Abstract
Focal cortical malformations comprise a heterogeneous group of disturbances of brain development, commonly associated with drug-resistant epilepsy and/or neuropsychological deficits. Electrophysiological studies on rodent models of cortical malformations demonstrated intrinsic hyperexcitability in the lesion and the structurally intact surround, indicating widespread imbalances of excitation and inhibition. Here, alterations in regional expression of GABAA receptor subunits were investigated immunohistochemically in adult rats with focal cortical malformations attributable to neonatal freeze-lesions. These lesions are morphologically characterized by a three- to four-layered cortex with microsulcus formation. Widespread regionally differential reduction of GABAA receptor subunits α1, α2, α3, α5, and γ2 was observed. Within the cortical malformation, this downregulation was most prominent for subunits α5 and γ2, whereas medial to the lesion, a significant and even stronger decrease of all subunits was detected. Lateral to the dysplastic cortex, the decrease was most prominent for subunit γ2 and moderate for subunits α1, α2, and α5, whereas subunit α3 was not consistently altered. Interestingly, the downregulation of GABAA receptor subunits also involved the ipsilateral hippocampal formation, as well as restricted contralateral neocortical areas, indicating widespread disturbances in the neocortical and hippocampal network. The described pattern of downregulation of GABAA receptor subunits allows the conclusion that there is a considerable modulation of subunit composition. Because alterations in subunit composition critically influence the electrophysiological and pharmacological properties of GABAA receptors, these alterations might contribute to the widespread hyperexcitability and help to explain pharmacotherapeutic characteristics in epileptic patients.
Keywords: cortical dysplasia, GABA, epilepsy, hyperexcitability, receptors, immunohistochemistry, developmental lesion
Cortical malformations comprise a heterogeneous group of genetic or acquired disturbances of cortical development that are frequently associated with drug-resistant epilepsies and/or neuropsychological deficits (Palmini et al., 1991;Raymond et al., 1995; Guerrini et al., 1999). Although recent research led to a better understanding of the pathogenesis (Gressens, 1998;Walsh, 1999), the pathophysiology of the resulting neurological abnormalities is only poorly understood. Electrophysiological studies on humans, as well as in vitro studies on rodent models of cortical dysgenesis, revealed an intrinsic epileptogenicity within the malformation and in widespread surrounding areas (Palmini et al., 1995;Jacobs et al., 1996; Luhmann and Raabe, 1996; Luhmann et al., 1998a;Redecker et al., 1998a). Altered intrinsic membrane properties or changes in network characteristics may contribute to this hyperexcitability. It is still debated to what extent and even in which direction the inhibitory function is altered by cortical malformations (Prince et al., 1997). An increase in GABA-mediated inhibitory efficacy was observed in layer V neurons within the paralesional zone (Prince et al., 1997; Jacobs and Prince, 1999). In pharmacological studies using 4-aminopyridine to induce epileptiform discharges, the inhibitory systems were at least not grossly impaired in the surround of cortical malformations (Hablitz and DeFazio, 1998). However, close to the dysplastic cortex, intracellular recordings in upper layers revealed a decrease in GABA-mediated inhibition (Luhmann et al., 1998b). Recent autoradiographic studies disclosed a significant reduction of binding to GABAA and GABAB receptors within the malformation and the surrounding neocortex (Zilles et al., 1998), pointing toward widespread changes in GABA receptor function.
To analyze whether alterations in distribution of specific GABAA receptor subunits help to understand the changes in GABAergic function, the expression of five major subunits were immunohistochemically investigated in adult rats with focal cortical malformations after neonatal freeze-lesions (Jacobs et al., 1996; Luhmann and Raabe, 1996; Luhmann et al., 1998a). So far, at least 19 different subunit subtypes of the pentameric GABAA receptors have been sequenced from the mammalian nervous system, comprising six α, three β, three γ, one δ, one ε, one θ, one π, and three ρ subunits (Barnard et al., 1998; Whiting et al., 1999). The majority of GABAA receptors contain a variable combination of α, β, and γ subunits, showing a specific regional and cellular distribution (Fritschy and Mohler, 1995). Although little is known about the specific properties of single subunits, functional studies demonstrated that the subunit composition of receptor subtypes determines their electrophysiological and pharmacological properties (Barnard et al., 1998; Narahashi, 1999), thus allowing a variety of adaptive changes (Olsen et al., 1999). Because different α subunits correspond to primarily distinct receptor subtypes (Fritschy and Mohler, 1995; Sieghart et al., 1999), this study concentrated on the regional distribution of four α subunits and one γ subunit. Hereby, a widespread regionally differential downregulation of GABAA receptor subunits was observed involving not only the cortical malformation but also structurally intact surrounding and remote brain regions.
MATERIALS AND METHODS
Lesion induction. Several litters of newborn Wistar rats (Experimental Animal Laboratory of the Heinrich-Heine-University, Düsseldorf, Germany) were used for the experiments. Focal freeze-lesions were induced at the day of birth (postnatal day 0, <24 hr) using a modification of the method of Dvorak and Feit (1977), as described previously in detail (Luhmann and Raabe, 1996; Luhmann et al., 1998a). In brief, newborn rats (n= 12; 8 females and 4 males) were anesthetized by hypothermia, and a liquid nitrogen cooled copper cylinder (diameter of 1 mm) was placed for 8 sec on the calvarium above the frontoparietal cortex. To create a longitudinal freeze-lesion, three identical freeze-lesions were placed in line parallel to the midline with a distance of 1.5 mm between the lesions. These lesions resulted in a 3- to 5-mm-long microsulcus in the rostrocaudal direction (Fig.1A). The wound was closed with histoacryl tissue glue (Braun-Dexon, Melsungen, Germany). Sham-operated rats (n = 7; 6 females and 1 male 1) were treated in the same way without cooling the copper cylinder. Freeze-lesioned and sham-operated rats were allowed to survive for 10–16 weeks before further immunohistochemical studies.
