | 0/30 | 0 /66 | 0 /54 |
Number of DRG neurons exhibiting spontaneous activity/total number of neurons in the group. Experimental groups: E1, GS and sural n. cut; E2, tibial, peroneal, sural and GS n. cut; E3, GS n. intact, tibial, peroneal, sural n. cut; E4, no nerve cut. A/C-neuron, DRG neuron with myelinated/unmyelinated axon.
Rate of ongoing activity and prevalence of bursting discharge pattern in lesioned DRG neurons increase with the total number of axotomized sensory neurons
The rate of ongoing activity in DRG neurons was obtained from time periods of at least 5 min. Axotomized A neurons in group E1 (small nerve lesion) exhibited a significantly lower mean discharge rate than those in group E2 [large nerve lesion; 2.7 ± 3.1 impulses (imp)/sec (mean ± SD) vs 6.0 ± 3.8 imp/sec;p < 0.001, t test; Fig.2A]. Across animals, the mean rate of spontaneous activity varied both in group E1 (2.7 ± 2.2 imp/sec; range, 0.9–5.8 imp/sec) and in group E2 (5.4 ± 1.3 imp/sec; range, 3.5–6.9 imp/sec). Two DRG neurons with unmyelinated axons discharged at <0.1 Hz.
Fig. 2.
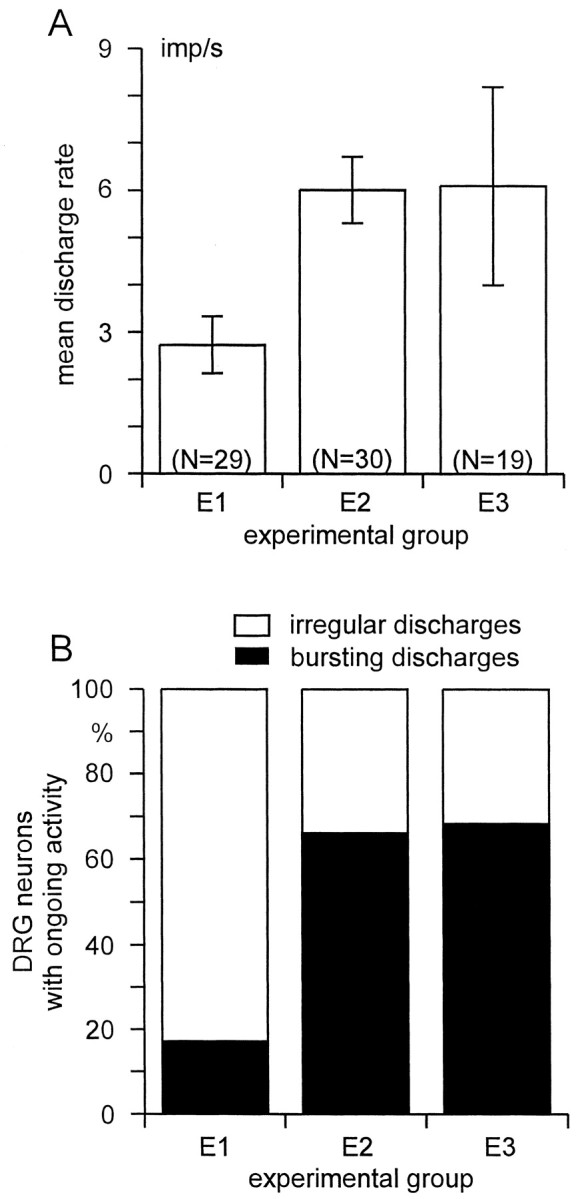
A, Mean discharge rate (±SEM) of spontaneously active DRG neurons with myelinated axons projecting into the GS nerve. B, Prevalence of different types of discharge pattern among DRG neurons that was significantly different between E1 and E2/E3 (p < 0.001, χ2 test). Experimental groups: E1, GS and sural nerve cut;E2, tibial, peroneal, sural, and GS nerve cut;E3, GS nerve intact, tibial, peroneal, and sural nerve cut.
During the observation periods the pattern of firing in single A neurons remained fixed. Among different A neurons, two main discharge patterns could be distinguished: (1) an “irregular” pattern with a broad unimodal distribution of interspike intervals (Fig.3A); and (2) a “bursting” discharge pattern, characterized by trains of action potentials interrupted by longer intervals (Fig.3B). Bursting neurons exhibited usually two, sometimes three separable peaks in their interspike interval histogram, as judged by visual inspection (Fig. 3, compare histograms in A, B). The first peak in these histograms represents intraburst interspike intervals and the second peak interburst intervals (Fig. 3). The majority (24 of 29; 82.8%) of axotomized A neurons in group E1 showed an irregular pattern, the remaining (5 of 29; 17.2%) fired in bursts. This was significantly different in group E2: here, more A neurons (19 of 30; 63.3%) exhibited a bursting discharge pattern than an irregular pattern (11 of 30, 36.7%; 24 of 29 vs 11 of 30; p < 0.001, χ2 test; Fig. 2B, Table 2). Axotomized A neurons with a bursting pattern exhibited a significantly higher overall discharge rate than those that fired irregularly (p < 0.01, ANOVA; Table 2).