Fig. 1.
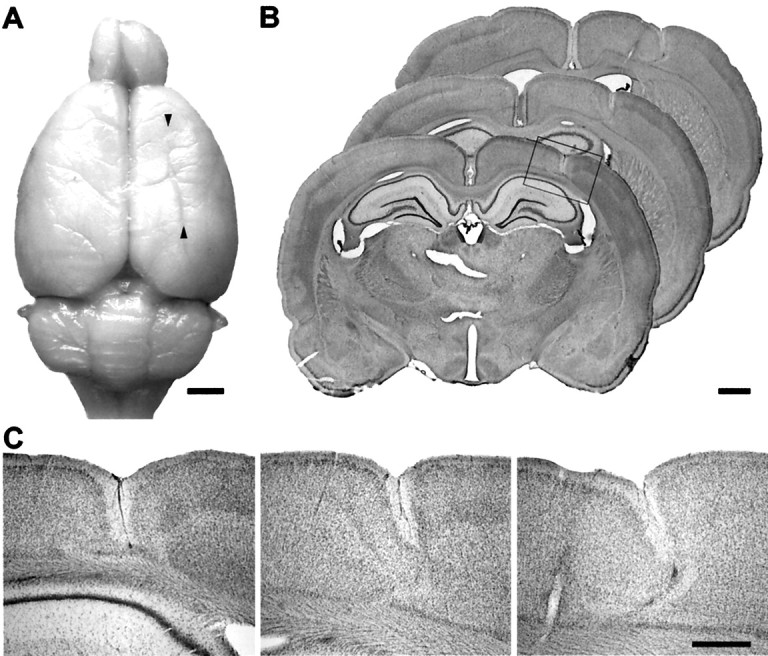
Morphology of freeze-lesion-induced focal cortical malformations. A, Adult rat brain that received a freeze-lesion at the day of birth, resulting in a longitudinal microgyrus. The microgyrus is macroscopically characterized by an infolding of the brain surface (arrowheads).B, Cresyl violet-stained coronal sections through the cortical malformation associating a loss of deep cortical layers and formation of a microsulcus (frame). The depth of the microsulcus increased in the anteroposterior direction.C, Higher magnifications of the sections displayed inB. In rostral parts of the brain, the dysplastic cortex is typically characterized by a three- to four-layered cortex. Because of the increase in depth, a nearly complete division of the neocortex is observed more occipitally. Scale bars: A, 2 mm;B, 1 mm; C, 500 μm.
Immunohistochemistry. Adult rats were deeply anesthetized with diethylether and perfused through the ascending aorta with 4% paraformaldehyde and 15% saturated picric acid solution in phosphate buffer (0.15 m), pH 7.4 (Fritschy and Mohler, 1995). Brains were removed immediately after the perfusion and post-fixed for 3 hr in the same fixative at 4°C. All samples were then cryoprotected in PBS containing 30% sucrose for 24 hr and stored at −75°C for further processing. To enhance the detection of synaptic receptor proteins in the subsequent immunohistochemical staining, the brains were processed with a modified antigen-retrieval procedure (Bohlhalter et al., 1996; Fritschy et al., 1998). The brains were incubated overnight at room temperature in 0.1m sodium citrate buffer, pH 4.5, and irradiated with microwaves (650 W, 135 sec) in the same buffer. Coronal sections were cut at 50 μm with a freezing microtome and collected in ice-cold 0.1 m PBS. A series of sections was Nissl-stained with cresyl violet for histological analysis of the freeze-lesion-induced cortical malformations.
The GABAA receptor subunits α1, α2, α3, α5, and γ2 were visualized using subunit-specific antisera raised in guinea pigs against synthetic peptides derived from rat subunit cDNA. These subunit-specific antisera have been extensively characterized biochemically by Western blotting and immunoprecipitation (for additional details, see Fritschy and Mohler, 1995), and their suitability for immunohistochemistry has been documented in several previous reports (Fritschy and Mohler, 1995; Fritschy et al., 1998;Neumann-Haefelin et al., 1998).
Free-floating sections were washed three times in Tris buffer (Tris saline, pH 7.4, and 0.05% Triton X-100) and incubated at 4°C overnight in primary antibody solution diluted in Tris-buffer containing 2% normal goat serum (NGS). The following dilutions of the antisera were used: GABAA receptor subunit α1, 1: 20,000; subunit α2, 1: 2000; subunit α3, 1: 2000; subunit α5, 1: 4000; and subunit γ2, 1: 3000. Sections were then washed three times in Tris buffer and incubated in biotinylated secondary antibody solution (Jackson ImmunoResearch, West Grove, PA) diluted 1: 300 in Tris buffer containing 2% NGS for 30 min at room temperature. After additional washing, sections were transferred to the avidin-peroxidase solution (Vectastain Elite kit; Vector Laboratories, Burlingame, CA) for 25 min, washed, and processed using diaminobenzidine hydrochloride (Sigma, St. Louis, MO) as chromogen. Sections were mounted onto gelatin-coated slides, air-dried, dehydrated with ascending series of ethanol, cleared, and coverslipped with toluene (Entellan; Merck, Darmstadt, Germany).
Changes in the regional and laminar distribution of GABAA receptor subunits were analyzed by light microscopy. For semiquantitative image analysis, sections were digitized with a charge-coupled device camera and processed with an imaging program (NIH Image). Measures of relative optical density of GABAA receptor subunit staining were performed on one section per animal and per antibody. The following brain regions were evaluated on both hemispheres (a scheme is illustrated in Fig. 4): the area of the cortical malformation, the frontal cortex (Fr), the hindlimb representation cortex (HL), the primary and secondary somatosensory cortex (Par1, Par2), the hippocampal formation, and the thalamus (Zilles, 1992). For background correction, the signal obtained in the corpus callosum was subtracted. Statistical significance of differences in mean optical densities between the experimental and control group was assessed using a two-samplet-test (p < 0.05). For display, images were contrast-enhanced and color-coded on a 256-level.