Fig. 3.
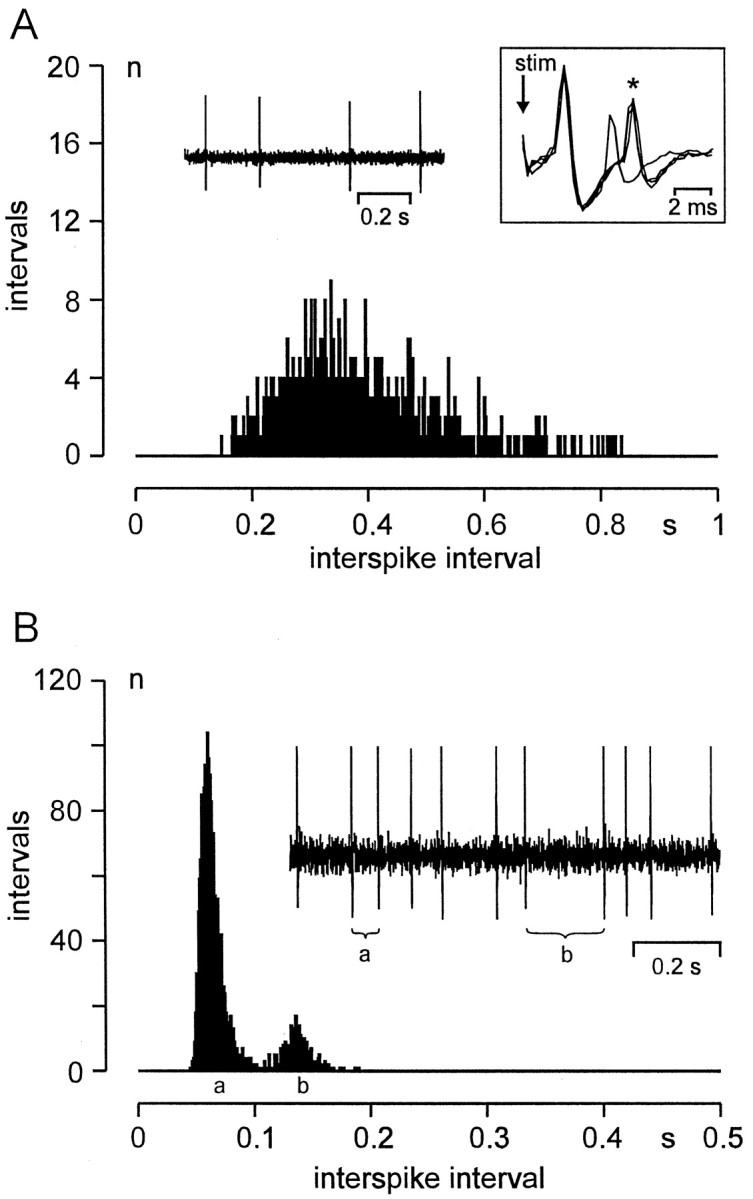
Representative examples of discharge patterns in DRG neurons with ongoing activity. Histograms show the distribution of temporal distances between successive spikes (interspike intervals); bin width, 1 msec. A, E1 neuron exhibiting an irregular discharge pattern and a unimodal distribution of interspike intervals. Left inset shows ongoing activity at low temporal resolution. Right inset shows the response of the unit (*) with a fixed latency after electrical stimulation: CV, 12.5 m/sec; note that immediately after a spontaneously occurring action potential the electrically evoked spike is missing because of to axonal membrane refractoriness. Ten days after nerve lesion.B, E2 neuron exhibiting bursting discharges. Intraburst (interburst) interspike interval marked with a(b) in the oscilloscope trace; corresponding peaks of the histogram likewise marked with a andb. Seven days after nerve lesion, CV 14.8 m/sec.
Table 2.
Discharge rate in A neurons projecting into GS nerve
| Exp. group | A neurons with irregular discharges | A neurons with bursting discharges | |||
|---|---|---|---|---|---|
| n | mean ± SEM (range) in imp/sec | n | Total frequency Intraburst frequency mean ± SEM (range) in imp/s | ||
| E1 | 24 | 2.2 ± 0.6 (0.1–14.7) | 5 | 5.4 ± 1.0 (2.1–7.7) | 14.2 ± 2.0 (9.7–21.3) |
| E2 | 11 | 2.7 ± 0.5 (0.2–4.3) | 19 | 7.9 ± 0.8 (2.7–13.2) | 20.6 ± 6.0 (10.9–100) |
| E3* | 5 | 3.1 ± 1.7 (0.4–9.6) | 13 | 4.6 ± 0.9 (1.7–11.6) | 19.0 ± 2.6 (8.6–36.4) |
A neuron, DRG neuron with myelinated axon. Experimental groups: E1, GS and sural n. cut; E2, tibial, peroneal, sural and GS n. cut; E3, GS n. intact, tibial, peroneal, sural n. cut.
* One A neuron with tonic discharges has not been not included in Table 2.
Uninjured DRG neurons develop ongoing activity after axotomy of neighboring DRG neurons
Without any preceding peripheral nerve lesion, 1 of 92 DRG neurons with myelinated axons projecting into the GS nerve was spontaneously active (Table 1, E4). In contrast, when major branches of the sciatic nerve (tibial, peroneal, and sural nerve, group E3) had been transected 6–12 d before the experiments, 19 of 101 A neurons projecting into the intact GS nerve exhibited ongoing discharges (p< 0.001, χ2 test; Table 1, E3). Their overall mean discharge rate and the distribution of discharge patterns resembled those obtained in neurons of group E2, where the GS nerve had been transected in addition to tibial, peroneal, and sural nerve (Fig.2). One unit fired tonically (40.7 Hz), i.e., its discharge had a highly regular interspike interval and was not interrupted by longer periods. Axotomized neurons (in group E1, E2) exhibiting bursting discharges had a higher mean firing frequency than nonaxotomized bursting neurons (in group E3; p < 0.05, ANOVA; Table2).
Most DRG neurons with ongoing activity have myelinated axons of medium conduction velocity
The distribution of conduction velocities among DRG neurons with myelinated axons exhibiting ongoing activity was similar in groups E1 and E2 (Fig. 4). Approximately one-third of the total number of units in both groups conducted faster than 30 m/sec (32.4% in group E1; 31.6% in group E2). Among these fast conducting units, <2.5% exhibited ongoing activity (1 of 46 in group E1; 1 of 43 in group E2; Fig. 4).
Fig. 4.
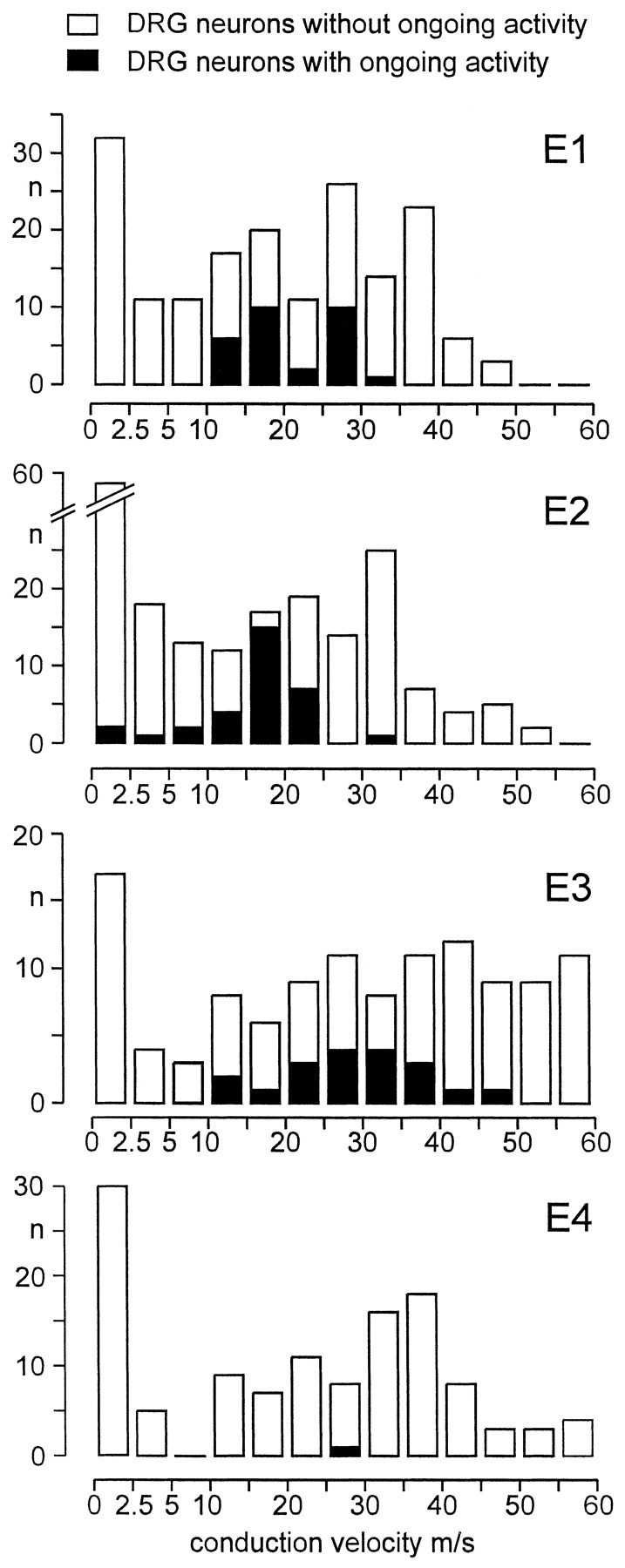
Conduction velocity distribution of DRG neurons projecting into the gastrocnemius soleus nerve in experimental groups E1–E4, separately for neurons with and without ongoing activity. Experimental groups: E1, GS and sural nerve cut;E2, tibial, peroneal, sural, and GS nerve cut;E3, GS nerve intact, tibial, peroneal, sural nerve cut;E4, no previous nerve lesion.