Fig. 4.
Semiquantitative analysis of regional alterations in immunoreactivity of GABAA receptor subunits. The relative differences of staining intensities measured as optical densities in sections from animals with focal cortical malformations compared with sham-operated animals are displayed for different neocortical areas. A schematic drawing of the evaluated regions is shown in the inset. Significant differences (p < 0.05) are indicated byasterisks. Within the microgyrus, receptor subunits α1, α5, and γ2 showed a significant decrease in immunoreactivity compared with sham-operated animals. In the adjacent Fr, a significant reduction is measured for all subunits, whereas in Par1 and Par2, subunits α2 and α5 were significantly decreased in Par2 and subunit γ2 was reduced in both areas.
RESULTS
Morphology of cortical malformations
All freeze-lesioned animals (n = 12) displayed typical cortical malformations consisting of a longitudinal microgyrus located in parallel to the midline with a length of 3–5 mm (Fig.1A). The microgyrus had a distance to midline of 1.5–3.5 mm (mean of 2.5 mm) and involved the forelimb (FL) and hindlimb representation cortex, as well as the secondary occipital cortex (Oc2) in most cases (Zilles, 1992). The laminar architecture of the cortical malformation varied to some extent. Most animals showed the formation of a small sulcus with an underlying three- to four-layered cortex as reported previously (Rosen et al., 1992, 1998; Jacobs et al., 1996; Luhmann et al., 1998a; Zilles et al., 1998). The depth of the microsulcus increased in the anteroposterior direction, extending to layer IV or V frontally and resulting in a complete division of the gray matter more occipitally (Fig. 1). In two animals, a complete division of the cortex throughout the microsulcus without a thin layer of remaining cells was observed, resembling the pathology of schizencephaly in humans. In addition to the longitudinal microgyrus, small clusters of ectopic cells were found in 4 of 12 freeze-lesioned animals in the molecular layer adjacent to the microsulcus. No structural change was observed in the hippocampal formation or other remote brain regions. In sham-operated animals, no abnormalities in cortical structure or lamination were detected.
Widespread dysregulation of GABAAreceptor subunits
The distribution of the GABAA receptor subunits α1, α2, α3, α5, and γ2 in sham-operated animals showed the same pattern as described previously (Fritschy and Mohler, 1995) (Fig. 2). Briefly, the most abundant subunits in the cerebral cortex were α1 and γ2, which displayed a nearly identical distribution pattern. In neocortex, these subunits showed particularly intense staining in layers III – IV, whereas the remaining layers exhibited a slightly lighter immunoreactivity. Subunits α2, α3, and α5 showed a more restricted distribution in the cerebral cortex, being primarily confined to certain layers. Whereas subunit α2 was strongly expressed in the upper layers (I–IV) and showed only slight staining in layers V–VI, subunits α3 and α5 were most abundant in deeper cortical layers and were almost absent in the outer layers. Subunit α3, and with a weaker staining also subunit α5, revealed a certain regional selectivity with a particularly intense immunoreactivity in deep layers of the Fr, the HL/FL, as well as the Oc1/Oc2. In contrast to the cerebral cortex, the hippocampal formation showed a differential pattern of GABAA receptor subunit expression with a particularly strong immunoreactivity of the subunits α2, α5, and to lesser extent of γ2. In the hippocampal formation, subunit α1 revealed a moderate and subunit α3 only a very weak staining.
Fig. 2.
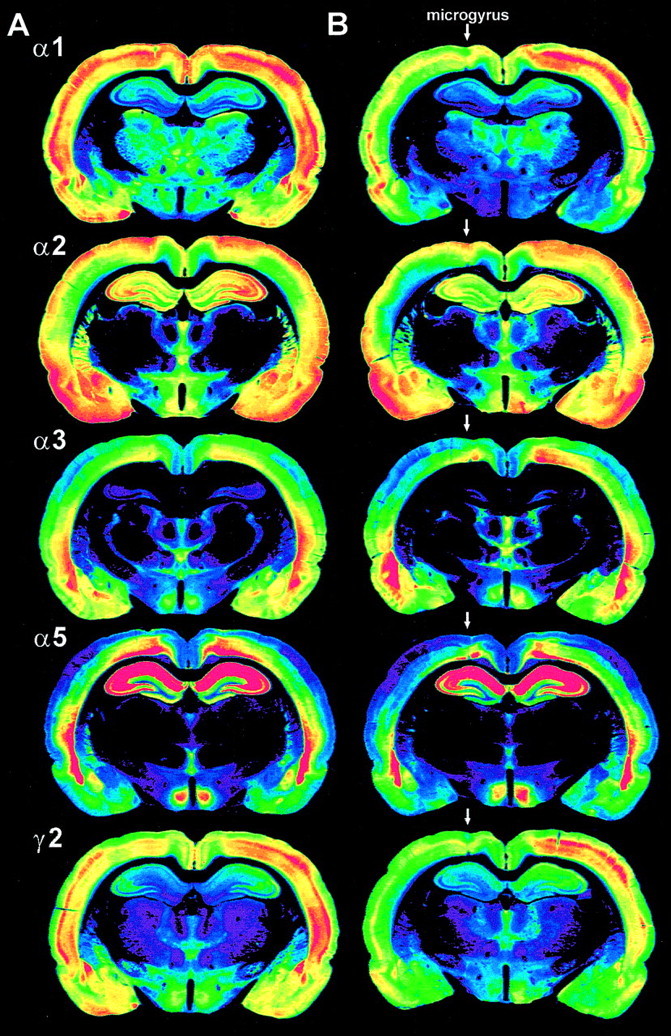
Distribution of GABAA receptor subunits α1, α2, α3, α5, and γ2 in sham-operated (A) and freeze-lesioned (B) rats. Color-coded images from immunohistochemically processed sections. For each subunit, the optical density of the immunoreactivity product was color-coded using a standard 256-level scale, ranging fromblack for background to violet,blue, green, yellow, andred for the most intense signals. Sham-operated rats (A) show the typical distribution pattern of subunits α1, α2, α3, α5, and γ2 with symmetric intensities on both hemispheres. Animals with freeze-lesion-induced cortical malformations (B, microgyrus marked with anarrow) display widespread reduction in immunoreactivity for all subunits, most prominently for subunits α1 and γ2, involving the area of the dysplastic cortex, but also surrounding neocortical areas and the ipsilateral hippocampal formation (see Fig.6).