It is well known that chronic axotomy induces a slowing in conduction velocity (Michaelis et al., 1996). This has also been observed in the present study: 39 of 193 (20.2%) A neurons in groups E3 and E4 that had not been chronically axotomized conducted faster than 45 m/sec in contrast to 10 of 278 (3.6%) axotomized A neurons in groups E1 and E2 (p < 0.001, χ2test; Fig. 4). Among the 29 axons in group E3 conducting faster than 45 m/sec, one was spontaneously active. Thus, in all groups, the vast majority of DRG neurons exhibiting ongoing activity had myelinated axons of medium conduction velocity.
The distributions of conduction velocity of A neurons exhibiting bursting discharges and of those that fired irregularly were not statistically different (p > 0.05, modified χ2 test of Brandt-Snedecor; Sachs, 1984).
DISCUSSION
After peripheral nerve transection, afferent neurons supplying skeletal muscle but not those supplying skin generated action potential activity originating within the DRG. Such ectopic activity was observed in axotomized neurons as well as in intact neurons. A large nerve lesion induced a higher rate of ectopic activity and a higher incidence of bursting discharge pattern than a small nerve lesion. Almost all neurons exhibiting ectopic discharges had myelinated axons of low and medium conduction velocity.
Ectopic ongoing activity originated within dorsal root ganglia
Action potential activity reported here very likely originated within the DRG, although an accurate localization of the site where the ongoing activity originated was not possible. Based on spike-triggered averaging, calculations were made under the assumption that the conduction velocity (CV) of peripheral and central axonal branch of the afferent neurons was identical, but for many afferent neurons the central CV in the dorsal root is in fact lower than the peripheral CV (Rindos et al., 1984). Taking this difference into account shifts the origin of ectopic activity to a more proximal location than the calculated one (Fig. 1, see example). However, the calculation error is probably small, first, because the difference in CV is small for myelinated afferent neurons compared with unmyelinated ones (Rindos et al., 1984), and, second, because the averaging electrode was positioned close (<15 mm) to the DRG while the distance of the single fiber recording electrode from the DRG was always >45 mm. Intracellular studies will have to elucidate whether the ectopic activity is generated by the membrane of the soma or whether it originates along the course of the axon within the DRG.
Muscle, not skin, afferents develop ongoing discharges of DRG origin
After peripheral nerve transection, ongoing discharges of DRG origin were exclusively present in muscle afferents with myelinated axons; all cutaneous afferents were silent. Apparently similar results have been obtained in studies investigating ectopic discharges originating within a neuroma: here, the prevalence of ongoing activity was much higher in the GS nerve neuroma than in the sural nerve neuroma (Proske et al., 1995; Michaelis et al., 1995; Tal et al., 1999) and in the superficial peroneal nerve neuroma (Blumberg and Jänig, 1984). Some fast-conducting myelinated muscle afferents ending in a neuroma exhibited ongoing activity (Proske et al., 1995), whereas ongoing activity originating in the DRG was virtually absent in fast-conducting myelinated muscle afferents, as shown in our study (Fig. 4). Therefore, the mechanisms underlying ectopic impulse generation in the neuroma and in the DRG are probably different (see below). Furthermore, it is unclear whether ongoing discharges of neuroma and DRG origin develop in the same group of muscle afferent neurons or in separate subgroups of neurons.
The present study implies that ongoing activity of DRG origin after complete sciatic nerve transection (Wall and Devor, 1983; Devor and Wall, 1990; Devor et al., 1994; Michaelis et al., 1996) and after spinal nerve lesion (Lee et al., 1999; Liu et al., 2000a,b) very likely also appeared in muscle afferents. The rate of ectopic ongoing activity in DRG neurons, 3–8 d after spinal nerve lesion (Liu et al., 2000a), was not significantly different from the rate of ongoing activity recorded in the present experiments after lesioning of most hindlimb nerves.
In the chronic constriction injury (CCI) nerve lesion model of the sciatic nerve, ectopic ongoing activity also originated in the DRG and occurred in afferent neurons with myelinated fibers but not in those with unmyelinated ones (Kajander and Bennett, 1992). This nerve lesion model is considered to be a combined mechanical and inflammatory lesion model (Maves et al., 1993; Clatworthy et al., 1995). Major differences of DRG pathophysiology have been implicated between the models of CCI and spinal nerve lesion (Ramer and Bisby, 1999). Therefore, in the CCI model, not only muscle afferents but also cutaneous afferents may develop ongoing discharges of DRG origin.
Finally most afferent neurons that could be activated and/or depressed by electrical stimulation of the lumbar sympathetic trunk via the DRG in rats with sciatic nerve lesion (McLachlan et al., 1993; Devor et al., 1994; Michaelis et al., 1996) and in rats with spinal nerve lesion (Häbler et al., 2000) had ongoing activity of DRG origin. It is therefore very likely that these afferent neurons originally innervated skeletal muscle, too.
Peripheral nerve lesion induces ongoing discharges of DRG origin in axotomized and nonaxotomized afferent neurons: indication for a paracrine intraganglionic signal
We found ongoing activity of DRG origin with a similar prevalence of ∼20% in axotomized and in nonaxotomized muscle afferent neurons when major branches of the sciatic nerve had been transected. Because there was almost no ongoing activity in muscle afferents in controls without any previous nerve lesion, it is tempting to assume that a paracrine signal induced or generated by axotomized DRG neurons triggered the ectopic activity also in nonaxotomized muscle afferent neurons. This idea is supported by our finding that the total number of lesioned afferent neurons significantly affected mean rate and discharge pattern of ongoing activity: the discharge frequency was high and mostly of bursting pattern when the total number of lesioned afferents was large, whereas the discharge rate was low and its pattern predominantly nonbursting when the nerve lesion was small. From our finding that the percentage of spontaneously firing neurons did not change with the extent of the nerve lesion, one can conclude that the amount of the paracrine signal that is liberated already by a relatively small nerve lesion is large enough to trigger spontaneous discharges in most sensitive neurons; an increased amount of this signal, e.g., after a larger nerve lesion, leads to increased discharge frequency but does not enhance the percentage of firing neurons.
The nature of this paracrine signal inducing the ectopic activity after nerve injury is unknown. It may be produced by the DRG cells or the satellite cells. Future investigations have to show whether these signals are related to other signals (neurotrophins, cytokines) that are at present discussed to be involved in sprouting of sympathetic fibers in the DRG after peripheral nerve lesion (Ramer et al., 1999).
Recently it has been demonstrated that nerve injury enhances the incidence of DRG neurons exhibiting high-frequency oscillations in their membrane potential and that ectopic spike activity is always based on the presence of these membrane potential oscillations (Amir et al., 1999). An intriguing question is whether the paracrine signal enhances the incidence of membrane potential oscillation after nerve injury.
Discharges in DRG neurons depolarize many nonspiking neurons in the same DRG (Utzschneider et al., 1992; Amir and Devor, 1996). Such “cross-excitation” induced by ectopic activity in neighboring lesioned nerves may also contribute to ongoing discharges in nonaxotomized muscle afferents, as observed in the present study.
Our finding that only muscle afferent neurons exhibited spontaneous ectopic activity in the DRG but not cutaneous afferent neurons argues that either the hypothetical paracrine signal specifically reacts with these muscle afferent neurons or the signal-induced mechanism leading to the ectopic ongoing activity in the DRG is specific for muscle afferent neurons.
A role of a TTX-sensitive Na+conductance for ectopic impulse generation in DRG neurons
Recent studies have shown that nerve injury-induced ectopic discharges of DRG origin, which, according to our results, have been very likely generated by muscle afferents could effectively be suppressed by low doses of lidocaine without concomitantly blocking impulse propagation (Devor et al., 1992; Omana-Zapata 1997a; Amir et al., 1999). Tetrodotoxin (TTX) was similarly effective (Omana-Zapata et al., 1997b; Amir et al., 1999). In concordance with this, sciatic nerve transection induced an upregulation of the TTX-sensitive type III Na+ channel in those DRGs that contained axotomized neurons (Waxman et al., 1994; Dib-Hajj et al., 1996); correspondingly, after sciatic nerve injury rapidly repriming Na+ currents were enhanced in some DRG neurons (Rizzo et al., 1995; Cummins and Waxman, 1997). This increases their excitability and may ultimately lead to ongoing action potential activity. From these studies it remains open whether the observed changes are restricted to axotomized neurons that comprise ∼50% of neurons in DRG L4 and L5 after sciatic nerve transection (Devor et al., 1985) or whether the type III Na+ channel is upregulated in nonlesioned neighboring afferent neurons, too. In any event, because enhanced Na+ currents have been found in axotomized cutaneous afferents (Rizzo et al., 1995), whereas axotomized cutaneous DRG neurons did not develop ongoing ectopic activity as shown here, additional mechanisms must exist that underlie the development of spontaneous firing in muscle DRG neurons.