In all animals with freeze-lesion-induced cortical malformations, the distribution pattern of GABAA receptor subunits was different compared with sham-operated controls (Fig. 2). Although the extent of changes in subunit distribution varied to some degree, a consistent pattern of alterations was observed in all freeze-lesioned animals. In the dysplastic cortex, qualitative and semiquantitative evaluation of the sections revealed a remarkable decrease in staining for all receptor subunits, with exception of subunit α2 (Figs. 2,3). Within the lesion, this downregulation was most prominent for subunits γ2 (−20%,p < 0.001) and α5 (−29%, p < 0.05), and less clear for subunits α1 (−11%, not significant) and α3 (−14%, not significant). In contrast, immunoreactivity of subunit α2 was slightly increased in the microgyrus. This increase was attributable to the infolding of superficial cortical layers that formed the microgyrus and also showed an intense staining in the surrounding cortex. Alterations in GABAA receptor subunit distribution also involved widespread neocortical areas surrounding the dysplastic cortex. In the adjacent frontal cortex, a strong decrease in immunoreactivity was observed for all receptor subunits. Interestingly, the degree of this decrease was more pronounced in the structurally mostly intact frontal cortex than in the dysplastic lesion itself (Fig. 4), showing the most prominent decrease in immunoreactivity for subunit α5 (−37%, p < 0.05) but also a clear decrease in staining for subunits α1 (−12%, p < 0.05), α2 (−24%, p < 0.001), α3 (−22%, p< 0.05), and γ2 (−23%, p < 0.05). In neocortical regions lateral to the microgyrus, in the Par1/Par2, the pattern of alterations was different compared with the lesion and medially adjacent areas. Whereas subunits α1, α2, α5, and γ2 showed a differentially intense reduction in immunoreactivity in these areas, subunit α3 revealed no consistent changes (Fig. 4). In these areas, the most prominent decrease was observed for subunits γ2 (Par1, −13%, p < 0.05; Par2, −13%, p < 0.05) and α5 (Par1, −13%, not significant; Par2, −20%,p < 0.05). In most animals, the alterations in subunit distribution did also involve the contralateral frontal cortex in which a decrease in immunoreactivity was found for all subunits that was slightly less pronounced than in ipsilateral frontal cortex (subunit α1, −13%, p < 0.05; subunit α2, −15%,p < 0.05; subunit α3, −15%, not significant; subunit α3, −31%, p < 0.05; subunit γ2, −21%,p < 0.05). Furthermore, in some animals, a mild reduction in staining was also observed in the contralateral area homotopic to the lesion, as well as in the contralateral somatosensory cortex, but these effects were neither as consistent nor as prominent as the changes found ipsilaterally.
Fig. 3.

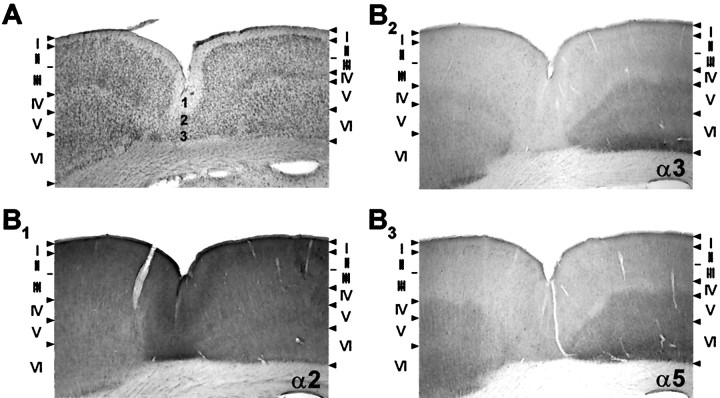
Comparison of neocortical distribution of GABAA receptor subunits α1, α2, α3, α5, and γ2 in sham-operated and freeze-lesioned rats. A, Photomicrographs of coronal sections at low magnification showing a freeze-lesion-induced cortical malformation (microgyrus marked with anarrow) and corresponding sections from a sham-operated animal. Animals with cortical malformations show a decreased immunoreactivity in the lesioned area and in adjacent neocortical areas, whereas the characteristic laminar distribution pattern of the different subunits is conserved. The reduction in staining intensity is most prominent for subunits α1 and γ2, moderate for subunits α2 and α5, and only mild for subunit α3. B, Higher magnification of the cortical malformation (framein A) showing the laminar distribution of GABAA receptor subunits within the lesion. Note the differential immunoreactivity for subunits α3 and α5 on the lateral and medial wall of the microgyrus.