Functional types of muscle afferents that generate ongoing activity of DRG origin
We found that almost all muscle afferents with spontaneous activity had medium or low CV; most of the 30–40% fastest conducting afferents of the total CV distribution were silent. This finding is not attributable to an activity-dependent reduction of axonal CV that is significant in C-fiber axons but does not exceed 5% of CV in myelinated axons (Torebjörk and Hallin, 1974). This result is different from that published by Wall and Devor (1983), who showed in Wistar-derived Sabra-strain rats with sciatic nerve lesion, that the CV distribution of myelinated axons was virtually identical for those with ongoing activity generated in the DRG and for silent ones.
Most muscle afferents conducting in the range of 5–30 m/sec are group III afferents. According to Mense and Meyer (1985), the majority of group III muscle afferents are non-nociceptive, being activated by low-threshold pressure (44%) or contraction (23%); 33% group III muscle afferents can only be activated by noxious mechanical or chemical stimuli. Therefore it is likely that some of the slowly conducting myelinated afferents with ectopic activity originating in the DRG had nociceptive function.
Possible sensory consequences of ectopic discharges
An obvious consequence of ectopic ongoing activity is that it can evoke sensations according to the functional type of sensory neurons that generate these discharges. Ongoing activity in group III muscle afferents as described in the present study may therefore generate ongoing muscle pain or abnormal proprioceptive sensations. Moreover, activity in group III muscle afferents may induce pain referred to the skin or other deep somatic structures (Torebjörk et al., 1984;Laursen et al., 1999).
Tactile allodynia is a cardinal symptom of neuropathic pain. One explanation for the generation of tactile allodynia is the establishment of central sensitization triggered by ectopic afferent input. It has been shown that muscle afferents are capable of producing a much greater central sensitization than cutaneous afferents (Wall and Woolf, 1984), although it is usually thought that input from C-fibers is necessary. However, there is recent evidence for rapid, nerve lesion-induced phenotypic changes, e.g., an increase in brain-derived neurotrophic factor (BDNF) expression in large-diameter DRG neurons (Michael et al., 1999; Zhou et al., 1999). BDNF can enhance the excitability of central neurons (Kerr et al., 1999). Thus, a phenotypic switch of myelinated muscle afferents for BDNF could explain how ectopic activity in these afferents induces central sensitization, even in the absence of C-fiber activity (Liu et al., 2000b).
In summary, we presented evidence that after nerve injury ongoing activity of DRG origin appears exclusively in small-diameter myelinated muscle afferents, both axotomized and unlesioned. This ectopic activity is probably induced by a paracrine signal released in the DRG and may contribute to neuropathic pain states.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (Mi 457/2-1, Ja 240/15-1). We thank Ms. Sigrid Augustin for technical assistance and Ms. Eike Tallone for expert help with the illustrations.
Correspondence should be addressed to Dr. Martin Michaelis, Physiologisches Institut, Christian-Albrechts-Universität, Olshausenstrasse 40, D-24098 Kiel, Germany. E-mail:M.Michaelis@physiologie.uni-kiel.de.
Prof. Liu's present address: Department of Physiology, Sun Yat-sen University of Medical Sciences, No.74 Zhongshan Road 2, 510089 Guangzhou, China.
REFERENCES
- 1.Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron R, Jänig W, Kollmann W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system of the rat. J Comp Neurol. 1988;275:460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- 4.Bennett GJ. Neuropathic pain. In: Wall PD, Melzack R, editors. Textbook of pain, Ed 3. Churchill Livingstone; Edinburgh: 1994. pp. 201–224. [Google Scholar]
- 5.Blumberg H, Jänig W. Discharge patterns of afferent fibers from a neuroma. Pain. 1984;20:335–353. doi: 10.1016/0304-3959(84)90111-8. [DOI] [PubMed] [Google Scholar]
- 6.Clatworthy AL, Illich PA, Castro GA, Walters ET. Role of periaxonal inflammation in the development of thermal hyperalgesia and guarding behavior in a rat model of neuropathic pain. Neurosci Lett. 1995;184:5–8. doi: 10.1016/0304-3940(94)11154-b. [DOI] [PubMed] [Google Scholar]
- 7.Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci. 1997;17:3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devor M, Wall PD. Cross-excitation in dorsal root ganglia of nerve-injured and intact rats. J Neurophysiol. 1990;64:1733–1746. doi: 10.1152/jn.1990.64.6.1733. [DOI] [PubMed] [Google Scholar]
- 9.Devor M, Seltzer Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, editors. Textbook of pain, Ed 4. Churchill Livingstone; Edinburgh: 1999. pp. 129–164. [Google Scholar]
- 10.Devor M, Govrin-Lippmann R, Frank I, Raber P. Proliferation of primary sensory neurons in adult rat dorsal root ganglion and the kinetics of retrograde cell loss after sciatic nerve section. Somatosens Res. 1985;3:139–167. doi: 10.3109/07367228509144581. [DOI] [PubMed] [Google Scholar]
- 11.Devor M, Wall PD, Catalan N. Systemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conduction. Pain. 1992;48:261–268. doi: 10.1016/0304-3959(92)90067-L. [DOI] [PubMed] [Google Scholar]
- 12.Devor M, Jänig W, Michaelis M. Modulation of activity in dorsal root ganglion (DRG) neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- 13.Dib-Hajj S, Black JA, Felts P, Waxman SG. Down-regulation of transcripts for Na channel alpha-SNS in spinal sensory neurons following axotomy. Proc Natl Acad Sci USA. 1996;93:14950–14954. doi: 10.1073/pnas.93.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster C, Handwerker HO. Automatic classification and analysis of microneurographic spike data using a PC/AT. J Neurosci Methods. 1990;31:109–118. doi: 10.1016/0165-0270(90)90155-9. [DOI] [PubMed] [Google Scholar]
- 15. Häbler H-J, Eschenfelder S, Brinker H, Grunow B, Jänig W, Liu X-G. Neurogenic vasoconstriction in the dorsal root ganglion may play a crucial role in sympathetic-afferent coupling after peripheral nerve injury. Proceedings of the ninth world congress on pain Devor M, Rowbotham M, Wiesenfeld-Hallin Z. 2000. IASP; Seattle, in press. [Google Scholar]
- 16.Jänig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. Prog Brain Res. 1996;113:161–184. doi: 10.1016/s0079-6123(08)61087-0. [DOI] [PubMed] [Google Scholar]
- 17.Kajander KC, Bennett GJ. Onset of a painful peripheral neuropathy: a partial and differential deafferentation and spontaneous discharge in Aβ and Aδ primary afferent neurons. J Neurophysiol. 1992;68:734–744. doi: 10.1152/jn.1992.68.3.734. [DOI] [PubMed] [Google Scholar]
- 18.Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- 19.Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SWN. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laursen RJ, Graven-Nielsen T, Jensen TS, Arendt-Nielsen L. The effect of compression and regional anaesthetic block on referred pain intensity in humans. Pain. 1999;80:257–263. doi: 10.1016/s0304-3959(98)00214-0. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Liu X, Kim HT, Chung K, Chung JM. Receptor subtype mediating the adrenergic sensitivity of pain behavior and ectopic discharges in neuropathic Lewis rats. J Neurophysiol. 1999;81:2226–2233. doi: 10.1152/jn.1999.81.5.2226. [DOI] [PubMed] [Google Scholar]
- 22.Liu X-G, Eschenfelder S, Blenk K-H, Jänig W, Häbler H-J. Spontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in rats. Pain. 2000a;84:309–318. doi: 10.1016/s0304-3959(99)00211-0. [DOI] [PubMed] [Google Scholar]
- 23.Liu C-N, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M (2000b) Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain, in press. [DOI] [PubMed]
- 24.Maves TJ, Pechman PS, Gebhart GF, Meller ST. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seen in man. Pain. 1993;54:57–69. doi: 10.1016/0304-3959(93)90100-4. [DOI] [PubMed] [Google Scholar]