In the area of the cortical malformation, the laminar distribution of GABAA receptor subunits was conserved, showing consistent similarities with adjacent structurally intact neocortex (Figs. 3, 5). The most superficial layer of the three- to four-layered microgyrus (Fig. 5A,layer 1) displayed the same distribution of GABAA receptor subunits compared with the corresponding layer I of the surrounding cortex, and the second and third layer of the dysplastic cortex (Fig. 5A, layers 2, 3) revealed very similar immunoreactivity as found in layers II–III of adjacent neocortical areas (Fig. 5). In addition, regional selectivity in distribution of certain GABAA receptor subunits was also conserved in the dysplastic cortex. In animals in which the microgyrus was located at the border between the sensorimotor hindlimb and motor frontal cortex (n = 3) (Fig. 3), the medial and lateral wall of the microgyrus exhibited a differential immunoreactivity of GABAA receptor subunits. In particular, the staining of subunits α3 and α5 showed a clear difference in staining intensity between the lateral and medial wall of the microgyrus corresponding to layers II–III of adjacent frontal cortex for the medial part and to layers II–III of hindlimb cortex on the lateral part of the microgyrus (Figs. 3, 5B).
Fig. 5.
Laminar distribution of GABAA receptor subunits α2, α3, and α5 within the cortical malformation compared with the underlying histology. A, Freeze-lesion-induced microgyrus showing a deep infolding of the cortex with an underlying three-layered cortex. B, The microgyrus is lined with an intense staining of subunit α2 reflecting a continuation of the superficial layers I, II, and III of adjacent cortex. Subunits α3 and α5 within the microgyrus also display a very similar immunoreactivity within the microgyrus compared with superficial layers of surrounding neocortical areas.
Alterations in distribution of GABAAreceptor subunits in animals with freeze-lesion-induced cortical malformations were not restricted to the neocortex but also involved the ipsilateral hippocampal formation (Figs. 2, 3,6). Qualitative and semiquantitative evaluation of the ipsilateral hippocampal formation revealed a clear reduction in immunoreactivity for subunits α1 (−28%,p < 0.05), α2 (−12%, p < 0.05), α5 (−12%, p < 0.05), and most strikingly for subunit γ2 (−39%, p < 0.05). This decrease in GABAA receptor subunit immunoreactivity appeared to be pronounced in the CA1 region, as well as the dentate gyrus, but was less marked in the regions CA2 and CA3 (Fig. 6B). Subunit α3 also showed some decrease in staining in the hippocampal region, but the weak expression of this subunit in control and freeze-lesioned animals resulted in a poor signal-to-noise-ratio, which did not allow a reliable assessment of changes in this area. Within the thalamus and the contralateral hippocampal formation, no consistent alteration of GABAA receptor subunit immunoreactivity was found.
Fig. 6.

Alterations in the distribution of GABAA receptor subunits α1, α2, α5, and γ2 within the ipsilateral hippocampal formation in animals with focal cortical malformations. A, The relative differences of staining intensities measured as optical densities in sections from animals with microgyri compared with sham-operated animals. Significant differences (p < 0.05) are indicated byasterisks. B, Color-coded images of the hippocampal formation from an animal with a freeze-lesion-induced microgyrus and a sham-operated control using a standard 256-level scale, ranging from black for background toviolet, blue, green,yellow, and red for the most intense signals. Note the prominent downregulation for subunits α1 and γ2.
DISCUSSION
The present study clearly demonstrates that experimentally induced cortical malformations induce a widespread dysregulation in the distribution of GABAA receptor subunits in adult animals. These alterations comprise a regionally differential reduction of GABAA receptor subunits in the dysplastic cortex but also in extended structurally intact brain regions, involving adjacent and remote neocortical areas of the ipsilateral hemisphere and surprisingly also the ipsilateral hippocampus and the contralateral frontal neocortex. This pattern of changes points toward a considerable modulation of GABAA receptor subunit composition, and taking into account that five major subunits were investigated, these changes might also allude to an absolute reduction of GABAA receptors.
Topography of alterations in GABAA receptor subunit distribution
Evidence from receptor autoradiographic studies indicated that binding of the GABA analog muscimol, which selectively binds to GABAA receptors, is reduced in the dysplastic cortex and widespread surrounding and remote brain regions (Zilles et al., 1998). Reduction in binding to GABAAreceptors can be interpreted as decreases in density and/or changes in affinity of the receptor. The prominent downregulation of neocortically most abundant GABAA receptor subunits α1 and γ2 described here strongly points toward a reduction of receptor density. However, subunits α1, α2, α3, and α5 showed a regionally differential downregulation, indicating changes in subunit composition that are likely to alter the affinity of the receptor.
Interestingly, alterations in distribution of GABAA receptor subunits were not restricted to the ipsilateral hemisphere but also involve remote brain regions, including the contralateral frontal cortex and the ipsilateral hippocampal formation. These findings indicate widespread disturbances of the neocortical and hippocampal network. Similar remote alterations of GABAA receptor function have been reported after acute focal cortical lesions in adult rat brain using receptor autoradiographic techniques (Witte et al., 1997; Qu et al., 1998; Que et al., 1999).
The pattern of GABAA receptor subunit distribution found within the microgyrus, especially the finding that the lateral and medial wall of the microgyrus, when located between two distinct cortical regions showed a differential distribution of subunits, is in agreement with the hypothesis of microgyrus development proposed by Zilles et al. (1998), which is schematically illustrated in Figure 7. Initially, neonatal freeze-lesions induce a focal destruction of all layers of the developing cortex present at the cortical surface at day of birth. During the first postlesional days, migration of layer II–III neurons into adjacent parts of intact cortex continues and replaces, together with layer I and the pial surface, the lesioned area by tangential expansion (Suzuki and Choi, 1991). Layer 2 of the microgyrus therefore results from tangential growth of adjacent layers II–III.
Fig. 7.

Schematic illustration of the development of a freeze-lesion-induced microgyrus (modified from Zilles et al., 1998). Cytoarchitectonical layers of the adjacent neocortex are specified withroman numerals and with arabic numeralswithin the dysplastic cortex. Arrows indicate the direction of migration of layer II–III neurons during the first postnatal days. For details, see Discussion.