- 25.McLachlan EM, Jänig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- 26.Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol (Lond) 1985;363:403–417. doi: 10.1113/jphysiol.1985.sp015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michael GJ, Averill S, Shortland PJ, Yan Q, Priestley JV. Axotomy results in major changes in BDNF expression by dorsal root ganglion cells: BDNF expression in large trkB and trkC cells, in pericellular baskets, and in projections to deep dorsal horn and dorsal column nuclei. Eur J Neurosci. 1999;11:3539–3551. doi: 10.1046/j.1460-9568.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- 28.Michaelis M, Blenk K-H, Jänig W, Vogel C. Development of spontaneous activity and mechanosensitivity in axotomized afferent nerve fibers during the first hours after nerve transection in rats. J Neurophysiol. 1995;74:1020–1027. doi: 10.1152/jn.1995.74.3.1020. [DOI] [PubMed] [Google Scholar]
- 29.Michaelis M, Devor M, Jänig W. Sympathetic modulation of activity in dorsal root ganglion neurons changes over time following peripheral nerve injury. J Neurophysiol. 1996;76:753–763. doi: 10.1152/jn.1996.76.2.753. [DOI] [PubMed] [Google Scholar]
- 30.Omana-Zapata I, Khabbaz MA, Hunter JC, Bley KR. QX-314 inhibits ectopic nerve activity associated with neuropathic pain. Brain Res. 1997a;771:228–237. doi: 10.1016/s0006-8993(97)00770-1. [DOI] [PubMed] [Google Scholar]
- 31.Omana-Zapata I, Khabbaz MA, Hunter JC, Clarke DE, Bley KR. Tetrodotoxin inhibits neuropathic ectopic activity in neuromas, dorsal root ganglia and dorsal horn neurons. Pain. 1997b;72:41–49. doi: 10.1016/s0304-3959(97)00012-2. [DOI] [PubMed] [Google Scholar]
- 32.Petersen M, Zhang J, Zhang J-M, LaMotte RH. Abnormal spontaneous activity and responses to norepinephrine in dissociated dorsal root ganglion cells after chronic nerve constriction. Pain. 1996;67:391–397. doi: 10.1016/0304-3959(96)03146-6. [DOI] [PubMed] [Google Scholar]
- 33.Proske U, Iggo A, Luff AR. Mechanical sensitivity of regenerating myelinated skin and muscle afferents in the cat. Exp Brain Res. 1995;104:89–98. doi: 10.1007/BF00229858. [DOI] [PubMed] [Google Scholar]
- 34.Ramer MS, Bisby MA. Adrenergic innervation of rat sensory ganglia following proximal or distal painful sciatic neuropathy: distinct mechanisms revealed by anti- NGF treatment. Eur J Neurosci. 1999;11:837–846. doi: 10.1046/j.1460-9568.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 35.Ramer MS, Thompson SWN, McMahon SB. Causes and consequences of sympathetic basket formation in dorsal root ganglia. Pain Suppl. 1999;6:S111–S120. doi: 10.1016/S0304-3959(99)00144-X. [DOI] [PubMed] [Google Scholar]
- 36.Rindos AJ, Loeb GE, Levitan H. Conduction velocity changes along lumbar primary afferent fibers in cats. Exp Neurol. 1984;86:208–226. doi: 10.1016/0014-4886(84)90182-1. [DOI] [PubMed] [Google Scholar]
- 37.Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow and enhancement of fast Na+ currents in cutaneous afferent dorsal root ganglion neurones following axotomy. Neurobiol Dis. 1995;2:87–96. doi: 10.1006/nbdi.1995.0009. [DOI] [PubMed] [Google Scholar]
- 38.Sachs L. Applied Statistics: A handbook for techniques. Springer; New York: 1984. [Google Scholar]
- 39.Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65:235–242. doi: 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 40.Tal M, Wall PD, Devor M. Myelinated afferent fiber types that become spontaneously active and mechanosensitive following nerve transection in the rat. Brain Res. 1999;824:218–223. doi: 10.1016/s0006-8993(99)01190-7. [DOI] [PubMed] [Google Scholar]
- 41.Torebjörk HE, Hallin RG. Responses in human A and C fibres to repeated electrical intradermal stimulation. J Neurol Neurosurg Psychiatry. 1974;37:653–664. doi: 10.1136/jnnp.37.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torebjörk HE, Ochoa JL, Schady W. Referred pain from intraneural stimulation of muscle fascicles in the median nerve. Pain. 1984;18:145–156. doi: 10.1016/0304-3959(84)90882-0. [DOI] [PubMed] [Google Scholar]
- 43.Utzschneider D, Kocsis J, Devor M. Mutual excitation among dorsal root ganglion neurons in the rat. Neurosci Lett. 1992;146:53–56. doi: 10.1016/0304-3940(92)90170-c. [DOI] [PubMed] [Google Scholar]
- 44.Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- 45.Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in excitability of the flexion reflex in the rat. J Physiol (Lond) 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waxman SG, Kocsis JD, Black JA. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie Y, Zhang J, Petersen M, LaMotte RH. Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol. 1995;73:1811–1820. doi: 10.1152/jn.1995.73.5.1811. [DOI] [PubMed] [Google Scholar]
- 48.Zhang JM, Song XJ, LaMotte RH. An in vitro study of ectopic discharge generation and adrenergic sensitivity in the intact, nerve-injured rat dorsal root ganglion. Pain. 1997;72:51–57. doi: 10.1016/s0304-3959(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 49.Zhou XF, Chie ET, Deng YS, Zhong JH, Quing X, Rush RA, Xian CJ. Injured primary sensory neurons switch phenotype for brain-derived neurotrophic factor in the rat. Neuroscience. 1999;92:841–853. doi: 10.1016/s0306-4522(99)00027-5. [DOI] [PubMed] [Google Scholar]