Functional consequences of alterations in GABAAreceptor subunit distribution
In contrast to the widespread changes in GABAA receptor distribution and binding, electrophysiological investigations did not disclose a general impairment of GABAergic inhibition. In intracellular recordings, the conductance of stimulus-evoked polysynaptic GABAA-mediated IPSPs was reduced in layer II–III neurons close to the microgyrus (Luhmann et al., 1998b). However, pharmacologically isolated monosynaptic GABAA-mediated IPSPs were similar in dysplastic and control cortex in this study. More laterally in the paramicrogyral cortex, layer V neurons revealed evoked and spontaneous IPSCs with significant larger amplitudes (Prince et al., 1997;Jacobs and Prince, 1999). These changes of inhibitory function were interpreted as alterations in excitatory innervation of GABAergic neurons in both studies, causing a decrease or loss of excitatory drive on inhibitory interneurons close to the microgyrus and an increase of these inputs in the paramicrogyral zone. However, these studies concentrated on two different areas and cortical laminae. The prominent downregulation of GABAA receptor subunits within the cortical malformation might contribute to the decrease in GABAergic function in upper layers close to the microgyrus, but the increase in inhibitory efficacy described in deep layers of the paramicrogyral zone coinciding with a downregulation of GABAA receptor subunits remains elusive. However, evidence for an increase in inhibitory efficacy also comes from studies on ibotenate-induced focal cortical malformations, showing very similar lesions and changes in cortical excitability (Redecker et al., 1998a,b). Ibotenate-induced cortical malformations induce a very similar reduction of GABAA receptor subunit distribution (Redecker et al., 1999), whereasin vitro extracellular recordings using the paired-pulse paradigm as a measure of functional inhibition reveal no significant impairment throughout the ipsilateral neocortex (Hagemann et al., 2000).
The reduction of the neocortically abundant receptor subunits α1 and γ2 in the paramicrogyral zone might represent a compensatory downregulation to account for the increased excitatory drive on GABAergic neurons (Prince et al., 1997; Jacobs and Prince, 1999). Interestingly, also less abundant subunits α2 and α5 were decreased in the paramicrogyral zone, whereas subunit α3 was not altered. This differential downregulation might represent modifications in subunit composition of the remaining receptors changing the function of the receptor and contributing to the increased GABAergic efficacy in this region. This hypothesis is supported by an increase in miniature IPSCs (Jacobs and Prince, 1999), likely reflecting a modification of the GABAA receptor. In addition, pharmacological studies point toward a switch in subunit composition in the paramicrogyral zone, showing a reduced sensitivity to the benzodiazepine receptor agonist zolpidem (DeFazio and Hablitz, 1999). GABAA receptors containing the α1 subunit exhibit a high affinity for zolpidem, whereas expression of subunits α2 or α3 give rise to less sensitive receptors (Luddens et al., 1995). The selective decrease of subunits α1, α2, and α5 indicates that the balance between α subunits is relatively changed in favor of subunit α3, likely decreasing the sensitivity to zolpidem. Because subunit α3 is more abundant during early postnatal development, even eclipsing the expression of subunit α1 (Laurie et al., 1992), this imbalance might reflect a delay in maturation of GABAA receptors.
Additional mechanisms
The underlying events causing these multiple modifications of GABAergic functions still warrant additional studies. Keeping in mind the complex subunit architecture of GABAAreceptors, changes of subunits not analyzed here are likely to be present, making the picture even more complicated. Furthermore, GABAergic transmission is regulated by a variety of additional factors affecting presynaptic GABA release and postsynaptic GABA efficacy. In particular, alterations in GABA synthesis and transport might contribute to an increased efficacy in the paramicrogyral zone. In this context, it has to be mentioned that the release of GABA from inhibitory terminals can be modulated by presynaptic GABAB autoreceptors (Bowery et al., 1980), which can inhibit the GABA release by as much as 40–60% (Davies et al., 1991; Mott et al., 1993; Thompson et al., 1993). Reduction in GABAB receptor binding in the surround of cortical malformations has been demonstrated recently (Zilles et al., 1998), probably contributing in part to the increase in GABAergic efficacy. Furthermore, additional alterations in density and function of GABAergic interneurons have to be considered. In the freeze-lesion model of cortical malformations, a reduction of parvalbumin-positive interneurons was described only temporarily in young animals (Jacobs et al., 1996; Rosen et al., 1998), whereas older animals, such as those used in this study, did not reveal a reduction of parvalbumin or calbindin immunoreactivity (P. Schwarz, C. C. Stichel, H. J. Luhmann, unpublished observations). Furthermore, the function of GABAA receptors is also modified via phosphorylation–dephosphorylation of specific subunits and a variety of endogenous and exogenous modulators, such as benzodiazepines, barbiturates, and neurosteroids, which potentiate the effects of GABA (Puia et al., 1990; Ito et al., 1996; Hevers and Luddens, 1998), as well as polyvalent cations like zinc, which diminishes the GABAergic efficacy (Buhl et al., 1996; Huang, 1997).
Clinical implications
The pattern of alterations in GABAA receptor subunit distribution corresponds well with clinical data on epileptic patients with focal cortical dysgenesis. In these patients, positron emission tomography was performed using11C-flumazenil as a tracer.11C-flumazenil is a neutral antagonist binding to the central benzodiazepine receptor, an allosteric modulatory site depending on the presence of both an α and a γ subunit. Using this method, widespread abnormalities in receptor binding were detected, involving not only the dysplastic area but also the structurally intact surround (Richardson et al., 1996).
The present findings strongly point toward widespread alterations in GABAA receptor subunit distribution. It has been shown recently using single-cell PCR techniques that, in a chronic model of mesial temporal lobe epilepsy, the development of behavioral seizures coincides with a decrease in mRNA for subunits α1 and γ2, no change for subunit α3, and an increase for subunits α4, β3, δ, and ε in dentate gyrus neurons. This switch in subunit composition altered the zinc sensitivity, providing therefore a possible mechanism for the generation of epileptic seizures (Buhl et al., 1996; Brooks-Kayal et al., 1998). A similar mechanism could play a role in this model; changes in GABAA receptor subunit distribution in widespread brain regions might aberrantly sensitize the receptors to endogenous modulators and therefore contribute to epileptogenesis. Alterations in GABAA receptor subunit distribution also have implications for antiepileptic drug therapy in patients with cortical malformations and help to explain the clinical finding that patients with cortical malformations frequently suffer from drug-resistant epilepsies (Palmini et al., 1994; Raymond et al., 1995).
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Grants SFB194/B4 (H.J.L.) and SFB194/B2 (O.W.W.). We thank S. Hamm and D. Steinhoff for excellent technical assistance.
Correspondence should be addressed to Dr. Otto W. Witte, Department of Neurology, Heinrich-Heine-University, Moorenstrasse 5, D-40225 Düsseldorf, Germany. E-mail: witteo@uni-duesseldorf.de.
REFERENCES
- 1.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 2.Bohlhalter S, Weinmann O, Mohler H, Fritschy JM. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. J Neurosci. 1996;16:283–297. doi: 10.1523/JNEUROSCI.16-01-00283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowery NG, Hill DR, Hudson AL, Doble A, Middlemiss DN, Shaw J, Turnbull M. (−)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 5.Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- 6.Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- 7.DeFazio RA, Hablitz JJ. Reduction of zolpidem sensitivity in a freeze lesion model of neocortical dysgenesis. J Neurophysiol. 1999;81:404–407. doi: 10.1152/jn.1999.81.1.404. [DOI] [PubMed] [Google Scholar]
- 8.Dvorak K, Feit J. Migration of neuroblasts through partial necrosis of the cerebral cortex in newborn rats-contribution to the problems of morphological development and developmental period of cerebral microgyria. Histological and autoradiographical study. Acta Neuropathol (Berl) 1977;38:203–212. doi: 10.1007/BF00688066. [DOI] [PubMed] [Google Scholar]
- 9.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 10.Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- 11.Gressens P. Mechanisms of cerebral dysgenesis. Curr Opin Pediatr. 1998;10:556–560. doi: 10.1097/00008480-199810060-00003. [DOI] [PubMed] [Google Scholar]
- 12.Guerrini R, Andermann E, Avoli M, Dobyns WB. Cortical dysplasias, genetics, and epileptogenesis. Adv Neurol. 1999;79:95–121. [PubMed] [Google Scholar]
- 13.Hablitz JJ, DeFazio T. Excitability changes in freeze-induced neocortical microgyria. Epilepsy Res. 1998;32:75–82. doi: 10.1016/s0920-1211(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 14.Hagemann G, Redecker C, Witte OW (2000) Intact functional inhibition in the surround of experimentally induced focal cortical dysplasias in rats. J Neurophysiol, in press. [DOI] [PubMed]
- 15.Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- 16.Huang EP. Metal ions and synaptic transmission: think zinc. Proc Natl Acad Sci USA. 1997;94:13386–13387. doi: 10.1073/pnas.94.25.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito T, Suzuki T, Wellman SE, Ho IK. Pharmacology of barbiturate tolerance/dependence: GABAA receptors and molecular aspects. Life Sci. 1996;59:169–195. doi: 10.1016/0024-3205(96)00199-3. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs KM, Prince DA. Postsynaptic currents in epileptogenic paramicrogyral cortex. Soc Neurosci Abstr. 1999;25:845. [Google Scholar]
- 19.Jacobs KM, Gutnick MJ, Prince DA. Hyperexcitability in a model of cortical maldevelopment. Cereb Cortex. 1996;6:514–523. doi: 10.1093/cercor/6.3.514. [DOI] [PubMed] [Google Scholar]
- 20.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luddens H, Korpi ER, Seeburg PH. GABAA/benzodiazepine receptor heterogeneity: neurophysiological implications. Neuropharmacology. 1995;34:245–254. doi: 10.1016/0028-3908(94)00158-o. [DOI] [PubMed] [Google Scholar]
- 22.Luhmann HJ, Raabe K. Characterization of neuronal migration disorders in neocortical structures expression of epileptiform activity in an animal model. Epilepsy Res. 1996;26:67–74. doi: 10.1016/s0920-1211(96)00041-1. [DOI] [PubMed] [Google Scholar]
- 23.Luhmann HJ, Raabe K, Qu M, Zilles K. Characterization of neuronal migration disorders in neocortical structures: extracellular in vitro recordings. Eur J Neurosci. 1998a;10:3085–3094. doi: 10.1046/j.1460-9568.1998.00311.x. [DOI] [PubMed] [Google Scholar]
- 24.Luhmann HJ, Karpuk N, Qu M, Zilles K. Characterization of neuronal migration disorders in neocortical structures. II. Intracellular in vitro recordings. J Neurophysiol. 1998b;80:92–102. doi: 10.1152/jn.1998.80.1.92. [DOI] [PubMed] [Google Scholar]
- 25.Mott DD, Xie CW, Wilson WA, Swartzwelder HS, Lewis DV. GABAB autoreceptors mediate activity-dependent disinhibition and enhance signal transmission in the dentate gyrus. J Neurophysiol. 1993;69:674–691. doi: 10.1152/jn.1993.69.3.674. [DOI] [PubMed] [Google Scholar]
- 26.Narahashi T. Chemical modulation of sodium channels and GABAA receptor channel. Adv Neurol. 1999;79:457–480. [PubMed] [Google Scholar]
- 27.Neumann-Haefelin T, Staiger JF, Redecker C, Zilles K, Fritschy JM, Mohler H, Witte OW. Immunohistochemical evidence for dysregulation of the GABAergic system ipsilateral to photochemically induced cortical infarcts in rats. Neuroscience. 1998;87:871–879. doi: 10.1016/s0306-4522(98)00124-9. [DOI] [PubMed] [Google Scholar]
- 28.Olsen RW, DeLorey TM, Gordey M, Kang MH. GABA receptor function and epilepsy. Adv Neurol. 1999;79:499–510. [PubMed] [Google Scholar]
- 29.Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y. Focal neuronal migration disorders and intractable partial epilepsy: results of surgical treatment. Ann Neurol. 1991;30:750–757. doi: 10.1002/ana.410300603. [DOI] [PubMed] [Google Scholar]
- 30.Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, Tampieri D, Robitaille Y, Paglioli E, Paglioli Neto E, Coutinho L, Kim HI. Operative strategies for patients with cortical dysplastic lesions and intractable epilepsy. Epilepsia. 1994;6 [Suppl 35]:S57–S71. doi: 10.1111/j.1528-1157.1994.tb05989.x. [DOI] [PubMed] [Google Scholar]
- 31.Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, Tampieri D, Gloor P, Quesney F, Andermann E, Paglioli E, Paglioli Neto E, Coutinho L, Leblanc R, Kim HI. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476–487. doi: 10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- 32.Prince DA, Jacobs KM, Salin PA, Hoffman S, Parada I. Chronic focal neocortical epileptogenesis: does disinhibition play a role. Can J Physiol Pharmacol. 1997;75:500–507. [PubMed] [Google Scholar]
- 33.Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 34.Qu M, Buchkremer-Ratzmann I, Schiene K, Schroeter M, Witte OW, Zilles K. Bihemispheric reduction of GABAA receptor binding following focal cortical photothrombotic lesions in the rat brain. Brain Res. 1998;813:374–380. doi: 10.1016/s0006-8993(98)01063-4. [DOI] [PubMed] [Google Scholar]
- 35.Que M, Witte OW, Neumann-Haefelin T, Schiene K, Schroeter M, Zilles K. Changes in GABA(A) and GABA(B) receptor binding following cortical photothrombosis: a quantitative receptor autoradiographic study. Neuroscience. 1999;93:1233–1240. doi: 10.1016/s0306-4522(99)00197-9. [DOI] [PubMed] [Google Scholar]
- 36.Raymond AA, Fish DR, Sisodiya SM, Alsanjari N, Stevens JM, Shorvon SD. Abnormalities of gyration, heterotopias, tuberous sclerosis, focal cortical dysplasia, microdysgenesis, dysembryoplastic neuroepithelial tumour and dysgenesis of the archicortex in epilepsy. Clinical, EEG and neuroimaging features in 100 adult patients. Brain. 1995;118:629–660. doi: 10.1093/brain/118.3.629. [DOI] [PubMed] [Google Scholar]
- 37.Redecker C, Lutzenburg M, Gressens P, Evrard P, Witte OW, Hagemann G. Excitability changes and glucose metabolism in experimentally induced focal cortical dysplasias. Cereb Cortex. 1998a;8:623–634. doi: 10.1093/cercor/8.7.623. [DOI] [PubMed] [Google Scholar]
- 38.Redecker C, Hagemann G, Witte OW, Marret S, Evrard P, Gressens P. Long-term evolution of excitotoxic cortical dysgenesis induced in the developing rat brain. Dev Brain Res. 1998b;109:109–113. doi: 10.1016/s0165-3806(98)00065-0. [DOI] [PubMed] [Google Scholar]
- 39.Redecker C, Luhmann HJ, Lutzenburg M, Hagemann G, Fritschy JM, Witte OW. Widespread alterations in distribution of GABAA-receptor-subunits in experimentally induced cortical malformations. Soc Neurosci Abstr. 1999;25:846. [Google Scholar]
- 40.Richardson MP, Koepp MJ, Brooks DJ, Fish DR, Duncan JS. Benzodiazepine receptors in focal epilepsy with cortical dysgenesis: an 11C-flumazenil PET study. Ann Neurol. 1996;40:188–198. doi: 10.1002/ana.410400210. [DOI] [PubMed] [Google Scholar]
- 41.Rosen GD, Press DM, Sherman GF, Galaburda AM. The development of induced cerebrocortical microgyria in the rat. J Neuropathol Exp Neurol. 1992;51:601–611. doi: 10.1097/00005072-199211000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Rosen GD, Jacobs KM, Prince DA. Effects of neonatal freeze lesions on expression of parvalbumin in rat neocortex. Cereb Cortex. 1998;8:753–761. doi: 10.1093/cercor/8.8.753. [DOI] [PubMed] [Google Scholar]
- 43.Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki M, Choi BH. Repair and reconstruction of the cortical plate following closed cryogenic injury to the neonatal rat cerebrum. Acta Neuropathol. 1991;82:93–101. doi: 10.1007/BF00293950. [DOI] [PubMed] [Google Scholar]
- 45.Thompson SM, Capogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- 46.Walsh CA. Genetic malformations of the human cerebral cortex. Neuron. 1999;23:19–29. doi: 10.1016/s0896-6273(00)80749-7. [DOI] [PubMed] [Google Scholar]
- 47.Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann NY Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- 48.Witte OW, Buchkremer-Ratzmann I, Schiene K, Neumann-Haefelin T, Hagemann G, Kraemer M, Zilles K, Freund HJ. Lesion-induced network plasticity in remote brain areas. Trends Neurosci. 1997;20:348–349. [PubMed] [Google Scholar]
- 49.Zilles K. The cortex of the rat. Springer; Berlin: 1992. [Google Scholar]
- 50.Zilles K, Qu M, Schleicher A, Luhmann HJ. Characterization of neuronal migration disorders in neocortical structures: quantitative receptor autoradiography of ionotropic glutamate, GABA(A) and GABA(B) receptors. Eur J Neurosci. 1998;10:3095–3106. doi: 10.1046/j.1460-9568.1998.00322.x. [DOI] [PubMed] [Google Scholar]